Abstract
The impact of shipping temperatures and preservation media used during transport of either peripheral blood mononuclear cells (PBMCs) or Jurkat cells was assessed, in view of implementing of a proficiency testing scheme on mononuclear cell viability. Samples were analyzed before and after shipment at different temperatures (ambient temperature, dry ice, and liquid nitrogen) and in different preservation media (serum with cryoprotectant, commercial cryopreservation solution, and room temperature transport medium). Sample quality was assessed by viability assays (Trypan Blue dye exclusion, flow cytometry, Cell Analysis System cell counting (CASY)), and by ELISpot functional assay. The liquid nitrogen storage and shipment were found to be the most stable conditions to preserve cell viability and functionality. However, we show that alternative high quality shipment conditions for viable cells are dry ice shipment and commercial cryopreservation solution. These were also cost-efficient shipment conditions, satisfying the requirements of a proficiency testing scheme for viable mononuclear cells. Room temperature transport medium dramatically and adversely affected the integrity of mononuclear cells.
Introduction
Shipment of viable cells between research laboratories and biobanks is necessary for many collaborative projects. Storage and specimen transport conditions need to be considered since they can influence biological specimen viability and functionality and lead to pre-analytical bias.1–3 The International Society for Biological and Environmental Repositories (ISBER) Best Practices include detailed guidelines on sample transport.4 The ISBER Biospecimen Science Working Group (BSWG) has published a standard biospecimen research experimental protocol for the study and reduction of pre-analytical variability related to sample processing.5 This Working Group has also published specific recommendations on logistics and sample transport.6 ISBER and the Integrated Biobank of Luxembourg (IBBL) have developed a Proficiency Testing (PT) program for biorepositories to enable external quality assessment of the methods used by biobanks as biospecimen Quality Control (QC) methods.7,8
Viable mononuclear cells are the object of one of the biorepository PT schemes. Viable mononuclear cells are important biospecimens because they allow researchers to identify circulating disease biomarkers. Examples include lymphocyte subset-specific gene expression signatures in cancer9 or autoimmune diseases,10 lymphocyte subset-specific miRNA signatures in multiple sclerosis,11 or T cell subset-specific flow cytometric signatures in Parkinson's disease.12 Frozen viable PBMCs are fit-for-purpose, not only for immunomagnetic sorting of purified monocyte and lymphocyte populations, following cryopreservation,13 but also for functional studies,14 immunophenotyping,15 establishment of lymphoblastoid cell lines (LCL) by Epstein Barr virus (EBV) transformation,16 and purification of CD34+ cells.17
The surrogate QC assay for either EBV transformation success18 or immunophenotyping and proliferation assays14 has shown cell viability, with a qualification cut-off at around 70% viability. Therefore, implementation of a PT scheme on cell viability for repositories, which process and cryopreserve mononuclear cells for all the above-mentioned end-uses, is of critical importance. Implementation of such a PT scheme includes shipment of viable mononuclear cells (as “PT test items”) to different participants, around the world. These PT test items should have percent viability (an assigned value), calculated after cell thawing. Furthermore, the test items should be homogeneous and stable before and after shipment.
Some early studies have demonstrated how storage2,3 and cryopreservation13,19–22 may influence PBMC viability and functionality.23–26 Previous studies established the practice to cryopreserve PBMCs within the first hours after blood collection1,14,27 in order to preserve PBMCs functionality for immunological assays. Several studies28–30 have also focused on handling and storage of cryopreserved PBMCs addressing the importance of blood shipment conditions in infectious disease studies. The effect of ambient temperature during shipment of fresh PBMCs on subsequent processing and recovery has been evaluated.31
However, there are a lack of data cross-investigating cell type, cryopreservation medium, transatlantic shipment conditions, and assessment methods. Our current study is the first to evaluate multiple variables affecting PT specimen integrity: viability (including early stage of apoptosis), functionality of peripheral blood mononuclear cells (PBMCs) and Jurkat cell line (an immortalized line of T lymphocyte cells), preserved in different preservation media (serum with cryoprotectant, commercial cryopreservation solution, and room temperature transport medium), shipped under different conditions (liquid nitrogen (LN), dry ice (DI) for frozen cells) or stored and shipped at ambient temperature.
Usually PBMCs are cryopreserved in LN using 10% DMSO27 and shipped in LN or DI.28 In our study, we have additionally assessed the viability and function of cells stored in commercially available preservation media CryoStor® CS1032,33 and AQIX® RS-I.34,35 The first medium, CryoStor® CS10, is pre-formulated with 10% DMSO,33 and provides a protective environment for cells during the freezing, storage, and thawing process. The second medium, AQIX® RS-I, is designed35 to simulate the composition of human interstitial fluid and thereby afford isolated cells to maintain homeostasis of biophysical and metabolic parameters during periods of both hypothermic and normothermic preservation. It could therefore allow a PT provider to reduce shipment costs while performing transport at ambient temperature.
Stability testing is necessary before implementation of a PT scheme in order to (i) assess the most cost-efficient shipment mode for the PT test items (viable mononuclear cells), and (ii) verify that there is no consequential instability of the test items. Furthermore, since a PT program requires a value assessment after cell thawing, under the same conditions as those employed by the PT participants, the baseline viability values used for normalization were calculated after cell thawing.
Methods
Participating laboratories
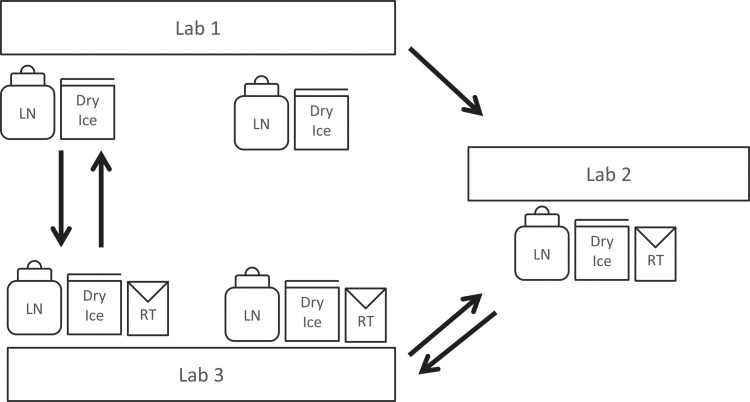
Three facilities participated in the study, all operating in compliance with ISO17025, CLIA, and Good Laboratory Practice guidelines (GLP). Three laboratories were used for this study: one laboratory was located on the West Coast (Lab 1) and one on the East Coast (Lab 2) of the United States and one in central Europe (Lab 3). Each facility was responsible for preparing samples for their testing and shipping to other facilities for testing (Fig. 1). Shipping was performed according to standard operating procedures (SOPs) including continuous temperature monitoring. Two shipment rounds were performed.
