Abstract
Background
The growing rates of ductal carcinoma in situ (DCIS) and evidence that Latinas may underuse breast-conserving surgery (BCS) compared with white women highlight the need to better understand how treatment decisions are made in this understudied group. To help address this gap, this study compared surgery and radiation treatment decision making among white and Spanish-speaking and English-speaking Latina women with DCIS recruited from eight population-based cancer registries from 35 California counties.
Methods
Women aged ≥18 who self-identified as Latina or non-Latina white diagnosed with DCIS between 2002 and 2005 were selected from eight California Cancer Registry (CCR) regions and surveyed about their DCIS treatment decision making by telephone approximately 24 months after diagnosis. Survey data were merged with CCR hospital-based records to obtain tumor and treatment data.
Results
Mean age was 57 years. Multivariate analysis indicated no differences by ethnicity or language in the receipt of mastectomy vs. BCS after controlling demographic, health, and personal preferences. English-speaking Latinas were more likely to receive radiation than their Spanish-speaking or white counterparts, controlling for demographic and other factors. Among women receiving BCS, physician recommendation was the strongest predictor of receipt of radiation.
Conclusions
Ethnic disparities in surgical treatment choices after breast cancer diagnosis were not seen in this cohort of women diagnosed with DCIS. Physicians play an essential role in patients' treatment choices for DCIS, particularly for adjuvant radiation.
Introduction
Before widespread use of mammography, the diagnosis of ductal carcinoma in situ (DCIS) was relatively infrequent.1 Coinciding with the upsurge in mammography screening in the 1980s, incidence rates for DCIS rose faster than for any other type of breast cancer,2 now accounting for up to 30% of all mammographically detected cancers.3,4 Currently, the overall incidence of DCIS is lower among Latinas compared with white women. As more Latinas undergo yearly screening, however, incidence rates are likely to increase in this group. Supporting this supposition, recent data for California indicate a doubling in the incidence of DCIS among Latinas, from 9.9% in 1990 to 20.1% in 2005.5
DCIS is highly curable, with a 10-year overall survival rate reaching almost 100%.6 Treatment goals for women diagnosed with DCIS are to control local disease and prevent the development of invasive cancer. However, there is controversy about the most appropriate treatment approach.7 With a limited understanding of markers of disease progression, almost all patients undergo some form of surgery. However, many lesions may never become clinically significant, rendering the surgery of negligible benefit.8 Until the early 1990s, mastectomy was the standard procedure, but since then, studies have shown that breast-conserving surgery (BCS) with radiation therapy is effective in reducing DCIS recurrence and the development of invasive carcinoma.9,10 Nevertheless, the necessity of radiation therapy for all women with DCIS undergoing BCS continues to be debated.11,12
Many variables influence whether women undergo BCS or mastectomy for DCIS. From the literature on invasive breast cancer, we know that BCS has increased in the last decades, but diffusion has not been uniform, and rates have varied by insurance status,13,14 geographic location,15 distance to radiotherapy facilities,16 urban vs. rural location, and proximity to teaching hospitals.17–19 Patient characteristics of age, socioeconomic status (SES), and ethnicity also play a role in treatment selection.20–23 There are few data on breast cancer treatment for Latinas with DCIS. Some studies of Latinas with invasive breast cancer suggest that they are less likely to receive BCS than white women24,25; others have found no such difference.26
Compared to invasive breast cancer, the standard of care for the treatment of DCIS is less clear. The use of radiation therapy following BCS for DCIS remains an area of active controversy. Although radiation clearly reduces local recurrence rates in this population by >50%, there are likely subsets of DCIS that gain little absolute benefit while incurring risk of treatment-related morbidity.27,28 Almost half of patients who currently undergo BCS for DCIS do not receive radiation.29 Generally, the discussion about choice of radiation after BCS for DCIS requires education, communication, and careful consideration of risks, benefits, and values. In this study, we sought to determine if this complex choice of treatment was affected by race/ethnicity.
Treatment decision making among Latinas and white women with DCIS may differ for several reasons. Less acculturated, monolingual Latinas may be at increased risk of poorer quality care because of socioeconomic factors, language differences,30 lack of familiarity with the healthcare system, and poor clinician-patient communication.31 They may be less likely to understand the distinctions between DCIS and invasive breast cancer, information that is critical in making an informed treatment decision.31 There is also evidence that English-speaking and Spanish-speaking Latinas may differ in screening behaviors and treatment decisions.32 Thus, the extent to which ethnic differences exist in choice of radiation is an understudied area of great interest and likely reflects the complex interplay between these related factors.
Personal and cultural preferences, as well as physician's recommendations, play an essential role in shaping women's treatment decisions. One previous study found an independent effect of personal and demographic factors, such as patient concern or surgeon discussion, in DCIS treatment selection,33 but the relative impact of each of these factors on treatment selection, particularly in the Latina population, is still not clear. Other studies of treatment decision making that have included Latinas did not address DCIS specifically, were limited to a small geographical area, and had a limited number of Latinas in their samples.26 The current study was designed to address these gaps in knowledge about treatment selection and practice patterns by conducting a population-based study of Latina and non-Latina white women with DCIS from 35 counties in California. We included both the personal and sociodemographic factors previously shown to be associated with treatment decisions to assess their impact in surgical and radiation therapy treatment selection.
