Abstract
Background
Brain regions critical to episodic memory are altered during the preclinical stages of Alzheimer’s disease (AD). However, reliable means of identifying cognitively-normal individuals at higher risk to develop AD have not been established.
Objective
To examine whether fMRI can detect early functional changes associated with scene encoding in a group of presymptomatic Presenilin-1 (PSEN1) E280A mutation carriers.
Methods
Participants were 39 young, cognitively-normal individuals from an autosomal dominant early-onset AD kindred, located in Antioquia, Colombia. Participants performed an fMRI scene encoding task and a post-scan subsequent memory test.
Results
PSEN1 mutation carriers exhibited hyperactivation within medial temporal lobe regions during successful scene encoding (hippocampal formation, parahippocampal gyrus) compared to age-matched non-carriers.
Conclusion
Hyperactivation in medial temporal lobe regions during scene encoding is seen in individuals genetically-determined to develop AD years before their clinical onset. Our findings will guide future research with the ultimate goal of using functional neuroimaging in the early detection of preclinical AD.
Keywords: Alzheimer’s disease, autosomal-dominant, Presenilin-1, fMRI, memory encoding
Introduction
For the first time since Alzheimer’s disease (AD) was described in 1906, we now have disease-modifying drugs under testing. These new drugs hold the promise of changing the course of the disease. Recent research has found that treatments are ineffective when initiated at the symptomatic stages of AD, which are associated with irreversible neuronal damage [1, 2]. As a result, preclinical detection of the disease, as a means to enable early intervention and eventual disease prevention, is a priority in AD research [1]. Examining carriers of AD-causing mutations, including carriers of the Presenilin-1 (PSEN1) mutation, the focus of the study described here, provides a unique opportunity to study the pre-symptomatic stages of AD.
Disruptions to the episodic memory system are among the earliest AD-related changes. Episodic memory deficits are mainly due to the degeneration of brain regions crucial for the encoding and consolidation of new memories, including the hippocampus and entorhinal cortex within the medial temporal lobe (MTL) and regions that comprise a more distributed memory network [3, 4]. fMRI paradigms have been developed in healthy populations that reliably activate MTL regions during memory encoding, including the scene encoding task used here [5].
fMRI studies have reported increased MTL activation, or hyperactivation, in mild cognitive impairment (MCI) patients compared to matched controls [6–11]. MTL hyperactivation during memory encoding has also been shown in individuals at genetic risk to develop AD [12–15], and in cognitively-normal older adults [16] and mildly impaired with positive amyloid imaging [17]. The variability in these fMRI results may be explained by differences in the degree of cognitive impairment, severity of underlying neuropathology, or task performance.
Carriers of AD-causing mutations provide a unique opportunity to characterize the brain changes associated with the predisposition to AD. Presenilin-1 (PSEN1) mutation carriers will develop early-onset AD in adulthood (mean age of clinical onset: 44 years) with complete penetrance. While the examination of genetic forms of AD may not translate directly to sporadic forms of the disease, examination of these carriers allows the AD community to study and understand the structural and functional brain changes that occur in the absence of cognitive symptoms.
We have reported that young PSEN1 mutation carriers (mean age 39 years) demonstrated significantly greater anterior hippocampal activation compared to non-carrier family members while performing a face-name paired-associate learning task [15]. This pattern of hippocampal hyperactivation was also observed in even younger mutation carriers (mean age 23 years) from the same kindred [18]. Taken together, these studies favor the hypothesis that hippocampal hyperactivation is one of the earliest markers of AD-related degeneration. In both of our earlier studies we considered fMRI activation regardless of subject’s performance on a post-scan subsequent memory test. In the current study, we examined whether this pattern of MTL hyperactivation extends to the more specific contrast of successful memory encoding. To accomplish this goal, 39 members of a Colombian kindred with familial early-onset AD performed a fMRI scene encoding task followed by a subsequent memory test. We used a hypothesis-driven approach to focus specifically on the fMRI activation within MTL regions. In addition, we further investigated the data using an exploratory whole-brain analysis to examine whether the PSEN1 mutation carriers exhibited a different pattern of global brain activation compared to non-carriers. For the fMRI analyses, all scenes presented during the encoding phase were classified as subsequently remembered or subsequently forgotten, individually for each subject. Based on our previous research with familial AD, we hypothesized that successful memory encoding would result in MTL hyperactivation in presymptomatic mutation carriers compared to non-carriers.
