Abstract
Purpose
The goal of this exploratory study was to investigate longitudinally the changes in facial kinematics, vowel formant frequencies, and speech intelligibility in individuals diagnosed with bulbar amyotrophic lateral sclerosis (ALS). This study was motivated by the need to understand articulatory and acoustic changes with disease progression and their subsequent effect on deterioration of speech in ALS.
Method
Lip and jaw movements and vowel acoustics were obtained for four individuals with bulbar ALS during four consecutive recording sessions with an average interval of three months between recordings. Participants read target words embedded into sentences at a comfortable speaking rate. Maximum vertical and horizontal mouth opening and maximum jaw displacements were obtained during corner vowels. First and second formant frequencies were measured for each vowel. Speech intelligibility and speaking rate score were obtained for each session as well.
Results
Transient, non-vowel-specific changes in kinematics of the jaw and lips were observed. Kinematic changes often preceded changes in vowel acoustics and speech intelligibility.
Conclusions
Nonlinear changes in speech kinematics should be considered in evaluation of the disease effects on jaw and lip musculature. Kinematic measures might be most suitable for early detection of changes associated with bulbar ALS.
Keywords: ALS, speech kinematics, formant frequencies, speech intelligibility, compensatory articulation
Introduction
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease known to affect speech musculature and result in dysarthria and, eventually, loss of intelligible speech. The pathophysiology of ALS includes progressive voluntary muscle weakness and atrophy, as well as spasticity and loss of reflexes. The speech bulbar system is complex and composed of a large number and variety of muscles and muscle groups (e.g., facial, lingual, laryngeal, velopharyngeal). The presentation of bulbar ALS may vary substantially between individuals depending on disease severity and which muscles are affected (Brooks et al., 1991).
This study explores changes in vowel-related facial movements, vowel acoustics, and speech intelligibility over the course of one year in four persons diagnosed with ALS. Vowels are important to study because they contribute significantly to speech intelligibility in healthy talkers and individuals with dysarthria (Kent et al., 1989; Kewley-Port, Burkle, & Lee, 2007). Changes in vowel acoustics have been consistently linked to deterioration of speech intelligibility in ALS (Kent et al., 1992; Mulligan et al., 1994; Turner, Tjaden, & Weismer, 1995; Weismer, Jeng, Laures, Kent, & Kent, 2001; Weismer, Kent, Hodge, & Martin, 1988; Weismer, Martin, Kent, & Kent, 1992). Research, however, is needed to understand the movement bases for speech intelligibility loss in ALS. This information may have broad implications for understanding the mechanisms that degrade speech intelligibility across neurogenic speech impairments and for identifying efficacious articulatory-based treatments in these populations.
Changes in the acoustic characteristics of vowels observed in ALS have been attributed to the reduction in the range and speed of articulatory movements (see Weismer, 2008). The few existing kinematic case studies suggest that over the course of the disease lip and tongue movements become smaller and slower whereas, at least in some individuals, jaw movements become exaggerated (DePaul & Abbs, 1987; DePaul, Abbs, Caligiuri, Gracco, & Brooks, 1988; Hirose, Kiritani, & Sawashima, 1982; Kent, Netsell, & Bauer, 1975). In contrast, a recent study of articulatory kinematics of the lower lip, jaw, and tongue reported data for eight talkers with ALS during vowels and showed that the movement deficits exist across articulators (Yunusova, Weismer, Westbury, & Lindstrom, 2008). Although the tongue was the most affected organ among oral articulators in this study, the jaw showed consistent vowel-specific movement changes such as reduction in movement size in some words and increases in other words as compared to a group of healthy controls. Only a small number of investigations have examined the effect of ALS on lip and jaw movements during speech.
Knowledge about the effects of aberrant jaw and lip movements on vowel formant frequencies is currently very limited but essential for understanding the articulatory basis of speech impairment. Jaw and lip movements affect both acoustic vowel quality (Fant, 1960) and contribute to the phonetic identity of a vowel (Fromkin, 1964; Montgomery & Jackson, 1983). For example, the height of the jaw plays a prominent role in determining the height of the tongue in the oral cavity, which is closely associated with the frequency of the first vocal tract resonance, F1 (Lindblom & Sundberg, 1971). An additional motivation for investigating the jaw and lips is the critical need for objective, but clinic-friendly measures of bulbar involvement. Unlike the tongue, facial movements are relatively easy to quantify non-invasively using modern optical motion technologies that are becoming affordable and relatively easy to use (Green, Wilson, Wang, & Moore, 2007).
To examine the longitudinal changes in vowel kinematics, vowel acoustics, and speech intelligibility with ALS progression we asked the following questions: (1) What is the time course of lip and jaw performance decline over the course of disease progression? Gradual changes in articulatory movements might be expected due to the progressive nature of the disease, which in the past has been modeled using linear functions (Gordon et al., 2007; Pradas et al., 1993); (2) Are the changes in kinematic performance similar across vowels or vowel specific? If the changes are similar across vowels, then they are most likely due to the effects of the disease on speech musculature as opposed to vowel-specific compensations produced to preserve speech quality (see Maeda, 1990; Perkell, Matthies, Svirsky, & Jordan, 1993); and (3) Do changes in vowel acoustics parallel or precede changes in articulatory kinematics in relation to the time of intelligibility loss? A better understanding of the time course of articulatory and acoustic changes will elucidate the articulatory basis of speech decline in ALS and may have value for predicting the loss of speech intelligibility. If, for example, changes in orofacial movements predate changes in vowel acoustics, these measures might be useful for early detection of disease onset in the speech (bulbar) system and for predicting impending losses of speech intelligibility.