FIG. 1.
Shipment schema and participating laboratories: Three facilities participated in the study, all operating in compliance with ISO17025, CLIA, and good lab practice guidelines: IBBL (Integrated BioBank of Luxembourg), PPD (PPD Vaccines and Biologics Laboratory), UCSF (University of California San Francisco AIDS Specimen Bank).
Samples used
Normal PBMC and Jurkat cell line samples were used and prepared as follows.
PBMC suspension was prepared by pooling blood from 12 8-mL ACD tubes, either from the same or from different HIV-negative donors, in order to obtain a minimum of 109 cells. All donors signed informed consent. PBMCs were separated on Ficoll gradients (EUROBIO, ref. CMSMSL01-01) and washed three times in phosphate-buffered saline (PBS).
Jurkat samples (Clone E6-1) were purchased from the American Tissue and Cell Collection (ATCC, ref. TIB-152). Cells were cultured at 37°C, 5% CO2 in RPMI 1640 (Invitrogen, ref. RPMI A10491-01) supplemented with 10% fetal bovine serum (FBS, Invitrogen, ref. 26140-079). Routine passage was carried out every 2 or 3 days.
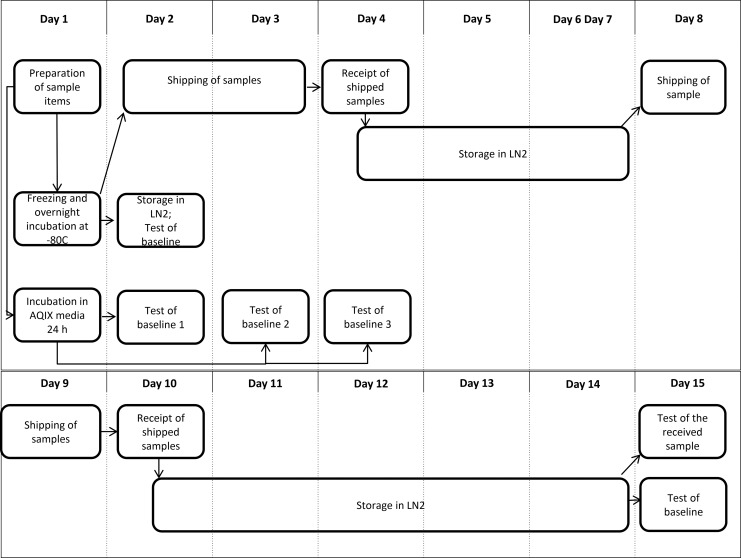
Both cell cultures were divided in two halves. Each half was processed as “high viability suspension” (HV) and as “intermediate viability suspension” (IV) for the first shipment round, or as “high viability suspension” and as “low viability suspension” (LV) for the second shipment round. The HV suspension corresponded to the Jurkat cells incubated according to manufacturer's instructions (cell passage 1–8) or freshly isolated cells from whole blood for PBMCs; the IV suspension was obtained, following incubation of HV suspension of Jurkat cells (cell passage 1–8) or fresh isolated PBMCs for one week at +4°C; the LV suspension was obtained, following incubation of Jurkat cells (cell passage 10–18) for one week at +4°C.
Each cell suspension was divided into three parts (Fig. 2) and culture medium was completely replaced with cold (+2°–8°C) preservation medium, using either:
• 90% heat inactivated FBS containing 10% DMSO freezing media (Sigma, ref. D2438),
• CryoStor® CS10 freezing media (BioLife Solutions, ref. 210102)
• AQIX® RS-I preservation media (Aqix Ltd, ref. RSI/KIT)
FIG. 2.
Flow charts illustrating the distribution and testing of specimens for one shipment round. Sample items included HV, IV, or LV cell suspensions, each in duplicates, in order to ship back one duplicate to the original production laboratory.
with a final cell concentration of 5×106 cells/mL.
One mL aliquots in FBS with 10% DMSO or CryoStor® CS10 were placed in freezing isopropanol containers (Mr Frosty, Nalgene®) for freezing at −80°C, kept 18 h (overnight), and then transferred to LN vapor for storage until shipment. After at least 6 h of LN storage, samples were shipped in LN or dry ice (DI). The aliquots made in AQIX® RS-I, were kept at +4°C for 24 h until shipment. For all study centers, the shipment took place simultaneously for all conditions (Fig. 2), while the stock of the same sample aliquots was kept for baseline testing on site.
Definition of sample quality value categories
The quality of the initial cell suspension before storage and shipment was set as: HV=high viability, IV=intermediate viability, LV=low viability. The definition of HV, IV, LV was based on Trypan Blue assessment of the Jurkat cell suspensions cryopreserved with 10% DMSO and stored in LN in Lab 3: 0<LV<25%, 25%<IV<80%, 80%<HV<100% (Table 1).
Table 1.
Baseline Values of Viability of Jurkat Cells Cryopreserved with 10% DMSO and Stored in LN2 Assessed by Various Methods
| Viability (%) of cells cryopreserved in 10% DMSO | |||
|---|---|---|---|
| Method | HV | IV | LV |
| Trypan Blue | 80.0 | 48.1 | 5.8 |
| CASY | 82.8 | 68.2 | 65.1 |
| Flow cytometry | 67.8 | 24.8 | 5.0 |
| Viability (%) of cells cryopreserved in CryoStor 10 | |||
|---|---|---|---|
| Method | HV | IV | LV |
| Trypan Blue | 86.1 | 48.4 | 12.7 |
| CASY | 85.2 | 77.5 | 68.5 |
| Flow cytometry | 78.5 | 29.7 | 6.3 |
Initial cell suspension quality before storage was set as: HV=high viability, IV=intermediate viability, LV=low viability.
Logistics
Logistics of the sample shipments to and from the three study centers is shown in Figure 1. Lab 1 participated only in the first shipment round and testing run, while Lab 2 and Lab 3 participated in both shipment rounds and testing runs. All study centers used the same references of consumables and protocols in both cell processing and testing experiments. The shipments were operated by Federal Express. The receiving laboratory was responsible for return shipment or testing of samples (Fig. 2).
