Abstract
Background
The influence of surgical margin status on long-term outcomes of patients undergoing adrenal resection for ACC remains not well defined. We studied the impact of surgical tumor margin status on recurrence-free survival (RFS) and overall survival (OS) of patients undergoing resection for ACC.
Methods
A total of 165 patients who underwent adrenal resection for ACC and met inclusion criteria were identified form a multi-institutional database. Clinicopathological data, pathologic margin status, and long-term outcomes were assessed. Patients were stratified into two groups based on margin status: R0 (margin >1 mm) versus R1.
Results
R0 resection was achieved in 126 patients (76.4 %), whereas 39 patients (23.6 %) had an R1 resection. Median and 5-year OS for patients undergoing R0 resection were 96.3 months and 64.8 % versus 25.1 months and 33.8 % for patients undergoing an R1 resection (both p < 0.001). On multivariable analysis, surgical margin status was an independent predictor of worse OS (hazard ratio [HR] 2.22, 95 % confidence interval [CI] 1.03–4.77; p = 0.04). The incidence of recurrence also differed between the two groups; 5-year RFS was 30.3 % among patients with an R0 resection versus 13.8 % among patients who had an R1 resection (p = 0.03). Lymph node metastasis (N1) was an independent predictor of RFS (HR 2.70, 95 % CI 1.04–6.99; p = 0.04).
Conclusions
A positive margin after ACC resection was associated with worse long-term survival. Patient selection and an emphasis on surgical technique to achieve R0 margins are pivotal to optimizing the best chance for long-term outcome among patients with ACC.
Adrenocortical carcinoma (ACC) is a rare, heterogenous endocrine malignancy with an incidence of 0.7–2 per million.1,2 Similar to other solid malignancies, such as colon cancer, the development of ACC likely proceeds through an adenoma to carcinoma sequence.3,4 Arising from the adrenal cortex, ACC tumors are classified either as functional or nonfunctional according to the hormonal activity of the tumor. Many nonfunctional tumors are sporadic and are diagnosed as incidental findings on cross-sectional imaging. Occasionally, ACC can be associated with hereditary syndromes, such as Li-Fraumeni, Beckwith-Wiedemann, multiple endocrine neoplasia type 1, congenital adrenal hyperplasia, familial adenomatous polyposis, Lynch syndrome, and Carney complex.5–10 Regardless of etiology, the cornerstone of treatment for ACC involves surgical resection.11–14 Despite the refinement of surgical technique and better selection of surgical candidates, the prognosis of patients with ACC can be guarded. Specifically, depending on the stage of disease, 5-year overall survival (OS) can range from 13 to 81 %.15 Several tumor-specific factors have been associated with outcome, including tumor size and high mitotic index/Ki67.11,16–19 The impact of operative factors, such as margin status, has been less well studied.
The effect of margin status on outcome has been well documented for several cancers, including primary colorectal cancer, colorectal liver metastasis, as well as hepatocellular carcinoma.20–24 In contrast, many studies investigating prognostic factors associated with ACC did not evaluate surgical margin status.18,25,26 Those few studies that did examine the effect of surgical margin status on long-term outcomes were limited to small, single-center case series.11,17 Given this, the purpose of the current study was to investigate the impact of margin status on recurrence-free (RFS) and overall (OS) survival of patients undergoing resection for ACC using a large, multi-center national collaborative database.
METHODS
Study Design
Patients who underwent curative intent resection for ACC between January 1993 and December 2014 at 13 tertiary academic centers in the United States were identified. The 13 institutions participating in the study included Johns Hopkins Hospital, Baltimore, MD; Emory University, Atlanta GA; Stanford University, Palo Alto, CA; Washington University, St. Louis, MO; Wake Forest University, Winston-Salem, NC; University of Wisconsin, Madison, WI; The Ohio State University, Columbus, OH; Medical College of Wisconsin, Milwaukee, WI; New York University, New York, NY; University of California at San Diego, San Diego, CA; University of California at San Francisco, San Francisco, CA; University of Texas Southwestern Medical Center, Dallas, TX; and Vanderbilt University Medical Center, Nashville, TN. Patients with distant metastasis and patients who underwent an incomplete macroscopic resection (R2) were excluded (n = 10). Only adult patients (≥18 years old) were included in the study cohort. The Institutional Review Board of the participating institutions approved the study.
