SUMMARY
Alzheimer’s disease (AD) is closely associated with synaptic dysfunction and thus, current treatments often aim to stimulate neurotransmission to improve cognitive impairment. While the formation of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex is essential for synaptic transmission, the correlation between SNAREs and AD neuropathology is unknown. Here we report that intracellular amyloid-β (Aβ) oligomers directly inhibit SNARE-mediated exocytosis by impairing SNARE complex formation. We observe abnormal reduction of SNARE complex levels in the brains of APP/PS1 transgenic (TG) mice compared to age-matched wild types. We demonstrate that Aβ oligomers block SNARE complex assembly through the direct interaction with a target membrane (t-)SNARE syntaxin 1a in vitro. Furthermore, the results of the in vitro single-vesicle content-mixing assay reveal that Aβ oligomers inhibit SNARE-mediated fusion pores. Thus, our study identifies a potential molecular mechanism by which intracellular Aβ oligomers hamper SNARE-mediated exocytosis, likely leading to AD-associated synaptic dysfunctions.
eTOC Blurb
The role of Aβ in cognitive impairment in Alzheimer’s disease still remains elusive. Yang et al. now show that Aβ oligomers binds to syntaxin 1a to impair SNARE-complex formation to inhibit exocytosis. The resulting inhibition of neurotransmission, could therefore lead to cognitive impairments.

INTRODUCTION
Cognitive deficits in Alzheimer’s disease (AD) are closely associated with synaptic dysfunction in the hippocampus and cortex (Huang and Mucke, 2012; Selkoe, 2002; Sheng et al., 2012). Stimulation of impaired synapses with acetylcholinesterase inhibitors improves symptoms and delays disease progression. Despite such clinical benefits, increasing acetylcholine (Ach) levels lacks disease-modifying properties and becomes less effective as brain atrophy reaches severe stages (Colovic et al., 2013; Jack et al., 2010; Kozauer and Katz, 2013). Given that amyloid-β (Aβ) oligomers are strongly suspected to influence the progression of AD, diverse pathological roles of the aggregated protein in the synapse have been reported (Benilova et al., 2012; Haass and Selkoe, 2007; Hardy, 2009; LaFerla et al., 1995; McLean et al., 1999; Puzzo et al., 2008; Shankar et al., 2008). However, the direct link between abnormal Aβ aggregation and synaptic dysfunction remains controversial. Thus, identification of the mechanistic link may provide a key therapeutic target to effectively treat AD.
Recent clinical investigations indicate that formation of the soluble N-ethylmaleimide sensitive factor attachment receptors (SNARE) complex is substantially reduced in the postmortem brains of AD patients (Sharma et al., 2012). In neurons, vesicle (v)-SNARE VAMP2 binds to target membrane (t-) SNAREs syntaxin 1a and SNAP-25 on the presynaptic membrane, whereby assembling into a stable four-helical bundle that catalyzes membrane fusion for exocytosis (Chen and Scheller, 2001; Jahn and Scheller, 2006; Sudhof and Rothman, 2009; Weber et al., 1998). Aberration in SNARE complex formation elicits erratic neuronal activity, which could lead to cognitive deficits (Corradini et al., 2009; Honer et al., 2002; Morton and Edwardson, 2001). Interestingly, it has been suggested that misfolded proteins cause cognitive impairments in relation to neurodegeneration by impairing presynaptic chaperones or directly inhibiting SNARE complex formation (Burre et al., 2010; Choi et al., 2013).
Remarkably, there is a strong correlation between intracellular Aβ (iAβ) and the progression of AD symptoms in the early stages of AD, indicating that iAβ-induced presynaptic dysfunction might be one of the pathophysiological origins of early AD (LaFerla et al., 2007; Li et al., 2007; Parodi et al., 2010). Though Aβ is normally found in the extra-cellular region, it is shown that Aβ is often cleaved off prematurely in the ER and Golgi. Furthermore, Aβ is trafficked back into the cytosol via the endocytotic pathway or passive transport, leading to the accumulation of iAβ (LaFerla et al., 2007). Recently, studies have focused on the presynaptic toxicity of Aβ oligomers in relation to neuronal communication. Genetic alterations favoring cytosolic Aβ have shown to elicit the depression of long term potentiation and the increase of long term depression. Furthermore, it was previously shown that exogenously administered Aβ in the presynapse inhibits neurotransmitter release from the neurons. Also, in agreement with these observations, iAβ accumulation precedes neuronal loss and extracellular Aβ deposition (Gouras et al., 2005; Moreno et al., 2009).