All ambient and dry ice shipments were sent with a Sensitech Temptale 4 (Sensitech Inc, ref. D4400-01) to monitor the temperature of the package through a shipment. SafTPak packaging (SafTPak Category B insulated shipping carton: for cryo- and dry ice shipments, ref. STP-320; for ambient shipments, ref. STP-309) was used and the Sensitech Template 4 was placed with the samples inside the inner box. Frozen, dry ice shipments had the shipper box filled to capacity with dry ice before being sealed and weighed. Upon receipt of the shipments at their destinations, samples and dataloggers were removed.
All cryogenic shipments were sent utilizing a Chart MVE Cryo Shipper equipped with a Cryolid with Datalogger (Chart MVE, ref. 10508967). The datalogger was started after the cryo shipper was charged with LN and stopped once the samples were removed from the cryo shipper. SafTPak packaging was used to safeguard the samples during transport.
Viability assessment
Viability of cryopreserved PBMCs and Jurkat cells was measured immediately after thawing on the day of shipment and after 1 and 2 weeks of storage (storage baseline values) in the laboratory of origin, simultaneously with samples having been shipped and tested by the recipient laboratory. For ambient transport media, the baseline measures included the measures on the day of shipment and after 1 and 2 days of storage (Fig.2). Sample quality was measured by determining the levels of viability and early apoptosis, whereas sample functionality was assessed by ELISpot. The viability parameters were assessed by Trypan Blue dye exclusion test, flow cytometry (by Guava, Guava Technologies and Influx, Becton Dickinson) and CASY cell counter (Cell Analysis System),36 for all applied conditions as described below. Cell recovery and cell viability were assessed immediately after thawing (within 1 h). Each sample was measured 2–3 times.
Thawing procedure
Upon removal from LN storage, no more than two cryovials were thawed at a time by gentle agitation in a 37°C water bath. When the last crystal dissolved, the contents of the vials were transferred immediately to a 15 mL tube and slowly diluted in 9 mL RPMI 1640 containing 10% FBS. The tubes were centrifuged at 150–200 g for 10 min and gently re-suspended in fresh complete RPMI medium.
Trypan Blue
The Trypan Blue exclusion assay was used to determine cell viability and membrane integrity. The cell suspension was mixed with 0.04% Trypan Blue dye. The live cells (negative for staining) and dead cells (positive for staining) were counted using a hemocytometer. The analytical uncertainty of the method, as previously established in Lab 3 corresponded to a CV% of 6.8% (p<0.05).
CASY cell counter
Using the CASY cell counter, an electric field multi-channel cell counting system, cell viability can be assessed based on the integrity of plasma membrane; the living cells have intact plasma membranes, whereas membranes of dead cells are disrupted. The analytical uncertainty of the method, as previously established in Lab 3 corresponded to a CV% of 2.1% (p<0.05).
Flow cytometry by Guava
Viability was assessed using the Guava PCA-96 system (Millipore) with the Guava ViaCount™ kit (ref. 4000-0040) utilizing DNA-binding dyes that stain viable and nonviable cells based on permeability. Sample analysis was automatically performed by the CytoSoft software and results were obtained as viable and total cell counts per mL. Apoptosis was also assessed by using the Guava Nexin™ kit (ref. 4500-0450) to identify early and late stages of apoptosis of the cells based on the staining with two dyes: Annexin V-PE and 7-ADD. Sample analysis was automatically performed by the CytoSoft software and results were obtained as viable, early apoptotic, late apoptotic cells, and cell debris. Both assays were performed according to the manufacturer's instructions. The analytical uncertainty of the method, as previously established in Lab 2 corresponded to a CV% of 4% (p<0.05).
Flow cytometry by Influx
The percentage of necrotic, early apoptotic and viable cells was determined with a BD Influx flow cytometer37–39 by staining with Annexin V and Sytox green (Invitrogen, Molecular Probes®, ref. A13202, S7020) according to manufacturer's instructions. For each sample, two measures of up to 50,000 events were acquired and the data were analyzed using the FlowJo software program (Treestar, Ashland, OR). Regions were drawn to identify the percentage of cells in each of the three possible populations: viable, necrotic, or early apoptotic. Cells that were negative for Sytox/Annexin V were considered viable as the membranes were intact enough to exclude the dyes; cells that were bright Sytox/Annexin V positive were considered dead/necrotic as very permeable to the dye; cells undergoing early apoptosis were positive for Annexin V staining. The analytical uncertainty of the method, as previously established in Lab 3 corresponded to a CV% of 1.27% and 2.56% for viable and early apoptotic cells, respectively (p<0.05).
T cell functionality assessment by ELISpot assay
Thawed PBMC cells were cultured overnight in R10 and viable cells were counted and tested for IFN-γ ELISpot responses to a peptide pool of twenty-three 10-mer and 11-mer CD8-restricted viral epitopes derived from influenza viruses (CEF) peptide pool, and phytohemagglutinin (PHA). Ninety-six-well plates with PVDF membranes (Millipore) were coated overnight at 4°C with anti-IFN-γ monoclonal antibody (MabTech). Cells were added to the blocked plates at 1×105 and 2×105 viable cells per well with CEF peptide pools diluted to approximately 2.5 μg/mL final concentration per peptide and PHA at a final concentration of 5.0 μg/mL. After overnight incubation, bound IFN-γ was detected with biotinylated anti-IFN-γ antibody (MAbTech), followed by alkaline phosphatase-conjugated anti-biotin antibody (Vector Laboratories) and BCIP/NBT substrate (Pierce). Spots were analyzed using a digital imager and automated counting system (AutoImmun Diagnostika). The assay response can vary by as much as two-fold, as previously established in Lab 2.
Data analysis
The data evaluation was carried out according to the International Harmonized Protocol for the Proficiency Testing of Analytical Laboratories,40 ISO 13528.41
The analysis was based on comparison of the mean values (across cell batches) of all participating laboratories to the mean values of the laboratory producing the test items. The percentage of viability was normalized by calculating the ratio of viable cells to the number of viable cells cryopreserved with 10% DMSO and stored in LN at the laboratory producing the test items. Significant differences were considered to be those exceeding each method's analytical uncertainty.
For the evaluation of the instability in the scope of the PT program results, the assigned value and the PT target standard deviation were calculated. The assigned values corresponded to viability values obtained from cells cryopreserved with 10% DMSO and stored in LN. SigmaPlot 11.0 software was used for calculations of mean and standard deviation values.