Methods
Participants
From a larger pool of participants, one female and three male talkers with multiple recording sessions were included in the study. All participants were diagnosed with ALS by a clinical neurologist using the revised El Escorial criteria (Brooks, Miller, Swash, & Munsat, 2000). Prior to the onset of ALS, none of the participants had significant histories of speech, language, hearing, vision, or neurological problems. All participants were taking low doses (30–60 mg) of an experimental compound R(+) pramipexole, as well as riluzole, vitamins, and various health supplements. R(+) pramipexole did not show a significant effect on rate of decline in ALS (Wang et al., 2008). Riluzole showed small beneficial effect on bulbar and limb function but not muscle strength (Miller, Mitchell, Lyon, & Moore, 2007).
Participants were seen nearly every three months for a longitudinal study of speech deterioration in ALS. Talker characteristics such as time course of disease, ALS-FRS scores (Cedarbaum et al., 1999) and speech related changes are summarized in Table 1. Speech intelligibility and speaking rate scores were obtained following procedures for the Sentence Intelligibility Test (SIT; Beukelman, Yorkston, Hakel, & Dorsey, 2007). Sentences of variable length were transcribed by a single judge, an unfamiliar listener. The listener was a 35-year-old female with negative history of speech, language, or hearing disorders. She was employed as a research assistant at the speech production laboratory and had no formal training in motor speech disorders or experience with dysarthria. The severity of the intelligibility deficit varied between participants. All participants had perceptible dysarthria at the time of study initiation as determined by a perceptual evaluation by an experienced speech-language pathologist.
Table 1. Demographic and Longitudinal Disease Related Changes for Each Participant.
| ID, Sex, Age | Characteristics | Session 1 | Session 2 | Session 3 | Session 4 |
|---|---|---|---|---|---|
| A1, M, 44 | Months postonset | 10 | 16 | 19 | 22 |
| ALSFRS-R Scores | 35 | 27 | 24 | 22 | |
| Intelligibility (%) | 94 | 92 | 88 | 61 | |
| Speaking Rate (WPM) | 157 | 127 | 115 | 91 | |
| Speech Characteristics | Slightly strained voice (Sessions 1–2) progressed to moderately severe dysarthria (Sessions 3–4) characterized by monopitch, hypernasality, imprecise articulation, distorted vowels, vocal fry, voicing breaks; Spastic-predominant mixed dysarthria | ||||
| A2, M, 46 | Months postonset | 15 | 17 | 18 | 21 |
| ALSFRS-R Scores | 41 | 40 | missing | 28 | |
| Intelligibility (%) | 93 | 92 | 86 | 61 | |
| Speaking Rate (WPM) | 149 | 65 | 64 | 60 | |
| Speech Characteristics | Dysarthria clearly perceived (all sessions): slow rate, short phrases, imprecise articulation (labial & lingual), hypernasal, breathy, vowel distortions; Flaccid-predominant mixed dysarthria | ||||
| A6, M, 49 | Months postonset | 22 | 25 | 28 | 31 |
| ALSFRS-R Scores | 50 | 49 | 31 | 24 | |
| Intelligibility (%) | 100 | 98 | 94 | 38 | |
| Speaking Rate (WPM) | 223 | 189 | 120 | 109 | |
| Speech Characteristics | Asymptomatic (Sessions 1–2), progressed to imprecise articulation, hypernasality, vowel distortions, breathy, vocal fry (Sessions 3–4); Mixed spastic-flaccid dysarthria | ||||
| A7, F, 53 | Months postonset | 33 | 37 | 40 | 43 |
| ALSFRS-R Scores | 39 | 36 | 33 | 27 | |
| Intelligibility (%) | 97 | 97 | 99 | 99 | |
| Speaking Rate (WPM) | 193 | 188 | 177 | 205 | |
| Speech Characteristics | Strained, breathy voice (S1), audible inspirations; breathiness increased, short phrases (S2); increased vocal strain- laryngospasm (S3), no subsequent perceptual change (S4); Mixed spastic-flaccid dysarthria | ||||
Note. Speech intelligibility is expressed as the percent of total words transcribed correctly in sentences. Speaking rate was calculated as the number of words produced per minute
Speech Sample
During the experiment, the participants read the words BEET, BOOT, BAT, and BOBBY embedded in a reading passage (see Green, Nip, Mefferd, Wilson, & Yunusova, 2010). These words were used because they contained the phonetically distinctive corner vowels /i, u, ae, a/. The sentences were read at a comfortable reading rate and loudness. The average number of repetitions per word and session was seven. Missing data occurred because some participants were unable to complete the recording session due to fatigue and because of occasional technical difficulties during data acquisition. These occurrences appeared to be random.
Recording Procedures and Data Postprocessing
Talkers were positioned in a comfortable chair with their head supported. A 3-D motion capture system (Motion Analysis Corporation—Eagle Cameras) was used to track movements of 15 small (approximately 3–4 mm) sphere-shaped reflective markers attached to each talker's head and face at specific anatomic landmarks with double-sided tape. Four markers were attached to the head, two—to the eyebrows, two—on the nose, four—around the lips, and three—on the chin (Figure 1). Movements of these markers were recorded with eight infrared video cameras at a sampling rate of 120 Hz. Markers used in this study are identified on Figure 1. They included two markers attached to the vermilion borders of the upper and lower lips at midline (UL and LL), two markers attached at the corners of the mouth (RC and LC), a single marker on the right side of the chin (JR), located approximately 2 cm from the chin midline, and a single head marker (RTH), located on the forehead. During postprocessing, movements of the markers were checked for tracking errors and low-pass filtered at 10 Hz using a zero-phase digital filter (8-pole Butterworth).
Figure 1.
