Abstract
Over the past decade, a wealth of experimental evidence has accumulated supporting the importance of Fc receptor (FcR) ligation in antibody-mediated pathology and protection in many disease states. Here we present the diverse evidence base that has accumulated as to the importance of antibody effector functions in the setting of HIV prevention and therapy, including clinical correlates, genetic associations, viral evasion strategies, and a rapidly growing number of compelling animal model experiments. Collectively, this work identifies antibody interactions with FcR as important to both therapeutic and prophylactic strategies involving both passive and active immunity. These findings mirror those in other fields as investigators continue to work toward identifying the right antibodies and the right effectors to be present at the right sites at the right time.
Introduction
Over the past several years, there has been significant effort in HIV research to analyze the natural antibody response to infection in order to enable the development of effective strategies for infection prevention and post-exposure eradication. These studies have led to the discovery of a number of highly potent broadly neutralizing antibodies whose effectiveness as potential therapeutics does not derive from virus strain coverage and neutralization potency alone(1). Evidence from natural infection, vaccination and antibody passive transfer studies in humans, non-human primates (NHP), and mouse models have each provided evidence of the critical roles antibody Fc receptors (FcRs) can play in infection prevention and post-exposure therapy. These receptors drive effector functions such as antibody-dependent cellular cytotoxicity (ADCC), virus inhibition (ADCVI), phagocytosis (ADCP), antigen presentation, B-cell activation, complement dependent cytotoxicity (CDC) and dictate antibody half-life and biodistribution in vivo. This review will highlight some of the recent studies performed to elucidate the role that FcRs play in the context of HIV with the goal to inform and promote further exploration of vaccine and therapeutic strategies designed to exploit FcR-dependent mechanisms for improved clinical outcomes.
A landmark passive transfer study in rhesus macaque revealed that engagement of FcγRs contributes to the protective activity of broadly neutralizing antibodies in prevention of HIV infection (1, 2). In humans, FcγR receptors FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa and FcγRIIIb are widely expressed on immune cells including macrophages, neutrophils, natural killer (NK) cells, eosinophils, basophils, and dendritic cells. These cell types have varying receptor expression profiles and engage in different effector functions such as ADCVI or ADCC against cells with viruses entering or budding and phagocytosis of Ab-virus immune complexes or infected cells, among others. Functional relationships between receptors and cell types are complex as differential engagement between activating and inhibitory FcγRs modulates cellular effector function(3). Receptors can also play additional roles in antigen presentation(4), B-cell inhibition and activation(5) and potentially HIV neutralization or infection inhibition on susceptible cells(6, 7).
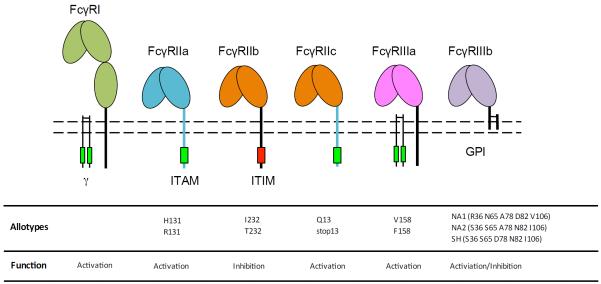
The classical FcγR consist of a family of immunoglobulin superfamily cell surface receptors with specificity to IgG, among which affinity, IgG subclass specificity, expression, signaling mechanisms, and ligation outcomes differ (Figure 1). Briefly, FcγRI is an activating receptor comprising of an α-chain with an intracellular domain which forms a complex with the signal transducing common FcRγ-chain dimer. FcγRIIa is an activating receptor with an intracellular immunoreceptor tyrosine-based activating motif (ITAM). A soluble form of FcγRIIa is found in serum and is either shed from the surface of cells or expressed in a truncated form missing the transmembrane domain secreted from Langerhans cells(8). FcγRIIb is an inhibitory receptor comprising an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM). FcγRIIc is an activating receptor with the extracellular domain identical to FcγRIIb and an intracellular ITAM domain identical to FcγRIIa. FcγRIIIa is an activating receptor reliant on the common FcRγ-chain for signal transduction. FcγRIIIb is exclusively expressed on neutrophils and is tethered to the cell membrane through a glycophosphatidylinositol (GPI) anchor; despite lacking an intracellular domain, FcγRIIIb is capable of triggering release of oxidative species(9), driving phagocytosis(10), as well as impairing FcγRIIa-driven ADCC(11). Soluble FcγRIII is also found in serum by shedding from macrophages, natural killer cells, some T cells and neutrophils, and has been found to increase early in HIV infection and decrease in patients with late stage AIDS(12). Soluble FcγRIII has also been shown to inhibit HIV infection of monocytes/macrophates by blocking CR3(13). The biophysical characteristics of FcγR and their allotypic variants, which will be discussed later, are summarized in Figure 1 (adapted from(14)).
Figure 1.
FcγR structural and functional characteristics.
Collectively, these Fc receptors underlie the landscape of antibody-mediated anti-viral effector activity. Here we describe the experimental evidence base as to the role of FcR in HIV vaccination, infection, progression, and therapy. Beyond classical FcγR, a few recent studies have demonstrated the value of more inclusive examination of the role of diverse FcR in prevention and therapy. We close by discussing recent findings relating the efficacy of antibody-based prevention with interactions with the neonatal Fc receptor (FcRn) responsible for IgG recycling and mucosal delivery; the potential role of IgA and FcαRs in protection from infection, and the growing potential for the use of novel therapeutic strategies used vectored antibody delivery to prevent or treat HIV infection.
Correlates associated with FcR-mediated effector function
Correlates of FcR-mediated effector function have been increasingly prevalent in the HIV literature in recent years, both in the setting of natural infection and vaccine-induced immunity. Collectively, evidence from multiple human vaccine trials and a number of NHP studies have identified a role for ligation of FcR in protection from infection.
Human vaccine trials
The most well-studied example of the importance of FcR function in vaccination is the modestly protective RV144 vaccine trial, for which a binding antibody response against the V1/V2 region of the viral envelope was shown to correlate with reduced risk of infection(15), and modulation in breakthrough virus sequence characteristics(16). Conversely, IgA titer against the virus was shown to correlate with an increased risk of infection; whereas higher levels of ADCC activity in low IgA responders was shown to be associated with lower risk of infection(15). These findings imply either a competitive mechanism of protection: whereby IgA, with different capacity to engage FcRs, can actually block a more productive IgG response(17); or a more broadly qualitative mechanism of protection: whereby qualitative characteristics of the overall humoral response dictate the degree of protection. In both cases, the isotype signals observed point toward a relevance of FcR-mediated activities. These findings were additionally strengthened last year with the finding that envelope-specific IgG3 responses, associated with polyfunctional effector activity, were also a correlate of reduced risk of infection(18, 19).
Additional evidence for the importance of FcR-mediated effector function is provided by several earlier trials. Though non-protective overall, in the Vax004 trial ADCC activity was shown to correlate inversely with infection risk(20). In the related Vax003 trial the envelope specific response was shown to have a strong IgG4 bias, associated with reduced antibody effector function(18). These findings support the RV144 antibody effector quality hypothesis, in that the presence of poorly functional Ig, whether IgA or IgG4, seems to reduce the ability of more functional IgG1/3 responses to provide protection from infection. In a modeling study, a negative impact of HIV-specific IgG2 and IgG4 responses could be observed in predictions of ADCP and ADCC activities(21), and this hypothesis was further strengthened by depletion studies in which removal of the IgG4 fraction caused an increase in the effector activity of the remaining antibody pool(18).
Nonhuman primate studies
Several non-human primate vaccine regimens with varying levels of protective efficacy have suggested that Fc-mediated effector function plays a role in either reduced risk of infection or lower viral load post infection (summarized in (22)). These studies have evaluated the effect of FcR-dependent antibody function against whole envelope, mosaic envelope and specific envelope conformations such as the CD4-induced state. In addition, these outcomes have been shown both in the context of subunit protein and adenovirus vector derived antibody responses against both SIV and SHIV.
Antibody effector correlates such as ADCC have long been associated with reduced viremia in the rhesus macaque model(23). In this study an adenoviral vector prime was followed by a homologous boost with SIV gp120 protein. While the authors were unable to demonstrate protection against the homologous SIVmac239 challenge strain, they showed strong ADCC responses, which correlated with reduced acute viral load. A more recent trial with an adenoviral vector expressed mosaic Env/Gag/Pol antigen showed significant reduction in per-exposure acquisition risk upon multiple rectal challenges against a similar neutralization resistant challenge model (SHIV-SF162-P3)(24). In this case, the protective response was entirely against the Env portion of the antigen, as the challenge strain contained SIV Gag/Pol with low homology to the HIV antigens in the mosaic being tested. A number of neutralizing and non-neutralizing Ab measurements correlated with protection, including binding titer, neutralizing titer, ADCP and complement deposition. A further interesting study was published this year using CD4-induced gp120 as an immunogen in rhesus macaques, in which protection correlated with antibody titer and ADCC activity, but not neutralization, and was inversely correlated with high vaccine elicited T-cell responses(25). This antigen induced primarily non-neutralizing but ADCC-capable antibodies, which showed significant protection against multiple low dose SHIV and SIV challenges. Addition of tetanus toxoid to the vaccination regime increased T-cell responses but decreased the level of protection seen. This result is hypothesized to occur similarly to the increase in infectivity found in the Step trials, where HIV-specific T-cell responses are thought to have increased susceptibility to infection(26). Intriguingly, a different protective vaccine study in rhesus macaques suggested that protection may have been due to inhibited recruitment of CD4+ T cells to the site of infection based on immune complex binding to the inhibitory FcγRIIb (27). It will be interesting to determine whether similar functional assessments in new or existing vaccine efficacy studies with established antibody correlates(28, 29) might complement and reinforce these associations with antibody functions.
Passive transfer studies
NHP models
Many passive transfer studies, in which neutralizing antibodies were administered intravenously in advance of viral challenge, have conclusively demonstrated that antibodies can protect from HIV infection. In a landmark study aimed at investigating the mechanism of this protection, the neutralizing antibody b12 was mutated to ablate interactions with FcR and or/complement. Despite equivalent neutralization potency and biodistribution, the silencing of antibody effector function caused half of the protective capacity to be lost(1). While a great deal of clinical data from human and NHP vaccine studies and from natural infection cohorts supports the significance of antibody effector function in impacting risk of infection, viral load, and progression(30), and points to the promise of rational optimization of effector function(14), an initial attempt to explore enhancing protection via differential binding to FcγR receptors proved unsuccessful. Passive transfer of a nonfucosylated broadly neutralizing antibody with enhanced binding to FcγRIIIa on NK cells did not augment protection from vaginal challenge(31). While many theories as to the lack of enhanced efficacy have been proposed, later studies have begun to point to a limited role of NK cells in controlling HIV transmission and containment during acute infection in the mucosa, in favor or other effector cell types that may possess greater potential to impact mucosal transmission.
Following up on these studies, several attempts have been made to evaluate the ability of passively transferred antibodies to provide protection, with varying success. Highly functional, but non-neutralizing antibodies isolated from human HIV controllers were unable to protect macaques against high dose SHIV challenge(32). This experiment highlights some of the difficulties in achieving effective protection with passive transfer of human antibodies to macaques: no human antibody was found at the site of challenge (vaginal mucosa), and the authors were only able to transfer a single dose at a concentration estimated to be less than half that necessary for b12 to provide protection in this model. In another study SHIVIG, a polyclonal antibody mixture isolated from macaques with high neutralizing titers, was evaluated for protection against low-dose intra-rectal challenge. While lower viral titers were observed at peak viremia for the high IgG dose, a greater number of transmitted founder viruses were observed at lower doses, suggesting a potential danger of antibody dependent enhancement(33), which is also dependent on FcR interactions. In contrast, vaginal application of a non-neutralizing cocktail of 246-D and 4B3 with potent Fc-mediated in vitro virus inhibition did not confer protection from a vaginal SHIV challenge in rhesus macaques but did display a reduction plasma viral load likely through FcγR-mediated effector function (34).
In other recent studies, passively transferred antibodies have exceeded expectations in remarkable ways. For example a cocktail of monoclonal neutralizing antibodies or even the single highly neutralizing monoclonal antibody PGT121 showed significant ability to suppress viremia in infected macaques(35), reinvigorating the notion that antibodies might be therapeutically useful. Similarly, an IgG1 Fc-mutant with increased FcRn affinity was shown to enhance protection against SHIV intra-rectal challenge(36), bolstering the possibility of using passively transferred antibodies as prevention. While FcRn binding does not directly enhance effector function it was shown to increase serum half-life threefold and enhance distribution to and persistence of antibody at the rectal mucosa. These recent findings highlight the diverse routes whereby FcR-mediated responses could help enhance antibody-based protection and therapy.