According to IUPAC and International Harmonized Protocol for the Proficiency Testing,40 the instability (Δ) of a test item is not consequential if the difference between the mean assigned value (or baseline) and the post-shipment mean is lower than 0.3 σp (Δ<0.3 σp), where σp is the PT standard deviation. The PT coefficient of variations (CV%) were 15%, 20%, and 25% for high viability test items, measured by Trypan Blue, CASY, and flow cytometry, respectively. They were 20%, 25%, and 30% for intermediate and low viability test items, measured by Trypan Blue, CASY, and flow cytometry, respectively. The Proficiency Testing σp is obtained by multiplying the CV% by the mean value of viability (assigned value).
Results
Shipping process
All samples were shipped and received within 48 h. Results of the temperature monitoring devices did not differ among testing laboratories and showed similar fluctuations during flight and vehicle travel. The highest temperatures occurred during one shipment, excluding one dry ice shipment that thawed during transit.
Effect of cryopreservation and storage in LN with different cryopreservation media on cell viability
Viability of cryopreserved and stored in LN cell suspensions was measured on the day of shipment (after 1 day of storage) and after 2 weeks of storage in different cryopreservation media (Fig. 2, Fig. 3).
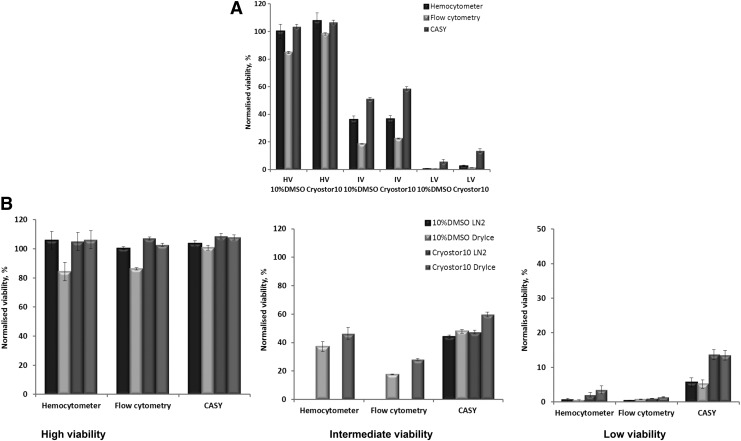
FIG. 3.
Effect of 2-week-storage in LN2 (<-130°C) in different cryopreservation media on Jurkat cells viability. HV=high viability, IV=intermediate viability, LV=low viability; A=after storage, B=after shipment.
Figure 3A summarizes the behaviour of Jurkat cells of the three viability levels (HV, IV, and LV) when stored in LN. The comparison between samples showed certain differences from the initial sample conditions as assessed by either Trypan Blue staining or flow cytometry. Comparison of the viability of the cells cryopreserved in 10% DMSO and CryoStor® CS10 showed no significant differences as measured by Trypan Blue and significant differences as measured by flow cytometry (Fig. 3A), with higher viability observed in CryoStor® CS10.
Effect of shipment in LN and Dry Ice with different cryopreservation media on cell viability
Viability of cryopreserved Jurkat cell suspensions shipped in LN and DI was measured after two shipments (Fig. 3B). A protective effect of CryoStor® CS10 was observed after shipment. After two shipments, there were differences between LN and DI, especially for Jurkat cells cryopreserved in 10% DMSO, with higher viability in LN. Such differences between LN and DI shipment were not observed in CryoStor® CS10. The difference in viability between cells in DMSO shipped in LN and those shipped in DI was observed by hemocytometry and flow cytometry, but not by CASY.
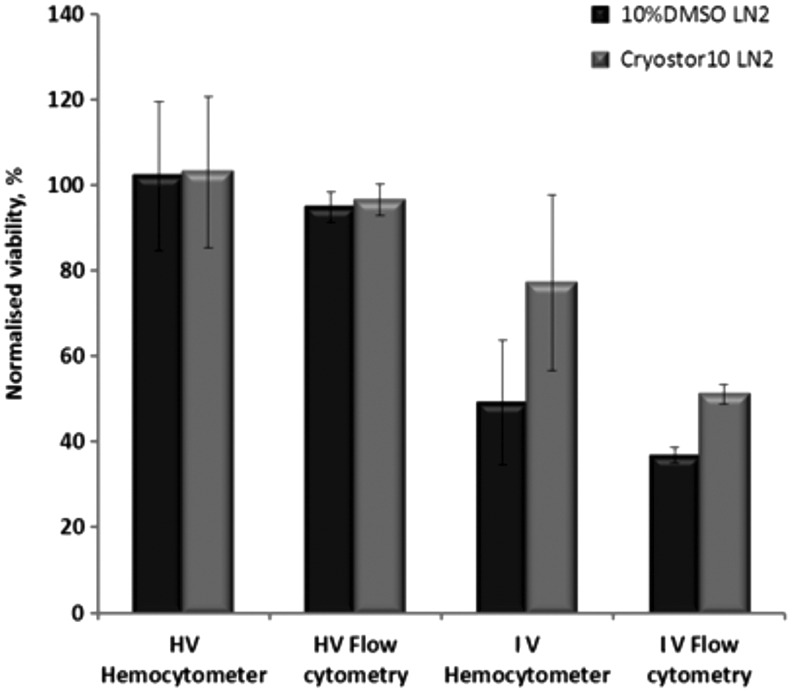
Figure 4 summarizes the viability results of PBMCs of different viability status. High viability PBMCs reacted similarly to Jurkat cells in the media used. Similar to the Jurkat cells, PBMCs had higher viability when cryopreserved and shipped in CryoStor® CS10 than in DMSO, as assessed by flow cytometry. However, this difference was not significant, when assessed by hemocytometry.
FIG. 4.
Effect of shipment in LN2 (<-130°C) with different cryopreservation media on PBMCs viability. Initial cell suspension quality before storage was set as: HV=high viability, IV=intermediate viability. Flow cytometry performed by Guava Nexin assay.
We have also determined if a consequential instability occurred.40,41 A consequential instability occurs when the viability of the samples, after shipment, is more than 0.3σp. Table 2 shows the results in terms of consequential instability (Δ>0.3σp) for the shipped Jurkat cells in DI. We showed that consequential instability was dependent on both the initial cell viability status and the assessment method used. Thus, for Jurkat:
(1) HV cell suspension PT testing by Trypan Blue and flow cytometry, all medium/transport mode combinations were sufficiently stable except for DMSO/ DI; for PT testing by CASY, all combinations were sufficiently stable;
(2) IV cell suspension PT testing by Trypan Blue, flow cytometry, or CASY, cells were sufficiently stable; however flow cytometry showed consequential instability;
(3) LV cell suspension: only flow cytometry and CASY testing of cells in CryoStor® CS10 showed acceptable stability.