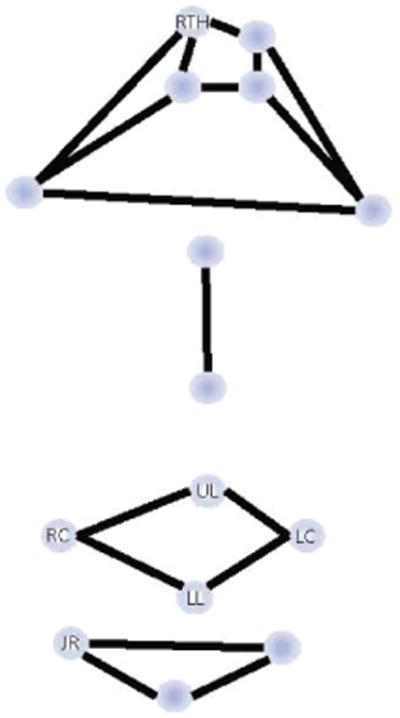
Marker array used to record facial kinematics with markers reported in the current study identified by letter-based code (RTH = right top head; UL = upper lip; LL = lower lip; RC = right mouth corner, LC = left mouth corner; JR = jaw right).
Acoustic signals were recorded simultaneously with kinematic signals directly onto a hard drive of a computer at the sampling rate of 32 kHz and 16 bit resolution. A high quality lapel microphone (Audio-Technica AT831R) was mounted on a head marker array, which was positioned on the forehead, approximately 15 cm from the mouth during the recordings.
For each CVC word, the jaw and lips moved from a high position for a consonant to a low position for the vowel. The intervals at which the measures were obtained during this movement were defined based on the lip aperture time history; the measurement interval was identified between the points of minimum UL to LL distance during the initial (/b/) and final consonants. Three derived signals were calculated for each word: (1) the distance between the stationary head marker (RTH) and JR represented head-corrected jaw movement; (2) the vertical lip aperture was defined as the distance between UL and LL; (3) the horizontal lip aperture was defined as the distance between RC and LC. The vertical lip opening and the distance between the RTH and JR for participant A1 producing BOBBY are plotted on Figure 2.
Figure 2.
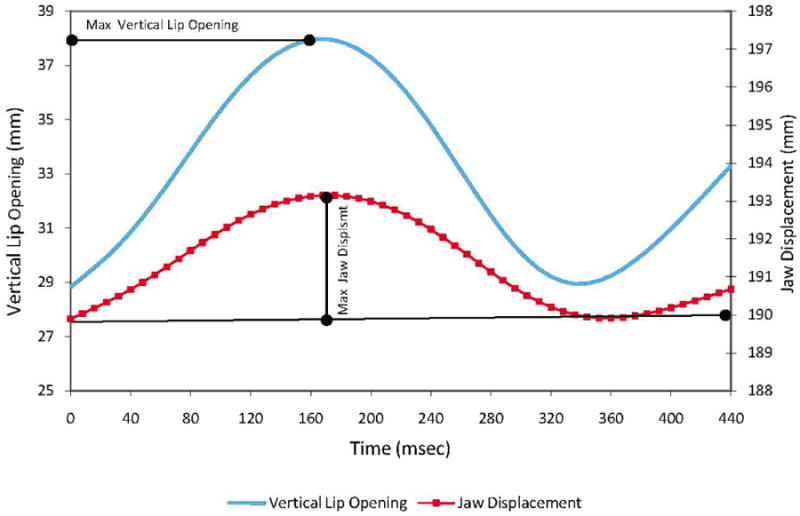
Vertical lip opening and jaw displacement traces in BOBBY produced by talker A1 during session 1.
Measures
Kinematic measures were taken from the three derived signals described above. They included:
J displacement (see Figure 2), defined as the straight-line distance between minimum and maximum in JR positions in the RTH-JR movement trajectory. The measure was used to represent the size of jaw movements during vowels.
Vertical mouth opening (lip separation), defined as the maximum inter-lip distance between UL and LL achieved during the vowel interval (see Figure 2), was calculated based on the vertical lip aperture trajectory. This measure represented the mouth opening in the vertical dimension. When viewed simultaneously with J displacement, this measure allowed for an indirect evaluation of the lip contribution to the vertical mouth aperture.
Horizontal mouth opening (lip spread), defined as the maximum interlip distance between RC and LC in the measured interval, was calculated based on the horizontal lip aperture trajectory. This measure represented the measure of lip spread during vowels and thus, the mouth shape in the horizontal dimension.
These kinematic measures were selected because they describe the change in the extent of jaw opening (measure 1) and lip shapes (measures 2 & 3) associated with each vowel. Each of these parameters contributes to the acoustic quality of the vowels (Lindblom & Sundberg, 1971) and vowel identification (Fromkin, 1964; Montgomery & Jackson, 1983; Plant, 1980). Because the exact position of the lip markers varied somewhat from session to session, the lip shape measures were expressed relative to their rest position by subtracting the distance between lip markers at rest from the distance during vowels. The rest position was defined as the position in which each participant maintained a still posture with their jaw in a normal bite and their lips closed.
Acoustic measures included first and second formants of each vowel (F1 and F2, Hz). F1 was used as it has been said to directly relate to the jaw motion as a determinant of tongue height (Lindblom & Sundberg, 1971). A derived measure of F2 difference was calculated between the pair of high vowels (F2 /i/ – F2 /u/) and low vowels (F2 /ae/ – F2 /a/)/. This measure was used as an indirect measure of tongue mobility in the anterio-posterior dimension (Stevens & House, 1955), which is affected in bulbar ALS. The mobility of the tongue over time was examined in parallel to changes in lip and jaw kinematics.
Acoustic measures were performed with TF32 (Milenkovic, 2001), using the wide-band (300 Hz; number of coefficients = 26) spectogram display as well as the LPC spectrum calculated for a 30-ms window centered at the temporal midpoint of the vowels. F1 and F2 tracks were hand-edited interactively as needed. The measurements were performed by a single measurer. Approximately 10% of the randomly chosen vowels were re-measured to obtain intrajudge reliability. The average error was 18.04 (SD = 27.8) Hz for F1 and 18.3 (SD = 25.09) Hz for F2 values.