Mouse models
The ability to increase protective efficacy by enhancing affinity to activating FcγRs has been compellingly demonstrated in vivo in protection using a humanized FcγR mouse model for HIV(37). This mouse model exploits FcγR chimeras consisting of human extracellular domains and mouse intracellular signaling domains(38). Conveniently the mice appear to recapitulate human FcγR structural features, cellular expression patterns and effector functions. Using this model, the finding that neutralization alone is insufficient for the in vivo protection achieved by neutralizing antibodies has been generalized across many of the most potent and broad neutralizing antibodies(37). Further, a broadly neutralizing Fc point mutant with increased affinity to activating FcγR displayed enhanced protection over wild type IgG.
Similarly, this model has been used to study the role of antibody-FcR interactions in the setting of antiviral antibody therapy. Here, antibodies were found to have strong potential in driving viral clearance based on their Fc domain and FcR-dependent ability to delay viral rebound following latent virus reactivation(39). In a subsequent study, this effect on delaying viral rebound was even more definitively linked to effective FcγR ligation via use of Fc domain point mutants with alternatively enhanced or ablated FcγR binding(37). These studies highlight that differential FcγR engagement may be a key factor in therapeutic efficacy based on impacting effector function. Such studies potentially offer a tractable means to decipher the FcγR and cell types involved in HIV protection and elimination.
Human passive transfer studies
The setting of mother to child HIV transmission presents a passive transfer experiment conducted naturally, in which IgA and IgG antibodies are delivered through placental transport prior to birth and via ingestion of breast milk after birth. Although early studies did not find an association between infant or maternal ADCC and infection risk(40, 41), better clinical status and delayed progression was sometimes observed(42, 43). Two more recent studies that assessed ADCC activity in the setting of maternal to child transmission have found associations with reduced viral transmission or mortality. In the first(44), a trend toward increased ADCC activity among uninfected versus infected infants was observed, whereas no difference in IgG1, IgG3, or neutralization activities was observed. Survival was strongly impacted by ADCC activity, and an inverse trend between ADCC and set point viral load was noted. In another study, breast milk supernatant ADCC activity, but not HIV envelope-specific IgG titers were found to correlate inversely with transmission(45).
Rapid growth in our understanding of the role that passively transferred antibodies could play in HIV therapy or eradication is expected over the next few years on the heels of the discovery of more and more broad and potently neutralizing antibodies and results from pre-clinical models discussed above. Early studies with first generation neutralizing antibodies demonstrated that viral rebound could be delayed after treatment cessation (46), and could exert selective pressure on the sequence of circulating virus (47), but that antibodies did not appear to be strong candidates to supplant small molecule therapies. The more impressive suppression results observed recently in NHP and mouse models have triggered re-evaluation of the utility of antibodies in treatment.
In the first major experimental human passive transfer study to be published in many years, the broadly neutralizing antibody 3BNC117 was evaluated in HIV infected and uninfected subjects to determine pharmacokinetics, safety, and impact on viral load(48). Among HIV+ individuals, a mean decrease in viral load of 1.5 log10 was observed. The impact of another antibody, KD-247, on viral RNA load was also recently reported, demonstrating an ability to reduce viral RNA by up to 1 log, with long-term viral suppression observed in one subject(49). These results, and the many broad and potent neutralizing antibodies now available for evaluation, point toward the possibility for use in combination in treatment and prevention strategies.
Findings in natural infection
A number of studies point toward the relevance of antibody interactions with FcγR in relation to disease progression (summarized in(30)). Briefly, in both humans and NHP, post-infection ADCC/ADCVI activity has been associated with viremic control and/or rate of disease progression(50–52), and conversely impaired ADCC and ADCP have been associated with progression(53, 54). In natural infection, Fc and Fab mediated HIV specific antibody activities appear to evolve independently(55), and in vivo, natural FcγR affinity variants are generated by IgG subclass switching between IgG1, IgG2, IgG3, and IgG4 as well as the tuning the Fc glycan structures. Recent studies among infected subjects have indicated these subclass, glycan, and FcγR-binding features can all impact the effector activity of HIV-specific antibodies (56–59), and further support the potential value of Fc tuning by antigenic cues in providing a new handle for modulating effector function by vaccination or in antibody therapy.
Associations with Genetic Variation of FcγR
Across the human population, a number of FcγR genetic variants, including both copy number variants (CNV) as well as single nucleotide polymorphic variants (SNP) exist; this genetic diversity has been associated with functional consequences ranging from different cell surface expression levels, differing signaling capacity, to differential binding affinities to IgGs. Numerous relationships have been described relating FcγR CNV and SNP variation in diverse clinical cohorts in the setting of monoclonal antibody therapy(60), autoimmunity(61), and susceptibility to and resulting outcomes from diverse infections(62–66). A summary of FcγR alleles and their associations in HIV infection, vaccination and disease progression are summarized in Table 1.
Table 1. FcγRIIa and FcγRIIIa alleles and their associations in HIV infection, vaccination and disease progression.
NE represents allele combinations that were not evaluated.
| Observation | HH | HR | RR | VV | VF | FF | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|
| Decline in proportion of subjects with CD4+ cells >=200/mm2 over time | − | − | + | − | − | − | RR faster decline | (67) |
| Decline in proportion of subjects without AIDS over time | − | − | + | − | − | − | RR faster decline | |
| Risk of P. jiroveci pneumonia as an AIDS-defining illness | + | − | − | − | − | − | HH higher risk, R lower risk | |
| HIV viral RNA/ml setpoint at ~18 mo | − | − | − | NE | NE | NE | No association | |
| Monocyte internalization of HIV immune complexes | +++ | ++ | + | NE | NE | NE | HH highest internalization | |
| Risk of Kaposi's sarcoma | − | − | − | + | − | − | VV higher risk | |
| Risk of perinatal HIV-1 transmission based on infant allotype | + | − | (+) | NE | NE | NE | HH higher risk, RR lower risk | (68) |
| Risk of perinatal HIV-1 transmission based on maternal allotype | − | − | − | NE | NE | NE | No association | |
| Lower HIV replication post vaccination | + | + | − | NE | NE | NE | HH or H/R smaller increase | (69) |
| Increased risk of infection | − | − | − | + | + | − | V increased risk | (70) |
| Risk of disease progression | − | − | + | + | − | + | VV and RR/FF increased risk | |
| Rate of infection for placebo recipients (Vax004) | − | − | − | − | − | − | No association | (71) |
| Rate of infection for low risk vaccine recipients (Vax004) | − | − | − | + | − | − | VV increased rate | |
| Risk of infection for vaccine trial participants (Step) | − | − | − | − | − | − | No association | (72) |
| Risk of infection (RV144) | − | − | − | − | − | − | No association | (73) |
FcγRII
While many studies have identified associations, the significance and possible mechanisms of these genetic variations on outcomes in HIV infection, disease progression, and vaccine responses remains unclear (recently reviewed in (74)). For example, among the two known allotypes of FcγRIIa (H131, R131), the H131 allotype has higher affinity to IgG, particularly IgG2(75), and these allotypes have been implicated as factors in virus acquisition, disease progression and susceptibility to AIDS related infections. However, these associations are often somewhat contradictory and appear variable across different subject cohorts. For example HIV-infected men with the RR genotype had a faster rate of decline in CD4+ T cells than HR or HH subjects, implying that enhanced IgG recognition may be protective(67). However, in the same study, a statistically significant difference in progression to AIDS in HH subjects was not observed, as HH subjects were more likely to be diagnosed with AIDS based on Pneumocystis jiroveci pneumonia, despite being less likely to be diagnosed with P. jiroveci subsequent to AIDS diagnosis. In contrast to this protective association with disease progression in adults, the HH genotype has been associated with increased perinatal HIV transmission(68). These apparent discrepancies are not easily resolved, and may arise due to the multiple and interacting factors that may impact clinical outcomes. For example, there are known associations between FcR genotype with different levels responses across IgG subclasses. These subclass differences could then reinforce or undermine relationships between FcR genotype and effector cell activity. Evidence of these types of interactions can be found in a small therapeutic vaccine study, in which subjects with both the HH or HR genotype better able to recognize IgG2 and antigen-specific IgG2 production exhibited enhanced HIV control(69).
These examples highlight that interpreting associations observed between FcγR allotypes and HIV outcomes can be perplexing. As a challenge in particular to the antibody-centric mechanisms presumed to be marked by genetic FcγR associations, a recent comprehensive phenotypic analysis of 78,000 traits in 669 female twins revealed that FcγRII polymorphisms are associated with numerous canonical traits on major immune cell subsets including T cells, B cells and dendritic cells(76). Associations were observed for CD27 expression on IgA+ B-cells, IgG+ B-cells, CD4+ T-cells and CD8+ T-cells, CD161 expression on CD4+ T cells, expression of FcγRII on CD1c+ myeloid dendritic cells (mDC) and inflammatory myeloid dendritic cells (iMDC). Interestingly, CD27 is capable of inducing apoptosis(77) and high expression is associated with long-term survival of CD8+ T cells in HIV-infected patients(78) while a loss of CD27+ B cells is associated with HIV infection and inversely correlated with CD70+ (CD27 ligand) T cells(79). Additionally, CD161+ CD4+ T cells are significantly reduce during HIV infection and have recently been identified as a precursor to gut homing Th17 T cells which are profoundly depleted potentially leading to overall impairment of mucosal immunity during infection(80). Evidently, FcγRIIa allotypes have profound associations beyond their impact on IgG subclass specificity and further work must be performed to elucidate the mechanisms behind the associations with T cell, B cells and dendritic cells and the effect on infection susceptibility, disease progression, and outcomes of vaccination.
FcγRIIb has no known allotypic variation in the extracellular domain, however it does have two known allotypes, I232 or T232, in the transmembrane helix. The T232 allotype is unable to activate intracellular inhibitory receptors due to T232 exclusion from sphingolipid rafts(81). The resulting lack of B cell receptor signal inhibition leads to unopposed pro-inflammatory signaling linked to systemic lupus erythematosus (SLE)(82). Interestingly, autoantigens associated with SLE have been identified to have sequence homology with HIV retroviral machinery such as gag and/or env as well as confirmed antibody cross-reactivity(83). Additionally, a broadly neutralizing antibody was isolated from an SLE/HIV patient was cross-reactive with both SLE associated dsDNA and the CD4 binding site of gp120(84), resulting in increasing interest in understanding how host tolerance mechanisms may impact on bnAb development(85). As more patients are identified that have concomitant autoimmunity and HIV it would be interesting to genotype FcγRIIb allotypes to parse any associations with disease susceptibility and progression.
In 20% of healthy individuals(86, 87), FcγRIIc is expressed on NK cells and B cells due to an open reading frame (ORF) allele. On NK cells it is capable of eliciting ADCC independent of FcγRIIIa(86) and on B cells it can enhance B cell activation(5). Additionally a nonclassical ORF allele in FcγRIIc drives expression of full length FcgRIIb on NK cells, and is capable of inhibiting FcγRIIIa driven ADCC(87). Intriguingly, an FcγRIIc SNP resulting in a premature stop codon was recently associated with reduced risk of infection in the RV144 vaccine trial where efficacy varied from an estimated 15% to 91% across polymorphic vaccinees(73). Given the limited information about FcγRIIc expression and activity, the functional association of this SNP remains undetermined.
FcγRIII
FcγRIIIa has two allotypes, V158 or F158, where V158 has higher affinity to all IgG subclasses than F158(88). Like FcγRIIa, clinical associations of the FcγRIIIa allotypes in the context of HIV are not entirely clear. One study concluded that V/F genotypes were not associated with differences in HIV progression(67); however another study indicated the V and VV alleles were risk factors for both HIV infection and progression, respectively(70). This result is also consistent with the Vax004 trial, in which subjects with the VV allele had a higher risk of HIV infection than subjects with either VF or FF alleles(71). In contrast, in the Step trial, no FcγRIIIa allotype alone impacted likelihood of infection(72). Collectively, these findings are somewhat surprising, and counter to run the simplest expectations one might form based on studies in antibody therapy of numerous cancer types where the high affinity V allele is associated with better outcomes.
FcγRIIIb has three known allotypes NA1, NA2 and SH where allotypic differences in the population have been correlated to autoimmunity such as SLE(89) or suseptibility to pathogenic infections such as malaria(9). Few studies have evaluated FcγRIIIb allotypes in the context of HIV, however one study indicated that a CNV allele in FcγRIIIb had an effect where a deletion was more frequent in HIV-tuberculosis co-infection(90). Despite FcγRIIIb's ability to modulate neutrophil effector function, sufficient evidence has not accumulated to define clear roles for FcγRIIIb in HIV protection or infection.