Information on patient demographics, tumor characteristics, perioperative characteristics, pre/postoperative chemo/radiotherapy, and tumor recurrence were recorded. All operative specimens underwent standard histopathological evaluation. Data on T stage, tumor size, tumor laterality, capsular invasion, and lymph node status were collected. R0 resection was defined as the absence of macroscopic or microscopic extension of the tumor at the surgical margin, while R1 resection was defined as microscopic extension of the tumor along the line of resection. Data on surgical approach, intraoperative estimated blood loss (EBL), intraoperative blood transfusions, and reoperation were obtained. Postoperative complications were defined based on the Clavien–Dindo classification: minor complications were classified as grade I and II, major complications as grade III and IV, whereas in-hospital mortality within the first 30 days after surgery was considered a grade V complication.27 Among those patients who experienced multiple complications, the highest grade complication was used for analytic purposes. Long-term outcomes included RFS and OS. Recurrence was defined as the presence of a biopsy-proven mass or the presence of a highly suspicious lesion on cross-sectional imaging.
Statistical Analysis
Demographic, clinicopathologic, and perioperative features of the study population were stratified according to margin status (R0 vs. R1). Continuous variables were presented as medians (interquartile range (IQR)) and baseline characteristics were described as frequencies and distributions. The differences between groups were assessed by the χ2, Student’s t test, and Mann–Whitney test, as appropriate. A relatively small subset of the cohort had missing data, and analyses suggested that these data were missing at random (MAR); as such, multiple imputation was performed for missing covariates. Univariate and multivariate models were assessed to determine the association of relevant clinicopathological factors with RFS and OS. Factors that were included in the univariate/multivariate model were selected based on clinical interest and scientific knowledge deriving from the scientific literature. Kaplan–Meier survival curves were utilized to estimate the median, 1-, 3-, and 5-year RFS and OS, whereas the log-rank test was used to compare differences in survival. All analyses were performed with Stata version 12.0 (StataCorp, College Station, TX), and p < 0.05 (two tailed) was considered statistically significant.
RESULTS
Demographic, Clinicopathologic, and Perioperative Characteristics
A total of 165 patients who underwent curative intent adrenal resection for ACC met the inclusion criteria and were included in the analytic cohort. Baseline characteristics of the cohort are summarized in Table 1. The median age at diagnosis was 53 years (IQR 45.0–63.0), and there was a female predominance with a male:female ratio of 1:2. The majority of patients were Caucasian (n = 129, 78.2 %). Almost half of the patients had an adrenal tumor that penetrated through the gland to the surface of nearby organs/structures or the fat that surrounds the gland (T3–T4 tumors: n = 74, 46.5 %); nodal metastasis was relatively uncommon (n = 14, 8.5 %). Almost one third of the tumors were clinically or biochemically considered as functional (n = 54, 34.5 %), often producing cortisol (n = 28, 17.9 %) and androgens (n = 18, 11.5 %). Mineralocorticoid-producing tumors comprised only 5.1 % (n = 8) of the tumors. Median tumor size was 11 cm (IQR 7.7–15.0); 87 patients presented with a left adrenal tumor versus 75 patients who presented with a right adrenal tumor.
TABLE 1.
Clinicopathologic characteristics of the study group according to margin status