Statistical Analysis
Because each subject showed a unique pattern of decline in our measures, we evaluated changes in vowel-related movements and acoustics for each subject's data separately. To study the pattern of change over time, pairwise differences between visits were calculated for each vowel and measured within a subject. There was a maximum of four visits and thus six possible pairwise differences/ contrasts. For each subject, we used t-tests to test whether each of the pairwise differences between visits were significantly different from zero. A significant result indicated a change for that subject between the two time points involved. Because the paired differences were correlated as were the measures, we adjusted the resulting p-values using the Bonferroni method. p-values were adjusted so that the reported .05 represents the reference value for statistically significant results.
Results
Jaw and Lips During Corner Vowels in the Course of Disease
Because a large number of comparisons were performed in this study, only significant results are displayed in tables and figures. Figure 3 shows significant changes in movement parameters over time relative to the values obtained at session 1. Results of the statistical analyses (t and adjusted p-values) are summarized in Table 2.
Figure 3.
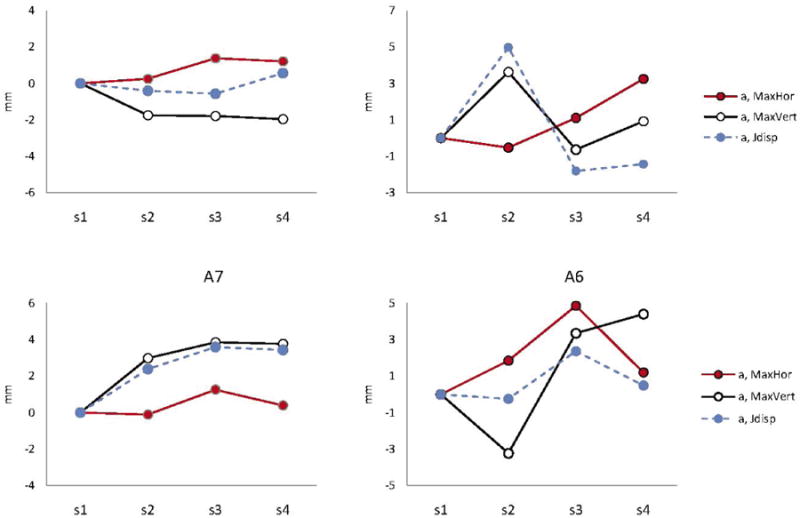
Changes in kinematic parameters with disease progression for each participant. Only statistically measures that changed significantly over time are plotted. The acronyms include: MaxHor = Maximum horizontal opening; MaxVert = maximum vertical opening; and Jdisp = Jaw displacement.
Table 2. Summary of the Statistically Significant Results for Each Talker and Measure.
| ID | Vowel | Measure | Session | t | P | ID | Vowel | Measure | Session | t | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | /a/ | MaxHor | 3-1 | 10.02 | 0.0001 | AM6 | /i/ | MaxHor | 2-1 | 25.48 | 0.004 |
| 3-2 | 12.23 | 0.0001 | 3-1 | 16.94 | 0.0134 | ||||||
| 4-1 | 10.22 | 0.0001 | MaxVert | 2-1 | −12.33 | 0.0345 | |||||
| 4-2 | 10.47 | 0.0001 | /u/ | MaxHor | 3-1 | 15.05 | 0.0191 | ||||
| MaxVert | 4-1 | −5.12 | 0.0076 | 4-2 | −18.94 | 0.0096 | |||||
| Jdisp | 4-3 | 5.06 | 0.0084 | 4-3 | −10.97 | 0.0486 | |||||
| A2 | /i/ | MaxVert | 2-1 | 8.89 | 0.045 | MaxVert | 2-1 | −12.13 | 0.0362 | ||
| Jdisp | 2-1 | 14.39 | 0.0109 | 3-1 | −11 | 0.0482 | |||||
| /u/ | MaxHor | 4-1 | 9.91 | 0.0328 | /a/ | MaxHor | 2-1 | 10.64 | 0.0001 | ||
| MaxVert | 2-1 | 10.3 | 0.0293 | 3-1 | 19.5 | 0.0001 | |||||
| /a/ | MaxHor | 4-1 | 14.39 | 0.0006 | 3-2 | 13.13 | 0.0001 | ||||
| 4-2 | 14.23 | 0.0028 | 4-3 | −13.79 | 0.0001 | ||||||
| MaxVert | 2-1 | 9.91 | 0.0036 | MaxVert | 2-1 | −5.83 | 0.0024 | ||||
| Jdisp | 2-1 | 10.96 | 0.0022 | 3-1 | 5.12 | 0.01 | |||||
| /ae/ | MaxHor | 4-3 | 6.93 | 0.0121 | 3-2 | 12.24 | 0.0001 | ||||
| MaxVert | 2-1 | 10.96 | 0.0439 | 4-1 | 5.58 | 0.005 | |||||
| 3-2 | −10.62 | 0.0481 | 4-2 | 10.79 | 0.0001 | ||||||
| A7 | /a/ | MaxHor | 3-1 | 8.2 | 0.0002 | Jdisp | 3-1 | 4.41 | 0.0256 | ||
| 3-2 | 7.1 | 0.0006 | 3-2 | 4.32 | 0.0299 | ||||||
| MaxVert | 2-1 | 7.74 | 0.0002 | 4-3 | −4.45 | 0.024 | |||||
| 3-1 | 7.44 | 0.0004 | /ae/ | MaxHor | 2-1 | 8.62 | 0.0017 | ||||
| 4-1 | 7.88 | 0.0002 | 3-1 | 17.92 | 0.0114 | ||||||
| Jdisp | 2-1 | 5.35 | 0.007 | 4-2 | −12.37 | 0.0001 | |||||
| 3-1 | 6.98 | 0.0007 | 4-3 | −17.52 | 0.0122 | ||||||
| 4-1 | 8.7 | 0.0001 | MaxVert | 2-1 | −6.71 | 0.0083 |
Note. MaxHor = Maximum horizontal opening; MaxVert = maximum vertical opening; and Jdisp = Jaw displacement.
