Abstract
OBJECTIVE
Otitis media is a common problem in the pediatric population. Despite antibiotic therapy, post-tympanostomy otorrhea can be difficult to treat. Biofilms have been shown to play a role in chronic and recurrent otitis media and are implicated in otorrhea. This study investigated both the microbial composition and the presence of biofilm fragments rich in extracellular DNA (eDNA) and the bacterial DNA-binding protein, integration host factor (IHF) in post-tympanostomy tube otorrhea
STUDY DESIGN
clinical samples
METHODS
Institutional review board approval was obtained and samples were recovered from pediatric patients with tympanostomy tubes and persistent otorrhea for both microbial culture and biofilm analysis. For biofilm assessment, frozen samples were sectioned then labeled using a rabbit anti-IHF which was detected with goat anti-rabbit IgG conjugated to AlexaFluor 594. Samples were then counterstained with DAPI to detect DNA and images were captured by inverted light microscopy.
RESULTS
Of 15 pediatric otorrhea samples analyzed, 9 (60%) contained solids that were positive for labeling of IHF in association with a lattice of eDNA, and 75% yielded positive bacterial cultures. Bacterial culture results included H. influenzae, MRSA, S. pneumoniae, M. catarrhalis, and P. aeruginosa.
CONCLUSION
Positive labeling of otorrhea solids for eDNA and IHF in combination with microbiological culture results indicated that biofilms likely played a key role in chronic otorrhea. Moreover, as a known critical structural component of biofilms, these findings suggest that DNABII proteins in association with eDNA may serve as an important therapeutic target in post-tympanostomy tube otorrhea.
Keywords: post-tympanostomy tube otorrhea, anti-IHF, biofilms, tympanostomy tube, acute otitis media, chronic otitis media with effusion
INTRODUCTION
Otitis media with effusion and recurrent acute otitis media are common problems in children, with a cumulative incidence of up to 80% by the age of 4 years [1]. Treatment of otitis media has consisted of systemic antibiotics, and for those resistant to medical treatment, surgical placement of tympanostomy tubes is performed. Tympanostomy tubes are placed in 667,000 children younger than 15 years every year, making this the most common ambulatory surgery performed on children in the United States [2]. Tympanostomy tubes do not typically prevent otitis media, but can relieve symptoms such as conductive hearing loss and pain associated with infection. Patients with tympanostomy tubes are treated with topical antibiotics and steroids, alleviating the need for a systemic antibiotic. However, complications associated with ear tube placement include tympanic membrane perforation, occurring in 1-2% of patients, extrusion, tympanosclerosis, and post-tympanostomy tube otorrhea [3].
Post-tympanostomy tube otorrhea can lead to tube occlusion and discomfort and can be described as acute, lasting less than 6-8 weeks, or chronic, lasting greater than 6-8 weeks [4]. The proportion of children who have experienced post-tympanostomy tube otorrhea has been cited as reaching 74% within 12 months and can be difficult to treat. Failure of topical antibiotic therapy and persistent otorrhea may lead to the additional use of oral antibiotic therapy and possibly tympanostomy tube removal [5,6,7,8]. Culture results from post-tympanostomy tube otorrhea has implicated common microorganisms associated with acute otitis media including non-typeable Haemophilus influenzae (NTHI), Streptococcus pneumoniae, and Moraxella catarrhalis [9,10,11]. In addition, Staphylococcus aureus and Pseudomonas aeruginosa are other bacteria implicated in chronic otorrhea and are likely to have entered into the middle ear via the auditory canal through the tympanostomy tube [12]. The formation of biofilms within the middle ear and in association with tympanostomy tubes have been investigated as a possible source for chronic otitis media and post- tube insertion otorrhea. Hall-Stoodley et al. were able to identify biofilms in association with middle ear mucosal biopsies within a pediatric population suffering from chronic otitis media . Generic stains and specific probes identified NTHI, S. pneumoniae, and M. catarrhalis thus correlating these predominant otopathogens with biofilm formation [11]. Furthermore, in an effort to decrease the incidence of post-tympanostomy tube otorrhea, numerous studies have been dedicated to identifying strategies to either transform the composition of the tube or the coating of the tube in order to inhibit biofilm growth. While multiple variables have been altered, there has not been a consensus as to which modification may provide the most benefit to this surgical treatment modality [12,13,14,15,16,17].