Overall, the variation in associations observed in these studies highlight the context dependence of allelic effects across different populations, different age groups, and different disease states. Different FcγR genotypes may be optimal pre-infection, post-infection, or in the setting of vaccination based on their widespread and pleiotropic effects. The impact of FcγR genotype on multiple key immune phenotypes, including variance in their ability to interact with acute phase proteins, and their linkage disequilibrium confound analysis and interpretation. Clearly, caution must be taken when attempting to glean generalizable trends, particularly from small studies. Overall, there appears to be limited evidence to support simple interpretations of these associations as directly mechanistically associated with variation in effector activity due to differential interactions with IgG. Hopefully, as more human passive transfer experiments are conducted, the role of FcγR allotypic variation in modulating at least antibody-mediated protection will become clearer.
Evidence of virologic evasion
Evidence as to the importance of FcR-mediated anti-viral activities can be found in viral evasion strategies. Beyond the well-known antigenic variation strategies HIV employs based on its low fidelity polymerase, which serves to remove the link between Ab and virus upstream of Fc-dependent activities, several viral genes have been characterized as having roles in modulating downstream antibody-FcR biology. For example, HIV negative factor (Nef) has been reported to influence class switching in trans in B cells, resulting in inhibition of class switching to IgA or IgG (91, 92).
Similarly, the Viral protein U (Vpu) is thought to act as a virulence factor by interfering with the host response to viral infection in a number of ways. Recently, Vpu was shown to directly interact with and antagonize the interferon-induced host protein tetherin (BST-2)(93)), whose native function is to inhibit release of enveloped viruses by securing budding virions to the cell surface. When tetherin expression was knocked down via RNAi, infected cells were resistant to ADCC(94). Conversely, when tetherin and CD4 expression were increased via interferon treatment, cells were sensitized to ADCC. Perhaps most compellingly, when specific mutations were made in Vpu that impaired the interaction with tetherin, cells were likewise sensitized to antibody-mediated cytolysis. A final aspect of immune evasion by Vpu may be found in its role in combination with Nef in downregulating CD4, which has been found to increase exposure of CD4-induced conformations of the HIV envelope protein, thought by some to be particularly relevant to ADCC(95, 96). Support for this notion was recently found in the ability of small molecule CD4 mimetics to also sensitize HIV-infected cells to anti-viral antibodies(97).
Lastly, HIV possesses extensive strategies for the evasion of complement-mediated lysis, while at the same time maintaining recognition by numerous complement receptors and several other lectins that may facilitate establishment of reservoirs of viral particles (summarized in(98, 99)). A long-standing body of literature describes modulation of lysis by means of complement regulatory factors including Decay Accelerating Factor (CD55) and membrane inhibitor of reactive lysis (MIRL, CD59)(100), and interactions with complement factor H (fH) in serum(101). More recent work has identified a role of complement opsonization in reducing antiviral inflammatory responses in immature dendritic cells(102). It is interesting to consider the possibility that the lack of dependence of b12-mediated protection from infection on complement may be partially dependent on factors such as these.
The role of viral recognition in antibody activity
Diverse envelope protein epitopes have been identified that permit ADCC including sites on gp41 such as the membrane proximal external region (MPER) or sites on gp120 such as glycosylation sites, the CD4 binding site, the V2/V3 loops and CD4-induced epitopes (CD4i) capable of binding envelope upon a conformational change due to gp120 binding to CD4 (recently reviewed(103)). However, the ability for an antibody to bind an epitope alone does not alone gaurantee efficient engagement of FcRs. Recent studies in HIV and other infectious diseases as well as oncology have revealed the interplay between epitope location and structure, the valency of antibody epitope binding, Fc orientation and accessibility towards FcR as well as IgG avidity (104) and resulting immune complex size(105) that can dramatically impact FcR mediated effector function.
CD4i epitopes are of partcular interest in HIV given the antibodies they produce are prevalent in patients undergoing HAART treatment during acute infection (106), dominant determinants of serum-mediated ADCC in chronically infected individuals(96) as well as highly prevalent among RV144 vaccinees(107), highlighting the strong immunogenicity of CD4i complexes, their involvement as an effective ADCC mediator and their potential value as vaccine immunogens. A recent study comparing two CD4i antibodies has raised additional considerations for epitope targeting and immunogen design where antibodies 2.2c and N5-i5 had similar monovalent affinities however their bivalent affinities towards antigen on the target cell surface differed 18-fold due to differential ability to crosslink antigen. Additionally, a VH-VL domain swap which reoriented the antibody Fc domain altered ADCC activity by an order of magnitude (108). Monoclonal antibody studies such as this highlight the importance of antibody valency towards antigen as well as the criticality of antigen binding geometry based on impacting Fc orientation and acessibility to FcγR.
Effector function depends on efficient opsonization, and antibody crosslinking of HIV antigen on a target cell surface has been previously demonstrated to enhance neutralization potency(109). However, isolation of naturally occuring antibodies that can crosslink antigen on free HIV virus has been a challenge. Bivalent and highly avid antibody binding to free virus has been previously demonstrated for icosahedral rhinovirus(110), poliovirus(111), and more recently for dengue virus (112, 113). A major distinction from most viruses is that an HIV virus contains as few as 7–14 envelope spike protein trimers on its surface(114) and it has been proposed that the virus has evolved to sparsely display trimers to evade bivalent antibody interactions, amplifying the challenge HIV imposes on the humoral immune system's ability to develop potent neutralizing antibodies(115). Additionally, because efficient ligation of most FcγR requires avid interactions, one could also speculate that this scarcity of of trimer targets additionally increases resistance to Fc mediated effector functions which requires immune complex formation. Analysis of cryo-electron tomography data revealed the most frequent nearest neighbor distance between trimer spikes was 15 nm, within the paratope to paratope reach of a typical antibody(116), resulting from a clustered trimer formation which was shown to be necessary for efficient viral entry(117–119). If it is indeed necessary for trimers to cluster for efficient viral entry, then the viruses most critical to block and clear will opportunely have a skewed trimer distribution more amenable to bivalent antibody binding, and avid opsonization.
A previous study examining antibody architectures with altered distances between variable regions indicated binding one antibody between two spikes (inter-spike) is possible for CD4 binding site antibodies like b12 but unlikely for gp41-specific antibodies like 4E10; increasing distance and flexibility between variable regions was associated with enhanced neutralization, suggesting both a lack of trimer mobility, and the importance of an avid interaction(120). Additionally, a recent study using a “molecular ruler” technique based on linking two Fabs by DNA showed intra-spike crosslinking strongly potentiated virus neutralization. There is additional support that avid Fab binding is advantageous from studying natural antibodies, where it has been previously shown that divalent Fab'2 posess superior neutralization activity relative to monovalent Fab(121). Interesting, a subclass-dependent difference in Fab'2 neutralization was also noted in this study. HIVIG-derived divalent Fab'2 from the IgG3 fraction provided better neutralization than either IgG1 or IgG2 Fab'2, despite equivalent performance as a monovalent Fab. Interestingly, IgG3 antibodies have been characterized to have increased conformational flexibility relative to other subclasses(122), supporting the notion that better bivalent recognition may underlie the enhanced activity of this subclass.
The development of intra-spike bivalent binding antibodies is thought to be uncommon, however carbohyrdate constellations on HIV trimer provide a unique opportunity. For instance 2G12 binds di-mannose residues effectively due to a VH-VL domain exchange leading to bivalent binding and potent neutralization(123). Additionally a naturally dimeric form of 2G12 that contains 2 Fc regions has also been shown to have improved neutralization potency and ADCC(124) highlighting the powerful effector function potentiation dimeric IgG binding can elicit. Further evidence as to the importance of avidity and epitope-dependent exposure of the Fc to effector activity can be found in a 2G12 isotype-switch experiment. 2G12 and gp41 targeting 2F5 antibodies were isotype switched to polymeric IgM, resulting in an increase in complement activation for 2G12 but a reduction for 2F5(125). This study highlights how the nature of how an antibody binds to its epitope can determine the inducibility of complement due to Fc clustering, orientation and steric hinderance. Interestingly, it was recently discovered that IgGs are capable of forming noncovalent hexamers when binding antigen on cells, and this higher order hexameric Fc structure enables avid engagment with C1q(126), depending on the presence or absence of C-terminal lysines(127). This finding corroborates previous speculation that antibodies binding at the surface of the cell may participate in Fc:Fc interactions(128) and may also explain notable modulation of complement deposition among anti-CD20 antibodies which target different epitopes based on variance in the ability to arrange Fc units for optimal engagement of C1q(129, 130).
Role of FcRn in HIV
The neonatal Fc receptor (FcRn) binds IgG in a pH dependent manner and modulates IgG half-life as well as biodistribution in vivo. A recent study in rhesus macaques using an IgG1 Fc mutant with increased pH sensitive affinity to FcRn improved transcytosis of IgG across cellular monolayers in vitro, increased serum half-life three fold, increased distribution and persistence of IgG in the rectum, and most importantly, significantly improved protection from SHIV intrarectal challenges(36). This study points to several new and important considerations: first, rather than exclusively serving as a means to decrease dose or extend the dosing interval for a mAb, enhanced FcRn recognition can provide specific transport to relevant sites of viral exposure. This study also raises an important consideration regarding the IgG3 correlate observed in the RV144 trial(19). There are numerous IgG3 allotypes in the human population, two of which (G3m15, G3m16), exhibit FcRn binding and plasma half-life similar to that of IgG1(131, 132), rather than the greatly reduced half-life of most IgG3 allotypes. Thus, depending on allotype, naturally induced or recombinant IgG3 serum half-life and compartmental distribution could be dramatically impacted. It is interesting to note that the RV144 trial occurred in Thailand and the G3m15 and G3m16 allotypes, uncommon in Europe (~1%), are quite prevalent in Asia (10–50%)(133). In passive immunization studies, additional exploration of the effects of enhanced binding to FcRn as well as FcγRs can be achieved by combining antibody Fc amino acid mutations or glycosylation engineering strategies(134, 135) to maximize both biodistribution and anti-viral bioactivity.
Beyond half-life and biodistribution, FcRn plays novel roles in IgG mediated phagocytosis(136) and immune complex processing. Recent in vitro data suggests FcRn enhances opsonized HIV transcytosis across epithelial layers(137, 138); however, it has also been described to divert immune complexes to lysosomes for antigen processing(139). In dendritic cells, FcRn regulates IgG immune complex processing into peptides compatible with MHC I and II and enables activation of CD4+ and CD8+ T cells linking the humoral and cellular branches of the adaptive immune response (140). Further research into the potential role of FcRn in in HIV dissemination and antigen processing could enable therapeutic strategies to better exploit these properties.
Role of IgA and its receptors in HIV
IgA is the most abundant antibody in the mucosa and can drive effector functions such as phagocytosis, ADCC, and release of cytokines and activated oxygen species through FcαR present on neutrophils, monocytes, eosinophils and some macrophages and dendritic cells(141). Dimeric IgA (dIgA) attachment to the polymeric Ig receptor (pIgR) enables transport to mucosal surfaces and results in release of secretory IgA (sIgA). Although their biological relevance in HIV is unknown, additional receptors for IgA have been reported in humans such as Fcα/μR, which binds both IgA and IgM, and the transferrin receptor, which is selective for monomeric IgA1(142). Overall, IgA is potentially an appealing candidate for HIV protection given its relevant localization in the mucosa as well as its potential in both virus neutralization and effector function. A number of studies have characterized the distinguishing features of the IgA subclasses, their compartmentalization and their potential relation to HIV protection as well as effector function relative to IgG.
Thus far, however, data has not converged on the possible role of IgA in HIV(143). The recent RV144 trial conferred 31% protective efficacy; however, HIV-specific IgA levels were higher in vaccine recipients who became infected, implying a negative role for IgA in vaccine-mediated protection (15). Whether this effect was a marker of other differences in subject immune responses, or a direct indicator of mechanisms of protection is unclear. A follow up study aimed at addressing this correlation found that IgA /IgG ratios were higher among infected subjects, that vaccine-induced IgA could block IgG mAb mediated ADCC, and that an IgA mAb isolated from a vaccinee could inhibit the ADCC activity of vaccine-induced IgG responses(17).