The data revealed that movements during vowel /a/ were consistently affected for all four individuals, when other vowels changed only for two talkers A2 and A6 (see Figure 3). The plots displayed in Figure 3 show that among measures, vertical and horizontal mouth openings, which reflect the combined motions of the jaw and lips, tended to be affected more often than the measures associated with jaw movements alone (J displacement). Within talker, the kinematic measures tended to change similarly across vowels. For example, the direction of change in the vertical mouth opening was similar across all vowels for A2. The same tendency was observed for mouth opening measures in A6 with an exception of J displacement and vertical mouth opening in /a/.
Vowel Acoustics: F1
Only a few of the F1 contrasts showed significant differences between session pairs with disease progression (Table 3). The t-statistic and p-values for these contrasts are reported in Table 4.
Table 3. Means and (Standard Deviations) of Statistically Significant F1 (Hz) Contrasts.
| ID | Vowel | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| A1 | i | 275 (16) | 294 (26) | 300 (32) | 386 (8) |
| A1 | a | 763 (34) | 707 (24) | 682 (26) | 725 (15) |
| A2 | a | 656 (49) | 658 (39) | 623 (25) | 394 (63) |
Table 4. Statistical Results for the Significant F1 Contrasts Reported in Table 3.
| ID | Vowel | Session | t | p |
|---|---|---|---|---|
| A1 | /i/ | 4-1 | 18.4 | 0.0105 |
| /a/ | 2-1 | −4.95 | 0.0102 | |
| 3-1 | −5.81 | 0.0025 | ||
| 4-3 | 5.12 | 0.0076 | ||
| A2 | /a/ | 4-1 | −7.72 | 0.0117 |
| 4-2 | −6.94 | 0.0453 | ||
| 4-3 | −2.59 | 0.0488 |
Vowel Acoustics: F2 and F2dif
Statistically significant changes in F2 were observed in 5 out of 96 comparisons across talkers. They were detected for vowels /i, a, ae/ produced by A1 and vowel /u/ produced by A2, with significant changes occurring mostly at session 4 with t-values ranging between − 12.92 and 4.19 and p-values < 0.03.
Figure 4 shows F2 difference (in Hz) computed by subtracting the average F2 of /u/ and /a/ from the average F2 in /i/ and /ae/, respectively. Note that during session 1, three of the four participants (i.e., A1, A6, and A7) showed relatively large contrasts for each pair. They were approximately 500 to 600 Hz for low vowels and between 900 and 1200 Hz for high vowels. The high vowel contrast decreased gradually over time for A6 and precipitously between the last two sessions for A1. For low vowels, the F2 contrast dropped gradually across all sessions, but only for A1. Both of the contrasts were notably reduced in A2 (200 and 400 Hz, respectively) at session 1, indicating significant tongue impairment (i.e., advancement/ retraction) from the beginning of the study.
Figure 4.
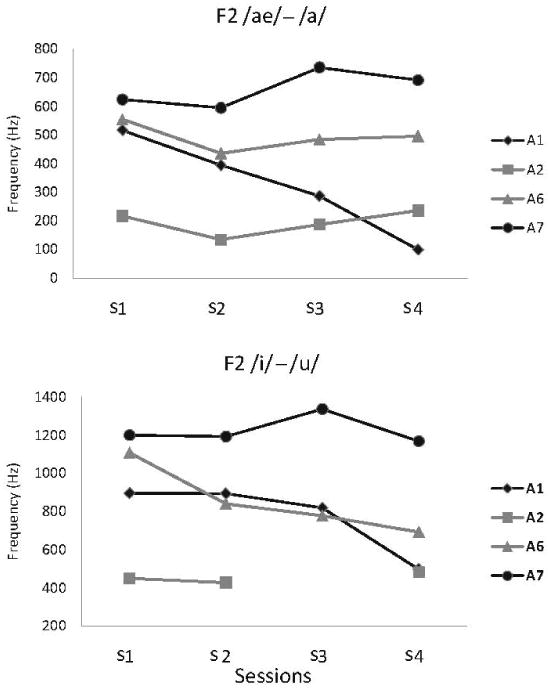
Changes in the F2 dif measure obtained for the high and low vowel contrast for each participant.
Movement, Acoustics, and Speech Intelligibility with Disease Progression
Figure 5 shows changes in all of the parameters that showed significant changes in F1 and kinematic measures for the vowel (/a/) for each talker. Speech intelligibility and speaking rate scores are also given together with the F2 /ae/ – /a/ contrast. The data are expressed in percent change relative to the first session. Positive values show an increase in these measures relative to its value for session 1; negative values show a decrease in each measure relative to its value in session 1. The results show that intelligibility decreased precipitously at session 4 for talkers A1, A2, and A6. For these talkers, speaking rate showed a much more gradual decline (but see A2). F1 in /a/ declined significantly in A1 and A2 only. For A1, the decline was gradual and occurred prior to the intelligibility drop between sessions 1 and 3. This decline was followed by an increase in F1 at session 4. For A2, the decline was precipitous and occurred at session 4, together with an intelligibility drop. F1 did not change for A6 or A7.
Figure 5.