Table 2.
Evaluation of Consequential Instability
| Dry Ice | After shipment | |||||
|---|---|---|---|---|---|---|
| DMSO | 0.3 σp | Δ | ||||
| Method | HV | IV | LV | HV | IV | LV |
| Trypan Blue | 3.8 | 2.9 | 0.4 | 16.4* | 1.4 | 2.3* |
| CASY | 5.1 | 5.1 | 4.9 | 4.4 | 4.4 | 8.6* |
| Flow cytometry | 5.8 | 2.2 | 0.5 | 8.7* | 1.3 | 3.0* |
| DryIce | After shipment | |||||
|---|---|---|---|---|---|---|
| CryoStor | 0.3 σp | Δ | ||||
| Method | HV | IV | LV | HV | IV | LV |
| Trypan Blue | 3.9 | 3.5 | 0.8 | 1.2 | 3.0 | 4.9* |
| CASY | 5.1 | 5.8 | 5.1 | 1.0 | 1.4 | 0.4 |
| Flow cytometry | 6.2 | 3.0 | 0.6 | 0.2 | 4.2* | 0.0 |
σp, PT standard deviation, expressed as percent viability; Δ, viability percent difference between baseline and post-shipment conditions. *correspond to consequential instability.
Effect of storage and ambient shipment in AQIX® RS-I preservation medium on cell viability
Viability levels of PBMC and Jurkat cells preserved in room temperature transport medium AQIX® RS-I were compared at different time points. There was a decrease of viability for both types of cells, independent of their initial viability status, during 2–7 day storage in the medium.
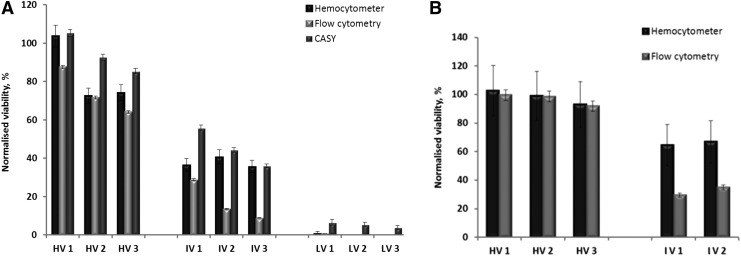
There was a significant decrease in viability for Jurkat cells preserved in the AQIX® RS-I on day 2 and 3 (Fig. 5) compared to 1 day of storage. A similar decrease in viability was observed after shipment and measurement on day 3 and 4 in the AQIX® RS-I medium (data not shown). An additional limiting factor for experimental measurements was a clotted cell pellet which formed after shipment or after 3 days in the AQIX® RS-I medium (Fig. 6).
FIG. 5.
Effect of short-term storage and shipment at RT in AQIX® RS-I room temperature transport medium (in the medium 1, 2, or 3 days, indicated as HV1, HV2, or HV3, etc. respectively) on: (A) Jurkat cells viability, (B) PBMCs viability (flow cytometry, performed by Guava Nexin assay). Initial cell suspension quality before storage was set as: HV=high viability, IV=intermediate viability, LV=low viability. Viability was normalized to the viability of cell suspension cryopreserved with 10% DMSO and stored in LN2 (baseline).
FIG. 6.
PBMCs after room temperature shipment in AQIX RS-I. The white cloud represents cell pellet/clot.
Functional assessment of cryopreserved and shipped cells
ELISpot assay, which measures specific T cell responses by counting T cells secreting cytokine (IFN-γ) after peptide stimulation, was used to monitor cell functionality. The PBMCs and Jurkat cells showed equivalent functionality in the ELISpot regardless of the preservation media used. The only limitation of the study was the cell quantity in the “low viability” (LV) cell suspension (Table 3). Relative to the IV suspension of PBMCs, the IV suspension of Jurkat cells resulted in a more important decrease in the number of IFN-γ producing cells in response to CEF and PHA. Lower spot counts to PHA and to CEF were observed with PBMCs of intermediate viability compared to PBMCs of high viability. PHA-stimulated PBMCs preserved in room temperature transport medium showed a decrease in the number of IFN-γ producing cells during the period of storage. The Jurkat cells had comparable functionality in the ELISpot when stored in either 10% DMSO or CryoStor® CS10, and a lower response when stored in AQIX® RS-I. The shipment method did not have an impact on the cell function measured by ELISpot. The treatment applied to both types of cells, to produce the LV or IV cell suspensions, had a negative impact on the function measured by ELISpot.
Table 3.
Effects of Cryopreservation Media and Temperature Conditions on IFN-γ ELISPOT Responses of Cell Suspensions of Different Initial Quality
| A. PBMCs | ||||
|---|---|---|---|---|
| ELISPOT Results (mean spots/10^6 cells) | ||||
| Sample Type | Storage media | Temp condition | CEF | PHA |
| HV PBMC | Cryostor 10 | Stored LN2 | 564 | tmtc |
| HV PBMC | Cryostor 10 | Shipped LN2 | 643 | tmtc |
| HV PBMC | 10% DMSO +FBS | Stored LN2 | 614 | tmtc |
| HV PBMC | 10% DMSO+FBS | Shipped LN2 | 433 | tmtc |
| HV PBMC | AQIX | Stored RT 1d | 703 | tmtc |
| HV PBMC | AQIX | Stored RT 2d | 663 | tmtc |
| IV PBMC | Cryostor 10 | Stored LN2 | 13 | 541 |
| IV PBMC | Cryostor 10 | Shipped LN2 | 10 | 649 |
| IV PBMC | 10% DMSO+FBS | Stored LN2 | 18 | 543 |
| IV PBMC | 10% DMSO+FBS | Shipped LN2 | 6 | 570 |
| IV PBMC | AQIX | Stored RT 1d | 11 | 473 |
| IV PBMC | AQIX | Stored RT 2d | 28 | 393 |
| B. Jurkat cells | ||||
|---|---|---|---|---|
| ELISPOT Results (mean spots/10^6 cells) | ||||
| Sample type | Storage media | Temp condition | CEF | PHA |
| IV Jurkat | Cryostor 10 | Shipped LN2 | 1 | 215 |
| IV Jurkat | Cryostor 10 | Shipped Dry Ice | 1 | 229 |
| IV Jurkat | 10% DMSO+FBS | Shipped LN2 | 0 | 139 |
| IV Jurkat | 10% DMSO+FBS | Shipped Dry Ice | 0 | 183 |
| IV Jurkat | AQIX | Shipped RT | 1 | 31 |
| LV Jurkat | Cryostor 10 | Shipped LN2 | QNS | 43 |
| LV Jurkat | Cryostor 10 | Shipped Dry Ice | 0 | 10 |
| LV Jurkat | 10% DMSO+FBS | Shipped LN2 | QNS | 28 |
| LV Jurkat | 10% DMSO+FBS | Shipped Dry Ice | QNS | 13 |
HV=high viability, IV=intermediate viability, LV=low viability; A. PBMCs, B. Jurkat cells;
QNS, quantity not sufficient; tmtc, too many to count.