Material and Methods
Study population
Women were sampled from eight California Cancer Registry (CCR) regions, which represents 35 of 58 counties in California. Inclusion criteria were women aged ≥18 who self-identified as either Latina or non-Latina white and were diagnosed with DCIS between 2002 and 2005 without a subsequent diagnosis of invasive breast cancer.
Sample
Study recruitment took place between January 2005 and September 2006. Within each region and county, we sampled all Latina women. Given their large numbers, white women were selected randomly and matched to the Latina cases by age (within 5-year increments), diagnosis period (within 6-month intervals), and county of diagnosis. From January 2005 to March 2006, white women and Latinas were selected with a 1:1 ratio. However, because of a lower than anticipated response rate among Latinas, we adjusted procedures, selecting white women on a 1:2 ratio to Latinas beginning in April 2006 until the end of recruitment. Three women who opted for no surgery were dropped from the analysis.
Data collection
Telephone interviews were conducted approximately 24 months after diagnosis in English or Spanish, per participant's preference. This was the time necessary to receive complete information from the CCR and process and reach participants for interviews. Participants received a $20 gift certificate for completing the interview. CCR data on initial course of treatment (which is collected from hospital medical records) were merged with the survey data for all participants to obtain or verify information (tumor grade, time since diagnosis, initial treatment, and radiation). Survey data were compared with the data obtained from the CCR on surgery type and whether or not they received radiation. Informed consent was obtained from all participants, and all study procedures were approved by the UCSF Committee on Human Research.
Measures
The main outcome variable was self-reported surgical treatment type: mastectomy vs. BCS. Specific survey questions included: Did the doctor remove only part of the breast; sometimes they call this a lumpectomy? and Did the doctor remove the entire breast; this is also called a mastectomy? A second outcome was whether radiation therapy was used among those who had BCS. Patients were asked: Did you have radiation therapy? There was a 92% (kappa = 0.82) agreement on surgery type. Radiation had a lower agreement rate (84%, kappa = 0.63), which largely came from 14% of cases who were categorized in the CCR data as not having radiation but reported having radiation through the survey.
Demographic indicators
Based on self-identification, patients were classified as white or Latina. Latinas were further classified as English-speaking Latinas (ESL) or Spanish-speaking Latinas (SSL) by their preferred interview language. Other indicators included age at interview, relationship status (never married, legally separated or divorced, or widowed vs. married or with a long-term partner), and highest year of school completed. The cases were regrouped into five geographic regions: San Francisco Bay Area, Central/Sacramento, Riverside/San Bernardino, Los Angeles/Tri counties, and San Diego/Imperial.
Access to care
Participants with public insurance (Medicare, MediCal, Veterans Administration), no insurance, or unknown insurance were classified as one group and compared to those with private insurance (HMO or private non-HMO). Women with no insurance were grouped this way because there were not enough respondents to treat as a separate category (n = 33).
Health-related indicators
To assess family history of breast cancer, participants were classified into two categories: those who had a mother, sister, daughter, grandmother, or aunt with a history of breast cancer vs. all others. To measure comorbidities, participants were given a list of health conditions and asked to indicate if they had ever been diagnosed with each condition. Any participant with lung, heart, kidney, or liver disease; blood clots; or strokes was considered as having a major health comorbidity that might impact the treatment decision. This resulted in a dichotomous variable of major comorbidity vs. none. Other indicators included whether or not a patient obtained a second opinion and her self-rated health assessment (excellent/very good vs. good/fair/poor/very poor). A histological grade indicator was based on registry data (grades 1, 2, and 3 or missing). Finally, we calculated time from diagnosis to interview in months.
Influences on surgical decision
Expanding on items used previously by Katz et al.,33 participants were asked to indicate if any of 20 items influenced their choice of surgery (not at all, somewhat, a lot). Based on maximum likelihood principal component analysis, we created six scales to assess potential influences on a woman's surgical decision. The Concerns about Survival Scale included two items: chances of surviving and reducing worry about getting the breast disease again (Cronbach's alpha = 0.68). The Concerns about Radiation Scale items were: wanting to avoid radiation and concerns about the side effects of radiation (Cronbach's alpha = 0.87). The Concerns about Appearance Scale used concerns about the amount of breast removed and concerns about appearance (Cronbach's alpha = 0.65). The Family Influences Scale combined the influence of partner's preference and that of family (Cronbach's alpha = 0.76). The Surgical Consequences Scale included three items: influence of recovery time, fear of pain, and resuming usual activities as soon as possible (Cronbach's alpha = 0.65). The Cost Scale included cost and health insurance problems (Cronbach's alpha = 0.64). Physician's recommendation about treatment and family's breast cancer history did not load on any factor and were used as single indicators. Scores ranged from 1 = not at all to 3 = a lot, with a higher score meaning greater influence.
Discussion of radiation with physician
Patients were asked if their physician talked to them about radiation therapy and if the physician said it was a necessary, optional, or unnecessary part of their treatment.