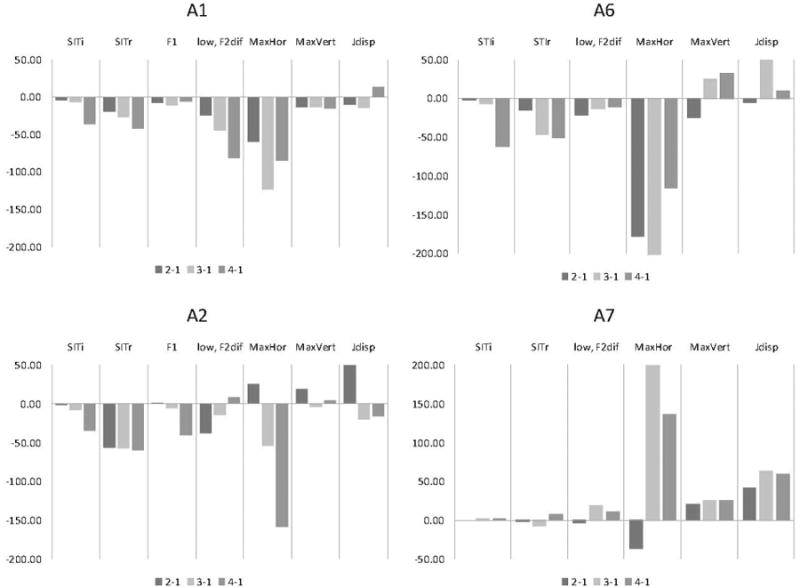
Each participant's percent (%) change (vertical axes) in each significant measure computed between session 1 and each subsequent session. Except for the overall SITi and SITr (= SIT intelligibility and rate) scores and low, F2diff = second formant difference between low vowels, all other measures are reported for vowel /a/ only (F1 = first formant; MaxHor = Maximum horizontal opening; MaxVert = maximum vertical opening; and Jdisp = Jaw displacement.
F2 difference between a pair of the low vowels (/ae/ – /a/) changed substantially for A1 only, showing a gradual reduction of this vowel contrast over time. F2 of vowel /ae/ contributed most to the change (about 400 Hz decline between sessions 1 and 4). For the other three talkers, the F2 difference did not change to the same degree with disease progression as for A1 (see Figure 4).
Among kinematic measures horizontal lip opening changed to a greatest degree. Note that the horizontal opening is shown as negative when the horizontal component decreased (i.e., movement toward lip protrusion) and shown as positive when it increased (i.e., movement toward lip spreading). The data revealed a tendency across talkers for the lip corners to move somewhat forward during /a/ as disease progressed. The jaw displacement tended to fluctuate over time, increasing for specific sessions for A1, A2, and A6 and all sessions for A7. Changes in the vertical mouth opening were relatively small and subject-specific.
Discussion
This investigation was designed to determine how vowel-related movements of the jaw and lip were affected by ALS longitudinally, and when these changes occurred relative to changes in vowel formant frequencies and speech intelligibility. All of the participants in this study demonstrated changes in lip and jaw kinematics over time. The most consistent kinematic finding across participants was with an increase in jaw displacement with disease progression. Changes in lip and jaw movements were similar across different vowels and often did not result in commensurate changes in vowel acoustic or speech intelligibility but preceded them in time. In contrast, acoustic changes paralleled changes in speech intelligibility.
Jaw Displacement Increased over time
Gradual reductions in the amplitude of speech movements over time might be expected with ALS due to progressive losses in articulatory muscle strength and/ or increased muscle tone. Our findings, however, show a more complicated course of motor change with all four talkers demonstrating an increase in jaw displacements rather than a decrease. In some participants, this change was transient (see A2 session 2, vowels /a/ and /i/ and A6 session 3, vowel /a/), but in others more gradual (i.e., A7).
Similar to the jaw displacement, changes in the variables related to mouth opening in talkers A6 and A2 appeared to be transient. Data for talkers A2 and A7 showed similarities in the direction and extent of change over time in jaw displacement and vertical mouth opening, suggesting a significant contribution of jaw to mouth opening throughout the disease for these talkers. In contrast, the data obtained from A6 and A1 showed some disassociation between the jaw and lip movements, suggesting a greater contribution of the lips to mouth opening over time than jaw for these two talkers. Although healthy talkers vary considerably in how they use their lips and jaw during speech (Kent et al., 1975), the observed changes across time in our participants suggest a differential expression or response to the disease.
Changes in tongue performance were inferred from examining the associated between acoustic and orofacial kinematic changes. The acoustic measure of F2 was used because of its sensitivity to potential reductions in the acoustic distinction particularly between low vowels (i.e., /ae/ – /a/). Two of the talkers (A1 and A2) showed this contrast to be substantially different from what might be expected in healthy talkers (Hillenbrand, Getty, Clark, & Wheeler, 1995); specifically, vowel distinction was poor for participant A2 across all sessions and vowel distinction decreased significantly over time for participant A1. In contrast, the other two talkers (A6 and A7) showed only small changes in this measure between sessions (e.g., drop at session 2 for A6 followed by a return to the initial values). As displayed in Figure 5, reductions in this acoustic measure did not coincide with the decreases in jaw or lip displacements in participants A1 between sessions 1 and 4, A2 between sessions 1 and 2, A6 between sessions 1 and 3, and A7 between sessions 1 and 2. This articulatory and acoustic disassociation is possibly due to significant changes in tongue performance. Tongue movement data will be required, in the future, to determine how tongue movement change longitudinally with disease progression and if the acoustic measure of F2 difference is an effective metric of tongue performance.
Kinematic Changes Are not Vowel-Specific
The observed increase in jaw displacement could be either due to the loss of the ability to efficiently scale oral movements because of motor neuron loss (Weismer et al., 1992), or compensatory responses to a weakening tongue, as previously suggested by other investigators (DePaul & Abbs, 1987; Hirose et al., 1982). Determining if the observed changes in articulatory performance are compensatory, however, is a difficult empirical problem and, consequently, highly speculative. We reasoned that if preserving acoustic vowel quality is the primary goal of speech production (see Guenther et al., 1999; Maeda, 1990; Perkell et al., 1993), then compensatory articulatory adjustments are expected to be vowel specific. For example, the adjustments necessary to preserve the quality of /i/ would be expected to be different from those for /u/ and/ or /a/. In our study, however, articulatory changes were similar across all vowels for two out of the four participants (i.e., see A2 and A6). These findings suggest that the increase in the extent of jaw movement that was observed in these subjects are not compensatory but due to the pathological changes occurring in motor neurons or physiologic responses to the disease such as muscle reinnervation via axonal sprouting, motor unit enlargement, and fiber type grouping (Brown, 1973). The resulting loss of fine motor control might lead to the changes in relatively small, precise movement of the jaw and lips during speech.