| Variable | Total (N = 165) |
R0 (N = 126) |
R1 (N = 39) |
p Value |
|---|---|---|---|---|
| Age (years) median (IQR) | 53.0 (45.0–63.0) | 51.0 (45.0–59.5) | 57.0 (48.0–67.0) | 0.11 |
| Gender | 0.63 | |||
| Female | 109 (66.1) | 82 (65.1) | 27 (69.2) | |
| Male | 56 (33.9) | 44 (34.9) | 12 (30.8) | |
| Race | 0.79 | |||
| White | 129 (78.2) | 100 (79.4) | 29 (74.4) | |
| Black | 10 (6.1) | 7 (5.6) | 3 (7.7) | |
| Others | 26 (15.8) | 19 (15.1) | 7 (17.9) | |
| Size (cm) median (IQR) | 11.0 (7.7–15.0) | 11.2 (7.8–15.0) | 10.8 (7.4–13.5) | 0.60 |
| Body mass index (kg/m2) | 27.0 (24.0–33.0) | 26.9 (24.0–31.0) | 31.0 (22.0–34.0) | 0.31 |
| Capsular invasion (n = 121) | <0.001 | |||
| No | 53 (43.8) | 49 (52.7) | 4 (14.3) | |
| Yes | 68 (56.2) | 44 (47.3) | 24 (85.7) | |
| Laterality (n = 162) | 0.47 | |||
| Left | 87 (53.7) | 68 (55.3) | 19 (48.7) | |
| Right | 75 (46.3) | 55 (44.7) | 20 (51.3) | |
| Nodal status | 0.08 | |||
| Negative | 37 (22.4) | 33 (26.2) | 4 (10.3) | |
| Positive | 14 (8.5) | 9 (7.1) | 5 (12.8) | |
| Not harvested | 114 (69.1) | 84 (66.7) | 30 (76.9) | |
| T stage (n = 159) | 0.05 | |||
| T1/T2 | 85 (53.5) | 70 (57.9) | 15 (39.5) | |
| T3/T4 | 74 (46.5) | 51 (42.1) | 23 (60.5) | |
| ASA (N = 118) | 0.12 | |||
| I | 22 (18.6) | 17 (18.1) | 5 (20.8) | |
| II | 31 (26.3) | 29 (30.9) | 2 (8.3) | |
| III | 57 (48.3) | 43 (45.7) | 14 (58.3) | |
| IV | 8 (6.8) | 5 (5.3) | 3 (12.5) | |
| Hormonal secretion (n = 156) | 0.17 | |||
| Glucocorticoid | 28 (17.9) | 17 (14.3) | 11 (29.7) | |
| Mineralocorticoid | 8 (5.1) | 7 (5.9) | 1 (2.7) | |
| Virilizing/feminizing | 18 (11.5) | 15 (12.6) | 3 (8.1) | |
| Nonsecreting | 102 (65.4) | 80 (67.2) | 22 (59.5) | |
| Operation approach (n = 162) | 0.78 | |||
| Open abdominal or posterior | 103 (63.6) | 80 (65.0) | 23 (59.0) | |
| Minimally invasive surgery | 34 (21.0) | 25 (20.3) | 9 (23.1) | |
| Open thoracoabdominal surgery | 25 (15.4) | 18 (14.6) | 7 (17.9) | |
| Transfusion | 36 (21.8) | 24 (19.0) | 12 (30.8) | 0.12 |
| Complication | 42 (25.4) | 32 (25.4) | 10 (25.6) | 0.98 |
| Grade of complications | 0.14 | |||
| I and II | 29 (69.0) | 24 (75.0) | 5 (50.0) | |
| III and IV | 13 (31.0) | 8 (25.0) | 5 (50.0) | |
| Neoadjuvant chemotherapy | 2 (1.2) | 1 (0.79) | 1 (2.6) | 0.38 |
| Adjuvant chemotherapy | 17 (10.3) | 12 (9.5) | 5 (12.8) | 0.55 |
| Postoperative mitotane | 47 (28.5) | 35 (27.8) | 12 (30.8) | 0.72 |
| Estimated blood loss (ml) | 500 (150–1400) | 500 (150–1200) | 600 (200–2000) | 0.46 |
At the time of surgery, the majority of patients underwent an adrenalectomy using an open approach (n = 128, 79 %), whereas one fifth of patients underwent a minimally invasive adrenal resection (n = 34, 21.0 %). Median EBL was 500 mL (IQR 150–1400) and a minority (n = 36, 21.8 %) of patients received an intraoperative blood transfusion. Postoperatively, approximately one fourth of patients experienced a complication within 30 days after surgery (n = 42, 25.4 %); the majority of complications were classified as minor/grade 1–2 (n = 29, 69.0 %). Only 1 patient died within 30 days after surgery for a postoperative mortality of 0.6 %. Use of preoperative (n = 2, 1.2 %) and postoperative (n = 17, 10.3 %) chemotherapy was uncommon; 47 (28.5 %) patients received postoperative mitotane.
An R0 margin status was achieved in 126 (76.4 %) patients and an R1 margin in 39 (23.6 %) patients. The majority of demographic, clinical, and tumor characteristics were similar between the two groups (Table 1). For example, tumor size and T stage were comparable between the two groups. In contrast, patients who underwent R1 resections were more likely to have tumors with capsular invasion (85.7 %) compared with patients who had R0 resections (47.3 %; p < 0.001).