Although research has shown that biofilms are present on middle ear mucosal surfaces and on tympanostomy tubes, there continues to be an increased incidence of post-tympanostomy tube otorrhea despite tailoring medical regimen and tympanostomy tube alterations to the presence of these specific microbes. We theorize that multiple physical and biological properties of the biofilms produced by these bacteria make them resistant to these various treatment modalities. Supporting this assertion is the fact that bacteria residing within a biofilm are known to be 1,000-fold more resistant to standard antibiotic treatment compared to planktonic bacteria [18]. The foundation of biofilm communities is the extracellular polymeric substance (EPS) or matrix which is involved in facilitating adhesion to surfaces and provides a physical barrier that protects the bacterial cells that reside within the biofilm. The arrangement of the EPS is highly diverse; however, the EPS of many human pathogens contains a large amount of extracellular DNA (eDNA) of both bacterial and host origin. The eDNA derived from bacteria is found in association with a family of bacterial DNABII-binding proteins, one of which has been identified at Integration Host Factor (IHF). IHF has been confirmed as an essential structural support for extracellular DNA in bacterial biofilms [19,20,21]. Targeting these proteins via use of antibodies directed against them results in catastrophic collapse of the biofilm structure with release of the resident bacteria as mediated by an equilibrium shift as DNABII proteins are titrated away from the biofilm [19,22]. Furthermore, there is a known synergistic effect between anti-IHF antibodies and traditional antibiotic treatment, wherein bacteria newly released from the biofilm matrix in this manner are 4 to 8-fold more sensitive to the killing action of several first-line antibiotics for treatment of OM which are ineffective on their own against bacteria residing within a biofilm [19]. The ability of anti-IHF antibodies to weaken the structural support of the biofilm matrix increases the efficacy of antibiotics and immune modulators to the resident bacterial cells, thus enhancing clearance of the biofilm [19].
To effectively devise a novel strategy to combat post-tympanostomy tube insertion associated otorrhea will require an clearer understanding of the microbiological basis for the extended drainage of fluids from the middle ear space. The basis of this investigation is grounded in the fact that biofilms continue to provide structural support and resistance to resident pathogens despite attempts to customize antibiotic therapy and modify tube structure and composition in an attempt to eradicate these biofilms. Since typanostomy tube insertion continues to be one of the most common surgical interventions for the pediatric population, targeting the actual components that confer stability to the biofilm matrix may prove to be an optimal treatment modality. Toward this goal, we first needed to determine if post-tympanostomy tube otorrhea samples contained fragments of bacterial biofilms and if these biofilms would contain both a lattice of crossed strands of eDNA as well as an associated DNABII protein (e.g. IHF) positioned at the vertices of these crossed eDNA strands, as we have shown in several other disease models [19,21,23,24] Microbiological cultures were also obtained from specimens to correlate the presence of specific pathogens with that of biofilm fragments contained within otorrhea samples that fulfilled the aforementioned criteria.
MATERIALS AND METHODS
Post-tympanostomy Tube Otorrhea Collection
Institutional review board approval was obtained and pediatric population with tympanostomy tubes and persistent otorrhea were identified in the Pediatric Otolaryngology clinic. Samples were collected through the tympanostomy tube via a sterile Juhn Tymp-Tap middle ear fluid aspirator/collector (Medtronic Xomed Inc, Jacksonville, Fl). Specimens were labeled according to laterality of collection, gender of the patient from which specimen was obtained from, age of patient, color, and relative consistency of the sample collected . After completion of the collection, the remainder of the otorrhea exudate was cultured using sterile BBL Culture Swab collection and transportation system (Becton, Dickinson and Company, Sparks, MD) and sent to Nationwide Children's Hospital Microbiology department for further speciation. The remainder of the specimen was removed from the collection tube and frozen in optimum cutting temperature compound (OCT) for immunohistochemical analysis.