Kinematic Change Predates Changes in Vowel Acoustics and Intelligibility
One of the primary goals of this work is to identify measures of bulbar performance that are highly sensitive to the onset of intelligibility decline. The results of this analysis revealed that changes in orofacial movements tended to predate changes in both vowel acoustics and speech intelligibility. We reported similar findings for articulatory speed (Yunusova et al., 2010). In contrast to articulatory movements, format frequencies and speech intelligibility showed a relatively steady rate of decline with the greatest change in both types of variables occurring at session 4, the time when speech became severely impaired in three of the talkers. Our longitudinal observations agree with prior research showing a moderate association between formant frequency values and speech intelligibility (Turner et al., 1995; Weismer et al., 2001). It is notable, however, that changes in formant frequencies were detected relatively rarely in this study, considering the degree of impairment in speech intelligibility in three of the talkers. Perceptual studies of vowels showing that vowels might contribute a relatively small component to intelligibility impairment in ALS (Klasner, Yorkston, & Strand, 1999; Weismer & Martin, 1992). Consonants, prosody, and paralinguistic factors might be significant contributors to speech deficit in ALS and should be accounted for when modeling speech intelligibility decline.
Conclusions
The data presented in this study showed longitudinal changes in kinematic, acoustic and intelligibility measures with disease progression in ALS. The time course of these changes revealed that orofacial movements followed an early and, sometimes transient course, suggesting that these measures might be more suitable than measure of vowel acoustics or speech intelligibility for early detection of disease onset in the speech (bulbar) system. The reasons for the changes in the jaw/ lip musculature remain unclear, however. The differences in the effect of the disesase on various speech organs needs to be further investigated, in order to determine if the observed changes in jaw/ lip kinematics are the consequence of neuromuscular deficit or is due to compensatory adjustments.
Acknowledgments
This research was supported by NIH-NIDCD Grant #1R01DC009890-01A1, funding from the Canada Foundation for Innovation (CFI-LOF), and Connaught New Investigator Award. We thank Cynthia Didion and Megan Wobken for their assistance in data collection and measurements.
Contributor Information
Yana Yunusova, Department of Speech-Language Pathology, University of Toronto, Sunnybrook Health Science Centre, Canada.
Jordan R. Green, Department of Special Education and Communication Disorders, University of Nebraska Munroe-Meyer Institute, Nebraska Medical Center
Mary J. Lindstrom, Department of Biostatistics, University of Wisconsin
Gary L. Pattee, Munroe-Meyer Institute, Nebraska Medical Center
Lorne Zinman, Department of Speech-Language Pathology, University of Toronto, Sunnybrook Health Science Centre, Toronto, Canada
References
- Beukelman D, Yorkston K, Hakel M, Dorsey M. Speech Intelligibility Test [Computer software] Lincoln, NE: Madonna Rehabilitation Hospital; 2007. [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Sufit RL, DePaul R, Tan YD, Sanjak M, Robbins J. Design of clinical therapeutic trials in amyotrophiclateral sclerosis. Advanced Neurology. 1991;56:521–546. [PubMed] [Google Scholar]
- Brown WF. Functional compensation of human motor units in health and disease. Journal of the Neurological Sciences. 1973;20:199–209. doi: 10.1016/0022-510x(73)90030-0. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of Neurological Sciences. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- DePaul R, Abbs JH. Manifestations of ALS in the cranial motor nerves: Dynametric, neuropathologic, and speech motor data. Neurologic Clinics. 1987;5:231–250. [PubMed] [Google Scholar]
- DePaul R, Abbs JH, Caligiuri MP, Gracco VL, Brooks BR. Differential involvement of hypoglossal, trigeminal and facial motoneurons in ALS. Neurology. 1988;38:281–283. doi: 10.1212/wnl.38.2.281. [DOI] [PubMed] [Google Scholar]
- Fant G. Acoustic theory of speech production. Paris, France: Mouton, The Hague; 1960. [Google Scholar]
- Fromkin V. Lip positions in American English vowels. Language and Speech. 1964;7:215–225. [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Tandan R. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase III randomized trial. Lancet Neurology. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Green JR, Nip I, Mefferd AS, Wilson EM, Yunusova Y. Lip movement exaggerations during infant directed speech. Journal of Speech, Language, and Hearing Research. 2010;53:1529–1542. doi: 10.1044/1092-4388(2010/09-0005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Wilson EM, Wang Y, Moore CA. Estimating mandibular motion based on chin surface targets during speech. Journal of Speech, Language, and Hearing Research. 2007;50:928–939. doi: 10.1044/1092-4388(2007/066). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Espy-Wilson CY, Boyce SE, Matthies ML, Zandipour M, Perkell JS. Articulatory trade-offs reduce acoustic variability during American English /r/ production. Journal of the Acoustical Society of America. 1999;105:2854–2865. doi: 10.1121/1.426900. [DOI] [PubMed] [Google Scholar]
- Hillenbrand J, Getty LA, Clark MJ, Wheeler K. Acoustic characteristics of American English vowels. Journal of the Acoustical Society of America. 1995;97:3099–3111. doi: 10.1121/1.411872. [DOI] [PubMed] [Google Scholar]