This human vaccine trial data conflicts with results from IgA passive transfer experiments and some vaccine studies in animal models. For example, to evaluate the neutralizing abilities of IgA, an intrarectal antibody administration and virus challenge study in infant rhesus macaques using dIgA1, dIgA2 and IgG1 with the same neutralizing Fv was conducted. In this study dIgA1 demonstrated the best protective capacity, which was associated with higher virion capture and inhibition of virus transcytosis(144). Interestingly, dimeric IgA has previously been shown to elicit more potent FcR mediated effector functions over both monomeric IgA and IgG1 in cell based assays(145). While the result of this IgG/A in vivo comparative experiment was surprising, it is confounded by potential differences associated with intrarectal IgG/A delivery, distribution, or persistence, and potential differences in effector populations in infant animals, as much as it may be driven by differences in the protective capacity of isotypes. Thus, to more directly assess whether IgA and IgG responses might either compete or constructively reinforcewith each other, a follow up study combined intravenous IgG1 with mucosal administration of dIgA1. The combination demonstrated superior protection to both IgG1 alone and dIgA alone, indicating that an immune response with both serum IgG1 and mucosal IgA1, even to the same epitope, could provide enhanced protection from SHIV(146).
Similar support for a protective role for IgA exists in a mucosal vaccination study in rhesus macaques in which sIgA titers in the rectum correlated with delayed acquisition in addition to anti-envelope antibody avidity, and ADCC titers correlated with the number of exposures required for infection(147). In humans, mucosal HIV-specific IgA responses have been observed among exposed but uninfected subjects at multiple sites of transmission(148–151). While there is long-standing evidence that HIV-specific IgA can mediate ADCC(152), recent attention has been paid to the potential ability of IgA to aggregate and trap virions at mucosal sites(153, 154). Consistent with the possible importance of aggregation and trapping, in a humanized mouse study in which a b12-IgA transgene was expressed in hematopoietic stem/progenitor cells, polymeric IgA demonstrated better protection of CD4+ cells than monomeric b12-IgA, and thus established an inhibitory effect of HIV-specific dimeric neutralizing IgA on mucosal transmission of HIV in humanized mice(155).
In order to better resolve the role of IgA and it's receptors, further passive transfer experiments are likely to be enlightening, and could leverage protein engineering efforts directed at this subclass. For example, a therapeutic IgA2/IgG hybrid antibody capable of forming J-chain polymers that bind pIgR, activate complement and bind to FcγRI has been generated(156). A more recent engineering effort produced an “IgGA” capable of binding FcγRI, FcγRII, FcαR and activating complement(157). There appears to be significant opportunity to tune binding to FcαR through Fc engineering as well as to the less characterized Fcα/μR found on follicular dendritic cells, a subset of tonsilar B cells and activated macrophages(158). Additionally, IgA is heavily glycosylated therefore clarifying potential lectin interactions on target or effector or on mucosal surfaces cells could prove valuable. A broader IgA toolkit could be useful for exploring the protective potential of IgA through systemic or mucosal passive immunization studies and gaining insight into compartmental dissemination and effector functions driven by IgA FcRs.
Like the FcγRs there are a number of genetic variants including 10 splice variants as well as SNPs in the FcαR gene and its associated promoter. Three SNPs are found within the promoter, two of which affect promoter activity(159). An SNP within the FcαR gene leads to an aspartic acid or asparagine in the extracellular domain close to the binding region of IgA though this effect of this is unknown(160). An SNP in the intracellular tail of FcαR leads to either a serine or glycine at residue 248 and has functional relevance where G248 has significantly more IL-6 release upon IgA crosslinking and is able to retain cytokine release in the absence of the of the common FcγR-chain. Because the role of FcαR allotypes in HIV is unknown, evaluation of these genetic markers in relation to HIV protection and disease progression could prove valuable.
Frontiers in exploiting FcRs for HIV protection and eradication
The development of vectored immuno-prophylaxis (VIP) using andeno-associated virus (AAV) vectors(161) provides the opportunity to circumvent classical vaccination and the expense and logistical challenges of passive immunization with neutralizing antibodies. With VIP, an AAV vector encoding a broadly neutralizing antibody is injected into muscle tissue, which then produces sustained levels of antibody systemically(161). The technology has previously demonstrated sustained protection in rhesus macaques(161) as well as in an FcγR humanized mouse model(162, 163) and is now in the recruiting stage of a Phase I clinical trial. If proven safe, one could envision applying multi-epitopic antibody cocktails via VIP to capture the breadth and global diversity of HIV and mediate against future virus escape variants. Moreover, programming isotype, subclass and amino acid Fc variants with differential binding to FcγR, FcαR, complement, or FcRn into the AAV vector could tune effector function and compartmental distribution for optimal virus protection.
Development of antibodies to move beyond treatment and towards viral elimination from infected individuals are also rapidly progressing(164). The fundamental challenge to strategies to “cure” HIV is the persistence of latent infection in the viral reservoir. Recent studies in rhesus macaque have shown the potential of preventing virus rebound in a fraction of subjects by administering multi-epitopic antibody cocktails with potent virus neutralization(35, 165). AAV technologies may also have a place in virus eradication: in one experiment addressing this possibility, after viral load was suppressed with anti-retroviral therapy a vector encoding a single broadly neutralizing antibody was capable of controlling viremia(166). Studies are underway on how to activate latently infected cells(167, 168) in order to promote their clearance, and a recent study in humanized mice explored a “kick and kill” method of reactivating latent viruses with a mixture of viral inducers in combination with a multi-epitopic antibody mixture and found this strategy could significantly delay rebound of viremia(39). Interestingly, control antibodies with ablated FcγR binding permitted much more rapid rebound, even when present at a 50 fold higher concentration—again indicating interactions with FcR are a necessary aspect of neutralizing antibody activity in vivo.
Conclusion
Collectively, the studies described here encompass evidence from the setting of natural infection, vaccination, passive transfer, human genetics, and virology to build a strong case as to the role of Fc receptors in the prevention, treatment, and course of HIV infection. As continued efforts to treat and prevent HIV via vaccination and passive transfer of antibody progress, there is strong motivation to pursue optimization of interactions with FcR as a means to enhance anti-viral activity.
Acknowledgements
This work was conducted with support from the Bill and Melinda Gates Foundation OPP1032817 and NIH 1R01AI102691.
References
- 1.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–4. doi: 10.1038/nature06106. Epub 2007/09/07. doi: 10.1038/nature06106. PubMed PMID: 17805298. [DOI] [PubMed] [Google Scholar]
- 2.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–4. doi: 10.1038/nm.1974. Epub 2009/06/16. doi: 10.1038/nm.1974. PubMed PMID: 19525965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115(10):2914–23. doi: 10.1172/JCI24772. Epub 2005/09/17. doi: 10.1172/JCI24772. PubMed PMID: 16167082; PubMed Central PMCID: PMC1201664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yada A, Ebihara S, Matsumura K, Endo S, Maeda T, Nakamura A, Akiyama K, Aiba S, Takai T. Accelerated antigen presentation and elicitation of humoral response in vivo by FcgammaRIIB- and FcgammaRI/III-mediated immune complex uptake. Cell Immunol. 2003;225(1):21–32. doi: 10.1016/j.cellimm.2003.09.008. Epub 2003/12/04. PubMed PMID: 14643301. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Wu J, Ptacek T, Redden DT, Brown EE, Alarcon GS, Ramsey-Goldman R, Petri MA, Reveille JD, Kaslow RA, Kimberly RP, Edberg JC. Allelic-dependent expression of an activating Fc receptor on B cells enhances humoral immune responses. Sci Transl Med. 2013;5(216):216ra175. doi: 10.1126/scitranslmed.3007097. Epub 2013/12/20. doi: 10.1126/scitranslmed.3007097. PubMed PMID: 24353158; PubMed Central PMCID: PMC3982386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez LG, Costa MR, Todd CA, Haynes BF, Montefiori DC. Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane-proximal external region of gp41. J Virol. 2009;83(15):7397–410. doi: 10.1128/JVI.00656-09. Epub 2009/05/22. doi: 10.1128/JVI.00656-09. PubMed PMID: 19458010; PubMed Central PMCID: PMC2708617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holl V, Peressin M, Schmidt S, Decoville T, Zolla-Pazner S, Aubertin AM, Moog C. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood. 2006;107(11):4466–74. doi: 10.1182/blood-2005-08-3490. Epub 2006/02/14. doi: 10.1182/blood-2005-08-3490. PubMed PMID: 16469871; PubMed Central PMCID: PMC1895798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astier A, de la Salle H, de la Salle C, Bieber T, Esposito-Farese ME, Freund M, Cazenave JP, Fridman WH, Teillaud JL, Hanau D. Human epidermal Langerhans cells secrete a soluble receptor for IgG (Fc gamma RII/CD32) that inhibits the binding of immune complexes to Fc gamma R+ cells. J Immunol. 1994;152(1):201–12. Epub 1994/01/01. PubMed PMID: 8254192. [PubMed] [Google Scholar]
- 9.Adu B, Jepsen MP, Gerds TA, Kyei-Baafour E, Christiansen M, Dodoo D, Theisen M. Fc gamma receptor 3B (FCGR3B-c.233C>A-rs5030738) polymorphism modifies the protective effect of malaria specific antibodies in Ghanaian children. J Infect Dis. 2014;209(2):285–9. doi: 10.1093/infdis/jit422. Epub 2013/08/13. doi: 10.1093/infdis/jit422. PubMed PMID: 23935200. [DOI] [PubMed] [Google Scholar]
- 10.Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Exp Hematol. 2009;37(3):309–21. doi: 10.1016/j.exphem.2008.11.006. Epub 2009/02/17. doi: 10.1016/j.exphem.2008.11.006. PubMed PMID: 19218011. [DOI] [PubMed] [Google Scholar]
- 11.Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, Desjarlais JR, Humpe A, Valerius T, Peipp M. Increasing FcgammaRIIa affinity of an FcgammaRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. MAbs. 2014;6(2):409–21. doi: 10.4161/mabs.27457. Epub 2014/02/05. doi: 10.4161/mabs.27457. PubMed PMID: 24492248; PubMed Central PMCID: PMC3984330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khayat D, Soubrane C, Andrieu JM, Visonneau S, Eme D, Tourani JM, Beldjord K, Weil M, Fernandez E, Jacquillat C. Changes of soluble CD16 levels in serum of HIV-infected patients: correlation with clinical and biologic prognostic factors. J Infect Dis. 1990;161(3):430–5. doi: 10.1093/infdis/161.3.430. Epub 1990/03/01. PubMed PMID: 2138203. [DOI] [PubMed] [Google Scholar]
- 13.Bouhlal H, Galon J, Kazatchkine MD, Fridman WH, Sautes-Fridman C, Haeffner Cavaillon N. Soluble CD16 inhibits CR3 (CD11b/CD18)-mediated infection of monocytes/macrophages by opsonized primary R5 HIV-1. J Immunol. 2001;166(5):3377–83. doi: 10.4049/jimmunol.166.5.3377. Epub 2001/02/24. PubMed PMID: 11207294. [DOI] [PubMed] [Google Scholar]