Discussion
Increasingly, biological specimens are transported domestically and internationally in the context of clinical trials or research projects. Many variables can influence the sample integrity during the transport process, including: temperature, packaging, courier, sample type, import/export requirements, seasons, and transit time days.6
In our study, we focused mainly on the impact of critical factors, including shipping temperatures, different applied preservation media, different sample types, and different assays to assess their impact on cell viability and function, for the purpose of establishing a mononuclear cell viability PT scheme for biorepositories.7,42 Our results show the trends of these impacts.
PT test items have different assigned values of percentage viability, and the assigned values should ideally cover the whole range of the viability measures (from 0 to 100%), with test items of different assigned values being sent to participants from one PT round to the other. Therefore, initial cell suspension viability levels before storage and shipment were set as: HV=high viability, IV=intermediate viability, LV=low viability. Common methods applied for assessing viable cell quality after storage include Trypan Blue dye exclusion43 and apoptotic assays.44 In addition, we used CASY cell counting, a method based on the impedance principle. Our results showed differences between different methods. The CASY cell counting method overestimated the viability, especially for intermediate and low viability cell suspensions. The lower the viability, the more important the CASY cell counter mis-estimation was. The most likely explanation is that the impedance-based method can detect only late apoptotic cells, when the cell membrane is already severely compromised. Trypan Blue exclusion assay distinguishes only between cells with intact and disrupted membranes, therefore this method does not give an indication of the cell death state. Our data suggest flow cytometry as an analytical method of choice, because of its higher specificity relative to Trypan Blue assessment and to CASY cell counting, especially in the case of compromised cell viability levels. However, when studying HV cell suspensions, all three methods gave comparable results.
In previously published literature, different procedures have been proposed, including storage in LN or −70°C and shipment of cells in DI or LN.1,29 Those studies have used −70°C storage and detected bias in viability or function measures due to the difference in the time of storage. Additionally, it has been shown that LN/DI or −70°C/DI storage/shipment of cryopreserved PBMCs is associated with a decrease in viability and viable cell recovery compared with LN/LN. However, those studies did not assess the impact of different preservation media during shipment or ambient temperature shipment. The room temperature transport medium (AQIX® RS-I) has been shown to be very efficient in preserving organs and tissues for several hours.45 However, its efficiency in maintaining mononuclear cell viability of individual cell suspensions has not been studied. Additionally, if such a medium maintained stable levels of viability at room temperature, it would be very cost efficient for shipment of the PT viable mononuclear cell test items.
Previous studies demonstrated that DMSO-containing freezing medium affects the level of apoptosis,46 and impacts PBMC function and viability.47 Other studies reported that storage of the specimens at higher temperatures than LN could increase apoptosis and reduce viability measured by Trypan Blue.44 The current study has addressed the effect of 10% DMSO in serum and as a component of the commercially available medium CryoStor® CS10 on the viability and function of cryopreserved and shipped PBMCs and Jurkat cells. Earlier publications have reported the cryoprotective and anti-apoptotic effects of CryoStor® CS10 on specific cell types.23,32 Similarly, our results have confirmed CryoStor® CS10's protective effect on Jurkat and PBMCs; there was higher post-thaw viability when cells were stored or shipped in CryoStor® CS10 and DI. Medium with serum and 10% DMSO resulted in lower cell viability and AQIX® RS-I resulted in a significant decrease of cell viability. Our results are in concordance with previously published data27 that reported a deleterious effect of submitting the cryopreserved cells to large temperature variations, all below 0°C.
Our results show that LN storage and LN shipment using cryoshippers ensures higher viability of cryopreserved mononuclear cells and therefore better stability of the shipped biospecimens, applying either serum with 10% DMSO or CryoStor® CS10. Additionally, the main advantage of cryoshippers is that they have a much longer cooling capacity compared to the dry ice. However, the use of cryoshippers significantly increases the shipment costs. First, a cryoshipper charged with liquid nitrogen vapor in its protective shipment packaging weighs around 25 kg, while most dry ice shipments can be done with about 10–15 kg of dry ice. This has a direct impact on the cost of the shipments, with most shipping companies based on standard transport rates. Second, dry ice shipments are usually made in single-use boxes that require no return shipment, while the cryoshippers need to be returned to the station of origin, requiring a return shipment that increases the price of the overall transport of samples by 30%–50%. Third, dry ice polyvinylchloride boxes are cheap compared to a fully equipped cryoshipper that costs about €400–800, and cases of damage during transport are not uncommon. To evaluate correctly the overall cost of cryoshipper-based shipments, one has to add the amortized cost of the cryoshipper to the cost of the shipment. With this in mind, we may conclude that LN shipments are impractical for PT program purposes. LN storage followed by dry ice shipment of viable mononuclear cells, cryopreserved in CryoStor® CS10, was shown to be most cost-efficient solution. AQIX® RS-I and ambient shipment cannot be recommended for the examined sample types. However, more investigations are required for use of AQIX® RS-I on other types of biospecimens and its use for short term preservation (24 h) at ambient temperature could be efficient.
The impact of the type of preservation medium was less obvious on PBMCs than it was on Jurkat cells. The IV PBMCs displayed a significant drop in viability when AQIX® RS-I medium was used. The different impact could be due to the different nature of the cells (multi-passaged/tumor vs. fresh/normal). Flow cytometry showed that the reduction in viability for the HV, IV, and LV cells was due to an increase in apoptotic/dead cells. The results obtained by CASY cell counter showed an overestimation of viable cells.
The baseline values were used for the PT-consequential instability calculations. Instability was not consequential in the scope of the PT program for Jurkat cells of high viability, shipped in CryoStor® CS10/DI, and tested by all methods; for Jurkat cells of intermediate viability, shipped in CryoStor® CS10/DI, and tested by Trypan Blue and CASY; for Jurkat cells of low viability, shipped in CryoStor® CS10/DI and tested by CASY and flow cytometry. For cells shipped in DMSO/DI instability was inconsequential only for the intermediate viability samples.