Associations of Margin Status with Recurrence-free Survival
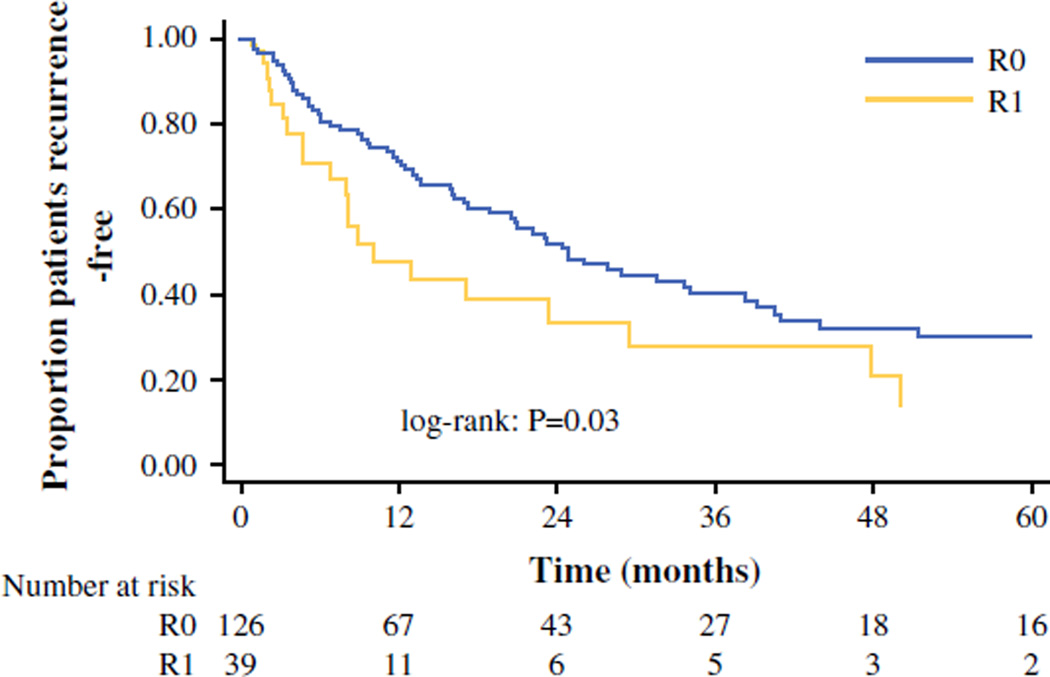
At a median follow-up of 22.9 months, 53.9 % of the overall cohort (n = 89) had developed a recurrence. Median, 1-, 3-, and 5-year RFS for the entire cohort was 23.2 months, 66.3, 37.6, and 27.0 %, respectively (Fig. 1; Table 2). Five-year RFS of patients who underwent a R0 resection was 30.3 versus 13.8 % of patients who underwent an R1 resection (p = 0.03). On univariate analysis, presence of a positive surgical margin was a predictor of shorter RFS (hazard ratio [HR] 1.71, 95 % CI 1.05–2.78; p = 0.03; Table 2); however, after adjusting for other clinicopathologic factors, only the presence of lymph node metastasis (N1) remained an independent predictor of RFS (HR 2.70, 95 % CI 1.04–6.99; p = 0.04; Table 2), whereas margin status was no longer associated with RFS (HR 1.06, 95 % CI 0.58–1.94).
FIG. 1.
Recurrence-free survival after ACC resection stratified by margin status
TABLE 2.
Predictive factors of recurrence-free survival among patients undergoing adrenal resection for ACC
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p Value | HR | 95 % CI | p Value | |
| Margin | ||||||
| R0 | Ref | |||||
| R1 | 1.71 | (1.05–2.78) | 0.03 | 1.06 | (0.58–1.94) | 0.84 |
| Age | 1.00 | (0.98–1.02) | 0.99 | 1.00 | (0.98–1.02) | 0.99 |
| Male gender | 1.16 | (0.75–1.80) | 0.49 | 1.16 | (0.69–1.96) | 0.57 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 0.84 | (0.36–1.94) | 0.68 | 0.8 | (0.33–1.95) | 0.63 |
| Other | 1.19 | (0.67–2.11) | 0.56 | 0.79 | (0.40–1.54) | 0.48 |
| Laterality | ||||||
| Left | Ref | Ref | ||||
| Right | 1.09 | (0.71–1.66) | 0.69 | 1.03 | (0.63–1.67) | 0.91 |
| Functional tumor | 1.58 | (1.03–2.42) | 0.04 | 1.02 | (0.52–1.96) | 0.96 |
| Cortisol-secreting tumor | 2.18 | (1.27–3.72) | 0.004 | 2.14 | (0.96–4.72) | 0.06 |
| T stage | ||||||
| I/II | Ref | Ref | ||||
| III/IV | 2.06 | (1.33–3.19) | 0.001 | 1.70 | (0.95–3.04) | 0.07 |
| Capsular invasion | 1.63 | (1.03–2.58) | 0.04 | 1.19 | (0.64–2.23) | 0.58 |
| Tumor size | 1.04 | (1.00–1.08) | 0.08 | 1.02 | (0.97–1.08) | 0.34 |
| Postoperative chemotherapy | 1.58 | (0.84–2.98) | 0.16 | 1.35 | (0.62–2.94) | 0.45 |
| Postoperative mitotane | 1.54 | (0.98–2.43) | 0.06 | 1.21 | (0.68–2.14) | 0.52 |
| N stage | ||||||
| N0 | Ref | Ref | ||||
| N1 | 3.00 | (1.30–6.95) | 0.01 | 2.70 | (1.04–6.99) | 0.04 |
| Nx | 1.19 | (0.72–1.98) | 0.5 | 1.31 | (0.72–2.35) | 0.37 |
Associations of Margin Status with Overall Survival
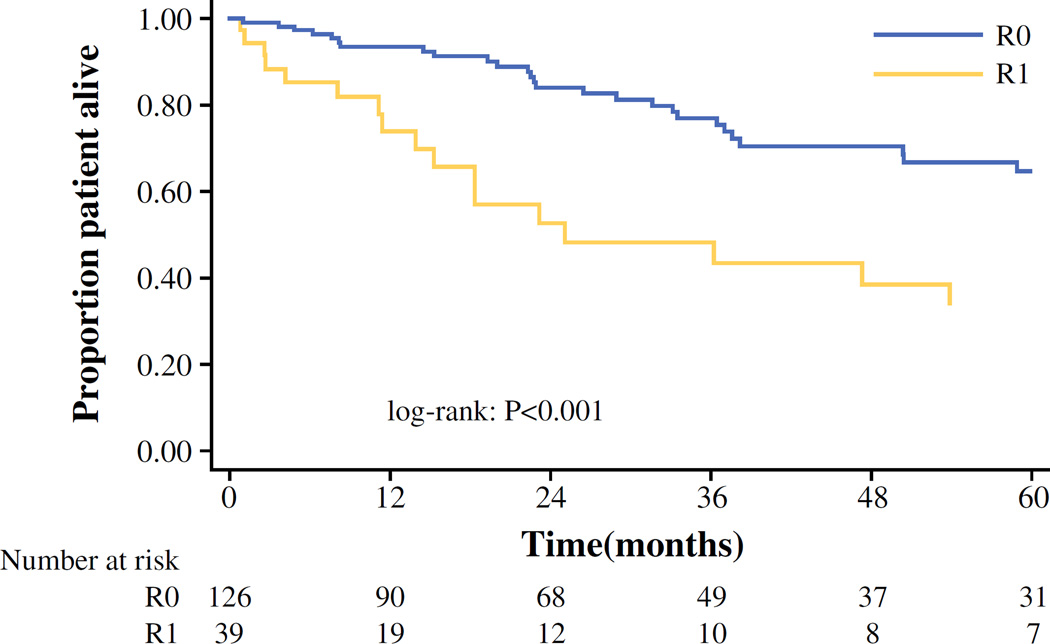
Median and 1-, 3-, and 5-year OS for the entire cohort was 86.3 months, 89.2, 70.9, and 57.7 %, respectively. Patients who underwent an R0 resection had a median survival and a 5-year OS of 96.3 months (95 % CI 60.7–207.4) and 64.8 % respectively, compared with only 25.1 months (95 % CI 13.9–76.1) and 33.8 % for patients who underwent an R1 resection (both p < 0.001; Fig. 2). While on univariate analysis, both the presence of positive margins (HR 2.65, 95 % CI 1.49–4.73; p = 0.001) and nodal metastasis (HR 3.46, 95 % CI 1.09–10.97; p = 0.04) were associated with worse OS (Table 3); on multivariate analysis, only the presence of positive surgical margin (HR 2.22, 95 % CI 1.03–4.77; p = 0.04) remained an independent predictor of worse OS (Table 3).
FIG. 2.
Overall survival after ACC resection stratified by margin status