- 14.Boesch AW, Alter G, Ackerman ME. Prospects for engineering HIV-specific antibodies for enhanced effector function and half-life. Curr Opin HIV AIDS. 2015;10(3):160–9. doi: 10.1097/COH.0000000000000149. Epub 2015/02/24. doi: 10.1097/COH.0000000000000149. PubMed PMID: 25700208; PubMed Central PMCID: PMC4419828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. doi: 10.1056/NEJMoa1113425. Epub 2012/04/06. doi: 10.1056/NEJMoa1113425. PubMed PMID: 22475592; PubMed Central PMCID: PMC3371689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490(7420):417–20. doi: 10.1038/nature11519. doi: 10.1038/nature11519. PubMed PMID: 22960785; PubMed Central PMCID: PMC3551291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110(22):9019–24. doi: 10.1073/pnas.1301456110. doi: 10.1073/pnas.1301456110. PubMed PMID: 23661056; PubMed Central PMCID: PMC3670311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra38. doi: 10.1126/scitranslmed.3007736. Epub 2014/03/22. doi: 10.1126/scitranslmed.3007736. PubMed PMID: 24648341. [DOI] [PubMed] [Google Scholar]
- 19.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra39. doi: 10.1126/scitranslmed.3007730. Epub 2014/03/22. doi: 10.1126/scitranslmed.3007730. PubMed PMID: 24648342; PubMed Central PMCID: PMC4116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178(10):6596–603. doi: 10.4049/jimmunol.178.10.6596. PubMed PMID: 17475891. [DOI] [PubMed] [Google Scholar]
- 21.Choi I, Chung AW, Suscovich TJ, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, O'Connell RJ, Francis D, Robb ML, Michael NL, Kim JH, Alter G, Ackerman ME, Bailey-Kellogg C. Machine learning methods enable predictive modeling of antibody feature:function relationships in RV144 vaccinees. PLoS computational biology. 2015;11(4):e1004185. doi: 10.1371/journal.pcbi.1004185. doi: 10.1371/journal.pcbi.1004185. PubMed PMID: 25874406; PubMed Central PMCID: PMC4395155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis GK. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology. 2014;142(1):46–57. doi: 10.1111/imm.12232. Epub 2014/05/21. PubMed PMID: 24843871; PubMed Central PMCID: PMC3992047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174(4):2185–9. doi: 10.4049/jimmunol.174.4.2185. PubMed PMID: 15699150. [DOI] [PubMed] [Google Scholar]
- 24.Barouch DH, Liu J, Peter L, Abbink P, Iampietro MJ, Cheung A, Alter G, Chung A, Dugast AS, Frahm N, McElrath MJ, Wenschuh H, Reimer U, Seaman MS, Pau MG, Weijtens M, Goudsmit J, Walsh SR, Dolin R, Baden LR. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001) J Infect Dis. 2013;207(2):248–56. doi: 10.1093/infdis/jis671. doi: 10.1093/infdis/jis671. PubMed PMID: 23125443; PubMed Central PMCID: PMC3532832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fouts TR, Bagley K, Prado IJ, Bobb KL, Schwartz JA, Xu R, Zagursky RJ, Egan MA, Eldridge JH, LaBranche CC, Montefiori DC, Le Buanec H, Zagury D, Pal R, Pavlakis GN, Felber BK, Franchini G, Gordon S, Vaccari M, Lewis GK, DeVico AL, Gallo RC. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A. 2015;112(9):E992–9. doi: 10.1073/pnas.1423669112. doi: 10.1073/pnas.1423669112. PubMed PMID: 25681373; PubMed Central PMCID: PMC4352796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fauci AS, Marovich MA, Dieffenbach CW, Hunter E, Buchbinder SP. Immunology. Immune activation with HIV vaccines. Science. 2014;344(6179):49–51. doi: 10.1126/science.1250672. doi: 10.1126/science.1250672. PubMed PMID: 24700849; PubMed Central PMCID: PMC4414116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang L, Smith AJ, Duan L, Perkey KE, Qu L, Wietgrefe S, Zupancic M, Southern PJ, Masek-Hammerman K, Reeves RK, Johnson RP, Haase AT. NK cell responses to simian immunodeficiency virus vaginal exposure in naive and vaccinated rhesus macaques. J Immunol. 2014;193(1):277–84. doi: 10.4049/jimmunol.1400417. Epub 2014/06/06. doi: 10.4049/jimmunol.1400417. PubMed PMID: 24899503; PubMed Central PMCID: PMC4083479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. doi: 10.1038/nature10766. doi: 10.1038/nature10766. PubMed PMID: 22217938; PubMed Central PMCID: PMC3271177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505(7484):502–8. doi: 10.1038/nature12893. doi: 10.1038/nature12893. PubMed PMID: 24352234; PubMed Central PMCID: PMC3946913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackerman ME, Dugast AS, Alter G. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annual review of medicine. 2012;63:113–30. doi: 10.1146/annurev-med-050310-085221. doi: 10.1146/annurev-med-050310-085221. PubMed PMID: 22077718. [DOI] [PubMed] [Google Scholar]
- 31.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86(11):6189–96. doi: 10.1128/JVI.00491-12. Epub 2012/03/30. doi: 10.1128/JVI.00491-12. PubMed PMID: 22457527; PubMed Central PMCID: PMC3372207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugast AS, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, Streeck H, Suscovich TJ, Ghebremichael M, Ackerman ME, Barouch DH, Alter G. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS One. 2014;9(5):e97229. doi: 10.1371/journal.pone.0097229. doi: 10.1371/journal.pone.0097229. PubMed PMID: 24820481; PubMed Central PMCID: PMC4018276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sholukh AM, Byrareddy SN, Shanmuganathan V, Hemashettar G, Lakhashe SK, Rasmussen RA, Watkins JD, Vyas HK, Thorat S, Brandstoetter T, Mukhtar MM, Yoon JK, Novembre FJ, Villinger F, Landucci G, Forthal DN, Ratcliffe S, Tuero I, Robert-Guroff M, Polonis VR, Bilska M, Montefiori DC, Johnson WE, Ertl HC, Ruprecht RM. Passive immunization of macaques with polyclonal anti-SHIV IgG against a heterologous tier 2 SHIV: outcome depends on IgG dose. Retrovirology. 2014;11:8. doi: 10.1186/1742-4690-11-8. doi: 10.1186/1742-4690-11-8. PubMed PMID: 24444350; PubMed Central PMCID: PMC3905655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, Van Ham G, Heyndrickx L, Van Dorsselaer A, Katinger D, Vcelar B, Zolla-Pazner S, Mangeot I, Kelly C, Shattock RJ, Le Grand R. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7(1):46–56. doi: 10.1038/mi.2013.23. Epub 2013/04/18. doi: 10.1038/mi.2013.23. PubMed PMID: 23591718. [DOI] [PubMed] [Google Scholar]
- 35.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–8. doi: 10.1038/nature12744. Epub 2013/11/01. doi: 10.1038/nature12744. PubMed PMID: 24172905; PubMed Central PMCID: PMC4017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD, Todd JP, Penzak SR, Saunders KO, Nason MC, Haase AT, Rao SS, Blumberg RS, Mascola JR, Nabel GJ. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5. doi: 10.1038/nature13612. doi: 10.1038/nature13612. PubMed PMID: 25119033; PubMed Central PMCID: PMC4433741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–53. doi: 10.1016/j.cell.2014.08.023. Epub 2014/09/13. doi: 10.1016/j.cell.2014.08.023. PubMed PMID: 25215485; PubMed Central PMCID: PMC4167398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A. 2012;109(16):6181–6. doi: 10.1073/pnas.1203954109. Epub 2012/04/05. doi: 10.1073/pnas.1203954109. PubMed PMID: 22474370; PubMed Central PMCID: PMC3341029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–99. doi: 10.1016/j.cell.2014.07.043. Epub 2014/08/19. doi: 10.1016/j.cell.2014.07.043. PubMed PMID: 25131989; PubMed Central PMCID: PMC4163911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins M, Landers D, Williams-Herman D, Wara D, Viscarello RR, Hammill HA, Kline MW, Shearer WT, Charlebois ED, Kohl S. Association between anti-human immunodeficiency virus type 1 (HIV-1) antibody-dependent cellular cytotoxicity antibody titers at birth and vertical transmission of HIV-1. J Infect Dis. 1994;170(2):308–12. doi: 10.1093/infdis/170.2.308. PubMed PMID: 8035015. [DOI] [PubMed] [Google Scholar]
- 41.Pugatch D, Sullivan JL, Pikora CA, Luzuriaga K. Delayed generation of antibodies mediating human immunodeficiency virus type 1-specific antibody-dependent cellular cytotoxicity in vertically infected infants. WITS Study Group. Women and Infants Transmission Study. J Infect Dis. 1997;176(3):643–8. doi: 10.1086/514085. PubMed PMID: 9291310. [DOI] [PubMed] [Google Scholar]
- 42.Broliden K, Sievers E, Tovo PA, Moschese V, Scarlatti G, Broliden PA, Fundaro C, Rossi P. Antibody-dependent cellular cytotoxicity and neutralizing activity in sera of HIV-1-infected mothers and their children. Clin Exp Immunol. 1993;93(1):56–64. doi: 10.1111/j.1365-2249.1993.tb06497.x. PubMed PMID: 8324904; PubMed Central PMCID: PMC1554739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ljunggren K, Moschese V, Broliden PA, Giaquinto C, Quinti I, Fenyo EM, Wahren B, Rossi P, Jondal M. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis. 1990;161(2):198–202. doi: 10.1093/infdis/161.2.198. PubMed PMID: 2299204. [DOI] [PubMed] [Google Scholar]
- 44.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. Passively Acquired Antibody-Dependent Cellular Cytotoxicity (ADCC) Activity in HIV-Infected Infants Is Associated with Reduced Mortality. Cell host & microbe. 2015;17(4):500–6. doi: 10.1016/j.chom.2015.03.002. doi: 10.1016/j.chom.2015.03.002. PubMed PMID: 25856755; PubMed Central PMCID: PMC4392343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. doi: 10.1371/journal.ppat.1002739. PubMed PMID: 22719248; PubMed Central PMCID: PMC3375288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Gunthard HF. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–22. doi: 10.1038/nm1244. doi: 10.1038/nm1244. PubMed PMID: 15880120. [DOI] [PubMed] [Google Scholar]
- 47.Mehandru S, Vcelar B, Wrin T, Stiegler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, Carrington M, Petropoulos C, Katinger H, Markowitz M. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–31. doi: 10.1128/JVI.01340-07. doi: 10.1128/JVI.01340-07. PubMed PMID: 17686878; PubMed Central PMCID: PMC2045579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015 doi: 10.1038/nature14411. Epub 2015/04/10. doi: 10.1038/nature14411. PubMed PMID: 25855300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsushita S, Yoshimura K, Ramirez KP, Pisupati J, Murakami T, Group KDS. Passive transfer of neutralizing mAb KD-247 reduces plasma viral load in patients chronically infected with HIV-1. Aids. 2015;29(4):453–62. doi: 10.1097/QAD.0000000000000570. doi: 10.1097/QAD.0000000000000570. PubMed PMID: 25630040. [DOI] [PubMed] [Google Scholar]
- 50.Asmal M, Sun Y, Lane S, Yeh W, Schmidt SD, Mascola JR, Letvin NL. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. J Virol. 2011;85(11):5465–75. doi: 10.1128/JVI.00313-11. Epub 2011/04/01. doi: 10.1128/JVI.00313-11. PubMed PMID: 21450829; PubMed Central PMCID: PMC3094968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–73. PubMed PMID: 8757343. [PubMed] [Google Scholar]