In this work we investigate the molecular mechanisms by which synaptic transmission is affected in AD. Given that the key clinical indication of AD is dementia, we hypothesized that misfolded Aβ might directly affect SNARE-mediated neuroexocytosis at the presynaptic terminal. The degree of SNARE complex formation within the brain of APP/PS1 transgenic (TG) mice was measured, and the in vitro binding assays were performed to provide the molecular basis for the reduced SNARE complex. Moreover, to investigate the effects of Aβ on the SNARE activity, we performed an in vitro single-vesicle content-mixing assay. Our results suggest that Aβ oligomers directly bind to the SNARE motif of syntaxin 1a such that SNARE complex formation is inhibited, which might lead to cognitive deficits in AD.
RESULTS
Reduced SNARE complexes in APP/PS1 transgenic mice
In a recent study, significant reduction of the SNARE complex was observed within the postmortem brains of AD patients (Sharma et al., 2012). This clinical observation indicates a possible link between SNARE-mediated exocytosis and AD. To determine if the reduced SNARE complex is associated with neuropathology of AD, we compared the level of SNARE complex formation in the cortical region of aged APP/PS1 TG (14-month-old, male, n=3) and age-matched wild-type (male, n=3) mice. The APP/PS1 TG mouse model overexpresses human Aβ, which begins to accumulate as the plaque forms within 6 months of age and induces AD-like abnormal behaviors. Accumulation of human Aβ in the brain is the pathogenic culprit for AD-associated symptoms such as dramatic deficits in synaptic plasticity (William et al., 2012), neurobiological damage and cognitive deficits in learning and memory tasks (Bornemann and Staufenbiel, 2000; German and Eisch, 2004; Morgan, 2003). The SDS-resistant property of SNARE complexes allowed us to determine the amount of complex formation using SDS-PAGE and western blot analysis. SNAP-25, VAMP2 and syntaxin 1a antibodies were used to individually quantify the amount of the SNARE complex in the brain homogenate of APP/PS1 TG and wild-type mice. While there were no apparent variations in the total amounts of SNAP-25, syntaxin 1a and VAMP2, the SNARE complex bands were significantly reduced in APP/PS1 TG mice (Figure 1A and Figure S1A). We note that higher molecular weight bands that represent oligomerized SNARE complexes were also detected (Kubista et al., 2004; Sakisaka et al., 2008). The SNARE complex bands were quantified and shown normalized to the total amount of SNAP-25, syntaxin 1a and VAMP2 from the boiled samples, respectively, and the results show ~50% decrease in the SNARE complex formation within APP/PS1 TG mice in all three cases (Figure 1A, right panel and Figure S1A, right panel). Thus, our results suggest that aggregation of Aβ may influence formation of the SNARE complex during the development of AD.
Figure 1. Aβ oligomers inhibit SNARE complex formation.
(A) SNARE complex assembly is reduced in aged-APP/PS1 TG mice. Western blot analysis of SNARE monomers and complexes in APP/PS1 TG mice and wild-type mice (14-month-old, n=3 mice,) was performed using anti-SNAP-25 antibody. Brain lysates were divided into two equal parts and immunoblotted without (−, left) or with (+, right) boiling prior to separation by SDS-PAGE. Page gels (12%) for unboiled (−) and boiled (+) samples were run separately, but side-by-side with markers. (+) and (−) membranes were aligned based on the markers. Ternary SNARE complexes were detectable only in unboiled samples. The SNARE complexes were quantified and normalized by the amount of SNARE proteins in the boiled sample (left, representative blot; right, quantitation from 5 independent blots). Data are represented as mean ± SD (**P<0.01 by Student’s t test). See also Figure S1A. (B) Aβ oligomers inhibit formation of the SDS-resistant SNARE complex from recombinant SNAREs while the monomers do not. The SNARE complexes were quantified and normalized by the total amount of SNARE proteins (left, representative SDS-PAGE gel; right, quantitation from 5 independent experiments). All Aβ oligomers concentrations are given as those of monomeric Aβ. (C) Schematic description of the co-floatation assay for Aβ oligomers’ binding to proteoliposomes (left). The amounts of Aβ bound to the individual vesicle samples were measured by dot blot using anti-amyloid oligomer (A11) antibody or anti-beta amyloid (6E10) antibody. Representative immunoblots (middle) and quantitation of liposome-bound proteins (right) are shown, respectively. See also Figure S1B. In (B) and (C), the data are represented as mean ± SD (*P<0.05, **P<0.01, and ***P<0.005 by Student’s t test; (B) n=5 and (C) n=3 independent experiments).