Materials and Methods
Participants
Thirty-nine volunteers were recruited from the University of Antioquia (Colombia) Registry, which currently includes more than 1,500 living members with familial AD. Nineteen participants were positive for the AD–associated PSEN1 mutation E280A (carriers) (M age = 34.04 years SD= 5.87) whereas twenty were PSEN1 mutation negative and served as controls (M age=34.45, SD 6.29). All participants belonged to a single extended family. Carriers from this kindred have an estimated median age of 44 years (95% CI 43–45) at onset of mild cognitive impairment (MCI) and 49 years (49–50) at onset of dementia [19]. Family members who lived in close proximity to the University of Antioquia and the Instituto de Alta Tecnologia Medica were invited to participate. Potential participants were screened in advance for the presence of neurological and psychological disorders, drug use, and MR scanner compatibility. Of 44 participants recruited, 2 were excluded because of claustrophobia, and 3 because of having metal in their bodies.
Clinical Assessments
All participants underwent comprehensive clinical and neuropsychological assessments [19]. These assessments included a structured interview, which focused on identification of memory complaints and their effect on everyday life, family life, social life, and working life of participants, as well as a medical and neuropsychological examination. Clinical history and medical and neurological examinations were performed by a neurologist or a physician trained in dementia assessment. Neuropsychological tests were conducted by neuropsychologists and psychologists trained in neuropsychology. Medical history and neuropsychological assessments were recorded on the Systematized Information System for the Neuroscience Group of Antioquia (SISNE). The neuropsychological protocol included the Mini-Mental State Examination (MMSE) and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery, which has been adapted to this Colombian population [20]. Normative data were previously generated for this battery from PSEN1 E280A non-carriers from the same kindred [19]. In addition, activities of daily living (ADL) were assessed with the Barthel index and the Lawton Instrumental ADL scale. The Global Deterioration Scale (GDS) was also used to rate level of impairment.
Most PSEN1 carriers in the current study were also participants in an fMRI study of face-name paired-associate encoding that has been previously reported [15]. Participants were selected based on the following criteria: 1) no clinically significant cognitive decline (based on the full clinical assessment, including no cognitive impairment within any domain as indicated by the most recent CERAD neuropsychological assessment, which was conducted within six months prior to the time of the scanning session, and GDS = 1); 2) no impairment in ADLs or IADLs (Barthel = 50; Lawton = 8). All participants were screened for MRI compatibility.
All participants provided written informed consent before participating in accordance with the regulations and approval of the ethics committee of the University of Antioquia (Colombia). Researchers were blind to the genetic status of the participants during data collection. The Colombian protocol allowed de-identified data collected in Colombia to be analyzed by researchers in the United States. The study was also approved by the Boston University IRB Committee.
Genetic Analysis
For genetic analyses, genomic DNA was extracted from blood by standard protocols, and PSEN1 E280A characterization was done as previously described [21]. Genomic DNA was amplified with the primers PSEN1-S 5′ AACAGCTCAGGAGAGGAATG 3′ and PSEN1-AS 5′ GATGAGACAAGTNCCNTGAA 3′. We used the restriction enzyme BsmI for restriction fragment length polymorphism analysis. Each participant was classified as a PSEN1 E280A carrier or non-carrier. Investigators were blind to the genetic status of the participants during data collection and analysis.
Scene Encoding Task
Participants underwent fMRI scanning while viewing and encoding complex visual scenes. This task was based on a paradigm developed by Stern and colleagues [5]. We used an fMRI mixed-design format with three conditions that alternated in blocks during each scanning run: 1) Novel stimuli: participants saw 14 novel scenes per block and were asked to try to remember them for later testing; 2) Repeated stimuli: participants saw four scenes, previously viewed during a practice trial, repeated in the same order, three times each per block; 3) Visual fixation: participants saw a white fixation cross-hair on a black background (see Figure 1). Each of six scanning runs consisted of the following blocks: fixation (10 seconds), novel (40 seconds), fixation (24 seconds), repeated (40 seconds), fixation (24 seconds), novel (40 seconds), fixation (24 seconds), repeated (40 seconds), fixation (10 seconds). Before these scanning runs, participants did a practice run to familiarize themselves with the scenes that were later used in the “repeated” condition. The visual scenes, presented for 2.5 seconds each, consisted of 148 complex color pictures (4 repeated scenes, 144 novel scenes). During the scene encoding task, subjects were instructed to indicate whether they thought the picture showed a cold or warm weather scene, and to remember the scenes for later testing. Responses were collected with a response button-box that subjects used with their dominant hand.
Figure 1. Scene encoding task.
During scanning, participants were presented with a series of visual scenes separated into blocks of novel scenes and familiar scenes. Each block started with a white fixation cross. In novel blocks, participants viewed single presentations of 12 novel scenes (upper). In repeated blocks, participants viewed four familiar scenes that were repeated in the same order three times per block (lower). Participants responded to each scene by indicating whether it was a picture taken in a warm or cool climate.