Preparation of sample and General Immunofluorescence staining for labeling IHF
The portion of the sample brought to the lab was embedded in OCT compound (Fisher Scientific, Pittsburgh, PA), snap frozen over liquid nitrogen and stored at −80 C until further analysis. Ten micron serial sections were cut using a Microm rotary cryotome, adhered to glass slides (Mercedes Medical, Sarasota, FL) and stored at −80° C. Sections were later stained to determine the presence of both eDNA and DNABII proteins in the samples. Briefly, slides were air-dried, fixed in cold acetone, then equilibrated in buffer (0.05M Tris-HCl, 0.15M NaCl and 0.05% Tween 20, pH 7.4). Sections were blocked with image-iT FX signal enhancer (Molecular Probes, Eugene, OR) and with Background Sniper (BioCare Medical, Concord, CA) per manufacturer's instructions. Sections were then incubated with a 1:200 dilution of polyclonal rabbit anti-IHFE. coli overnight at 4° C, in a humidified chamber. Slides were rinsed and incubated with goat anti-rabbit IgG conjugated to AlexaFluor 594 (Invitrogen, Eugene, OR) for 30 minutes at room temp. As a counterstain for eDNA, sections were incubated with DAPI and cover-slipped using ProLong Gold antifade reagent (Molecular Probes, Eugene, OR). Use of naive rabbit serum in place of immune serum and use of secondary antibody alone served as negative control preparations for immunolabeling studies. Sections were viewed with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss Inc., Thornwood, NY)
Statistical Analysis
Not applicable.
RESULTS
A total of 15 samples were collected and processed for immunofluorecent labeling for the presence of a DNABII protein (e.g. IHF) and counterstained for eDNA. Table 1.
Table 1.
Microbial culture results and positive labeling
| Specimen | Positive IHF labeling | Positive eDNA labeling | Culture Results |
|---|---|---|---|
| 1 | - | - | No growth |
| 2 | - | - | No growth |
| 3 | + | + | No growth |
| 4 | + | + | Alicaligenes faecalis, Achromobacter xylosoxidans |
| 5 | + | + | Alicaligenes faecalis, Achromobacter xylosoxidans |
| 6 | + | + | Haemophilus influenzae |
| 7 | + | + | Escherichia coli |
| 8 | + | + | Moraxella catarrhalis |
| 9 | - | + | Haemophilus influenzae |
| 10 | + | + | Methicillin-resistant Staphylococcus aureus (MRSA) |
| 11 | + | + | Methicillin-resistant Staphylococcus aureus (MRSA) Streptococcus pneumoniae |
| 12 | - | - | Yeast |
| 13 | - | - | Corynebacterium |
| 14 | + | + | Pseudomonas aeruginosa |
| 15 | - | - | Mixed bacteria/Normal flora |
Culture results
Of the 15 total specimens, 11 (73%) were culture positive for bacterial species whereas one yielded yeast. In four of 11 specimens that were culture positive for bacterial species, one of the three predominant otopathogens of otitis media was cultured (e.g. NTHI, S. pneumoniae and M. catarrhalis). Two additional cultures (15%) yielded methicillin-resistant S. aureus. The remaining specimens were of mixed microbial origin while three specimens were culture negative. The culture negative specimens were noted to have solid fragments of cerumen, which is suspected to have antimicrobial properties that could inhibit bacterial growth [25]. However, this culture negative status is the hallmark of middle ears that contain biofilms and thus was not unexpected [26,27,28]. Moreover, these children are typically treated with topical antibiotics which may have contributed to the culture negative status of these latter three specimens. The positive labeling for a DNABII protein in association with a dense lattice of eDNA within solids present in otorrhea samples recovered from children post-tympanostomy tube insertion suggested the contribution of bacterial biofilms to this chronic condition. Out of the 15 samples labeled with rabbit anti-IHFEE. coli and counterstained with DAPI, 9 were positive for both eDNA and the DNA binding protein IHF. Sixty percent of all specimens were positive for IHF and as additional support for the hypothesis that bacterial biofilms might be contributing to the chronic otorrhea in these children, eight of the eleven (73%) samples that were positive for microbial growth were also positive for labeling with antibodies directed against the DNABII protein, IHF. (Figure 1 and 2)
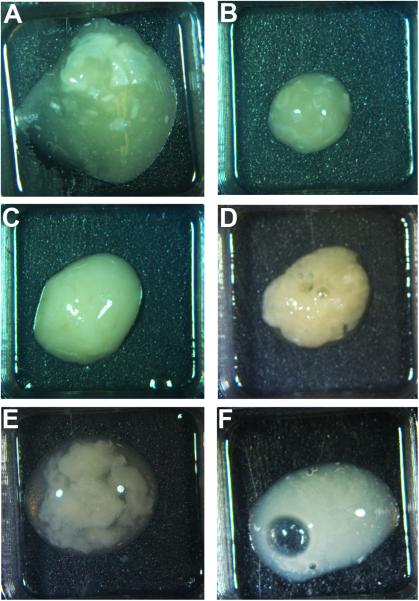
Figure 1.