Reasons for not having radiation
Patients who had BCS but did not receive radiation were asked to respond true or false to 12 possible reasons for not choosing radiation. Seven items were dropped from these analyses because of limited response variation (endorsed by <15%). The five items included in these analyses are: I thought it would not be helpful; concerned about side effects and complications; risk of recurrence was low; if DCIS came back, would not have radiation as an option; and my doctor did not recommend it.
Statistical analysis
Descriptive statistics were used to illustrate the characteristics of the total sample, and differences between the women in ethnic/language groups were assessed using chi-square or analysis of variance (ANOVA). Factors influencing surgery and reasons for not receiving radiation were graphically presented by ethnicity. We fit three logistic regression models. Model 1 modeled the probability of a woman selecting mastectomy. All demographic, access to care, and health-related indicators were included in the final models. All influences on surgical decision scales were tested and included except for the influences of family cancer history and cost, as family cancer history and insurance were already accounted for. Model 2, which estimated the likelihood of radiation therapy for women who received BCS, contained all the demographic, access to care, and health-related indicators. Model 3 was identical to model 2, with the inclusion of the measures of physicians' indication about discussion of radiation. In order to explain attenuations in effect magnitude between models 2 and 3, chi-square tests were performed to assess the associations between factors in model 2 and the physician discussion variable.
Results
The sampling frame consisted of 1404 women who were mailed the study invitation letter. Of these, 21 patients were not contacted at their physicians' request, 98 women were deemed ineligible after initial contact, and 54 had incorrect information. Interviewers' attempts to contact the remaining 1231 women resulted in 319 refusals (64 by postcard, 255 verbally), 167 nonrespondents, and 745 completed surveys. White women had a higher completion rate than Latinas (67% and 55%, respectively).
Sample characteristics
The mean age of the sample was 57 years (Table 1). The majority were married or in a permanent relationship. A greater proportion of white women had a college education compared to ESL and SSL women. The majority of white and ESL women were privately insured, but this was not true for SSL women. A larger proportion of white and ESL women reported having a relative with a history of breast cancer compared with SSL women.
Table 1.
Sample Characteristics, by Ethnicity Language Group
| White women n = 396 | English-speaking Latinas n = 156 | Spanish-speaking Latinas n = 193 | Total n = 745 | pa | |
|---|---|---|---|---|---|
| Background characteristics | |||||
| Mean age (SD) | 57.1 (10.0) | 56.7 (10.3) | 54.9 (10.2) | 56.5 (10.1) | <0.05 |
| Age | 0.53 | ||||
| <50 | 28.5 | 28.9 | 33.7 | 29.9 | |
| 50–64 | 47.5 | 44.9 | 46.6 | 46.7 | |
| ≥65 | 24.0 | 26.3 | 19.7 | 23.4 | |
| Relationship status | 0.11 | ||||
| Single | 32.0 | 34.4 | 24.7 | 30.6 | |
| Married or living with partner | 68.0 | 65.6 | 75.3 | 69.4 | |
| Education level | <0.0001 | ||||
| High school completed or less | 21.1 | 47.1 | 79.9 | 41.6 | |
| Any college or higher | 78.9 | 52.9 | 20.1 | 58.4 | |
| Geographic region | <0.01 | ||||
| San Francisco Bay Area | 32.6 | 26.9 | 17.6 | 27.5 | |
| Sacramento, Central California | 17.9 | 25.0 | 21.2 | 20.3 | |
| San Diego | 8.3 | 5.8 | 9.8 | 8.2 | |
| Los Angeles | 29.0 | 26.3 | 37.3 | 30.6 | |
| Riverside/San Bernardino | 12.1 | 16.0 | 14.0 | 13.4 | |
| Access to care | |||||
| Insurance | <0.0001 | ||||
| Private insurance | 82.3 | 76.3 | 45.6 | 71.5 | |
| All other insurance | 17.7 | 23.7 | 54.4 | 28.5 | |
| Health-related indicators | |||||
| Family history of breast cancer | 41.4 | 43.6 | 19.7 | 36.2 | <0.0001 |
| Major comorbidity | 25.5 | 29.5 | 21.8 | 25.4 | 0.26 |
| Requested second opinion | 45.3 | 41.7 | 20.2 | 38.0 | <0.0001 |
| Time from diagnosis, in months (SD) | 24.8 (7.6) | 22.8 (7.4) | 22.3 (8.4) | 23.8 (7.9) | 0.0003 |
| Health status | <0.0001 | ||||
| Excellent/very good | 57.2 | 41.7 | 22.8 | 45.0 | |
| Good/fair/poor/very poor | 42.8 | 58.3 | 77.2 | 55.0 | |
| Histology grade | |||||
| Grade 1 | 7.6 | 7.7 | 7.3 | 7.5 | 0.79 |
| Grade 2 | 30.3 | 31.4 | 32.6 | 31.1 | |
| Grade 3 | 40.1 | 42.3 | 44.0 | 41.6 | |
| Missing | 22.0 | 18.6 | 16.1 | 19.7 | |
| Treatment for DCIS | |||||
| Breast-conserving surgery | 68.5 | 69.0 | 65.3 | 67.8 | 0.68 |
| Mastectomy | 31.5 | 31.0 | 34.7 | 32.2 | |
| Breast-conserving surgery | |||||
| With radiation | 72.2 | 86.9 | 81.8 | 77.7 | 0.0038 |
| Without radiation | 27.8 | 13.1 | 18.3 | 22.3 | |
| Radiation | |||||
| Discussion with physicianb | <.0001 | ||||
| Physician indicated radiation optional | 32.2 | 18.7 | 10.3 | 23.9 | |
| Physician indicated radiation necessary | 52.2 | 72.9 | 75.4 | 62.4 | |
| Physician indicated radiation unnecessary or not discussed | 15.6 | 8.41 | 14.3 | 13.7 | |
Analysis of variance for mean age and time from diagnosis comparisons. Pearson chi-square for other comparisons.