TABLE 3.
Predictive factors of overall survival among patients undergoing adrenal resection for ACC
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p Value | HR | 95 % CI | p Value | |
| Margin | ||||||
| R0 | Ref | Ref | ||||
| R1 | 2.65 | (1.49–4.73) | 0.001 | 2.22 | (1.03–4.77) | 0.04 |
| Age | 0.99 | (0.97–1.02) | 0.63 | 0.99 | (0.97–1.01) | 0.39 |
| Male gender | 0.86 | (0.49–1.53) | 0.61 | 0.85 | (0.43–1.67) | 0.64 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 0.84 | (0.26–2.73) | 0.77 | 0.88 | (0.24–3.17) | 0.84 |
| Other | 0.85 | (0.38–1.90) | 0.69 | 0.42 | (0.15–1.17) | 0.1 |
| Laterality | ||||||
| Left | Ref | Ref | ||||
| Right | 1.36 | (0.79–2.35) | 0.27 | 1.45 | (0.79–2.65) | 0.23 |
| Functional tumor | 1.5 | (0.86–2.59) | 0.15 | 1.19 | (0.52–2.75) | 0.68 |
| Cortisol-secreting tumor | 1.72 | (0.86–3.48) | 0.13 | 1.19 | (0.42–3.37) | 0.74 |
| T stage | ||||||
| I/II | Ref | Ref | ||||
| III/IV | 1.74 | (0.96–3.16) | 0.07 | 1.30 | (0.61–2.77) | 0.50 |
| Capsular invasion | 1.82 | (0.91–3.62) | 0.09 | 1.39 | (0.55–3.49) | 0.48 |
| Tumor size | 1.05 | (0.99–1.10) | 0.09 | 1.04 | (0.98–1.11) | 0.2 |
| Postoperative chemotherapy | 0.92 | (0.34–2.52) | 0.87 | 0.61 | (0.15–2.42) | 0.48 |
| Postoperative mitotane | 1.21 | (0.64–2.28) | 0.55 | 0.91 | (0.42–2.00) | 0.82 |
| N stage | ||||||
| N0 | Ref | Ref | ||||
| N1 | 3.46 | (1.09–10.97) | 0.04 | 2.73 | (0.69–10.8) | 0.15 |
| Nx | 1.98 | (0.88–4.44) | 0.1 | 1.97 | (0.81–4.82) | 0.14 |
DISCUSSION
Adrenal resection represents the best treatment option for ACC, because it provides patients with potential for long-term cure and survival.28 While several reports have examined recurrence after resection in ACC, substantially fewer have exclusively focused on the effect of surgical margin.11,16,19,29 In a study by Ip et al. surgical margin status was not a significant prognostic factor for either OS or RFS in multivariable analysis.19 In a separate study by Ayala-Ramirez, surgical margin status was not significant for OS but was significant only for local RFS.11 Given these disparate findings, we sought to define the impact of margin status on long-term outcomes among patients with localized disease undergoing a curative-intent resection (R0/R1). In the present cohort, approximately one fourth of patients had an R1 resection, and perhaps more importantly, a resection with microscopically positive margins was associated with worse long-term outcome compared with an R0 resection. The incidence of R1 resection in the current study was relatively high (23.6 %). The high incidence of an R1 resection may seem somewhat surprising, especially given that patients were treated at specialized tertiary centers.11,17 However, quite often patients with ACC are referred to tertiary centers at an advanced stage, with large tumors that present technical difficulties.13 Consistent with this, the median tumor size of ACC lesions among patients treated in the current study was 11 cm. Of note, the incidence of R0 resection was similar among patients undergoing a minimally invasive (77.7 %) versus open (73.5 %) ACC resection (p = 0.62), suggesting that a minimally invasive approach can indeed achieve an R0 margin in appropriately selected patients. These data are important, because there is an ongoing debate on the best surgical approach for localized ACC.30–33