Post-scan Subsequent Memory Test
Twenty minutes following the scanning session, participants performed a subsequent memory test in which all previously viewed scenes were presented along with an equal number of distractors. Participants indicated by button-box whether each scene was either old or new.
Within group recognition accuracy was determined using a discrimination index Pr (percent hits – percent false alarms). Independent samples t-tests were used to compare the behavioral performance between groups (non-carriers, PSEN1 carriers).
MRI Data Acquisition
Anatomical and functional data were acquired on a 1.5 Philips Achieva MR scanner at the Instituto de Alta Tecnología Médica (IATM) in Medellin, Colombia. For each subject, two high-resolution T1-weighted structural MRI scans were collected to examine cortical neuroanatomy (MPRAGE; field of view, 256 x 256; 1.0 x 1.0 x 1.0 mm; 176 slices). Automatic shimming procedures were performed. We ran five functional T2*-weighted gradient echo, echo-planar blood oxygenation level-dependent (BOLD) scans [repetition time (TR): 2 sec; echo time: 40 msec; flip angle: 90 degrees; field of view: 200 x 200; 24 slices] and acquired 134 images during each scan (3.125 x 3.125 mm in-plane resolution; 5 mm slice thickness; 1 mm skip between slices; resolution 64 x 64). Slices were aligned parallel to the Anterior Commissure (AC) - Posterior Commissure (PC).
fMRI Analyses
fMRI pre-processing
fMRI data were pre-processed with SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). Structural and functional image data were passed through a series of preprocessing steps: 1) Functional images were reoriented such that the origin (ie, coordinate xyz =[0 0 0]) was at the AC. 2) BOLD images were realigned to the first image within a run using INRIAlign toolbox [22] to correct for variance due to susceptibility-by-movement interactions. 3) The high-resolution structural images were coregistered to the mean BOLD image created during the motion correction step. 4) The high-resolution structural images were segmented into white matter and gray matter images. A bias-corrected structural image was also created at this step using the default tissue probability maps as priors. 5) A group template was generated using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) tool [23] 6) The bias-corrected structural images were spatially normalized to this template. 7) The coregistered BOLD images were spatially normalized into standard Montreal Neurological Institute (MNI) space using the DARTEL template and resampled to 3mm x 3mm isotropic voxels. 8) Finally, all BOLD images were spatially smoothed using a 6mm full-width at half-maximum Gaussian filter.
Successful encoding analysis
For each participant, stimuli were individually classified into subsequent remembered (i.e., encoding trials that lead to an “old” recognition response) and subsequent forgotten trials (i.e., encoding trials that lead to a “new” recognition response) based on their responses during the post-scan subsequent memory recognition test. No response trials (response omissions) were included in the model but were not considered in later analyses.
The hemodynamic response at stimulus onset for each event type (subsequently remembered and subsequently forgotten trials) was modeled by two response functions and their temporal derivative. Temporal derivatives were included in the model to account for the residual variance resulting from small temporal differences in the onset of the hemodynamic response. The functions were convolved with stimuli onsets to create covariates in a general linear model. Movement parameters were included in the model as covariates of no interest to capture residual movement artifacts.
To test the hypothesis that the magnitude of MTL (hippocampal formation, parahippocampal gyrus) activation was different in the PSEN1 mutation carriers compared to non-carriers, anatomical masks were created of specific ROIs using an atlas-based tool, WFU_PickAtlas [24]. A combined mask of the MTL regions (left MTL, right MTL) was created. Subsequently, parameter estimates (beta weights) were extracted from each participant’s remembered versus forgotten contrast image, after applying the functional ROI masks. For each participant, only the voxels significantly active within the ROIs at a statistical threshold of p<0.001 uncorrected were included. Parameter estimates were then averaged across participants for each ROI. For exploratory purposes, a whole brain SPM analysis was also performed. Whole brain SPMs were thresholded at a p < 0.005 (extent threshold = 20 voxels) uncorrected.
Structural MRI analysis
T1 structural data were processed through a semi-automated anatomical reconstruction and labeling procedure, using the ITK-snap software package [25] (http://www.itksnap.org) and methods described in a previous study [26]. Additionally, the Duvernoy atlas [27] was used as a reference for anatomical details. The hippocampal tail was outlined from the first appearance of ovoid mass of gray matter inferiomedial to the trigone of the lateral ventricle, and boundary landmarks included the fasciolar gyrus and the crus of the fornix. The hippocampal head was evident by the emergence of the uncal recess in the superomedial region of the hippocampus. Important boundaries for identifying the hippocampal head included the uncal recess of the inferior horn of the lateral horn and the alveus.