For patients who received radiation (n = 503).
DCIS, ductal carcinoma in situ; SD, standard deviation.
Type of surgical treatment
The majority of respondents chose BCS (Table 1). Three participants reported no surgical procedure and were not included in further analysis. Among those who had BCS, 22% reported not receiving radiation therapy. A greater proportion of ESL and SSL women reported receiving radiation compared to white women. ESL and SSL women were more likely to indicate that their physician said radiation therapy was necessary (72.9% and 75.4% vs. 52.2% for white women). Similar differences were observed in age. Younger age groups were more likely to indicate that their physician said radiation was necessary (65.9% for those <50, 62.2% for those 50–64, and 59.5% for those ≥65).
Factors influencing surgical decision
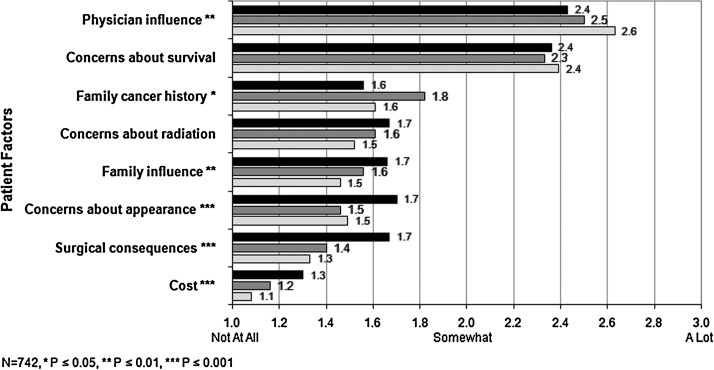
Figure 1 shows the unadjusted rates of patient-reported factors that influenced surgical decision. Regardless of surgery type, physician influence and concerns about survival were the two most important factors. White women rated the influence of physicians higher than did both Latina groups. ESL women rated the influence of family cancer history higher than did SSL or white women. SSL women gave higher ratings to family influences, appearance, surgical consequences, and cost than either white or ESL women, although these four influences were the least cited by all groups.
FIG. 1.
Factors influencing surgery, by ethnicity language. Average values reported by each ethnicity language group are shown for the patient factors that influenced their decision on the type of surgery to undergo. The scale was from 1(no influence at all) to 3 (a lot of influence).  , Spanish-speaking Latinas;
, Spanish-speaking Latinas;  , English-speaking Latinas;
, English-speaking Latinas;  , white women.
, white women.
Multivariate analysis
Surgical choice
Women were more likely to choose a mastectomy if they were younger (odds ratio [OR] 1.76, 95% confidence interval [CI] 1.03–2.98), had requested a second opinion (OR 1.51, 95% CI 1.04–2.19), or had reported less than excellent or very good health (OR 1.49, 95% CI 1.01–2.19) (Table 2). Greater concern about survival (OR 1.86, 95% CI 1.39–2.51) and radiation (OR 1.75, 95% CI 1.38–2.22) were positively associated with having had a mastectomy. A higher score on the physician recommendation item, indicating a greater degree of influence, was inversely associated with having a mastectomy (OR 0.70, 95% CI 0.55–0.90). Overall, the family influences scale was not associated with undergoing a mastectomy; however, examination of the interaction effect between ethnicity/language and each of the reported influences indicates that ESL women who reported family influences were less likely to undergo a mastectomy (OR 0.38, 95% CI 0.20-0.73).
Table 2.