Aβ oligomers inhibit the formation of the SDS-resistant SNARE complex
The inhibition of SNARE complex formation in APP/PS1 TG mice suggests a possible direct interaction between Aβ and SNARE proteins. The SNARE motifs of SNAP-25, syntaxin 1a and VAMP2 assemble to form a four-helix bundle within the ternary SNARE complex. Blockage or cleavage of these motifs reduces synaptic membrane fusion and neurotransmitter release which may lead to cognitive deficits in AD (O’Connor et al., 1997; Schiavo et al., 1992; Yang et al., 2010).
Here, we examined whether and how Aβ affects complex formation between purified recombinant SNAREs. We subjected Aβ monomers or oligomers (see Materials and Methods for details) to the equimolar mixture of SNAP-25, syntaxin 1a and VAMP2 and compared the formation of the SNARE complex in SDS-PAGE. Remarkably, while monomeric Aβ had no effect on SNARE complex formation in the range of 0–0.5 μM, a significant reduction was observed in the presence of Aβ oligomers (Figure 1B). In fact, we observed only trace amounts of the SNARE complex with 0.5 μM Aβ oligomers (concentrations given in moles of monomeric Aβ). Thus, our results reveal that, in contrast to monomers, Aβ oligomers have the capacity to inhibit SNARE complex formation. These results are in coherence with clinical observations that the development of AD correlates with conversion of non-toxic monomers to pathogenic oligomers (Benilova et al., 2012).
Because SNARE complex formation was significantly reduced by Aβ oligomers in a minimalistic environment, a direct physical interaction between Aβ oligomers and v-SNAREs or t-SNAREs could be anticipated. To test this idea, we performed a modified co-floatation assay (Figure 1C) in a cell-free membrane environment (Burre et al., 2010). Aβ oligomers were incubated with either t-vesicles or v-vesicles, which were reconstituted with syntaxin 1a and SNAP-25 or VAMP2 and synaptotagmin 1 (Syt1), respectively. Although Syt1 is not a SNARE protein, it is located on the synaptic vesicle and tightly regulates Ca2+-triggered synaptic exocytosis. Vesicles were then collected from the pellets after centrifugation, followed by the dot blot analysis using anti-beta amyloid (6E10) and anti-amyloid oligomer (A11) antibodies (Figure 1C). We observed significant physical binding between Aβ oligomers and t-vesicles. The dot blot binding intensity of t-vesicles was significantly higher than that of v-vesicles or protein-free liposomes, thus suggesting that Aβ oligomers preferentially bind to either or both syntaxin 1a or SNAP-25 in the membrane environment. Also, we confirmed the existence of both t- and v-SNAREs in the precipitated liposomes by western blot analysis (Figure S1B). Taken together, these results show that Aβ oligomers, but not the monomers, inhibit SNARE complex formation by interacting with t-SNAREs.
Aβ is a syntaxin 1a binding protein
Unless Aβ oligomers interact with t-SNAREs by conformation-specific binding, monomeric Aβ could also bind to t-SNAREs. To further dissect the molecular origin of the Aβ-SNARE interaction, we performed a pull-down assay employing glutathione S-transferase (GST)-SNAP-25, -VAMP2 and -syntaxin 1a in an attempt to specifically identify the target protein (Figure 2). The individual GST-SNARE proteins were separately attached to the glutathione agarose beads after which Aβ monomers/oligomers were added to the sample followed by thorough washing to remove unbound proteins (Figure 2A). Both Aβ monomers/oligomers exhibited a strong affinity to syntaxin 1a while the monomers show some weaker binding to SNAP-25 than the binding to syntaxin 1a (Figure 2C, D and E). In contrast, no interaction was observed with VAMP2, which is consistent with our modified co-floatation assay results.
Figure 2. Aβ monomers and oligomers bind to the SNARE motif of syntaxin 1a.