- Hirose H, Kiritani S, Sawashima M. Patterns of dysarthric movement in patients with amyotrophic lateral sclerosis and pseudobulbar palsy. Folia Phoniatrica et Logopaedica. 1982;34:106–112. doi: 10.1159/000265636. [DOI] [PubMed] [Google Scholar]
- Kent JF, Kent RD, Rosenbek JC, Weismer G, Martin RE, Sufit RL. Quantitative description of the dysarthria in women with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1992;35:723–733. doi: 10.1044/jshr.3504.723. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Weismer G, Martin RE, Sufit RL, Rosenbek JC. Relationships between speech intelligibility and the slope of second-formant transitions in dysarthric subjects. Clinical Linguistics and Phonetics. 1989;3:347–358. [Google Scholar]
- Kent RD, Netsell R, Bauer LL. Cineradiographic assessment of articulatory mobility in the dysarthrias. Journal of Speech and Hearing Disorders. 1975;40:467–480. doi: 10.1044/jshd.4004.467. [DOI] [PubMed] [Google Scholar]
- Kewley-Port D, Burkle TZ, Lee JH. Contribution of consonant versus vowel information to sentence intelligibility for young normal-hearing and elderly hearing-impaired listeners. Journal of the Acoustical Society of America. 2007;122:2365–2375. doi: 10.1121/1.2773986. [DOI] [PubMed] [Google Scholar]
- Klasner ER, Yorkston KM, Strand EA. Patterns of perceptual features in speakers with ALS: Prominence and intelligibility considerations. Journal of Medical Speech-Language Pathology. 1999;72(2):117–126. [Google Scholar]
- Lindblom B, Sundberg J. Acoustical consequences of lip, tongue, jaw and larynx movement. Journal of the Acoustical Society of America. 1971;50:1166–1179. doi: 10.1121/1.1912750. [DOI] [PubMed] [Google Scholar]
- Maeda S. Compensatory articulation during speech: Evidence from the analysis and synthesis of vocal-tract shapes using an articulatory model. In: Hardcastle W, Marchal A, editors. Speech production and modeling. The Netherlands: Kluwer; 1990. pp. 131–149. [Google Scholar]
- Milenkovic PH. TF32 [Computer software] Madison: University of Wisconsin; 2001. [Google Scholar]
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database of Systematic Reviews 2007. 2007;(1):CD001447. doi: 10.1002/14651858.CD001447.pub2. Art. No. [DOI] [PubMed] [Google Scholar]
- Montgomery AA, Jackson PL. Physical characteristics of the lips underlying vowel lip reading performance. Journal of the Acoustical Society of America. 1983;73:2134–2144. doi: 10.1121/1.389537. [DOI] [PubMed] [Google Scholar]
- Mulligan M, Carpenter J, Riddel J, Delaney MK, Badger G, Krusinski P. Intelligibility and the acoustic characteristics of speech in amyotrophic lateral sclerosis (ALS) Journal of Speech and Hearing Research. 1994;37:496–503. doi: 10.1044/jshr.3703.496. [DOI] [PubMed] [Google Scholar]
- Perkell JS, Matthies ML, Svirsky MA, Jordan MI. Trading relations between tongue-body raising and lip rounding in production of the vowel /u/: A pilot “motor equivalence” study. Journal of the Acoustical Society of America. 1993;93:2948–2961. doi: 10.1121/1.405814. [DOI] [PubMed] [Google Scholar]
- Plant GL. Visual identification of Australian vowels and diphthongs. Australian Journal of Audiology. 1980;2(2):83–91. [Google Scholar]
- Pradas J, Finison L, Andres PL, Thornell B, Hollander D, Munsat TL. The natural history of amyotrophic lateral sclerosis and the use of natural history controls in therapeutic trials. Neurology. 1993;43(4):751–755. doi: 10.1212/wnl.43.4.751. [DOI] [PubMed] [Google Scholar]
- Stevens KN, House AS. Development of a quantitative description of vowel articulation. Journal of the Acoustical Society of America. 1955;27:484–493. [Google Scholar]
- Turner G, Tjaden K, Weismer G. The influence of speaking rate on vowel space and speech intelligibility for individuals with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1995;38:1001–1014. doi: 10.1044/jshr.3805.1001. [DOI] [PubMed] [Google Scholar]
- Wang H, Larriviere KS, Keller KE, Ware K, Burns T, Conaway M, et al. Bennett J., Jr R+ pramipexole as a mitochondrially focused neuroprotectant: Initial early phase studies in ALS. Amyotrophic Lateral Sclerosis. 2008;9:50–58. doi: 10.1080/17482960701791234. [DOI] [PubMed] [Google Scholar]
- Weismer G. Speech intelligibility. In: Ball MJ, Perkins MR, Müller N, Howard S, editors. The handbook of clinical linguistics. Oxford, UK: Blackwell; 2008. pp. 568–582. [Google Scholar]
- Weismer G, Jeng JY, Laures JS, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica. 2001;53:1–18. doi: 10.1159/000052649. [DOI] [PubMed] [Google Scholar]
- Weismer G, Kent RD, Hodge M, Martin R. The acoustic signatures for intelligibility test words. Journal of the Acoustical Society of America. 1988;84:1281–1291. doi: 10.1121/1.396627. [DOI] [PubMed] [Google Scholar]
- Weismer G, Martin RE. Acoustic and perceptual approaches to the study of intelligibility. In: Kent RD, editor. Intelligibility in speech disorders: Theory, measurement, and management. Philadelphia, PA: John Benjamin; 1992. pp. 67–118. [Google Scholar]
- Weismer G, Martin R, Kent JF, Kent RD. Formant trajectory characteristics of males with amyotrophic lateral sclerosis. Journal of the Acoustical Society of America. 1992;91:1085–1097. doi: 10.1121/1.402635. [DOI] [PubMed] [Google Scholar]
- Yunusova Y, Green J, Lindstrom M, Ball L, Pattee G, Zinman L. Kinematics of disease progression in bulbar ALS. Journal of Communication Disorders. 2010;43:6–20. doi: 10.1016/j.jcomdis.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y, Weismer G, Westbury JR, Lindstrom M. Articulatory movements during vowels in speakers with dysarthria and normal controls. Journal of Speech, Language, and Hearing Research. 2008;51:596–611. doi: 10.1044/1092-4388(2008/043). [DOI] [PubMed] [Google Scholar]