- 52.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, Kent SJ, Stratov I. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58(2):127–31. doi: 10.1097/QAI.0b013e31822c62b9. Epub 2011/07/28. doi: 10.1097/QAI.0b013e31822c62b9. PubMed PMID: 21792067; PubMed Central PMCID: PMC3175260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dugast AS, Tonelli A, Berger CT, Ackerman ME, Sciaranghella G, Liu Q, Sips M, Toth I, Piechocka-Trocha A, Ghebremichael M, Alter G. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology. 2011;415(2):160–7. doi: 10.1016/j.virol.2011.03.012. Epub 2011/05/14. doi: 10.1016/j.virol.2011.03.012. PubMed PMID: 21565376; PubMed Central PMCID: PMC3112178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia M, Li D, He X, Zhao Y, Peng H, Ma P, Hong K, Liang H, Shao Y. Impaired natural killer cell-induced antibody-dependent cell-mediated cytotoxicity is associated with human immunodeficiency virus-1 disease progression. Clin Exp Immunol. 2013;171(1):107–16. doi: 10.1111/j.1365-2249.2012.04672.x. Epub 2012/12/04. doi: 10.1111/j.1365-2249.2012.04672.x. PubMed PMID: 23199330; PubMed Central PMCID: PMC3530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dugast AS, Stamatatos L, Tonelli A, Suscovich TJ, Licht AF, Mikell I, Ackerman ME, Streeck H, Klasse PJ, Moore JP, Alter G. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. Eur J Immunol. 2014;44(10):2925–37. doi: 10.1002/eji.201344305. Epub 2014/07/22. doi: 10.1002/eji.201344305. PubMed PMID: 25043633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai JI, Licht AF, Dugast AS, Suscovich T, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. Divergent antibody subclass and specificity profiles but not protective HLA-B alleles are associated with variable antibody effector function among HIV-1 controllers. J Virol. 2014;88(5):2799–809. doi: 10.1128/JVI.03130-13. Epub 2013/12/20. doi: 10.1128/JVI.03130-13. PubMed PMID: 24352471; PubMed Central PMCID: PMC3958053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–92. doi: 10.1172/JCI65708. Epub 2013/04/09. doi: 10.1172/JCI65708. PubMed PMID: 23563315; PubMed Central PMCID: PMC3637034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S, Alter G. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. Aids. 2014 doi: 10.1097/QAD.0000000000000444. Epub 2014/08/28. doi: 10.1097/QAD.0000000000000444. PubMed PMID: 25160934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013;87(10):5468–76. doi: 10.1128/JVI.03403-12. Epub 2013/03/08. doi: 10.1128/JVI.03403-12. PubMed PMID: 23468489; PubMed Central PMCID: PMC3648186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–7. doi: 10.1200/JCO.2003.05.013. Epub 2003/09/17. doi: 10.1200/JCO.2003.05.013. PubMed PMID: 12975461. [DOI] [PubMed] [Google Scholar]
- 61.van der Pol WL, van den Berg LH, Scheepers RH, van der Bom JG, van Doorn PA, van Koningsveld R, van den Broek MC, Wokke JH, van de Winkel JG. IgG receptor IIa alleles determine susceptibility and severity of Guillain-Barre syndrome. Neurology. 2000;54(8):1661–5. doi: 10.1212/wnl.54.8.1661. Epub 2000/04/13. PubMed PMID: 10762510. [DOI] [PubMed] [Google Scholar]
- 62.Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin Exp Immunol. 2009;157(2):244–54. doi: 10.1111/j.1365-2249.2009.03980.x. Epub 2009/07/17. doi: 10.1111/j.1365-2249.2009.03980.x. PubMed PMID: 19604264; PubMed Central PMCID: PMC2730850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rascu A, Repp R, Westerdaal NA, Kalden JR, van de Winkel JG. Clinical relevance of Fc gamma receptor polymorphisms. Annals of the New York Academy of Sciences. 1997;815:282–95. doi: 10.1111/j.1749-6632.1997.tb52070.x. PubMed PMID: 9186665. [DOI] [PubMed] [Google Scholar]
- 64.van de Winkel JG. Fc receptors: role in biology and antibody therapy. Immunol Lett. 2010;128(1):4–5. doi: 10.1016/j.imlet.2009.09.005. doi: 10.1016/j.imlet.2009.09.005. PubMed PMID: 19808048. [DOI] [PubMed] [Google Scholar]
- 65.Clatworthy MR. Fcg Receptor Polymorphisms and Susceptibility to Infection. In: Ackerman ME, Nimmerjahn F, editors. Antibody Fc: Linking adaptive and innate immunity. Elsevier; Philadelphia, PA: 2014. pp. 217–56. [Google Scholar]
- 66.Nimmerjahn F. Activating and inhibitory FcgammaRs in autoimmune disorders. Springer seminars in immunopathology. 2006;28(4):305–19. doi: 10.1007/s00281-006-0052-1. doi: 10.1007/s00281-006-0052-1. PubMed PMID: 17115158. [DOI] [PubMed] [Google Scholar]
- 67.Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179(11):7916–23. doi: 10.4049/jimmunol.179.11.7916. Epub 2007/11/21. PubMed PMID: 18025239. [DOI] [PubMed] [Google Scholar]
- 68.Brouwer KC, Lal RB, Mirel LB, Yang C, van Eijk AM, Ayisi J, Otieno J, Nahlen BL, Steketee R, Lal AA, Shi YP. Polymorphism of Fc receptor IIa for IgG in infants is associated with susceptibility to perinatal HIV-1 infection. Aids. 2004;18(8):1187–94. doi: 10.1097/00002030-200405210-00012. Epub 2004/05/29. PubMed PMID: 15166534. [DOI] [PubMed] [Google Scholar]
- 69.French MA, Tanaskovic S, Law MG, Lim A, Fernandez S, Ward LD, Kelleher AD, Emery S. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a `high-affinity' FcgammaRIIa genotype. Aids. 2010;24(13):1983–90. doi: 10.1097/QAD.0b013e32833c1ce0. Epub 2010/07/17. doi: 10.1097/QAD.0b013e32833c1ce0. PubMed PMID: 20634666. [DOI] [PubMed] [Google Scholar]
- 70.Poonia B, Kijak GH, Pauza CD. High affinity allele for the gene of FCGR3A is risk factor for HIV infection and progression. PLoS One. 2010;5(12):e15562. doi: 10.1371/journal.pone.0015562. Epub 2010/12/29. doi: 10.1371/journal.pone.0015562. PubMed PMID: 21187939; PubMed Central PMCID: PMC3004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forthal DN, Gabriel EE, Wang A, Landucci G, Phan TB. Association of Fcgamma receptor IIIa genotype with the rate of HIV infection after gp120 vaccination. Blood. 2012;120(14):2836–42. doi: 10.1182/blood-2012-05-431361. Epub 2012/08/24. doi: 10.1182/blood-2012-05-431361. PubMed PMID: 22915639; PubMed Central PMCID: PMC3466964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pandey JP, Namboodiri AM, Bu S, De Dieu Tapsoba J, Sato A, Dai JY. Immunoglobulin genes and the acquisition of HIV infection in a randomized trial of recombinant adenovirus HIV vaccine. Virology. 2013;441(1):70–4. doi: 10.1016/j.virol.2013.03.007. Epub 2013/04/16. doi: 10.1016/j.virol.2013.03.007. PubMed PMID: 23582638; PubMed Central PMCID: PMC3750738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, Pyo CW, Zolla-Pazner S, Montefiori D, Liao HX, Nabel G, Pinter A, Evans DT, Gottardo R, Dai JY, Janes H, Morris D, Fong Y, Edlefsen PT, Li F, Frahm N, Alpert MD, Prentice H, Rerks-Ngarm S, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Robb ML, O'Connell RJ, Haynes BF, Michael NL, Kim JH, McElrath MJ, Geraghty DE. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J Clin Invest. 2014;124(9):3879–90. doi: 10.1172/JCI75539. Epub 2014/08/12. doi: 10.1172/JCI75539. PubMed PMID: 25105367; PubMed Central PMCID: PMC4151214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cocklin SL, Schmitz JE. The role of Fc receptors in HIV infection and vaccine efficacy. Curr Opin HIV AIDS. 2014;9(3):257–62. doi: 10.1097/COH.0000000000000051. doi: 10.1097/COH.0000000000000051. PubMed PMID: 24670320; PubMed Central PMCID: PMC4120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991;147(4):1338–43. Epub 1991/08/15. PubMed PMID: 1831223. [PubMed] [Google Scholar]
- 76.Roederer M, Quaye L, Mangino M, Beddall MH, Mahnke Y, Chattopadhyay P, Tosi I, Napolitano L, Terranova Barberio M, Menni C, Villanova F, Di Meglio P, Spector TD, Nestle FO. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell. 2015;161(2):387–403. doi: 10.1016/j.cell.2015.02.046. Epub 2015/03/17. doi: 10.1016/j.cell.2015.02.046. PubMed PMID: 25772697; PubMed Central PMCID: PMC4393780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, Schlossman SF. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U S A. 1997;94(12):6346–51. doi: 10.1073/pnas.94.12.6346. Epub 1997/06/10. PubMed PMID: 9177220; PubMed Central PMCID: PMC21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ochsenbein AF, Riddell SR, Brown M, Corey L, Baerlocher GM, Lansdorp PM, Greenberg PD. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J Exp Med. 2004;200(11):1407–17. doi: 10.1084/jem.20040717. Epub 2004/12/08. doi: 10.1084/jem.20040717. PubMed PMID: 15583014; PubMed Central PMCID: PMC2211945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. Aids. 2001;15(8):957–64. doi: 10.1097/00002030-200105250-00003. Epub 2001/06/16. PubMed PMID: 11399977. [DOI] [PubMed] [Google Scholar]
- 80.Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, Goulder P, Klenerman P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24(4):491–502. doi: 10.1097/QAD.0b013e3283344895. Epub 2010/01/15. doi: 10.1097/QAD.0b013e3283344895. PubMed PMID: 20071976. [DOI] [PubMed] [Google Scholar]
- 81.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, Smith KG. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11(10):1056–8. doi: 10.1038/nm1288. Epub 2005/09/20. doi: 10.1038/nm1288. PubMed PMID: 16170323. [DOI] [PubMed] [Google Scholar]
- 82.Kono H, Kyogoku C, Suzuki T, Tsuchiya N, Honda H, Yamamoto K, Tokunaga K, Honda Z. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14(19):2881–92. doi: 10.1093/hmg/ddi320. Epub 2005/08/24. doi: 10.1093/hmg/ddi320. PubMed PMID: 16115811. [DOI] [PubMed] [Google Scholar]
- 83.Mody GM, Patel N, Budhoo A, Dubula T. Concomitant systemic lupus erythematosus and HIV: case series and literature review. Semin Arthritis Rheum. 2014;44(2):186–94. doi: 10.1016/j.semarthrit.2014.05.009. Epub 2014/06/11. doi: 10.1016/j.semarthrit.2014.05.009. PubMed PMID: 24913962. [DOI] [PubMed] [Google Scholar]
- 84.Bonsignori M, Wiehe K, Grimm SK, Lynch R, Yang G, Kozink DM, Perrin F, Cooper AJ, Hwang KK, Chen X, Liu M, McKee K, Parks RJ, Eudailey J, Wang M, Clowse M, Criscione-Schreiber LG, Moody MA, Ackerman ME, Boyd SD, Gao F, Kelsoe G, Verkoczy L, Tomaras GD, Liao HX, Kepler TB, Montefiori DC, Mascola JR, Haynes BF. An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1. J Clin Invest. 2014;124(4):1835–43. doi: 10.1172/JCI73441. Epub 2014/03/13. doi: 10.1172/JCI73441. PubMed PMID: 24614107; PubMed Central PMCID: PMC3973118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelsoe G, Verkoczy L, Haynes BF. Immune System Regulation in the Induction of Broadly Neutralizing HIV-1 Antibodies. Vaccines. 2014;2(1):1–14. doi: 10.3390/vaccines2010001. doi: 10.3390/vaccines2010001. PubMed PMID: 24932410; PubMed Central PMCID: PMC4053940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breunis WB, van Mirre E, Bruin M, Geissler J, de Boer M, Peters M, Roos D, de Haas M, Koene HR, Kuijpers TW. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. 2008;111(3):1029–38. doi: 10.1182/blood-2007-03-079913. Epub 2007/09/11. doi: 10.1182/blood-2007-03-079913. PubMed PMID: 17827395. [DOI] [PubMed] [Google Scholar]
- 87.van der Heijden J, Breunis WB, Geissler J, de Boer M, van den Berg TK, Kuijpers TW. Phenotypic variation in IgG receptors by nonclassical FCGR2C alleles. J Immunol. 2012;188(3):1318–24. doi: 10.4049/jimmunol.1003945. Epub 2011/12/27. doi: 10.4049/jimmunol.1003945. PubMed PMID: 22198951. [DOI] [PubMed] [Google Scholar]
- 88.Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol. 2012;188(9):4405–11. doi: 10.4049/jimmunol.1200090. Epub 2012/03/31. doi: 10.4049/jimmunol.1200090. PubMed PMID: 22461693. [DOI] [PubMed] [Google Scholar]
- 89.Morris DL, Roberts AL, Witherden AS, Tarzi R, Barros P, Whittaker JC, Cook TH, Aitman TJ, Vyse TJ. Evidence for both copy number and allelic (NA1/NA2) risk at the FCGR3B locus in systemic lupus erythematosus. Eur J Hum Genet. 2010;18(9):1027–31. doi: 10.1038/ejhg.2010.56. Epub 2010/05/06. doi: 10.1038/ejhg.2010.56. PubMed PMID: 20442749; PubMed Central PMCID: PMC2987408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Machado LR, Bowdrey J, Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Yimer G, Amogne W, Mugusi S, Janabi M, Aderaye G, Mugusi F, Viskaduraki M, Aklillu E, Hollox EJ. Copy number variation of Fc gamma receptor genes in HIV-infected and HIV-tuberculosis co-infected individuals in sub-Saharan Africa. PLoS One. 2013;8(11):e78165. doi: 10.1371/journal.pone.0078165. Epub 2013/11/20. doi: 10.1371/journal.pone.0078165. PubMed PMID: 24250791; PubMed Central PMCID: PMC3826734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nature immunology. 2006;7(3):302–10. doi: 10.1038/ni1302. doi: 10.1038/ni1302. PubMed PMID: 16429138. [DOI] [PubMed] [Google Scholar]