(A) Schematics of the GST pull-down assay for Aβ’s binding to individual SNARE proteins. (B, glutathione agarose bead) (B) The primary domain structure of syntaxin 1a. The region corresponding to the N-terminal Habc domain is depicted as the white bar, the SNARE motif as the red bar and the transmembrane (TM) region as the black bar. SynH3 refers to the syntaxin 1a mutant containing only the SNARE motif. (C) The amounts of the Aβ monomers bound to the SNARE proteins were measured by dot blot using anti-beta amyloid antibody (6E10, upper panel). SNARE proteins cleaved from GST were visualized by Coomassie blue staining after SDS-PAGE (lower panel). (D) The amounts of Aβ oligomers bound to the SNARE proteins were measured by dot blot using anti-amyloid oligomer antibody (A11, upper panel). SNARE proteins cleaved from GST were visualized by Coomassie blue staining after SDS-PAGE (lower panel). Also, see Figure S2C for precipitation by 6E10 that verifies the existence of Aβ. (E) The dot blots of the Aβ monomer and oligomers bound to SNAREs were quantified and normalized with respect to the Aβ input. The Aβ monomers and oligomers were detected by anti-beta amyloid (6E10) and anti-amyloid oligomer (A11) antibodies, respectively. Data are represented as mean ± SD (*P<0.05, **P<0.01 by Student’s t test). The experiments were performed three times with independently prepared samples.
We further investigated the Aβ-binding region within syntaxin 1a by performing the pull-down experiments with truncated syntaxin 1a, one containing only the SNARE motif (SynH3) and the other containing only the N-terminal Habc domain (Figure 2B). We found that Aβ monomers/oligomers bind only to SynH3, suggesting that both the monomeric and oligomeric form of Aβ bind specifically to the SNARE motif region of syntaxin 1a (Figure 2C, D and Figure S2). Notably, although only Aβ oligomers display any inhibitory effects on SNARE complex formation, both monomers and oligomers bind to the SNARE motif region of syntaxin 1a.
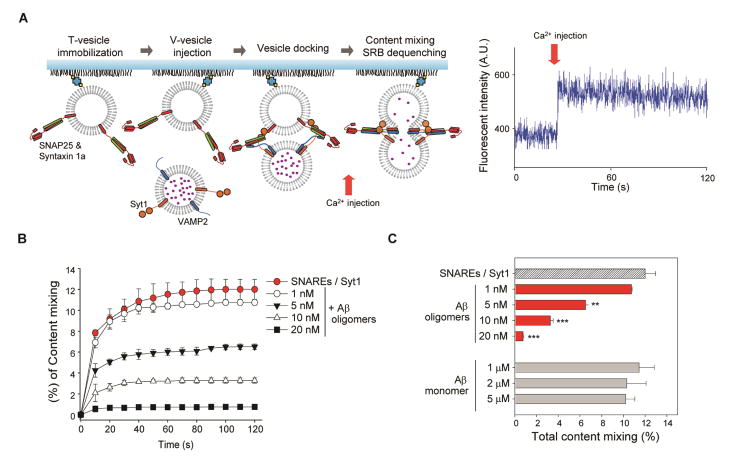
Aβ oligomers inhibit Ca2+-triggered content-mixing
Although the impairment of SNARE complex formation would reduce synaptic vesicle fusion, it is critical to confirm that the Aβ-syntaxin 1a interaction induces the inhibition of SNARE-mediated exocytosis. Thus, we performed a well-defined in vitro single-vesicle content-mixing assay that monitors SNARE-mediated Ca2+-triggered opening of single fusion pore, with a total internal reflection fluorescence (TIRF) microscope (Lai et al., 2013).
Experimentally, two populations of vesicles representing the synaptic vesicles and target membrane were prepared. The t-vesicles, reconstituted with syntaxin 1a and SNAP-25, were injected into the flow chamber and tethered on the polyethylene glycol (PEG) coated imaging surface. The v-vesicles, encapsulated with sulforhodamine B (SRB, 20 mM) and reconstituted with VAMP2 and Syt1, were flown into the flow chamber to allow vesicle docking. After sufficient buffer exchange to remove any unbound v-vesicles, 500 μM Ca2+ was injected into the flow cell to trigger vesicle fusion (Figure 3A). The stepwise increase in fluorescence intensity of the v-vesicles, due to the dequenching of SRB, was used to identify content-mixing from individual vesicle pairs (Figure 3A, right panel).
Figure 3. Aβ oligomers inhibit Ca2+-triggered exocytosis in the in vitro single-vesicle content-mixing assay.