Surgical Choice: Logistic Regression, Odds of Mastectomy (n = 742)
| Odds of mastectomya | |
|---|---|
| Background characteristics | |
| Ethnicity language (Ref = white women) | |
| English-speaking Latinas | 0.92 (0.57–1.47) |
| Spanish-speaking Latinas | 1.26 (0.74–2.15) |
| Age (Ref = 65 and over) | |
| <50 | 1.76 (1.03–2.98) |
| 50–64 | 0.94 (0.57–1.54) |
| Relationship status (Ref = married or living with partner) | |
| Single | 1.01 (0.68–1.52) |
| Education level (Ref = college or higher) | |
| High school completed or less | 0.83 (0.53–1.28) |
| Access to care | |
| Insurance (Ref = private insurance) | |
| All other insurance | 0.78 (0.49–1.24) |
| Health-related factors | |
| Family history of breast cancer (Ref = none) | 1.28 (0.89–1.86) |
| Major comorbidity (Ref = none) | 1.08 (0.71–1.63) |
| Requested second opinion | 1.51 (1.04–2.19) |
| Health status (Ref = excellent/very good) | |
| Good/fair/poor/very poor | 1.49 (1.01–2.19) |
| Histology grade (Ref = grade 1) | |
| Grade 2 | 0.78 (0.37–1.63) |
| Grade 3 | 1.28 (0.63–2.62) |
| Missing | 0.77 (0.36–1.68) |
| Influences on surgical decision | |
| Concerns about survival | 1.86 (1.39–2.51) |
| Concerns about radiation | 1.75 (1.38–2.22) |
| Concerns about appearance | 0.97 (0.72–1.31) |
| Family influences | 0.93 (0.70–1.22) |
| Surgical consequences | 1.16 (0.76–1.75) |
| Physician influence | 0.70 (0.55–0.90) |
Controlled for geographic region and time since diagnosis.
Receipt of radiation
Among the women who had BCS, ESL women were more likely to have received radiation than white women (OR 2.84, 95% CI 1.43-5.64) (Table 3). Compared with women aged ≥65, women <50 (OR 2.14, 95% CI 1.10–4.14) and those 50–64 (OR 1.87, 95% CI 1.08–3.25) were more likely to receive radiation. Women with a high-grade tumor were almost 5 times more likely to receive radiation than those with a grade 1 tumor (OR 4.75, 95% CI 2.06–10.95). When discussion of the necessity of radiation is included in the analysis, higher histology grade tumors remain significant, and women whose physicians indicated radiation was necessary were 8 times more likely to receive it than those who reported it was optional. An inverse relationship was observed for those whose physicians indicated it was unnecessary or did not discuss it with them. The relationship with language groups is attenuated with the inclusion of the discussion variable.
Table 3.
Logistic Regression, Odds of Radiation Therapy Among Women Who Received Breast Conserving Surgery (n = 503)
| Odds of radiationa | ||
|---|---|---|
| Without discussion with physician | Includes discussion with physician | |
| Background characteristics | ||
| Ethnicity language (Ref = white women) | ||
| English-speaking Latinas | 2.84 (1.43–5.64) | 2.09 (0.89–4.92) |
| Spanish-speaking Latinas | 1.51 (0.77–2.94) | 1.35 (0.54–3.37) |
| Age (Ref = 65 and over) | ||
| Less than 50 | 2.14 (1.10–4.14) | 1.45 (0.61–3.42) |
| 50–64 | 1.87 (1.08–3.25) | 1.85 (0.89–3.85) |
| Relationship status (Ref = married or living with partner) | ||
| Single | 1.21 (0.73–2.00) | 1.23 (0.64–2.38) |
| Education level (Ref = college or higher) | ||
| High school completed or less | 0.65 (0.38–1.10) | 0.64 (0.32–1.30) |
| Access to care | ||
| Insurance (Ref = private insurance) | ||
| All other insurance | 1.69 (0.95–3.02) | 1.63 (0.75–3.56) |
| Health-related factors | ||
| Family history of breast cancer (Ref = no) | 0.88 (0.54–1.41) | 1.22 (0.65–2.28) |
| Major comorbidity (Ref = no) | 1.24 (0.72–2.14) | 1.11 (0.55–2.26) |
| Requested second opinion | 1.05 (0.64–1.73) | 1.05 (0.55–1.98) |
| Health status (Ref = excellent/very good) | ||
| Good/fair/poor/very poor | 1.48 (0.92–2.38) | 1.33 (0.71–2.51) |
| Histology grade (Ref = grade 1) | ||
| Grade 2 | 1.70 (0.77–3.78) | 1.91 (0.69–5.28) |
| Grade 3 | 4.75 (2.06–10.95) | 5.03 (1.74–14.55) |
| Missing | 2.84 (1.18–6.82) | 2.77 (0.89–8.60) |
| Discussion with physician (Ref = radiation optional) | ||
| Physician indicated radiation necessary | – | 8.05 (4.04–16.03) |
| Physician indicated unnecessary or not discussed | – | 0.09 (0.04–0.19) |
Controlling for geographic region and time since diagnosis.
Reasons for not having radiation
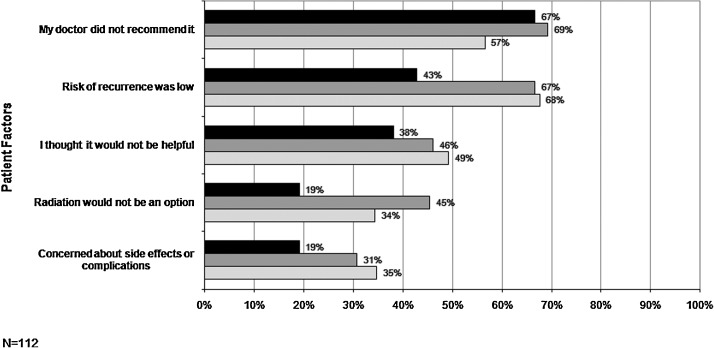
Among BCS patients who did not undergo radiation treatment, lack of physician recommendation was the most frequently cited reason, followed by (in descending order of importance) risk of recurrence was low, I thought it would not be helpful, radiation would not be an option, and concerned about side effects or complications (Fig. 2).