To examine possible confounding effects of the right and left hippocampal volumes on their respective right and left hippocampal activation during both novel and repeated conditions (as measured by the beta weights), we implemented two separate partial correlations with the hippocampal activation as the dependent variable and the hippocampal volume as the independent variable. We controlled for age and years of education by conducting partial correlations and including them in the model. The partial correlations were also corrected for multiple comparisons using the Bonferroni method. All statistical analyses were performed using statistical software (SPSS version 16.0).
Statistical analysis
Pearson correlation analyses were done to investigate the association between age, education, cognitive measures, post-scan subsequent memory performance, and MTL activation. An analysis of variance was used to compare the groups using the parameter estimates, adjusting for age.
Results
The presymptomatic PSEN1 mutation carriers and non-carriers did not differ in age, gender, education or MMSE. Furthermore, they did not differ in any of the CERAD neuropsychological battery tests (Table 1). There was no significant correlation between MTL activation and years of education (p=0.60).
Table 1.
Subject demographic and neuropsychological data
| Non-carriers (n= 19) | PSEN1 Carriers (n=20) | p-value | |
|---|---|---|---|
| Gender | 16 females/ 3 males | 15 females/ 5 male | |
| Age | 34.45 (6.29) | 34.04 (5.87) | 0.83 |
| Range | 24–49 | 25–44 | |
| Education | 10.80 (3.28) | 10.77 (2.30) | 0.79 |
| Range | 5–16 | 6–16 | |
|
| |||
| CERAD Tests | |||
| MMSE/ 30 | 29.5 (1.0) | 29.6 (0.5) | 0.51 |
| Verbal Fluency | 19.65 (5.38) | 18.57 (4.51) | 0.31 |
| Naming/ 15 | 13.55 (1.50) | 13.86 (1.08) | 0.52 |
| Memory Words | |||
| Reading | 10 (0.00) | 10 (0.00) | |
| Total Correct/ 30 | 19.35 (3.49) | 20.40 (3.34) | 0.32 |
| Total Intrusions | 1.65 (2.3) | 0.86 (0.99) | 0.17 |
| Recall of Words | |||
| Total Correct/ 10 | 7.15 (1.49) | 6.86 (1.58) | 0.32 |
| Total Intrusions | 0.35 (0.67) | 0.22 (0.42) | 0.35 |
| Recognition of Words | |||
| Correct “yes” /10 | 9.55 (0.82) | 9.81 (0.39) | 0.12 |
| Correct “no” /10 | 9.95 (0.22) | 10 (0.00) | 0.31 |
| Constructional Praxis/ 11 | 9.75 (0.96) | 9.86 (1.03) | 0.84 |
| Recall of Drawings/ 11 | 9.15 (1.92) | 8.31 (2.16) | 0.13 |
Values denote mean (+/− Standard Deviation)
Behavioral Results of the Subsequent Memory Test
There were no significant differences between the PSEN1 mutation carriers and controls for post-scan subsequent memory accuracy (p =0.52) or reaction time (p = 0.80). To confirm that subsequent memory effects were not influenced by response times during encoding, we analyzed response times during the scene encoding task (e.g. simple identification of the picture as a cold vs. warm weather scene) based on post-scan subsequent memory performance (e.g. correctly identifying a scene as previously viewed during scanning vs. forgotten scenes). This analysis revealed no significant differences between groups (p=0.91). The recognition accuracy on the discrimination index Pr was 0.46 +/− 0.11 for the non-carriers and 0.48 +/− 0.12 for the PSEN1 carriers. The overall average of median reaction time for correct responses was 1447 ms +/− 517ms for the non-carriers and 1493ms +/− 588ms for the PSEN1 carriers.
Imaging Results of Successful Encoding
A priori ROI analysis: MTL regions
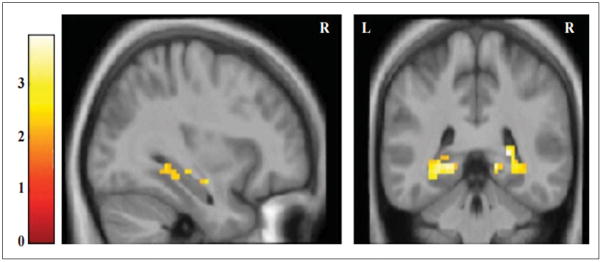
We examined the MTL regions for subsequently remembered versus forgotten scenes. Compared to non-carriers, PSEN1 mutation carriers demonstrated hyperactivation within MTL regions (p=0.005 uncorrected, p value was not significant after correcting for multiple comparisons, Table 2, Figure 2). Correlation analyses showed that performance on the post-scan recognition test was significantly correlated with MTL activation (R= 0.41).