Representative images of six otorrhea samples which contain solid components that were analyzed for the presence of eDNA and associated DNABII proteins. A-F corresponds to samples 1-6.
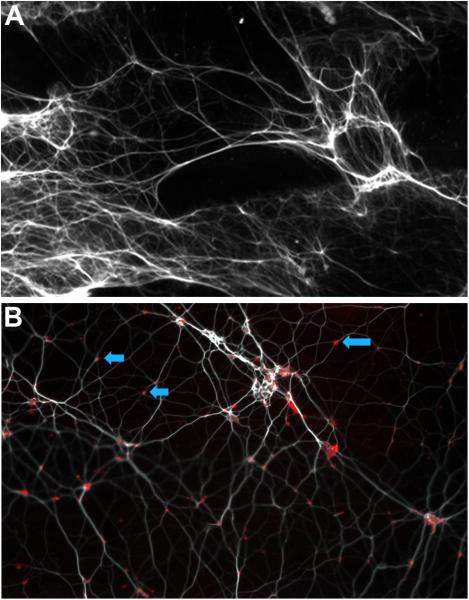
Figure 2.
A) Fluorescent microscopy image of otorrhea sample at 10× magnification: section labeled for presence extracellular DNA (eDNA) which appears as a lattice of strands of DNA (pseudocolored white). B) Fluorescent microscopy image of same otorrhea sample at 63× magnification: Section labeled for presence of IHF (RED). Positive labeling (RED) was observed at a majority of the vertices (BLUE arrows) formed by crossed strands of eDNA (pseudocolored white)
DISCUSSION
Most children have experienced at least one acute otitis media episode by the age of 3 years, and by 6 years, at least 40% have experienced 3 or more episodes [29]. The socioeconomic burden of acute otitis media is significant. In 2011, mean expenditures per child with expenses to treat otitis media were 427 dollar for the 7.5 million children treated. Simultaneously, the national expenditure for treatment and disability associated with otitis media exceeded 3.2 billion dollars [30,31]. For this reason otitis media continues to draw attention in the research community.
Tympanostomy tube insertion is the primary surgical treatment for otitis media. Tympanostomy tubes were introduced in 1952 by Beverly Armstrong as a new treatment for chronic secretory otitis media. Since the introduction, there has been a multitude of variations on the material, size, and shapes of the tympanostomy tube [32]. Tympanostomy tube insertion continues to be the mainstay treatment for otitis media with effusion and Eustachian tube dysfunction in which it has been associated with improved quality of life for both the child and care giver. A study by Boruk et al published in Otolaryngology Head Neck Surgery in 2007 showed that physical suffering during an episode of otitis media was a problem for 85% of children, emotional distress for 76%, and activity limitations for 57%. It was also notable for 31% of caregivers had to cancel family activities, 29% reported lack of sleep, and 12% missed work or school. [33]. The efficacy of tympanostomy tubes treating chronic otitis media with effusion, recurrent acute otitis media, or both have been studied in randomized control trials. Tympanostomy tube insertion decreases the prevalence of middle ear effusion in children suffering from chronic otitis media by 32% in the first year and improves average hearing level by 5 to 12 db [34,35]. The variations within the designs of tympanostomy tubes have sought to improve clinical efficacy through the ease of insertion into the tympanic membrane, duration of tube placement, decreased bacterial growth, and clearance of effusion. The difficulty in incorporating all of these variables is the reason no single type of tympanostomy tube has gained widespread acceptance. Furthermore, there is no one type of tympanostomy tube in which bacteria will not adhere [4].