FIG. 2.
Factors influencing radiation among breast-conserving surgery patients who did not receive radiation, by ethnicity language. Percentage of patients who cited each influence factor for the three ethnicity language groups for patients who did not receive radiation.  , Spanish-speaking Latinas;
, Spanish-speaking Latinas;  , English-speaking Latinas;
, English-speaking Latinas;  , white women.
, white women.
Discussion
This article examined treatment decisions among Latina and non-Latina white women diagnosed with DCIS in a population-based study. We explored the relative importance of various influences on women's choices related to surgery and radiation therapy, controlling for demographic characteristics, access to care, and health-related factors. We elected to study treatment decisions for DCIS because DCIS illustrates a health issue for which there is controversy over the risks and benefits of treatment, even among experts in the field.34 We also address the gaps in knowledge about DCIS treatment selection in the Latina population.
Our primary outcome was receipt of mastectomy for surgical treatment of DCIS. Although earlier studies have identified racial/ethnic differences as an important determinant of mastectomy rate for breast cancer,26 our results show no such differences between white women and either SSL or ESL women. Katz et al.,26 who also disaggregated the Latina group based on language, found similar results. This combined evidence indicates that prior ethnic disparities in treatment choices may have decreased over time.24,25
We found that age and self-reported health status were associated with surgical treatment selection. Similar to other DCIS studies, we found that younger age was associated with receipt of mastectomy.35 This is not surprising, given the high risk of recurrence among younger women.34 In contrast, among those diagnosed with invasive breast cancer, younger women tend to receive BCS over mastectomy.36,37 Our results also show that the likelihood of a mastectomy was greater among those who perceived themselves to be in poorer health. This may suggest that these women were not good candidates for prolonged radiation treatment. Surprisingly, another health indicator, presence of a major comorbidity, was not associated with choice of surgical treatment.
The influence of a medical second opinion on a breast surgical decision is not well established. Although several studies suggest that those seeking a second opinion tend to choose BCS,38 we found just the opposite. Bleicher et al.37 also found that women who consulted with a greater number of surgeons were more likely to choose mastectomy. The higher use of mastectomy in our sample could reflect visits to plastic surgeons after a decision to undergo mastectomy was already made. However, we did not have data on the specialty of the clinician providing the second opinion. Consistent with studies that have identified physician's recommendation as one of the most important influences on treatment selections for breast cancer patients,39 including type of surgery received,33 our study found that women who weighed their physician's recommendation more heavily in the decision were less likely to receive a mastectomy. Physician's recommendation was the strongest influence for all groups, and it was especially critical for white women.
Similar to other studies, we found that greater concerns about survival and the risks and benefits of radiation were associated with mastectomy40 and played a greater role in women's decisions than more concrete barriers, such as physical appearance and function. The lack of significant ethnic/language differences in these key factors suggests the universality of women's concerns, irrespective of ethnicity/language group. Although fairly well established as standard adjuvant therapy for women who receive BCS, the use of radiation among women with DCIS continues to be debated.34 The preponderance of evidence indicates a significant reduction in ipsilateral in situ as well as invasive cancer among women who receive radiation vs. those who do not.41 The proportion of women in our study who elected BCS and received radiation was somewhat higher than that reported for women with DCIS in other studies,26 although a significant proportion of women from all groups still appeared to have received no radiation therapy (13%–28%). ESL women were more likely to receive radiation than either one of the other groups, controlling for demographic and other factors. This is somewhat contrary to other studies in which a trend toward lower rates of radiation was observed among Latinas.26
Among women undergoing BCS, radiation was more likely to be given to those with a higher chance of a recurrence, such as younger women and those with high-grade disease. In cases where no radiation was given, physicians may have thought that the risk of recurrence was very low, thus not warranting the use of radiation. Physician influence is reflected in the significant effect of their characterization of radiation, that is, necessary, optional, or unnecessary/not discussed, on women's choice of radiation therapy. The physician's influence is also seen when examining the reasons women gave for not choosing radiation therapy, with lack of physician recommendation being the most common reason cited. The effects of ethnicity/language and age on the likelihood of having radiation were attenuated in the model that included the physician discussion variable. In order to explain this attenuation, bivariate analysis was conducted and showed significant relationships between ethnicity/language and age with physician discussion (p < 0.0001, p = 0.05, respectively) indicating that Latinas and younger women were more likely than white and older women to report that their physicians indicated that radiation was necessary.
Other reasons played a role, however. Over two thirds of ESL and white women who did not undergo radiation after BCS indicated that it was not warranted because of the low chance of recurrence. A smaller proportion (43%) of SSL women cited this reason for not having radiation, suggesting that language barriers may prevent a detailed discussion of the potential risks and benefits of radiation therapy. In fact, of the top five factors influencing radiation therapy treatment decisions, all were consistently endorsed to a lesser degree by SSL patients compared with the other two groups. The lack of significant differences may be due to the small number of participants who did not undergo radiation therapy and the fact that the items used a dichotomous scale as opposed to a Likert scale.