Table 2.
Within-group differences during successful encoding of complex scenes (Remembered scenes vs. forgotten scenes).
| Brain Region | Atlas Coordinates† Millimeters |
p-value (uncorrected) | ||
|---|---|---|---|---|
|
| ||||
| X | Y | Z | ||
| PSEN1 Mutation Carriers | ||||
| Prefrontal Cortex | ||||
| Left precentral gyrus | −36 | 8 | 31 | 0.001 |
| Right superior frontal gyrus | 24 | 12 | 47 | 0.001 |
| Parietal Cortex | ||||
| Right angular gyrus | 27 | −61 | 49 | 0.0001 |
| Right superior parietal gyrus | 24 | −70 | 49 | 0.002 |
| Medial Temporal Lobes | ||||
| Left medial temporal pole | −42 | 20 | −29 | 0.002 |
| Left hippocampus | −21 | −40 | 1 | 0.003 |
| Right hippocampus | 24 | −34 | 5 | 0.004 |
| Right parahippocampal gyrus | 30 | −25 | −20 | 0.002 |
| Right fusiform gyrus | 33 | −40 | −11 | 0.001 |
| Left fusiform gyrus | −36 | −43 | −23 | 0.002 |
| Non-Carriers | ||||
| Parietal Cortex | ||||
| Left inferior parietal lobule | −30 | −52 | 46 | 0.0001 |
| Medial Temporal Lobes | ||||
| Left parahippocampal gyrus | −30 | −28 | −19 | 0.0001 |
| Right parahippocampal gyrus | 36 | −28 | −14 | 0.002 |
| Left hippocampus | −24 | −34 | 5 | 0.002 |
| Right hippocampus | 24 | −40 | 10 | 0.001 |
| Left fusiform gyrus | −45 | −61 | −17 | 0.002 |
| Left inferior temporal gyrus | −48 | 5 | −35 | 0.002 |
Figure 2. PSEN1 carriers show greater MTL activity during successful encoding of complex scenes than non-carriers.
A priori region-of-interest analysis during successful encoding. Statistical Parametric Maps (SPMs) for the comparison PSEN1 mutation carriers vs noncarriers for the contrast remembered vs forgotten scenes. Functional images are displayed on anatomical images derived from an average obtained from the normalized structural images of all subjects using an anatomical mask of the combined bilateral hippocampal formation and parahippocampal gyri with a statistical threshold of p < 0.005 uncorrected (extent threshold 5 voxels). MNI coordinates for peak activated voxel: [x,y,z][33, −40, −8]. Color bar represents t values for all activated voxels within the anatomical mask.
Whole brain results
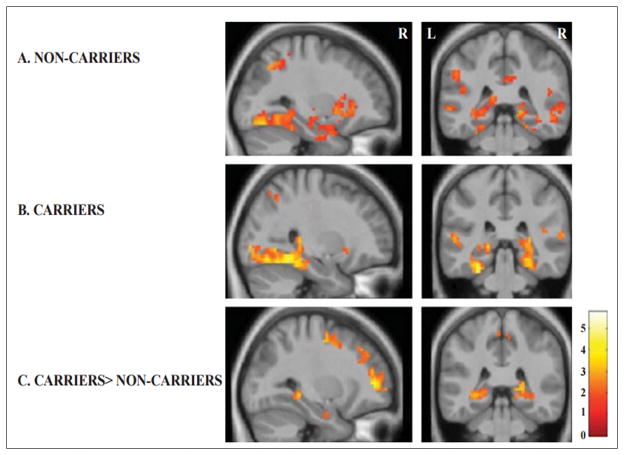
The whole-brain analysis showed that for both the PSEN1 and non-carrier groups there was an increase in the fMRI signal in the prefrontal cortex (precentral gyrus and superior frontal gyrus), parietal lobes (angular gyrus, inferior parietal lobule, superior parietal gyrus), and fusiform gyrus, parahippocampal gyrus, and hippocampal formation during the encoding of subsequently remembered versus subsequently forgotten trials (Table 2, Figure 3). The between-group analysis (PSEN1 carriers>non-carriers) revealed greater activations in the angular gyrus, precuneus, superior frontal gyrus, inferior frontal gyrus, parahippocampal gyrus, and hippocampal formation (p=0.005 uncorrected, p value was not significant after correcting for multiple comparisons, Table 3, Figure 3).
Figure 3. Whole-brain analyses of successful encoding and subsequent memory.