(A) Schematics of the in vitro content-mixing assay (left). A representative fluorescent intensity time trace is shown (right). (B) The cumulative time plot of the content-mixing percentage among total docked population at various concentrations of Aβ oligomers (Ca2+ injection at 10 s). The control, without Aβ oligomers, is depicted with red circles. See also Figure S3. (C) Histogram of total content-mixing percentage during the 120 s observation period. Data in (B) and (C) represent mean ± SD (**P<0.01, ***P<0.005 by Student’s t test; n=5 independent experiments).
As expected, a significant decrease in content-mixing population was observed as a function of Aβ oligomers concentration. While ~12% of total docked vesicle pairs displayed content-mixing, at t=120 s, in the absence of Aβ oligomers, we observed a 10.5%, 45.8%, 72.7% and 93.7% decrease in content-mixing population corresponding to 1 nM, 5 nM, 10 nM and 20 nM treatment of Aβ oligomers (concentrations given in moles of monomeric Aβ), respectively (Figure 3B). No such inhibitory effect was observed even at higher concentrations of Aβ monomers, consistent with the notion that Aβ oligomers are the neurotoxic agents (Figure 3C).
We further investigated whether Aβ monomers/oligomers affect synaptic vesicle docking by comparing the total docked vesicle pairs at various concentrations of Aβ monomers/oligomers (Figure S3B). The vesicle docking population displayed no variations, indicating that both Aβ monomers/oligomers do not affect vesicle docking in the presence or absence of Syt1. However, we observed a significant decrease of lipid-mixing in the presence of Aβ oligomers, while monomers exhibited no apparent difference (Figure S3C–H). Thus, our results suggest that Aβ oligomers, but not the monomers, specifically inhibit the fusion step between docking and lipid-mixing.
DISCUSSION
Aβ accumulation has been associated with the cholinergic dysfunction observed in AD, which is characterized by diminished neurotransmitter release (Itoh et al., 1996; Kar et al., 1998; Vaucher et al., 2001). However, the mechanism of Aβ-mediated toxicity is not clearly understood. In this study, we raise the possibility that the oligomeric form of Aβ directly inhibits SNARE-mediated exocytosis by binding to the SNARE motif of syntaxin 1a. APP/PS1 TG mice displayed a significant decrease in SNARE complex formation when compared with that of wild-type mice, which is in agreement with the results from the brains of AD patients (Sharma et al., 2012). Because APP/PS1 TG mice are specifically engineered to overexpress Aβ, but not other AD inducing proteins such as tau (Holcomb et al., 1998), our in vivo data establishes a possible connection between abnormal Aβ and SNARE proteins. Our in vitro experiments not only reveal that Aβ binds to syntaxin 1a but further narrow the binding region to the SNARE motif, which forms a four-helix bundle (Poirier et al., 1998; Sutton et al., 1998). Moreover, we demonstrate that Aβ oligomers have the capacity to inhibit SNARE-mediated exocytosis by monitoring fusion pore opening between a single-vesicle pair with TIRF microscopy. To the contrary, the Aβ monomers failed to exhibit any inhibitory effects on SNARE complex formation or membrane fusion, despite the fact that monomers also bind to syntaxin 1a. We speculate that steric hindrance of the Aβ monomers may not be sufficient to inhibit SNARE-mediated exocytosis. Collectively, our results are in coherence with previous studies suggesting that iAβ oligomers are the pathogens responsible for cognitive impairment in AD (Gouras et al., 2005; LaFerla et al., 2007; Li et al., 2007; Moreno et al., 2009; Parodi et al., 2010) and further identify the underlying molecular mechanisms and the target protein machinery.
Interestingly, in our previous work we found that α-synuclein (αSyn) oligomers, which are thought to be the primal cause of the Parkinson’s disease, inhibit vesicle fusion (Choi et al., 2013). In this case, the key interaction is between αSyn and v-SNARE, which is somewhat different from Aβ oligomers’ interaction with t-SNARE. Although the detailed underlying molecular interactions are different for αSyn and Aβ, the common pathophysiological theme for these two dementia-inducing proteins is their binding to SNAREs and the inhibition of SNARE-mediated vesicle fusion by their oligomers. Therefore, our results altogether might suggest that the inhibition of SNARE-dependent vesicle fusion is a common physiological origin shared by neurodegenerative diseases.