In one study, Ayala-Ramirez et al. reported that margin status was not an independent predictor of worse OS after surgery for ACC.11 In this study, however, only a small subset of patients who were referred underwent surgical resection and the analyses may have been underpowered to detect an effect of margin status on outcome (i.e., type II statistical error). In contrast, in a separate study involving a larger cohort of 1,400 different hospitals in the National Cancer Data Base (NCDB), Bilimoria et al. reported that an R1 resection was associated with an unfavorable prognosis (HR 1.81, 95 % CI 1.44–2.27; p < 0.0001).17 This study likely suffered from significant heterogeneity in how surgical margin status was determined among the 1400 hospitals, many of which did only a few cases. While some differences in surgical pathology interpretation also were likely in the current study, the variability was undoubtedly less, given that each of 13 centers were high-volume academic centers. By collaborating with 13 centers throughout the United States, we were able to obtain an adequate sample size to examine margin status compared with other single-center reports. Of note, we found that surgical margin status was an independent predictor of worse OS. Moreover, when stratifying the patients based on surgical margin status, 5-year survival among patients who underwent an R1 resection was almost one half of the long-term outcome of patients who had a R0 resection (33.8 vs. 64.8 %). The reason for the worse outcome among patients with R1 disease is undoubtedly multifactorial. In addition, unlike the NCDB study, we were able to assess microscopic vascular invasion. Interestingly, the presence of capsular invasion was the only factor associated with an increased risk of an R1 resection. Weiss et al. had previously noted that capsular invasion can be characterized by nests or cords of tumor cells extending into or through the capsule with stromal reaction and was a surrogate for an aggressive underlying tumor biology.34 Collectively, the data strongly suggest that surgical margin status is an important factor associated with long-term outcome. This finding is in accordance with previous studies on other solid malignancies that have reported a more favorable prognosis among patients who undergo complete tumor resection.23,35–39
Besides investigating the prognostic role of the surgical margin status, we also defined other patient- and tumor-related factors that were associated with a worse prognosis among patients who underwent curative intent surgical resection. For example, in the current study, 27 % of patients who underwent lymph node dissection had lymph node metastasis. In turn, lymph node metastasis was noted to be an important prognostic factor of RFS—consistent with previous reports on ACC.40,41 The finding that lymph node metastasis had an impact on staging and prognosis is clinically relevant in that lymph node dissection remains a topic of debate among surgeons, because it is not a routine component of radical adrenalectomy.13,42 For example, only 30 % of patients in our large nationwide collaborative had lymph node sampling performed. In contrast to lymph node metastasis, we did not find an association of patient age, tumor size, or tumor grade with prognosis. As such, these patient- and tumor-related characteristics should not preclude resection when an R0 margin can be achieved.