Statistical parametric maps (SPMs) for both groups, non-carriers and PSEN1 mutation carriers, for the subsequent memory contrast (successfully remembered vs subsequently forgotten stimuli) are displayed on anatomical images derived from an average obtained from the normalized structural images of all subjects with a statistical threshold of p < 0.005 uncorrected. Each group (A-Non Carriers and B- Carriers) showed an increase in the fMRI signal response within the MTL regions, posterior parietal regions, and fusiform gyrus for the subsequent memory contrast (successfully remembered vs subsequently forgotten stimuli). Compared to noncarriers, PSEN1 mutation carriers exhibited greater activity in bilateral hippocampi and parahippocampal gyrus and bilateral precuneus for subsequently remembered vs forgotten stimuli. Color bar represents t values for all activated voxels in the whole brain.
Table 3.
Between-group differences during successful encoding of complex scenes (Remembered scenes vs. forgotten scenes).
| Brain Region | Atlas Coordinates† Millimeters |
p-value (uncorrected) | ||
|---|---|---|---|---|
|
| ||||
| X | Y | Z | ||
| Carriers> Non Carriers | ||||
| Prefrontal Cortex | ||||
| Right inferior frontal gyrus | 45 | 26 | 22 | 0.001 |
| Left superior frontal gyrus | −24 | 47 | 7 | 0.002 |
| Parietal Cortex | ||||
| Right precuneus | 15 | −64 | 25 | 0.005 |
| Right angular gyrus | 36 | −61 | 37 | 0.002 |
| Medial Temporal Lobes | ||||
| Left hippocampus | −27 | −37 | −5 | 0.001 |
| Right hippocampus | 18 | −34 | 2 | 0.003 |
| Left parahippocampal gyrus | −21 | −40 | −5 | 0.003 |
Volumetric measurements
An ANOVA revealed no statistically significant differences in hippocampal volume between PSEN1 mutation carriers and non-carriers [F (1, 37)= 2.31, p= 0.13].
Discussion
Despite identical behavioral performance during the successful encoding of complex scenes, young presymptomatic PSEN1 mutation carriers demonstrate greater MTL activation (hyperactivation) within the hippocampal formation and parahippocampal gyrus, when compared to non-carrier family members.
It is now widely accepted that the neuropathological changes of Alzheimer's disease begin as early as 20 years before the clinical onset of disease [18, 28, 29]. In the past few decades, researchers have begun to characterize the biological and cognitive processes that precede the clinical onset of AD, which has led to proposals for new diagnostic criteria for preclinical AD, and has set the stage for the first presymptomatic clinical trials. These presymptomatic clinical trials seek to evaluate promising treatments, with the ultimate goal of delaying the emergence of symptoms, or completely prevent the clinical presentation of the disease.
Our group and others [15, 18, 30, 31] have shown evidence of functional and structural brain changes during preclinical stages of familial AD, and have suggested that they may provide a foothold for progressive physiological changes associated with the disease.
The aim of our current study was to define differences in MTL activation measured with fMRI during successful memory encoding in young presymptomatic PSEN1 mutation carriers from a Colombian kindred. Participants were examined using fMRI while they encoded complex scenes. Later in the same session (post-scan), participants performed a subsequent memory test in which they identified previously seen stimuli as remembered or forgotten (Old vs New). In the fMRI analysis, we examined MTL activation during successful encoding (activation for scenes which were subsequently correctly recognized as having been seen before in the post-scan memory test).
The current findings are consistent with the results from two of our previous studies. Compared to non-carrier family members, we showed that young carriers had hyperactivation of the right anterior hippocampal activation during encoding of face-name paired-associates [15]. In another study [18], we found a similar pattern of hippocampal hyperactivation, in addition to an increase in activation in the parietal cortex, in a group of much younger carriers from the same kindred, even before evidence of amyloid accumulation as measured by CSF. Together, these studies provide convergent support for the consistency of the MTL hyperactivation as a marker of preclinical AD and demonstrate that subtle evidence of neurodegenerative changes in brain function can be present decades before any evidence of clinical symptoms of the disease.
Whole brain analyses also demonstrated that PSEN1 mutation carriers had greater activation in the posterior parietal cortex during successful encoding, compared to non-carriers. In our previous work [18], we reported a similar finding with a younger cohort. This increased parietal activation in regions known to be less active during cognitive tasks may suggest a failure to deactivate the default network regions, which is likely related to the accumulation of brain amyloid. This finding has been interpreted as an early sign of degeneration as well [17].