One notable observation is that Aβ oligomers hinder the formation of the SNARE complex, while having no effect on vesicle docking. These two sets of data appear to be paradoxical because SNARE complex formation is necessary for synaptic vesicle docking. However, these results actually provide deep molecular-level insights into the mechanism by which Aβ oligomers inhibit the SNARE function. It might be that Aβ oligomers allow only partial SNARE assembly, to the point, sufficient for vesicle docking. In fact, the partially-zipped complex, in which only the N-terminal half is assembled, is considered a bona fide intermediate state along the SNARE complex formation pathway (Kim et al., 2012; Shin et al., 2014). Thus, we speculate that Aβ oligomers botch SNARE complex formation right at this intermediate stage by binding to the C-terminal half of the syntaxin 1a SNARE motif, whereby SNARE assembly is allowed only at the N-terminal half without further progression towards vesicle fusion (Figure 4).
Figure 4. A mechanistic model for the inhibition of Ca2+-triggered SNARE-mediated exocytosis by Aβ oligomers.
Normal SNARE-mediated exocytosis involves full zippering of the SNARE complex, which induces the fusion pore (left). A hypothetical mechanistic model shows that Aβ oligomers bind to t-SNAREs in such a way that vesicle docking is unaffected yet stops zippering of SNARE complex, thereby inhibiting pore formation (right).
SNARE proteins are constantly recycled from synaptic vesicles to the plasma membrane and back to vesicles (Chen and Scheller, 2001; Sollner et al., 1993). Although we attributed the decrease of the SNARE complex in AD-induced transgenic mice and post-mortem brains of AD-patients to the inhibition of SNARE-mediated vesicle fusion, the decrease could arise from other reasons. The SDS-resistant SNARE complex represents the cis-SNARE complex on the plasma membrane. Thus, if the disassembly of the cis-complex to individual SNAREs by α-SNAP and NSF on the plasma membrane is promoted or if the endocytic pathway is accelerated by Aβ oligomers, a similar reduction of the cis-SNARE complex would be observed. However, a recent study reported that Aβ oligomers induce depletion of synaptic vesicles (Parodi et al., 2010), suggesting that acceleration of the endocytotic pathway is highly unlikely, warranting further investigation. Also, if the expression of SNARE proteins was somehow impaired, a similar result would be observed even in the absence of Aβ oligomers’ inhibitory effect on SNARE complex assembly. However, the invariant total amount of SNAP-25, syntaxin 1a and VAMP2 in APP/PS1 TG mice suggests that this too is unlikely.
In conclusion, our study provides potential molecular mechanisms of the clinical notion that presynaptic Aβ inhibits synaptic transmission. Recently, the US Food and Drug Administration announced a new strategic plan to focus on the development of drugs which enhance cognition of AD patients in the early stage before overt dementia (Kozauer and Katz, 2013). Commercially available AD drugs have shown that increasing levels of Ach help daily activities of demented AD patients. Our findings suggest an additional target for such drugs to treat AD. Blocking the Aβ binding to syntaxin 1a could help restore Aβ oligomer-stricken SNARE-mediated exocytosis, which would help patients to regain normal cognition.
EXPERIMENTAL PROCEDURES
Preparation of Aβ oligomers
The synthetic peptide wild-type Aβ42 was purchased from American Peptide. As described previously (Ahmed et al., 2010; Dahlgren et al., 2002), the Aβ42 peptide was initially dissolved to 1 mM in hexafluoroisopropanol (Sigma-Aldrich) and separated into aliquots in sterile microcentrifuge tubes. Hexafluoroisopropanol was removed under vacuum in a SpeedVac, and the peptide film was stored desiccated at −80 °C. To make oligomers cold PBS was added to bring the peptide to a final concentration of 10 μM and incubated at 4 °C for 18 h (Ahmed et al., 2010). The size of the Aβ oligomers (penta/hexamers) was previously confirmed using size exclusion chromatography, transmission electron microscopy and dynamic light scattering (Ahmed et al., 2010; Bitan et al., 2003)
In vitro single-vesicle content-mixing assay
After surface coating with PEG molecules and PEG-biotin molecules (molar ratio 40:1, Laysan Bio), the quartz slide was assembled into a flow chamber and coated with streptavidin (0.2 mg/mL, Sigma-Aldrich). Following 10 min incubation at room temperature, the t-vesicles (125 μM) were immobilized on the PEG-coated surface for 20 min. After several rounds of washing in 200 μl of buffer, the v-vesicles (10 μM) containing 20 mM SRB were injected into the flow chamber for the 10 min pre-docking at room temperature (~25 °C). After washing out the unbound v-vesicles, additional 20 min incubation was followed by 500 μM of Ca2+ injection using motorized syringe pump. A stepwise jump in fluorescence intensity was detected with fusion pores, due to the dequenching SRB. The fluorescent signals from the immobilized individual vesicle pairs were obtained from TIRF microscope. The details of TIRF microscope imaging and single molecule data analysis have been reported in our previous work (Diao et al., 2012).