Several limitations should be considered when interpreting the current study. As with all retrospective analyses, there may have been selection bias regarding how patients were chosen for surgical resection. This bias may have led to the avoidance of surgery for certain patients with ACC who were perceived to have a high risk of a positive margin—thereby under-representing the number of patients who would have ultimately undergone an R1 resection. In addition, while the current study involved 13 major tertiary medical centers, the sample size remained relatively small (n ≈ 300), and this impacted our ability to do certain subset analyses. Moreover, all participating centers were tertiary centers where ACC is treated by experienced teams. As such, the data may not be completely generalizable (e.g., “true” incidence of R1 resection) to the community hospital setting. While the multi-institutional nature of the dataset strengthens the statistical analysis, potential differences in histopathologic processing and margin measurements of ACC specimens by different pathologists across the 13 institutions may have occurred.
CONCLUSIONS
A positive margin after resection of ACC was associated with worse long-term survival. In fact, while many patient and tumor related factors were not associated with RFS and OS, R0 margin had a strong impact on long-term outcome. Patient- or tumor-related factors, such as the tumor size, should not preclude patients from curative intent resection when an R0 resection is feasible. Patient selection and an emphasis on surgical technique to achieve R0 margins are pivotal to optimizing the best chance for long-term outcome.
Footnotes
DISCLOSURE None.
REFERENCES
- 1.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 2.Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49:2579–2586. doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Custodio G, Parise GA, Kiesel Filho N, Komechen H, Sabbaga CC, Rosati R, et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J Clin Oncol. 2013;31:2619–2626. doi: 10.1200/JCO.2012.46.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratakis CA. Adrenal cancer in 2013: Time to individualize treatment for adrenocortical cancer? Nat Rev Endocrinol. 2014;10:76–78. doi: 10.1038/nrendo.2013.263. [DOI] [PubMed] [Google Scholar]
- 5.Bougeard G, Sesboue R, Baert-Desurmont S, Vasseur S, Martin C, Tinat J, et al. Molecular basis of the Li-Fraumeni syndrome: an update from the French LFS families. J Med Genet. 2008;45:535–538. doi: 10.1136/jmg.2008.057570. [DOI] [PubMed] [Google Scholar]
- 6.Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012;166:269–279. doi: 10.1530/EJE-11-0679. [DOI] [PubMed] [Google Scholar]
- 7.Lapunzina P. Risk of tumorigenesis in overgrowth syndromes: a comprehensive review. Am J Med Genet C Semin Med Genet. 2005;137C:53–71. doi: 10.1002/ajmg.c.30064. [DOI] [PubMed] [Google Scholar]
- 8.Medina-Arana V, Delgado L, Gonzalez L, Bravo A, Díaz H, Salido E, et al. Adrenocortical carcinoma, an unusual extra-colonic tumor associated with Lynch II syndrome. Fam Cancer. 2011;10:265–271. doi: 10.1007/s10689-010-9416-8. [DOI] [PubMed] [Google Scholar]
- 9.Morin E, Mete O, Wasserman JD, Joshua AM, Asa SL, Ezzat S. Carney complex with adrenal cortical carcinoma. J Clin Endocrinol Metab. 2012;97:E202–E206. doi: 10.1210/jc.2011-2321. [DOI] [PubMed] [Google Scholar]
- 10.Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol. 2013;31:3012–3018. doi: 10.1200/JCO.2012.48.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayala-Ramirez M, Jasim S, Feng L, Ejaz S, Deniz F, Busaidy N, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169:891–899. doi: 10.1530/EJE-13-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livhits M, Li N, Yeh MW, Harari A. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery. 2014;156:1531–1540. doi: 10.1016/j.surg.2014.08.047. discussion 1540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihai R. Diagnosis, treatment and outcome of adrenocortical cancer. Br J Surg. 2015;102:291–306. doi: 10.1002/bjs.9743. [DOI] [PubMed] [Google Scholar]
- 14.Ronchi CL, Kroiss M, Sbiera S, Deutschbein T, Fassnacht M. EJE prize 2014: current and evolving treatment options in adrenocortical carcinoma: where do we stand and where do we want to go? Eur J Endocrinol. 2014;171:R1–R11. doi: 10.1530/EJE-14-0273. [DOI] [PubMed] [Google Scholar]
- 15.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 16.Beuschlein F, Weigel J, Saeger W, Kroiss M, Wild V, Daffara F, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100:841–849. doi: 10.1210/jc.2014-3182. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 18.Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99:455–461. doi: 10.1210/jc.2013-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ip JC, Pang TC, Glover AR, Soon P, Clarke S, Richardson A, et al. Improving outcomes in adrenocortical cancer: an Australian perspective. Ann Surg Oncol. 2014;22(7):2309–2316. doi: 10.1245/s10434-014-4133-4. [DOI] [PubMed] [Google Scholar]