The pattern of increased fMRI activation within MTL and parietal regions may constitute an early and subtle sign of neurodegeneration within episodic memory networks. The molecular and cellular bases of this activation, however, remain unclear. Previous work in animal models has suggested that at lower levels of amyloid pathology, presynaptic facilitation leads to synaptic potentiation and excitation, whereas at higher levels of amyloid, postsynaptic depression is observed [32]. There is also evidence indicating that hippocampal hyperactivity might possibly reflect inefficient synaptic transmission [33], or abnormal sprouting of cholinergic fibers [34]. Lastly, it has been suggested that hyperactivation may result from neuronal excitotoxicity caused by the impact of PSEN1 dysfunction in the amyloid precursor protein regulation of axonal pruning [35] resulting in over formation of synapses within cortical circuits [36]. In human neuroimaging studies, it is clear that the mechanisms leading to hyperactivation are transient and occur early. The fMRI hyperactivation then shifts to brain hypoactivation as the disease progressed to more severe stages [37]. Further evidence in the literature of this shift from hyperactivation to hypoactivation comes from several imaging studies, all using different imaging modalities, applied to separate cohorts of individuals. A recent study by Ryan and colleagues demonstrated decreased mean diffusivity in the hippocampus in presymptomatic PSEN1 mutation carriers but increased hippocampal mean diffusivity in symptomatic carriers [30]. Another recent paper, in this case using FDG-PET, showed hypermetabolism in the precuneus/posterior cingulate in young mutation carriers, but hypometabolism in mutation carriers who were closer to the expected age of disease onset [31]. Another study showed unexpected restricted diffusion and increased volume in the caudate and increased cortical thickness in precuneus and parieto-temporal areas of young mutation carriers [38]. Taken together, all of these studies have shown evidence that the direction of change in imaging markers may show dynamic alterations during progression of the disease.
Strengths of the present study are the use of fMRI with a unique, very homogeneous population with a single-gene mutation for early-onset AD. Studies of PSEN1 mutation carriers allow us to examine cognitively normal adult individuals who will go on to develop AD in the future with near certainty. The average age of our participants was 34 years, and the average age of disease onset in this cohort is 44 years and therefore reduces the likelihood that our participants would have any aging related pathology. Our findings support the need to start examining individuals at risk for AD as early as 10 years before possible clinical onset of the disease. Also, we demonstrated that fMRI memory paradigms known to recruit MTL structures are sensitive to preclinical changes. In particular, we showed that the scene encoding task has potential to be used as a cognitive test in the early detection of Alzheimer’s.
This study also has several limitations, including relatively small sample sizes, reduced statistical power, and uncertainty in the extent to which our findings may be generalizable to other AD-causing mutations or sporadic late-onset AD. While the present results are based on a relatively small number of participants compared to studies of sporadic AD, the present sample represents one of the largest of its kind in familial, early-onset AD. Although our imaging findings did not survive correction for multiple comparisons, and therefore should be considered as exploratory, the pattern of fMRI activation and the consistency of our findings with previous studies reduce the chance of Type 1 errors. At this point, we also cannot rule out that differences between mutation carriers and non-carriers may be developmental, and further studies are needed to explore this possibility.
Taken together, these findings complement our understanding of the neural correlates of memory dysfunction in presymptomatic stages of familial AD, and also extend our knowledge of the earliest brain functional changes that may predispose individuals to develop AD later in life. Further research is still needed to study and identify reliable preclinical markers of AD at its earliest stages. Functional neuroimaging studies of genetically predetermined individuals may help diagnose and treat AD much earlier and more accurately than would otherwise be possible.
Acknowledgments
This study was supported by the Boston University Center for Neuroscience (to CES), Department of Psychology (to CES and YTQ), a Boston University Alzheimer’s Disease Center pilot award (P30 AG13846 to CES and AB), the National Institute of Neurological Disorders and Stroke (F31-NS078786-01A1 to YTQ), the National Institute of Health- Office of the Director (DP5OD019833 to YTQ), COLCIENCIAS-Colombia (Projects: 1115-408-20512, 1115-545-31651 to FL), and an Anonymous Organization (FL). The authors thank the Instituto de Alta Tecnología Médica (IATM) staff for their help with data acquisition. We thank the PSEN1 Colombian families for contributing their valuable time and effort, without which this study would not have been possible.