Co-floatation assay for measuring the binding properties of Aβ oligomers
We measured the binding properties of Aβ oligomers to proteoliposomes (t- and v-vesicles) and protein- free vesicles using a modified co-flotation assay. Both t- and v-vesicles (200 μM) were incubated with 200 nM of Aβ oligomers for 30 min at room temperature, and then centrifuged at 199,000 × g for 30 min using airfuse centrifugation (Beckman). The amounts of vesicle-bound Aβ oligomers from pellet, compared with the initial input of Aβ oligomers, were measured by dot blot using anti-amyloid oligomer (A11) antibody (Millipore) and anti-beta amyloid (6E10) antibody (Covance).
GST pull-down assay
GST-tagged SNAREs bound to glutathione agarose bead (50 μL, 50% slurry equilibrated in the same buffer) were mixed with Aβ (1 μM) each in 500 μL PBST (PBS containing 0.1% Triton X-100), incubated at room temperature for 30 min with gentle agitation. After washing five times with buffer, bound GST-tagged SNARE proteins were eluted by thrombin cleavage, followed by dot blot and SDS-PAGE.
Supplementary Material
Highlights.
SNARE complex formation is reduced in APP/PS1 mice.
Aβ oligomers inhibit SNARE complex formation.
Aβ binds to the SNARE motif of syntaxin 1a.
Aβ oligomers inhibit SNARE-mediated vesicle fusion.
Acknowledgments
This work was supported by NIH grants (R01 GM051290, Y.-K.S.), KIST Institutional Programs (2E25520, 2E25023), KHIDI (HI14C3319), and RF and the WISET Grant funded by the Ministry of Science (Republic of Korea), MSIP under the Program for Returners into R&D (KW-2014-PPD-0076).
Abbreviations footnote
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- GST
Glutathione S-transferase
- iAβ
intracellular Aβ
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- PEG
Polyethylene glycol
- Syt1
synaptotagmin 1
- SRB
sulforhodamine B
Footnotes
AUTHOR CONTRIBUTIONS
Y. Y., J. K., N. R., H. R., and Y.-K. S. designed the experiments. Y. Y. and J. K. performed the experiments. H.Y.K. performed the animal preparation and S. L. prepared Aβ. Y. Y., J. K., Y. K., and Y.-K. S. wrote the paper with contributions from all of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO. Structural conversion of neurotoxic amyloid-β (1–42) oligomers to fibrils. Nat Struct Mol Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci USA. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann KD, Staufenbiel M. Transgenic mouse models of Alzheimer’s disease. Ann N Y Acad Sci. 2000;908:260–266. doi: 10.1111/j.1749-6632.2000.tb06653.x. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, Lee NK, Shin YK. Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013;110:4087–4092. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini I, Verderio C, Sala M, Wilson MC, Matteoli M. SNAP-25 in neuropsychiatric disorders. Ann N Y Acad Sci. 2009;1152:93–99. doi: 10.1111/j.1749-6632.2008.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Diao J, Ishitsuka Y, Lee H, Joo C, Su Z, Syed S, Shin YK, Yoon TY, Ha T. A single vesicle-vesicle fusion assay for in vitro studies of SNAREs and accessory proteins. Nat Protoc. 2012;7:921–934. doi: 10.1038/nprot.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Eisch AJ. Mouse models of Alzheimer’s disease: insight into treatment. Rev Neurosci. 2004;15:353–369. doi: 10.1515/revneuro.2004.15.5.353. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Bayer TA, Xie J, Hu L, Li HY, Arango V, Mann JJ, Dwork AJ, Trimble WS. Abnormalities of SNARE Mechanism Proteins in Anterior Frontal Cortex in Severe Mental Illness. Cereb Cortex. 2002;12:349–356. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Nitta A, Nadai M, Nishimura K, Hirose M, Hasegawa T, Nabeshima T. Dysfunction of cholinergic and dopaminergic neuronal systems in beta-amyloid protein--infused rats. J Neurochem. 1996;66:1113–1117. doi: 10.1046/j.1471-4159.1996.66031113.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Kar S, Issa AM, Seto D, Auld DS, Collier B, Quirion R. Amyloid beta-peptide inhibits high-affinity choline uptake and acetylcholine release in rat hippocampal slices. J Neurochem. 