Although this was a population-based study covering 35 counties, we acknowledge several limitations. Because the study was based on retrospective information, patients' recall of factors that influenced their treatment decisions may have changed over time or have been influenced by posttreatment experiences. However, this time interval allowed us to gather information on posttreatment care, which would otherwise not be available. Additionally, information about pathological characteristics of DCIS was limited by registry data availability. Namely, we lacked information on the presence of multifocal disease and margin status, and there were a large number of cases with missing tumor size and grade data.
In summary, mastectomy rates for DCIS among white women, English-speaking Latinas, and Spanish-speaking Latinas did not differ significantly. The small differences in treatment choices made among the three groups are encouraging, as this suggests that DCIS treatment disparities may be narrowing over time. Physician recommendation continues to play an important role in the treatment decision, evidenced both in the participants' reports and the association of more aggressive treatment with high-risk groups. The significance of personal factors in treatment choice across ethnic groups reflects the universal concerns of women, irrespective of ethnicity/language status, highlighting the importance of eliciting and incorporating their preferences in the treatment decision-making process.
Acknowledgments
This research was conducted with the support of the California Breast Cancer Research Program (grant 9PB-0157). The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred. We thank Susan Duffey, Jennifer Livaudais, and Jessica Quinn for their editing and project assistance.
Disclosure Statement
E.S.H. has received research funding from Merck & Co., Inc. C.P.K., A.M.N., J.B., S.S., L.K., and D.N. have no conflicts of interest to report.
References
- 1.Ernster V. Epidemiology and natural history of ductal carcinoma in situ. In: Melvin J, editor; Siverstein MD, editor. Ductal carcinoma in situ of the breast. Baltimore: Williams & Wilkins; 1998. pp. 23–33. [Google Scholar]
- 2.Simon MS. Lemanne D. Schwartz AG. Martino S. Swanson GM. Recent trends in the incidence in situ and invasive breast cancer in the Detroit Metropolitan area (1975–1988) Cancer. 1993;71:769–774. doi: 10.1002/1097-0142(19930201)71:3<769::aid-cncr2820710320>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Kerlikowske K. Grady D. Barclay J. Sickles EA. Eaton A. Ernster V. Positive predictive value of screening mammography by age and family history of breast cancer. JAMA. 1993;270:2444–2450. [PubMed] [Google Scholar]
- 4.Rebner M. Noninvasive breast cancer. Radiology. 1994;190:623–631. doi: 10.1148/radiology.190.3.8115600. [DOI] [PubMed] [Google Scholar]
- 5.California Cancer Registry. Cancer incidence/mortality rates in California. 2009.
- 6.Solin LJ. Yet I-T. Kurtz J. Ductal carcinoma in situ (intraductal carcinoma) of the breast treated with breast-conserving surgery and definitive irradiation. Correlation of pathologic parameters with outcome of treatment. Cancer. 1993;71:2532–2542. doi: 10.1002/1097-0142(19930415)71:8<2532::aid-cncr2820710817>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein MJ. Ductal carcinoma in-situ of the breast. Controversial issues. Oncologist. 1998;3:94–103. [PubMed] [Google Scholar]
- 8.Jones JL. Overdiagnosis and overtreatment of breast cancer: Progression of ductal carcinoma in situ: The pathological perspective. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16677423. Breast Cancer Res. 2006;8:204. doi: 10.1186/bcr1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher B. Costantino J. Redmond C, et al. Lumpectomy compared to lumpectomy and radiation therapy for the treatment of intraductal breats cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 10.Hillner B. Desch C. Carlson R. Smith T. Esserman L. Bear H. Trade-offs between survival and breast preservation for three initial treatments of ductal carcinoma in situ of the breast. J Clin Oncol. 1996;14:70–77. doi: 10.1200/JCO.1996.14.1.70. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb N. San Antonio Breast Cancer Symposium explores DCIS “battleground.”. J Natl Cancer Inst. 2000;92:292–297. doi: 10.1093/jnci/92.4.295. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein MJ. Lagios MD. Waisman JR, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;92:1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 13.Roetzheim RG. Gonzalez EC. Ferrante JM. Pal N. Van Durme DJ. Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000. www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&opt=r&uid=11147590. pp. 2202–2213.www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&opt=r&uid=11147590 [DOI] [PubMed]
- 14.Osteen RT. Steel GD. Menck H. Regional differences in the management of breast cancer. CA Cancer J Clin. 1992;42:39–43. doi: 10.3322/canjclin.42.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Guadagnoli E. Weeks JC. Shapiro CL. Gurwitz JH. Borbas C. Soumerai S. Use of breast-conserving surgery of treatment of stage I and stage II breast cancer. J Clin Oncol. 1998;16:101–106. doi: 10.1200/JCO.1998.16.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Nattinger AB. Kneusel RT. Hoffman RG. Gillingan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–1345. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 17.Johantgen ME. Rosanna CM. Harris RD. Treating early-stage breast cancer: Hospital characteristics associated with breast conserving surgery. Am J Public Health. 1995;85:1432–1434. doi: 10.2105/ajph.85.10.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satariano ER. Swanson GM. Moll PP. Nonclinical factors associated with surgery received for treatment of early-stage breast cancer. Am J Public Health. 1992;82:195–198. doi: 10.2105/ajph.82.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nattinger AB. Gottlieb BH. Veum J. Yahnke D. Goodwing JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–1107. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 20.Riley GF. Potosky AL. Klabunde CN. Warren JL. Ballard-Barbash R. Stage at diagnosis and treatment patterns among older women with breast cancer. JAMA. 1999;281:720–726. doi: 10.1001/jama.281.8.720. [DOI] [PubMed] [Google Scholar]