Good recovery, viability,48 and consistent IFN-γ ELISpot responses49 have been reported for PBMCs optimally stored in LN (or below −130°C). However, there is little information on the impact of shipment in different cryopreservation media and at different temperatures on frozen mononuclear cell sample quality, as assessed by IFN-γ ELISpot testing. Therefore, IFN-γ ELISpot responses to CEF and PHA were evaluated. Our data suggest that the initial quality of stored samples is a critical factor for the post-thaw results. Initial low quality of the cell suspensions lead to lower responses in the ELISpot assay, after stimulation with mitogens or T-cell-specific antigens.
Depending on the antigen used, the method showed different sensitivity to the conditions tested. Responses to CEF were only detected in the HV PBMC suspension. PHA stimulation was possible in both HV and IV PBMCs suspensions. Viable PBMCs generated high responses to PHA. Such high PHA responses make it difficult for the automated counter to distinguish individual spots resulting in a “tmtc” (too many to count) read-out (Table 3). However, PHA serves as a good qualitative indicator of PMBC functionality, as shown by the fact that the IV PBMCs demonstrated several-fold lower PHA responses as compared to the HV PBMCs. ELISpot also shows that long hypothermic treatment is detrimental to cell functionality. However, this method could not differentiate between cell physiological conditions at different days, as could the CASY cell counting and the flow cytometry methods.
Overall, the results obtained are in accordance with previously published results showing that the function of cryopreserved PBMCs is associated with viability,30 and indicating that cell viability <70% may introduce a bias in the responses measured by ELISpot.14,43 Our data also support that the baseline quality of biospecimens before storage and shipment influences their stability. Besides, the use of serum in a medium may have an impact on lymphocyte activation in T cell assays due to the presence of different cytokines in serum.50 As proposed by Smith et al.,27 the ELISpot cut-off value should be ≥1,000 SFC (spot-forming cells). However, functional assays are variable and require large sample sets to demonstrate significant differences.
Neither we nor others51 could identify systematic “shipping errors” for cryopreserved cells; however the applied preservation conditions (temperature, time, media, etc.) may be critical factors affecting biospecimen quality and analytical results. We conclude that CryoStor® CS10/Dry Ice combination gives higher viability by every method, for each viability level, and is the most cost-efficient shipment method. Another critical factor is the laboratory/operator performance. Therefore, whenever possible, laboratories should participate in the “inter-laboratory exercises” or Proficiency Testing Programs7,42 in order to control random or systematic laboratory errors, to establish quality assurance programs and guarantee the quality of cryopreserved and shipped biospecimens, and to increase standardization among biobanks and/or end-user laboratories.
Our study shows the trends of analytical results variations under the influence of different shipment temperatures and different preservation media. It is known that LN/LN storage/shipment ensures the highest quality of cryopreserved biospecimens, and this was confirmed. However, under limited funding conditions or in the context of large PT programs, LN shipments are impractical. In these cases, we show that alternative high quality shipment conditions for viable cells are dry ice shipment and CryoStor® CS10 cryopreservation medium. Indeed, for both Jurkat and PBMC cells, it is possible to use CryoStor® CS10 with dry ice shipment or when the initial cell suspension displays suboptimal viability, without dramatic impact on cell viability. Room temperature transport medium was not fit-for-purpose, it dramatically and adversely affected the integrity of mononuclear cells. Since shipment temperature is so critical, we propose monitoring of shipment temperatures.
Acknowledgments
We thank Wim Ammerlaan for excellent technical work, and Katy Beaumont and Jay Oustrich for organization of the shipments and other logistics.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bull M, Lee D, Stucky J, et al. . Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods 2007;322:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garraud O, Moreau T. Effect of blood storage on lymphocyte subpopulations. J Immun Methods 1984;75:95–98 [DOI] [PubMed] [Google Scholar]
- 3.Weiblen BJ, Debell K, Giorgio A, et al. . Monoclonal antibody testing of lymphocytes after overnight storage. J Immun Methods 1984;70:179–183 [DOI] [PubMed] [Google Scholar]
- 4.International Society for Biological and Environmental Repositories. 2012 Best Practices for Repositories. Collection, Storage, Retrieval, and Distribution of Biological Materials for Research. Biopreserv Biobanking 2012;10:79–161. http://c.ymcdn.com/sites/www.isber.org/resource/resmgr/Files/2012ISBERBestPractices3rdedi.pdf [DOI] [PubMed] [Google Scholar]
- 5.Betsou F, Barnes R, Burke T, et al. . Human biospecimen research: Experimental protocol and quality control tools. Cancer Epidemiol Biomarkers Prevention 2009;18:1017–1025 [DOI] [PubMed] [Google Scholar]
- 6.http://c.ymcdn.com/sites/www.isber.org/resource/resmgr/Files/ISBER-from_biospecimen_logis.pdf Accessed 5June2014
- 7.Poloni F, Ashton G, Coppola D, et al. . ISBER Proficiency Testing program for biorepositories: Pilot results for DNA and RNA schemes. Biopreserv Biobanking 2012;10:313–314 [Google Scholar]
- 8.Betsou F, Sobel M. The ISBER Proficiency Testing Program: Two successful years already, and new features to come. Biopreserv Biobanking 2013;11:255–256 [DOI] [PubMed] [Google Scholar]
- 9.Wolf B, Schwarzer A, Côté AL, et al. . Gene expression profile of peripheral blood lymphocytes from renal cell carcinoma patients treated with IL-2, interferon-α and dendritic cell vaccine. PLoS One 2012;7:e50221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugar PL, Love C, Grammer AC, et al. . Molecular characterization of circulating plasma cells in patients with active systematic lupus erythematosus. PLoS One 2012;7:e44362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Santis G, Ferracin M, Biondani A, et al. . Altered miRNA expression in T regulatory cells in course of multiple sclerosis. J Neuroimmunol 2010;226:165–171 [DOI] [PubMed] [Google Scholar]