1998;70:2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- Kim JY, Choi BK, Choi MG, Kim SA, Lai Y, Shin YK, Lee NK. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 2012;31:2144–2155. doi: 10.1038/emboj.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med. 2013;368:1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- Kubista H, Edelbauer H, Boehm S. Evidence for structural and functional diversity among SDS-resistant SNARE complexes in neuroendocrine cells. J Cell Sci. 2004;117:955–966. doi: 10.1242/jcs.00941. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G. The Alzheimer’s A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- Lai Y, Diao J, Liu Y, Ishitsuka Y, Su Z, Schulten K, Ha T, Shin YK. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin 1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1333–1338. doi: 10.1073/pnas.1218818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen L, Lee DH, Yu LC, Zhang Y. The role of intracellular amyloid beta in Alzheimer’s disease. Prog Neurobiol. 2007;83:131–139. doi: 10.1016/j.pneurobio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinas RR. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci USA. 2009;106:5901–5906. doi: 10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Learning and memory deficits in APP transgenic mouse models of amyloid deposition. Neurochem Res. 2003;28:1029–1034. doi: 10.1023/a:1023255106106. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Edwardson JM. Progressive depletion of complexin II in a transgenic mouse model of Huntington’s disease. J Neurochem. 2001;76:166–172. doi: 10.1046/j.1471-4159.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- O’Connor V, Heuss C, De Bello WM, Dresbach T, Charlton MP, Hunt JH, Pellegrini LL, Hodel A, Burger MM, Betz H, et al. Disruption of syntaxin-mediated protein interactions blocks neurotransmitter secretion. Proc Natl Acad Sci USA. 1997;94:12186–12191. doi: 10.1073/pnas.94.22.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi J, Sepulveda FJ, Roa J, Opazo C, Inestrosa NC, Aguayo LG. β-amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J Biol Chem. 2010;285:2506–2514. doi: 10.1074/jbc.M109.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nature structural biology. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Yamamoto Y, Mochida S, Nakamura M, Nishikawa K, Ishizaki H, Okamoto-Tanaka M, Miyoshi J, Fujiyoshi Y, Manabe T, et al. Dual inhibition of SNARE complex formation by tomosyn ensures controlled neurotransmitter release. J Cell Biol. 2008;183:323–337. doi: 10.1083/jcb.200805150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo GG, Benfenati F, Poulain B, Rossetto O, de Laureto PP, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burre J, Sudhof TC. Proteasome inhibition alleviates SNARE-dependent neurodegeneration. Sci Transl Med. 2012;4:147ra113. doi: 10.1126/scitranslmed.3004028. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Lou X, Kweon DH, Shin YK. Multiple conformations of a single SNAREpin between two nanodisc membranes reveal diverse pre-fusion states. Biochem J. 2014;459:95–102. doi: 10.1042/BJ20131668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Aumont N, Pearson D, Rowe W, Poirier J, Kar S. Amyloid beta peptide levels and its effects on hippocampal acetylcholine release in aged, cognitively-impaired and -unimpaired rats. J Chem Neuroanat. 2001;21:323–329. doi: 10.1016/s0891-0618(01)00120-x. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- William CM, Andermann ML, Goldey GJ, Roumis DK, Reid RC, Shatz CJ, Albers MW, Frosch MP, Hyman BT. Synaptic plasticity defect following visual deprivation in Alzheimer’s disease model transgenic mice. J Neurosci. 2012;32:8004–8011. doi: 10.1523/JNEUROSCI.5369-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shin JY, Oh JM, Jung CH, Hwang Y, Kim S, Kim JS, Yoon KJ, Ryu JY, Shin J, et al. Dissection of SNARE-driven membrane fusion and neuroexocytosis by wedging small hydrophobic molecules into the SNARE zipper. Proc Natl Acad Sci USA. 2010;107:22145–22150. doi: 10.1073/pnas.1006899108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.