A major factor is that tympanostomy tubes and middle ear mucosa represent ideal environments for attachment of bacteria and biofilm growth. Bacteria and biofilms have been shown to adhere to natural and synthetic materials via the use of a self-produced matrix of extracellular polymeric substances (EPS) comprised of extracellular DNA, proteins, and polysaccharides. The EPS is a semipermeable layer which comprises approximately 90% of the biofilm mass. It selectively allows diffusion of water, nutrients, and essential substances through the matrix, while protecting resident bacteria from exposure to antibiotics, host defense cells and soluble immune effectors, and other microorganisms [20]. These components allow the bacteria to have a 1,000 fold resistance to antibiotic therapies when compared to their planktonic form [18]. Bakaletz et al demonstrated that non-typeable Haemophilus influenza biofilms contained eDNA that is organized into a mesh-like lattice that confers stability to the biofilm [23].
The complex organization of the EPS indicates that each component is essential to biofilm survival. DNABII family proteins have also been exhibited as a critical component to biofilm structural stability [19,20,21,33]. Integration host factor (IHF), a member of the DNABII family proteins, binds extracellular DNA within the EPS of bacterial biofilms. Exposure of biofilms to antibodies directed against IHF was notable for its ability to significantly disrupt the EPS resulting in catastrophic collapse of the biofilm with release of the resident bacteria that were highly susceptible to the killing action of traditional antibiotics [22]. For this reason, antibodies directed against IHF might serve as a novel therapeutic to disrupt those biofilms responsible for chronic otorrhea, thus rendering them susceptible to antibiotic regimens. The synergistic effect of anti-IHF and topical antibiotic therapy would likely mediate a more effective clearance of bacterial biofilms from the middle ear space and tympanostomy tubes to decrease the incidence and chronicity of otorrhea.
Our study demonstrated that the majority of post-tympanostomy tube otorrhea specimens contained fragments of biofilms that included an extensive lattice of eDNA with integrated DNABII protein (e.g. IHF) within the biofilm matrix. This study is the first to examine chronic otorrhea specimens for the presence of a bacterial biofilm with specific structural elements. We also demonstrated that a majority of culture positive samples were also positive for the presence of IHF.
CONCLUSION
Post-tympanostomy tube otorrhea is a common pathological consequence that affects a high percentage of children. Sixty percent of specimens obtained in this study suggested the presence of biofilms through the positive labeling of both eDNA and IHF in the solid fragments within otorrhea samples. Our results suggest that biofilms may play an important role in the persistence of otorrhea after tympanostomy tube placement. Moreover, the presence of a DNABII protein responsible for biofilm structural integrity in the majority of these specimens provides an intriguing target for the development of a novel therapeutic that may be used at the bedside to improve quality of life and treatment of post-tympanostomy tube otorrhea
ACKNOWLEDGMENTS
This research was supported by grant from the NIH/NIDCD to LOB & SDG – R01DC011818. We thank our colleagues from the Research Institute at Nationwide Childrens Hospital and the Otolaryngology Department at Nationwide Childrens Hospital who provided insight and expertise that greatly assisted the research.
We thank the physicians, residents, nursing, and staff in the Pediatric Otolaryngology department at Nationwide Childrens Hospital for assistance in collection of specimens that greatly assisted in the completion of the manuscript.
Footnotes
Conflict of Interest: There are no conflicts of interest.
Contributor Information
Winslo Idicula, Wexner Medical Center at The Ohio State University, Department of Otolaryngology- Head and Neck Surgery Columbus, Ohio, Nationwide Children's Hospital, Department of Otolaryngology- Head and Neck Surgery Columbus, Ohio.
Joseph Jurcisek, The Research Institute at Nationwide Children's Hospital, Center for Microbial Pathogenesis Columbus, Ohio.
Nathan D Cass, The Ohio State University College Of Medicine, Columbus, Ohio.
Syed Ali, The Ohio State University College Of Medicine, Columbus, Ohio.
Steven D Goodman, The Research Institute at Nationwide Children's Hospital, Center for Microbial Pathogenesis Columbus, Ohio.
Charles Elmaraghy, Nationwide Children's Hospital, Department of Otolaryngology- Head and Neck Surgery Columbus, Ohio, Wexner Medical Center at The Ohio State University, Department of Otolaryngology- Head and Neck Surgery Columbus, Ohio.
Kris Jatana, Nationwide Children's Hospital, Department of Otolaryngology- Head and Neck Surgery Columbus, Ohio, Wexner Medical Center at The Ohio State University, Department of Otolaryngology- Head and Neck Surgery Columbus, Ohio.