- 12.Saunders JA, Estes KA, Kosloski LM, et al. . CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson's disease. J Neuroimmune Pharmacol 2012;7:927–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sleasman JW, Leon BH, Aleixo LF, et al. . Immunomagnetic selection of purified monocyte and lymphocyte populations from peripheral blood mononuclear cells following cryopreservation. Clin Diagn Lab Immunol 1997;4:653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg A, Song LY, Wilkening C, et al. . Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol 2009;16:1176–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimann KA, Chernoff M, Wilkening CL, et al. . Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. The ACTG Immunology Advanced Technology Laboratories. Clin Diagn Lab Immunol 2000;7:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay S, Khandjian EW. Successful use of long-term frozen lymphocytes for the establishment of lymphoblastoid cell lines. Clin Biochem 1998;31:555–556 [DOI] [PubMed] [Google Scholar]
- 17.Koizumi K, Nishio M, Endo T, et al. . Large scale purification of human blood CD34+ cells from cryopreserved peripheral blood stem cells, using a nylon-fiber syringe system and immunomagnetic microspheres. Bone Marrow Transplant 2000;26:787–793 [DOI] [PubMed] [Google Scholar]
- 18.Chang IC, Wu JY, Lu HI, et al. . High-potentiality preliminary selection criteria and transformation time-dependent factors analysis for establishing Epstein-Barr virus transformed human lymphoblastoid cell lines. Cell Prolif 2006;39: 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tollerud DJ, Brown LM, Clark JW, et al. . Cryopreservation and long-term liquid nitrogen storage of peripheral blood mononuclear cells for flow cytometry analysis: Effects on cell subset proportions and fluorescence intensity. J Clin Lab Analysis 1991;5:255–261 [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman M. Effects of cryopreservation on immune responses. Cryobiology 1996;33:581–588 [DOI] [PubMed] [Google Scholar]
- 21.Costantini A, Mancini S, Giuliodoro S, et al. . Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods 2003;278:145–155 [DOI] [PubMed] [Google Scholar]
- 22.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion 1996;36:303–308 [DOI] [PubMed] [Google Scholar]
- 23.Baust JM, Van Buskirk R, Baust JG. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev Dev Biol Animal 2000;36:262–270 [DOI] [PubMed] [Google Scholar]
- 24.Pratt Riccio EK, Nevers JI, Banic DM, et al. . Cryopreservation of peripheral blood mononuclear cells does not significantly affect the levels of spontaneous apoptosis after 24-h culture. Cryobiology 2002;45:127–134 [DOI] [PubMed] [Google Scholar]
- 25.Tre TI, Roep BO, Peakman M. Enhancing the sensitivity of assays to detect T cell reactivity: The effect of cell separation and cryopreservation media. Ann NY Acad Sci 2004;1037:26–32 [DOI] [PubMed] [Google Scholar]
- 26.Kreher CR, Dittrich MT, Guerkov R, et al. . CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immun Methods 2003;278:79–93 [DOI] [PubMed] [Google Scholar]
- 27.Smith JG, Joseph HR, Green T, et al. . Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions. Clin Vaccine Immunol 2007;14:527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg A, Betensky RA, Zhang L, et al. . Effect of shipment, storage, antigoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol 1998;5:804–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg A, Song LY, Wilkening CL, et al. . Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Methods 2010;363:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betensky RA, Connick E, Devers J, et al. . Shipment impairs lymphocyte proliferative responses to microbial antigens. Clin Diagn Lab Immunol 2000;7:759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson WC, Smolkin ME, Farris EM, et al. . Shipping blood to a central laboratory in multicenter clinical trials: Effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med 2011;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke DM, Yadock DJ, Nicoud IB, et al. . Improved post-thaw recovery of peripheral blood stem/progenitor cells using a novel intracellular-like cryopreservation solution. Cytotherapy 2009;11:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.http://biolifesolutions.com/cgmp-biopreservation-media-products/cryostor/ Accessed 5June2014
- 34.Newton K, Rees D. Summary Report: Assessment of a novel non-phosphate buffered solution, AQIX® RS-I, in the isolation and 24-hour culturing of human PBMC's in comparison to RPMI medium. AUDIT TRIAL REPORTS; [November, 2008] http://www.aqix.com/Documents/R23-AQIX%20website%20RF-data%20final.pdf Accessed 5June2014 [Google Scholar]
- 35.http://www.aqix.com/formulation.php Accessed 5June2014
- 36.CASY Technology (Online). Available: http://lifescience.roche.com/wcsstore/RASCatalogAssetStore/Articles/05966680001_04.10_US.pdf Accessed 5June2014
- 37.Holmes KL, Otten G, Yokoyama WM. Flow cytometry analysis using the Becton Dickinson FACS Calibur. Curr Proto Immunol 2002;49:5.4.1–5.4.22 [DOI] [PubMed] [Google Scholar]
- 38.Cummings BS, Wills LP, Schnellmann RG. Measurement of cell death in mammalian cells. Curr Proto Pharmacol 2012;56:12.8.1–12.8.24 [DOI] [PubMed] [Google Scholar]
- 39.Zhivotovsky B, Samali A, Orrenius S. Determination of apoptosis and necrosis. Curr Proto Toxicol 2001;00:2.2.1–2.2.34 [DOI] [PubMed] [Google Scholar]
- 40.Thompson M, Ellison SL, Wood R. The International Harmonized Protocol for the Proficiency Testing of Analytical Chemistry Laboratories. Pure Appl Chem 2006;78:145–196 [Google Scholar]
- 41.ISO 13528:2005(E). Statistical methods for use in proficiency testing by inter-laboratory comparison, 1st edition, 2005 [Google Scholar]
- 42.http://www.isber.org/?page=PTGI Accessed 5June2014
- 43.Weinberg A, Zhang L, Brown D, et al. . Viability and functional activity of cryopreserved mononuclear cells. Clin Diagn Lab Immunol 2000;7:714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowke KR, Behnke J, Hanson C, et al. . Apoptosis: A method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J Immunol Methods 2000;244:139–144 [DOI] [PubMed] [Google Scholar]
- 45.Rees D. Physiological medium for perfusing, preserving and storing isolated cell, tissue and organ samples. Patent US6946241, 2000 [Google Scholar]
- 46.Abrahamsen JF, Bakken AM, Bruserud O. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion 2002;42:1573–1580 [DOI] [PubMed] [Google Scholar]
- 47.Disis ML, Rosa Dela, Goodell V, et al. . Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immun Methods 2006;308:13–18 [DOI] [PubMed] [Google Scholar]
- 48.Kleeberger CA, Lyles RH, Margolick JB, et al. . Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immuno 1999; 6:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith JG, Liu X, Kaufhold RM, et al. . Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin Diagn Lab Immunol 2001;8:871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Froud SJ. The development, benefits and disadvantages of serum-free media. Dev Biol Stand 1999;99:157–166 [PubMed] [Google Scholar]
- 51.Whiteside TL, Griffin DL, Stanson J, et al. . Shipping of therapeutic somatic cell products. Cytotherapy 2011;13:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]