Footnotes
DISCLOSURES
YTQ: none
KW: none
GC: none
MM: none
FL: none
AB: none
CES: none
CONTRIBUTORS: Study conception and design: YTQ, KW, AB, FL, CES
Study supervision: YTQ, CES, FL
Acquisition of data: YTQ, GC, FL
Analysis and interpretation of data: YTQ, KW, AB, FL, CES
Statistical analysis: YTQ, KW, FL, CES
Drafting the manuscript: YTQ, KW, AB, CES
Preparation of figures and tables: YTQ, GC, MM
Obtained funding: YTQ, AB, CES
Critical revision of the manuscript for important intellectual content: YTQ, KW, GC, MM, FL, AB, CES
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiman EM, McKhann GM, Albert MS, Sperling RA, Petersen RC, Blacker D. Alzheimer's disease: implications of the updated diagnostic and research criteria. J Clin Psychiatry. 2011;72:1190–1196. doi: 10.4088/JCP.10087co1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer's disease. Nat Med. 2004;(10 Suppl):S34–41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- 4.Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, Johnson SC. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. J Am Geriatr Soc. 2008;56:920–934. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamalainen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007;78:812–818. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain. 2006;129:1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, Beckmann CF, Smith SM, Matthews PM, Mackay CE. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011;54:602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillon G, Lopera F, Stern CE. Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann Neurol. 2010;68:865–875. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum JB, Kosik KS, Tariot PN, Lopera F. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, Saldarriaga A, Lopera F. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 20.Aguirre-Acevedo DC, Gomez RD, Moreno S, Henao-Arboleda E, Motta M, Munoz C, Arana A, Pineda DA, Lopera F. Validity and reliability of the CERAD-Col neuropsychological battery. Rev Neurol. 2007;45:655–660. [PubMed] [Google Scholar]
- 21.Lendon CL, Martinez A, Behrens IM, Kosik KS, Madrigal L, Norton J, Neuman R, Myers A, Busfield F, Wragg M, Arcos M, Arango Viana JC, Ossa J, Ruiz A, Goate AM, Lopera F. E280A PS-1 mutation causes Alzheimer's disease but age of onset is not modified by ApoE alleles. Hum Mutat. 1997;10:186–195. doi: 10.1002/(SICI)1098-1004(1997)10:3<186::AID-HUMU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 25.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 27.Duvernoy HM. The human hippocampus, functional anatomy, vascularization and serial sections with MRI. Springer-Verlag; New York: 2005. [Google Scholar]
- 28.Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, Langbaum JB, Ayutyanont N, Roontiva A, Thiyyagura P, Lee W, Mo H, Lopez L, Moreno S, Acosta-Baena N, Giraldo M, Garcia G, Reiman RA, Huentelman MJ, Kosik KS, Tariot PN, Lopera F, Reiman EM. Florbetapir PET analysis of amyloid-beta deposition in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional study. Lancet Neurol. 2012;11:1057–1065. doi: 10.1016/S1474-4422(12)70227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Thornton JS, Mancini L, Hyare H, Yousry T, Ridgway GR, Zhang H, Modat M, Alexander DC, Rossor MN, Ourselin S, Fox NC. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain. 2013;136:1399–1414. doi: 10.1093/brain/awt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benzinger TL, Blazey T, Jack CR, Jr, Koeppe RA, Su Y, Xiong C, Raichle ME, Snyder AZ, Ances BM, Bateman RJ, Cairns NJ, Fagan AM, Goate A, Marcus DS, Aisen PS, Christensen JJ, Ercole L, Hornbeck RC, Farrar AM, Aldea P, Jasielec MS, Owen CJ, Xie X, Mayeux R, Brickman A, McDade E, Klunk W, Mathis CA, Ringman J, Thompson PM, Ghetti B, Saykin AJ, Sperling RA, Johnson KA, Salloway S, Correia S, Schofield PR, Masters CL, Rowe C, Villemagne VL, Martins R, Ourselin S, Rossor MN, Fox NC, Cash DM, Weiner MW, Holtzman DM, Buckles VD, Moulder K, Morris JC. Regional variability of imaging biomarkers in autosomal dominant Alzheimer's disease. Proc Natl Acad Sci U S A. 2013;110:E4502–4509. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palop JJ, Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin? Neuromolecular Med. 2010;12:48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer's and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Hasselmo ME. A computational model of the progression of Alzheimer's disease. MD Comput. 1997;14:181–191. [PubMed] [Google Scholar]
- 37.Prvulovic D, Van de Ven V, Sack AT, Maurer K, Linden DE. Functional activation imaging in aging and dementia. Psychiatry Res. 2005;140:97–113. doi: 10.1016/j.pscychresns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Fortea J, Sala-Llonch R, Bartres-Faz D, Bosch B, Llado A, Bargallo N, Molinuevo JL, Sanchez-Valle R. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J Alzheimers Dis. 2010;22:909–922. doi: 10.3233/JAD-2010-100678. [DOI] [PubMed] [Google Scholar]