Lauren O. Bakaletz, The Research Institute at Nationwide Children's Hospital, Center for Microbial Pathogenesis Columbus, Ohio.
Reference List
- 1.Infante-Rivard C, Fernandez A. Otitis media in children: frequency, risk factors, and research avenues. Epidemiol Rev. 1993;15:444–65. doi: 10.1093/oxfordjournals.epirev.a036129. [DOI] [PubMed] [Google Scholar]
- 2.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1–25. [PubMed] [Google Scholar]
- 3.Hochman J, Blakley B, Abdoh A, Aleid H. Post-tympanostomy tube otorrhea: a meta-analysis. Otolaryngol Head Neck Surg. 2006;135(1):8–11. doi: 10.1016/j.otohns.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Wang JC, Hamood AN, Saadeh C, et al. Strategies to prevent biofilm-based tympanostomy tube infections. International Journal of Pediatric Otorhinolaryngology. 2014;(78):1433–1438. doi: 10.1016/j.ijporl.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Kay DJ, Nelson M, Rosenfield RM. Meta analysis of tympanostomy tube sequelae. Otolaryngology-Head and Neck Surgery. 2001;(124):374–380. doi: 10.1067/mhn.2001.113941. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham MJ, Eavey RD, Krouse JH, et al. Tympanostomy tubes: experience with removal. Laryngoscope. 1993;103(6):659–62. doi: 10.1288/00005537-199306000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Adkins AP, Friedman EM. Surgical indications and outcomes of tympanostomy tube removal. Int. J Pediatr. Otorhinolaryngol. 2005;69(8):1047–105. doi: 10.1016/j.ijporl.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Dohar J, Giles W, Roland P, et al. Topical ciprofloxacin/dexamethasone superior to oral amoxicillin/clavulanic acid in acute otitis media with otorrhea through tympanostomy tubes. Pediatrics. 2006;118(3):e561–e569. doi: 10.1542/peds.2005-2033. [DOI] [PubMed] [Google Scholar]
- 9.Post JC, Stoodley P, Hall A, Stoodley L, Ehrlich GD. The role of biofilms in otolaryngologic infections. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:185–190. doi: 10.1097/01.moo.0000124936.46948.6a. [DOI] [PubMed] [Google Scholar]
- 10.Leibovitz E, Serebro M, Givon-Lavi N, Greenberg D, Broides A, Leiberman A. Epidemiologic and Microbiologic Characteristics of culture positive Spontaneous Otorrhea in Children with Acute Otitis Media. Pediatr. Infect. Dis. J. 2009;28:381–384. doi: 10.1097/INF.0b013e318194e783. [DOI] [PubMed] [Google Scholar]
- 11.Hall-Stoodley L, Hu F, Gieseke A, Nistico L, Nguyen D. Direct Detection of Bacterial Biofilms on the Middle-Ear Mucosa of Children with Chronic Otitis Media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saidi IS, Biedlingmaier JF, Whelan P. In Vivo resistance to bacterial Biofilm Formation on Tympanostomy Tubes as a Function of Tube Material. Otolaryngol. – Head Neck Surgery. 1999;120:621–627. doi: 10.1053/hn.1999.v120.a94162. [DOI] [PubMed] [Google Scholar]
- 13.Jang CH, Park H, Cho YB, Choi CH, Park IY. The use of piperacillin-tazobactam coated tympanostomy tubes against ciprofloxacin-resistant Pseudomonas biofilm formation: An in vitro study. Int. J. Pediatr. Otorhinolaryngol. 2009;73:295–299. doi: 10.1016/j.ijporl.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Licameli G, Johnston P, Luz J, Daley J, Kenna M. Phohporycholine-coated antibiotic tympanostomy tubes: Are post tube placement complications reduced. Int. J. Pediatr. Otorhinolaryngol. 2008;72:1323–1328. doi: 10.1016/j.ijporl.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Kinnari TJ, Rihkanen H, Laine T, Salonen E-M, Jero J. Role of albumin coating of tympanostomy tubes: long term clinical evaluation. The Laryngoscope. 2007;117:2213–2217. doi: 10.1097/MLG.0b013e3181468631. [DOI] [PubMed] [Google Scholar]
- 16.Gan CW, Chooi WH, Ng HCA, Wong YS, Venkatraman SS, Lim LHY. Development of a novel biodegradable drug eluting ventilation tube for chronic otitis media with effusion. Laryngoscope. 2013;123:1770–1777. doi: 10.1002/lary.23895. [DOI] [PubMed] [Google Scholar]