- 92.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, Dyer WB, Ketas TJ, Chadburn A, Cohen-Gould L, Knowles DM, Chiu A, Sanders RW, Chen K, Cerutti A. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nature immunology. 2009;10(9):1008–17. doi: 10.1038/ni.1753. doi: 10.1038/ni.1753. PubMed PMID: 19648924; PubMed Central PMCID: PMC2784687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McNatt MW, Zang T, Bieniasz PD. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog. 2013;9(4):e1003299. doi: 10.1371/journal.ppat.1003299. doi: 10.1371/journal.ppat.1003299. PubMed PMID: 23633949; PubMed Central PMCID: PMC3635990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 2014;111(17):6425–30. doi: 10.1073/pnas.1321507111. Epub 2014/04/16. doi: 10.1073/pnas.1321507111. PubMed PMID: 24733916; PubMed Central PMCID: PMC4035966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol. 2014;88(5):2633–44. doi: 10.1128/JVI.03230-13. Epub 2013/12/20. doi: 10.1128/JVI.03230-13. PubMed PMID: 24352444; PubMed Central PMCID: PMC3958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol. 2015;89(1):545–51. doi: 10.1128/JVI.02868-14. Epub 2014/10/24. doi: 10.1128/JVI.02868-14. PubMed PMID: 25339767; PubMed Central PMCID: PMC4301108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schon A, Freire E, Routy JP, Smith AB, 3rd, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A. 2015;112(20):E2687–94. doi: 10.1073/pnas.1506755112. doi: 10.1073/pnas.1506755112. PubMed PMID: 25941367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huber G, Banki Z, Lengauer S, Stoiber H. Emerging role for complement in HIV infection. Curr Opin HIV AIDS. 2011;6(5):419–26. doi: 10.1097/COH.0b013e3283495a26. doi: 10.1097/COH.0b013e3283495a26. PubMed PMID: 21825871. [DOI] [PubMed] [Google Scholar]
- 99.Stoiber H, Pruenster M, Ammann CG, Dierich MP. Complement-opsonized HIV: the free rider on its way to infection. Mol Immunol. 2005;42(2):153–60. doi: 10.1016/j.molimm.2004.06.024. doi: 10.1016/j.molimm.2004.06.024. PubMed PMID: 15488605. [DOI] [PubMed] [Google Scholar]
- 100.Saifuddin M, Parker CJ, Peeples ME, Gorny MK, Zolla-Pazner S, Ghassemi M, Rooney IA, Atkinson JP, Spear GT. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182(2):501–9. doi: 10.1084/jem.182.2.501. PubMed PMID: 7543140; PubMed Central PMCID: PMC2192116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stoiber H, Pinter C, Siccardi AG, Clivio A, Dierich MP. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55) J Exp Med. 1996;183(1):307–10. doi: 10.1084/jem.183.1.307. PubMed PMID: 8551237; PubMed Central PMCID: PMC2192395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellegard R, Crisci E, Burgener A, Sjowall C, Birse K, Westmacott G, Hinkula J, Lifson JD, Larsson M. Complement opsonization of HIV-1 results in decreased antiviral and inflammatory responses in immature dendritic cells via CR3. J Immunol. 2014;193(9):4590–601. doi: 10.4049/jimmunol.1401781. doi: 10.4049/jimmunol.1401781. PubMed PMID: 25252956; PubMed Central PMCID: PMC4201991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pollara J, Bonsignori M, Moody MA, Pazgier M, Haynes BF, Ferrari G. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr HIV Res. 2013;11(5):378–87. doi: 10.2174/1570162X113116660059. Epub 2013/11/07. PubMed PMID: 24191939; PubMed Central PMCID: PMC3878369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O'Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol. 2014;88(14):7715–26. doi: 10.1128/JVI.00156-14. Epub 2014/05/09. doi: 10.1128/JVI.00156-14. PubMed PMID: 24807721; PubMed Central PMCID: PMC4097802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcgammaRs. J Immunol. 2013;190(8):4315–23. doi: 10.4049/jimmunol.1200501. doi: 10.4049/jimmunol.1200501. PubMed PMID: 23509345. [DOI] [PubMed] [Google Scholar]
- 106.Robinson JE, Elliott DH, Martin EA, Micken K, Rosenberg ES. High frequencies of antibody responses to CD4 induced epitopes in HIV infected patients started on HAART during acute infection. Hum Antibodies. 2005;14(3–4):115–21. Epub 2006/05/25. PubMed PMID: 16720981. [PubMed] [Google Scholar]
- 107.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86(21):11521–32. doi: 10.1128/JVI.01023-12. Epub 2012/08/17. doi: 10.1128/JVI.01023-12. PubMed PMID: 22896626; PubMed Central PMCID: PMC3486290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acharya P, Tolbert WD, Gohain N, Wu X, Yu L, Liu T, Huang W, Huang CC, Kwon YD, Louder RK, Luongo TS, McLellan JS, Pancera M, Yang Y, Zhang B, Flinko R, Foulke JS, Jr., Sajadi MM, Kamin-Lewis R, Robinson JE, Martin L, Kwong PD, Guan Y, DeVico AL, Lewis GK, Pazgier M. Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J Virol. 2014;88(21):12895–906. doi: 10.1128/JVI.02194-14. Epub 2014/08/29. doi: 10.1128/JVI.02194-14. PubMed PMID: 25165110; PubMed Central PMCID: PMC4248932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cavacini LA, Emes CL, Power J, Duval M, Posner MR. Effect of antibody valency on interaction with cell-surface expressed HIV-1 and viral neutralization. J Immunol. 1994;152(5):2538–45. Epub 1994/03/01. PubMed PMID: 7510748. [PubMed] [Google Scholar]
- 110.Smith TJ, Olson NH, Cheng RH, Chase ES, Baker TS. Structure of a human rhinovirus-bivalently bound antibody complex: implications for viral neutralization and antibody flexibility. Proc Natl Acad Sci U S A. 1993;90(15):7015–8. doi: 10.1073/pnas.90.15.7015. Epub 1993/08/01. PubMed PMID: 8394005; PubMed Central PMCID: PMC47066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Icenogle J, Shiwen H, Duke G, Gilbert S, Rueckert R, Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983;127(2):412–25. doi: 10.1016/0042-6822(83)90154-x. Epub 1983/06/01. PubMed PMID: 6306918. [DOI] [PubMed] [Google Scholar]
- 112.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med. 2012;4(139):139ra83. doi: 10.1126/scitranslmed.3003888. Epub 2012/06/23. doi: 10.1126/scitranslmed.3003888. PubMed PMID: 22723463. [DOI] [PubMed] [Google Scholar]
- 113.Fibriansah G, Tan JL, Smith SA, de Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, de Silva AM, Crowe JE, Lok SM. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun. 2015;6:6341. doi: 10.1038/ncomms7341. Epub 2015/02/24. doi: 10.1038/ncomms7341. PubMed PMID: 25698059; PubMed Central PMCID: PMC4346626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chertova E, Bess JW, Jr., Crise BJ, Sowder IR, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2002;76(11):5315–25. doi: 10.1128/JVI.76.11.5315-5325.2002. Epub 2002/05/07. PubMed PMID: 11991960; PubMed Central PMCID: PMC137021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klein JS, Bjorkman PJ. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 2010;6(5):e1000908. doi: 10.1371/journal.ppat.1000908. Epub 2010/06/05. doi: 10.1371/journal.ppat.1000908. PubMed PMID: 20523901; PubMed Central PMCID: PMC2877745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu P, Liu J, Bess J, Jr., Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441(7095):847–52. doi: 10.1038/nature04817. Epub 2006/05/27. doi: 10.1038/nature04817. PubMed PMID: 16728975. [DOI] [PubMed] [Google Scholar]
- 117.Chojnacki J, Staudt T, Glass B, Bingen P, Engelhardt J, Anders M, Schneider J, Muller B, Hell SW, Krausslich HG. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science. 2012;338(6106):524–8. doi: 10.1126/science.1226359. Epub 2012/11/01. doi: 10.1126/science.1226359. PubMed PMID: 23112332. [DOI] [PubMed] [Google Scholar]
- 118.Roy NH, Chan J, Lambele M, Thali M. Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion. J Virol. 2013;87(13):7516–25. doi: 10.1128/JVI.00790-13. Epub 2013/05/03. doi: 10.1128/JVI.00790-13. PubMed PMID: 23637402; PubMed Central PMCID: PMC3700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muranyi W, Malkusch S, Muller B, Heilemann M, Krausslich HG. Super-resolution microscopy reveals specific recruitment of HIV-1 envelope proteins to viral assembly sites dependent on the envelope C-terminal tail. PLoS Pathog. 2013;9(2):e1003198. doi: 10.1371/journal.ppat.1003198. Epub 2013/03/08. doi: 10.1371/journal.ppat.1003198. PubMed PMID: 23468635; PubMed Central PMCID: PMC3585150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klein JS, Gnanapragasam PN, Galimidi RP, Foglesong CP, West AP, Jr., Bjorkman PJ. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc Natl Acad Sci U S A. 2009;106(18):7385–90. doi: 10.1073/pnas.0811427106. Epub 2009/04/18. doi: 10.1073/pnas.0811427106. PubMed PMID: 19372381; PubMed Central PMCID: PMC2678646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scharf O, Golding H, King LR, Eller N, Frazier D, Golding B, Scott DE. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J Virol. 2001;75(14):6558–65. doi: 10.1128/JVI.75.14.6558-6565.2001. Epub 2001/06/20. doi: 10.1128/JVI.75.14.6558-6565.2001. PubMed PMID: 11413323; PubMed Central PMCID: PMC114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159(7):3372–82. PubMed PMID: 9317136. [PubMed] [Google Scholar]
- 123.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. Antibody 2G12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the HIV-1 glycan shield if domain exchanged. J Virol. 2010;84(20):10690–9. doi: 10.1128/JVI.01110-10. Epub 2010/08/13. doi: 10.1128/JVI.01110-10. PubMed PMID: 20702629; PubMed Central PMCID: PMC2950587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klein JS, Webster A, Gnanapragasam PN, Galimidi RP, Bjorkman PJ. A dimeric form of the HIV-1 antibody 2G12 elicits potent antibody-dependent cellular cytotoxicity. Aids. 2010;24(11):1633–40. doi: 10.1097/qad.0b013e32833ad8c8. Epub 2010/07/06. PubMed PMID: 20597163; PubMed Central PMCID: PMC2898891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wolbank S, Kunert R, Stiegler G, Katinger H. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J Virol. 2003;77(7):4095–103. doi: 10.1128/JVI.77.7.4095-4103.2003. Epub 2003/03/14. PubMed PMID: 12634368; PubMed Central PMCID: PMC150633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–3. doi: 10.1126/science.1248943. Epub 2014/03/15. doi: 10.1126/science.1248943. PubMed PMID: 24626930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van den Bremer ET, Beurskens FJ, Voorhorst M, Engelberts PJ, de Jong RN, van der Boom BG, Cook EM, Lindorfer MA, Taylor RP, van Berkel PH, Parren PW. Human IgG is produced in a pro-form that requires clipping of C-terminal lysines for maximal complement activation. MAbs. 2015 doi: 10.1080/19420862.2015.1046665. 0. doi: 10.1080/19420862.2015.1046665. PubMed PMID: 26037225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saphire EO, Stanfield RL, Crispin MD, Parren PW, Rudd PM, Dwek RA, Burton DR, Wilson IA. Contrasting IgG structures reveal extreme asymmetry and flexibility. J Mol Biol. 2002;319(1):9–18. doi: 10.1016/S0022-2836(02)00244-9. Epub 2002/06/08. doi: 10.1016/S0022-2836(02)00244-9. PubMed PMID: 12051932. [DOI] [PubMed] [Google Scholar]
- 129.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, Parren PW, Glennie MJ, van de Winkel JG. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–71. doi: 10.4049/jimmunol.177.1.362. Epub 2006/06/21. PubMed PMID: 16785532. [DOI] [PubMed] [Google Scholar]
- 130.Wu Y, West AP, Jr., Kim HJ, Thornton ME, Ward AB, Bjorkman PJ. Structural basis for enhanced HIV-1 neutralization by a dimeric immunoglobulin G form of the glycan-recognizing antibody 2G12. Cell Rep. 2013;5(5):1443–55. doi: 10.1016/j.celrep.2013.11.015. Epub 2013/12/10. doi: 10.1016/j.celrep.2013.11.015. PubMed PMID: 24316082; PubMed Central PMCID: PMC3919625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, de Haas M, Jonsdottir I, van der Schoot CE, Vidarsson G. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. doi: 10.1038/ncomms1608. Epub 2011/12/22. doi: 10.1038/ncomms1608. PubMed PMID: 22186895; PubMed Central PMCID: PMC3247843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Einarsdottir H, Ji Y, Visser R, Mo C, Luo G, Scherjon S, van der Schoot CE, Vidarsson G. H435-containing immunoglobulin G3 allotypes are transported efficiently across the human placenta: implications for alloantibody-mediated diseases of the newborn. Transfusion. 2014;54(3):665–71. doi: 10.1111/trf.12334. Epub 2013/07/09. doi: 10.1111/trf.12334. PubMed PMID: 23829325. [DOI] [PubMed] [Google Scholar]