- 20.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 21.Margonis GA, Spolverato G, Kim Y, Ejaz A, Pawlik TM. Intraoperative surgical margin re-resection for colorectal liver metastasis: is it worth the effort? J Gastrointest Surg. 2015;19:699–707. doi: 10.1007/s11605-014-2710-2. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Wahab M, El-Husseiny TS, El Hanafy E, El Shobary M, Hamdy E. Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg. 2010;395:625–632. doi: 10.1007/s00423-010-0643-0. [DOI] [PubMed] [Google Scholar]
- 23.Schiffman SC, Woodall CE, Kooby DA, Martin RC, Staley CA, Egnatashvili V, et al. Factors associated with recurrence and survival following hepatectomy for large hepatocellular carcinoma: a multicenter analysis. J Surg Oncol. 2010;101:105–110. doi: 10.1002/jso.21461. [DOI] [PubMed] [Google Scholar]
- 24.Khan MA, Hakeem AR, Scott N, Saunders RN. Significance of R1 resection margin in colon cancer resections in the modern era. Colorectal Dis. 2015 doi: 10.1111/codi.12960. [DOI] [PubMed] [Google Scholar]
- 25.Loncar Z, Djukic V, Zivaljevic V, Pekmezovic T, Diklic A, Tatic S, et al. Survival and prognostic factors for adrenocortical carcinoma: a single institution experience. BMC Urol. 2015;15:43. doi: 10.1186/s12894-015-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanis KN, Kanthan R. Diagnostic and prognostic features in adrenocortical carcinoma: a single-institution case series and review of the literature. World J Surg Oncol. 2015;13:117. doi: 10.1186/s12957-015-0527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller BS, Doherty GM. Surgical management of adrenocortical tumours. Nat Rev Endocrinol. 2014;10:282–292. doi: 10.1038/nrendo.2014.26. [DOI] [PubMed] [Google Scholar]
- 29.Hwang EC, Hwang I, Jung SI, Kang TW, Kwon DD, Heo SH, et al. Prognostic factors for recurrence-free and overall survival after adrenalectomy for metastatic carcinoma: a retrospective cohort pilot study. BMC Urol. 2014;14:41. doi: 10.1186/1471-2490-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubbs EG, Callender GG, Xing Y, Perrier ND, Evans DB, Phan AT, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–270. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 31.Leboulleux S, Deandreis D, Al Ghuzlan A, Aupérin A, Goéré D, Dromain C, et al. Adrenocortical carcinoma: is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol. 2010;162:1147–1153. doi: 10.1530/EJE-09-1096. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi CP, Raffaelli M, De Crea C, Boniardi M, De Toma G, Marzano LA, et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery. 2012;152:1158–1164. doi: 10.1016/j.surg.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Porpiglia F, Garrone C, Giraudo G, Destefanis P, Fontana D, Morino M. Transperitoneal laparoscopic adrenalectomy: experience in 72 procedures. J Endourol. 2001;15:275–279. doi: 10.1089/089277901750161755. [DOI] [PubMed] [Google Scholar]
- 34.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Chen HW, Liao S, Lau WY, Wang FJ, Deng FW, Lai EC, et al. Prognostic impact of hepatic resection for hepatocellular carcinoma: the role of the surgeon in achieving R0 resection–a retrospective cohort study. Int J Surg. 2015;13:297–301. doi: 10.1016/j.ijsu.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Paik KY, Jung JC, Heo JS, Choi SH, Choi DW, Kim YI. What prognostic factors are important for resected intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol. 2008;23:766–770. doi: 10.1111/j.1440-1746.2007.05040.x. [DOI] [PubMed] [Google Scholar]
- 38.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi S, Doss C, Levy S, Levy R. TAPA-1, the target of an antiproliferative antibody, is associated on the cell surface with the Leu-13 antigen. J Immunol. 1990;145:2207–2213. [PubMed] [Google Scholar]
- 40.Gaujoux S, Brennan MF. Recommendation for standardized surgical management of primary adrenocortical carcinoma. Surgery. 2012;152:123–132. doi: 10.1016/j.surg.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Reibetanz J, Jurowich C, Erdogan I, Nies C, Rayes N, Dralle H, et al. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann Surg. 2012;255:363–369. doi: 10.1097/SLA.0b013e3182367ac3. [DOI] [PubMed] [Google Scholar]
- 42.Saade N, Sadler C, Goldfarb M. Impact of regional lymph node dissection on disease specific survival in adrenal cortical carcinoma. Horm Metab Res. 2015 doi: 10.1055/s-0035-1549877. [DOI] [PubMed] [Google Scholar]