- 17.Jang CH, Cho YB, Choi CH. Int. J. Effect of Ion-bombarded silicone tympanostomy tube on ciprofloxacin-resistant Pseudomonas aeruginosa biofilm formation. Pediatr. Otorhinolaryngol. 2012;76:1471–1473. doi: 10.1016/j.ijporl.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Slinger R, Chan F. Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagn Microbiol Infect Dis. 2006;56:247–253. doi: 10.1016/j.diagmicrobio.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Goodman SD, Obergfell KP, Jurcisek JA. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunology. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 20.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 21.Whitchurch C, Tolker-Nielsen T, Ragas P, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;(295):1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 22.Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, et al. Antibodies directed against integration host factor mediate biofilm clearance from nasopore. Laryngoscope. 2013;(123):2626–2632. doi: 10.1002/lary.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurcisek JA, Bakaletz LO. Biofiilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. 2007;189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakaletz LO. Bacterial biofilms in the upper airway-evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev. 2012;13(3):154–9. doi: 10.1016/j.prrv.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lum CL, Jeyanthi S, Prepageran N, et al. Antibacterial and antifungal properties of human cerumen. J Laryngol Otol. 2009;(123):375–378. doi: 10.1017/S0022215108003307. [DOI] [PubMed] [Google Scholar]
- 26.Post JC, Aul JJ, White GJ, et al. PCR-based detection of bacterial DNA after antimicrobial treatment is indicative of persistent, viable bacteria in the chinchilla model of otitis media. Am J Otolaryngol. 1996 Mar-Apr;17(2):106–11. doi: 10.1016/s0196-0709(96)90005-8. [DOI] [PubMed] [Google Scholar]
- 27.Aul JJ, Anderson KW, Wadowsky RM, et al. Comparative evaluation of culture and PCR for the detection and determination of persistence of bacterial strains and DNAs in the Chinchilla laniger model of otitis media. Ann Otol Rhinol Laryngol. 1998 Jun;107(6):508–13. doi: 10.1177/000348949810700609. [DOI] [PubMed] [Google Scholar]
- 28.Rayner MG, Zhang Y, Gorry MC, et al. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998 Jan 28;279(4):296–9. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 29.Casselbrant ML, Mandel EM. Epidemiology. In: Rosenfeld RM, Bluestone CD, editors. Evidence-Based Otitis Media. 2nd ed. BC Decker; Hamilton, Ontario, Canada: 2003. pp. 147–162. [Google Scholar]
- 30.Goldblatt EL, Dohar J, Nozza RJ, et al. Topical ofloxacin versus systemic amoxicillin/clavulanate in purulent otorrhea in children with tympanostomy tubes. Int J Pediatr Otorhinolaryngol. 1998;46(1-2):91–101. doi: 10.1016/s0165-5876(98)00150-5. [DOI] [PubMed] [Google Scholar]
- 31.Soni A. Agency for Healthcare Research and Quality; Rockville, MD: Apr, 2014. The Five Most Costly Children's Conditions, 2011: Estimates for U.S. Civilian Noninstitutionalized Children, Ages 0–17. Statistical Brief #434. http://www.meps.ahrq.gov/mepsweb/data_files/publications/st434/stat434.shtml. [PubMed] [Google Scholar]
- 32.Mudry The tympanostomy tube: An ingenious invention of the mid 19th century. International Journal of Pediatric Otorhinolaryngology. 2013;77:153–157. doi: 10.1016/j.ijporl.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Boruk M, Lee P, Faynzilbert Y, Rosenfeld RM. Caregiver wellbeing and child quality of life. Otolaryngol Head Neck Surg. 2007;136(2):159–168. doi: 10.1016/j.otohns.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010;(10):CD001801. doi: 10.1002/14651858.CD001801.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Rovers MM, Black N, Browning GG, Maw R, Zielhuis GA, Haggard MP. Grommets in otitis media with effusion: an individual patient data meta-analysis. Arch Dis Child. 2005;90(5):480–485. doi: 10.1136/adc.2004.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]