- 21.Dolan JT. Granchi TS. Miller CC., 3rd Brunicardi FC. Low use of breast conservation surgery in medically indigent populations. Am J Surg. 1999. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10670855. pp. 470–474.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10670855 [DOI] [PubMed]
- 22.Michalski TA. Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9010104. pp. 314–319.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9010104 [PubMed]
- 23.Mandelblatt JS. Hadley J. Kerner JF, et al. Patterns of breast carcinoma treatment in older women: Patient preference and clinical and physical influences. Cancer. 2000. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10931455. pp. 561–573.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10931455 [PubMed]
- 24.Legorreta AP. Liu X. Parker RG. Examining the use of breast-conserving treatment for women with breast cancer in a managed care environment. Am J Clin Oncol. 2000. www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=11039500. pp. 438–441.www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=11039500 [DOI] [PubMed]
- 25.Morris CR. Cohen R. Schlag R. Wright WE. Increasing trends in the use of breast-conserving surgery in California. Am J Public Health. 2000. www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=10667193. pp. 281–284.www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=10667193 [DOI] [PMC free article] [PubMed]
- 26.Katz SJ. Lantz PM. Paredes Y, et al. Breast cancer treatment experiences of Latinas in Los Angeles County. Am J Public Health. 2005. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16257945. pp. 2225–2230.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16257945 [DOI] [PMC free article] [PubMed]
- 27.Houghton J. George W. Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: Randomized controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin A. Parker S. Ghersi D. Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 2009. Jan 21, [DOI] [PubMed]
- 29.Baxter N. Virnig B. Durham S. Tuttle T. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez LE. Morales A. Language and use of cancer screening services among border and non-border Hispanic Texas women. Ethn Health. 2007. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17454099. pp. 245–263.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17454099 [DOI] [PubMed]
- 31.Napoles-Springer AM. Livaudais JC. Bloom J. Hwang S. Kaplan CP. Information exchange and decision making in the treatment of Latina and white women with ductal carcinoma in situ. J Psychosoc Oncol. 2007. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18032263. pp. 19–36.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18032263 [DOI] [PubMed]
- 32.Lara M. Gamboa C. Kahramanian MI. Morales LS. Bautista DE. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annu Rev Public Health. 2005. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15760294. pp. 367–397.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15760294 [DOI] [PMC free article] [PubMed]
- 33.Katz SJ. Lantz PM. Zemencuk J. Correlates of surgical treatment type for women with noninvasive and invasive breast cancer. J Womens Health Gend Based Med. 2001;10:659–669. doi: 10.1089/15246090152563533. [DOI] [PubMed] [Google Scholar]
- 34.Virnig BA. Shamliyan T. Tuttle TM. Kane RL. Wilt TJ. Diagnosis and management of ductal carcinoma in situ (DCIS). Evidence report/technology assessment No. 185 (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-10064-I) Agency for Healthcare Research and Quality. Sep, 2009.
- 35.Katz SJ. Lantz PM. Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma-in-situ. J Clin Oncol. 2005. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15860856. pp. 3001–3007.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15860856 [DOI] [PMC free article] [PubMed]
- 36.Hiotis K. Ye W. Sposto R. Skinner KA. Predictors of breast conservation therapy: Size is not all that matters. Cancer. 2005. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15641031. pp. 892–899.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15641031 [DOI] [PubMed]
- 37.Bleicher RJ. Abrahamse P. Hawley ST. Katz SJ. Morrow M. The influence of age on the breast surgery decision-making process. Ann Surg Oncol. 2008. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18058182. pp. 854–862.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18058182 [DOI] [PubMed]
- 38.Clauson J. Hsieh YC. Acharya S. Rademaker AW. Morrow M. Results of the Lynn Sage Second-Opinion Program for local therapy in patients with breast carcinoma. Changes in management and determinants of where care is delivered. Cancer. 2002. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11920455. pp. 889–894.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11920455 [DOI] [PubMed]
- 39.Smitt MC. Heltzel M. Women's use of resources in decision-making for early-stage breast cancer: Results of a community-based survey. Ann Surg Oncol. 1997;4:564–569. doi: 10.1007/BF02305537. [DOI] [PubMed] [Google Scholar]
- 40.Katz SJ. Lantz PM. Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16110013. pp. 5526–5533.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16110013 [DOI] [PubMed]
- 41.Bijker N. Meijnen P. Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: Ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853–-A study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006. www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16801628. pp. 3381–3387.www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16801628 [DOI] [PubMed]