- 133.Kapur R, Einarsdottir HK, Vidarsson G. IgG-effector functions: “the good, the bad and the ugly”. Immunol Lett. 2014;160(2):139–44. doi: 10.1016/j.imlet.2014.01.015. Epub 2014/02/06. doi: 10.1016/j.imlet.2014.01.015. PubMed PMID: 24495619. [DOI] [PubMed] [Google Scholar]
- 134.Liu Z, Gunasekaran K, Wang W, Razinkov V, Sekirov L, Leng E, Sweet H, Foltz I, Howard M, Rousseau AM, Kozlosky C, Fanslow W, Yan W. Asymmetrical Fc engineering greatly enhances antibody-dependent cellular cytotoxicity (ADCC) effector function and stability of the modified antibodies. J Biol Chem. 2014;289(6):3571–90. doi: 10.1074/jbc.M113.513366. Epub 2013/12/07. doi: 10.1074/jbc.M113.513366. PubMed PMID: 24311787; PubMed Central PMCID: PMC3916558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Monnet C, Jorieux S, Souyris N, Zaki O, Jacquet A, Fournier N, Crozet F, de Romeuf C, Bouayadi K, Urbain R, Behrens CK, Mondon P, Fontayne A. Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody. MAbs. 2014;6(2):422–36. doi: 10.4161/mabs.27854. Epub 2014/02/05. doi: 10.4161/mabs.27854. PubMed PMID: 24492301; PubMed Central PMCID: PMC3984331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108(10):3573–9. doi: 10.1182/blood-2006-05-024539. Epub 2006/07/20. doi: 10.1182/blood-2006-05-024539. PubMed PMID: 16849638. [DOI] [PubMed] [Google Scholar]
- 137.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, Corti D, Moldt B, Hel Z, Lanzavecchia A, Ruprecht RM, Burton DR, Mestecky J, Anderson DJ, Forthal DN. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9(11):e1003776. doi: 10.1371/journal.ppat.1003776. Epub 2013/11/28. doi: 10.1371/journal.ppat.1003776. PubMed PMID: 24278022; PubMed Central PMCID: PMC3836734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kinlock BL, Wang Y, Turner TM, Wang C, Liu B. Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. PLoS One. 2014;9(5):e96760. doi: 10.1371/journal.pone.0096760. Epub 2014/05/17. doi: 10.1371/journal.pone.0096760. PubMed PMID: 24830293; PubMed Central PMCID: PMC4022679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weflen AW, Baier N, Tang QJ, Van den Hof M, Blumberg RS, Lencer WI, Massol RH. Multivalent immune complexes divert FcRn to lysosomes by exclusion from recycling sorting tubules. Mol Biol Cell. 2013;24(15):2398–405. doi: 10.1091/mbc.E13-04-0174. Epub 2013/06/07. doi: 10.1091/mbc.E13-04-0174. PubMed PMID: 23741050; PubMed Central PMCID: PMC3727932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baker K, Rath T, Pyzik M, Blumberg RS. The Role of FcRn in Antigen Presentation. Front Immunol. 2014;5:408. doi: 10.3389/fimmu.2014.00408. Epub 2014/09/16. doi: 10.3389/fimmu.2014.00408. PubMed PMID: 25221553; PubMed Central PMCID: PMC4145246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–7. doi: 10.1038/mi.2011.39. Epub 2011/09/23. doi: 10.1038/mi.2011.39. PubMed PMID: 21937984. [DOI] [PubMed] [Google Scholar]
- 142.Moura IC, Centelles MN, Arcos-Fajardo M, Malheiros DM, Collawn JF, Cooper MD, Monteiro RC. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med. 2001;194(4):417–25. doi: 10.1084/jem.194.4.417. Epub 2001/08/22. PubMed PMID: 11514599; PubMed Central PMCID: PMC2193503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou M, Ruprecht RM. Are anti-HIV IgAs good guys or bad guys? Retrovirology. 2014;11(1):109. doi: 10.1186/s12977-014-0109-5. doi: 10.1186/s12977-014-0109-5. PubMed PMID: 25499540; PubMed Central PMCID: PMC4297362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, Reinherz EL, Gupta S, Forthal DN, Sattentau QJ, Villinger F, Corti D, Ruprecht RM. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. Aids. 2013;27(9):F13–20. doi: 10.1097/QAD.0b013e328360eac6. Epub 2013/06/19. doi: 10.1097/QAD.0b013e328360eac6. PubMed PMID: 23775002; PubMed Central PMCID: PMC4084966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JG, Valerius T. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol. 2007;179(5):2936–43. doi: 10.4049/jimmunol.179.5.2936. Epub 2007/08/22. PubMed PMID: 17709508. [DOI] [PubMed] [Google Scholar]
- 146.Sholukh AM, Watkins JD, Vyas HK, Gupta S, Lakhashe SK, Thorat S, Zhou M, Hemashettar G, Bachler BC, Forthal DN, Villinger F, Sattentau QJ, Weiss RA, Agatic G, Corti D, Lanzavecchia A, Heeney JL, Ruprecht RM. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: Complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine. 2015;33(17):2086–95. doi: 10.1016/j.vaccine.2015.02.020. Epub 2015/03/15. doi: 10.1016/j.vaccine.2015.02.020. PubMed PMID: 25769884; PubMed Central PMCID: PMC4411954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86(8):4644–57. doi: 10.1128/JVI.06812-11. Epub 2012/02/22. doi: 10.1128/JVI.06812-11. PubMed PMID: 22345466; PubMed Central PMCID: PMC3318604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirbod T, Kong X, Kigozi G, Ndyanabo A, Serwadda D, Prodger JL, Tobian AA, Nalugoda F, Wawer MJ, Shahabi K, Rojas OL, Gommerman JL, Broliden K, Kaul R, Gray RH. HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS Pathog. 2014;10(10):e1004416. doi: 10.1371/journal.ppat.1004416. doi: 10.1371/journal.ppat.1004416. PubMed PMID: 25275513; PubMed Central PMCID: PMC4183701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seaton KE, Ballweber L, Lan A, Donathan M, Hughes S, Vojtech L, Moody MA, Liao HX, Haynes BF, Galloway CG, Richardson BA, Karim SA, Dezzutti CS, McElrath MJ, Tomaras GD, Hladik F. HIV-1 specific IgA detected in vaginal secretions of HIV uninfected women participating in a microbicide trial in Southern Africa are primarily directed toward gp120 and gp140 specificities. PLoS One. 2014;9(7):e101863. doi: 10.1371/journal.pone.0101863. Epub 2014/07/24. doi: 10.1371/journal.pone.0101863. PubMed PMID: 25054205; PubMed Central PMCID: PMC4108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hirbod T, Broliden K, Kaul R. Genital immunoglobulin A and HIV-1 protection: virus neutralization versus specificity. Aids. 2008;22(17):2401–2. doi: 10.1097/QAD.0b013e328314e3a6. doi: 10.1097/QAD.0b013e328314e3a6. PubMed PMID: 18981783. [DOI] [PubMed] [Google Scholar]
- 151.Hirbod T, Kaul R, Reichard C, Kimani J, Ngugi E, Bwayo JJ, Nagelkerke N, Hasselrot K, Li B, Moses S, Kibera HIVSG, MacDonald KS, Broliden K. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. Aids. 2008;22(6):727–35. doi: 10.1097/QAD.0b013e3282f56b64. doi: 10.1097/QAD.0b013e3282f56b64. PubMed PMID: 18356602. [DOI] [PubMed] [Google Scholar]
- 152.Black KP, Cummins JE, Jr., Jackson S. Serum and secretory IgA from HIV-infected individuals mediate antibody-dependent cellular cytotoxicity. Clinical immunology and immunopathology. 1996;81(2):182–90. doi: 10.1006/clin.1996.0175. PubMed PMID: 8906750. [DOI] [PubMed] [Google Scholar]
- 153.Stieh DJ, King DF, Klein K, Liu P, Shen X, Hwang KK, Ferrari G, Montefiori DC, Haynes B, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Robb ML, Kim JH, Denny TN, Tomaras GD, Shattock RJ. Aggregate complexes of HIV-1 induced by multimeric antibodies. Retrovirology. 2014;11(1):78. doi: 10.1186/s12977-014-0078-8. doi: 10.1186/s12977-014-0078-8. PubMed PMID: 25274446; PubMed Central PMCID: PMC4193994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One. 2013;8(10):e76176. doi: 10.1371/journal.pone.0076176. doi: 10.1371/journal.pone.0076176. PubMed PMID: 24098437; PubMed Central PMCID: PMC3788792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hur EM, Patel SN, Shimizu S, Rao DS, Gnanapragasam PN, An DS, Yang L, Baltimore D. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120(23):4571–82. doi: 10.1182/blood-2012-04-422303. doi: 10.1182/blood-2012-04-422303. PubMed PMID: 23065154; PubMed Central PMCID: PMC3512234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chintalacharuvu KR, Vuong LU, Loi LA, Larrick JW, Morrison SL. Hybrid IgA2/IgG1 antibodies with tailor-made effector functions. Clin Immunol. 2001;101(1):21–31. doi: 10.1006/clim.2001.5083. Epub 2001/10/03. doi: 10.1006/clim.2001.5083. PubMed PMID: 11580223. [DOI] [PubMed] [Google Scholar]
- 157.Kelton W, Mehta N, Charab W, Lee J, Lee CH, Kojima T, Kang TH, Georgiou G. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol. 2014;21(12):1603–9. doi: 10.1016/j.chembiol.2014.10.017. Epub 2014/12/17. doi: 10.1016/j.chembiol.2014.10.017. PubMed PMID: 25500223. [DOI] [PubMed] [Google Scholar]
- 158.Yoo EM, Trinh KR, Lim H, Wims LA, Morrison SL. Characterization of IgA and IgM binding and internalization by surface-expressed human Fcalpha/mu receptor. Mol Immunol. 2011;48(15–16):1818–26. doi: 10.1016/j.molimm.2011.05.011. Epub 2011/06/03. doi: 10.1016/j.molimm.2011.05.011. PubMed PMID: 21632111. [DOI] [PubMed] [Google Scholar]
- 159.Bakema JE, van Egmond M. The human immunoglobulin A Fc receptor FcalphaRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011;4(6):612–24. doi: 10.1038/mi.2011.36. Epub 2011/09/23. doi: 10.1038/mi.2011.36. PubMed PMID: 21937986. [DOI] [PubMed] [Google Scholar]
- 160.Jasek M, Obojski A, Manczak M, Wisniewski A, Winiarska B, Malolepszy J, Jutel M, Luszczek W, Kusnierczyk P. Are single nucleotide polymorphisms of the immunoglobulin A Fc receptor gene associated with allergic asthma? Int Arch Allergy Immunol. 2004;135(4):325–31. doi: 10.1159/000082327. Epub 2004/11/27. doi: 10.1159/000082327. PubMed PMID: 15564774. [DOI] [PubMed] [Google Scholar]
- 161.Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15(8):901–6. doi: 10.1038/nm.1967. Epub 2009/05/19. doi: 10.1038/nm.1967. PubMed PMID: 19448633; PubMed Central PMCID: PMC2723177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–4. doi: 10.1038/nature10660. Epub 2011/12/06. doi: 10.1038/nature10660. PubMed PMID: 22139420; PubMed Central PMCID: PMC3253190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, Rao DS, An DS, Baltimore D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20(3):296–300. doi: 10.1038/nm.3471. Epub 2014/02/11. doi: 10.1038/nm.3471. PubMed PMID: 24509526; PubMed Central PMCID: PMC3990417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS research and human retroviruses. 2015;31(1):13–24. doi: 10.1089/aid.2014.0235. doi: 10.1089/AID.2014.0235. PubMed PMID: 25385703; PubMed Central PMCID: PMC4287163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Jr., Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503(7475):277–80. doi: 10.1038/nature12746. Epub 2013/11/01. doi: 10.1038/nature12746. PubMed PMID: 24172896; PubMed Central PMCID: PMC4133787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Buning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013;110(41):16538–43. doi: 10.1073/pnas.1315295110. Epub 2013/09/18. doi: 10.1073/pnas.1315295110. PubMed PMID: 24043801; PubMed Central PMCID: PMC3799352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Doyon G, Sobolewski MD, Huber K, McMahon D, Mellors JW, Sluis-Cremer N. Discovery of a small molecule agonist of phosphatidylinositol 3-kinase p110alpha that reactivates latent HIV-1. PLoS One. 2014;9(1):e84964. doi: 10.1371/journal.pone.0084964. Epub 2014/02/04. doi: 10.1371/journal.pone.0084964. PubMed PMID: 24489654; PubMed Central PMCID: PMC3906007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Klase Z, Yedavalli VS, Houzet L, Perkins M, Maldarelli F, Brenchley J, Strebel K, Liu P, Jeang KT. Activation of HIV-1 from latent infection via synergy of RUNX1 inhibitor Ro5-3335 and SAHA. PLoS Pathog. 2014;10(3):e1003997. doi: 10.1371/journal.ppat.1003997. Epub 2014/03/22. doi: 10.1371/journal.ppat.1003997. PubMed PMID: 24651404; PubMed Central PMCID: PMC3961356. [DOI] [PMC free article] [PubMed] [Google Scholar]