Abstract
Previous studies have found that differences in brain volume among older adults predict performance in laboratory tasks of executive control, memory, and motor learning. In the present study we asked whether regional differences in brain volume as assessed by the application of a voxel-based morphometry technique on high resolution MRI would also be useful in predicting the acquisition of skill in complex tasks, such as strategy-based video games. Twenty older adults were trained for over 20 hours to play Rise of Nations, a complex real-time strategy game. These adults showed substantial improvements over the training period in game performance. MRI scans obtained prior to training revealed that the volume of a number of brain regions, which have been previously associated with subsets of the trained skills, predicted a substantial amount of variance in learning on the complex game. Thus, regional differences in brain volume can predict learning in complex tasks that entail the use of a variety of perceptual, cognitive and motor processes.
Keywords: Aging, brain volume, MRI, skill acquisition, video game, cognitive training, older adults
1. Introduction
It is well established in the cognitive aging literature that as we age, particularly after 65 years of age, we exhibit differential decline in both brain structure and cognitive functions. Regarding grey matter brain volume, effects of aging are greater for frontal cortex (Jernigan et al., 2001), basal ganglia, hippocampus, caudate and cerebellum than for the entorhinal, anterior cingulate and primary visual cortex (Kennedy et al., 2009; Raz, 2000; Wong et al., 1984). Age-related cortical thinning is also found to be differential with temporal cortex being spared more than other cortices; the greatest rate of regional thinning was found in primary motor cortex whereas the greatest magnitude was found in inferior prefrontal, precentral, and supramarginal regions (Salat et al., 2004). Regarding cognitive functions, fluid abilities decline more rapidly with age, such as processing speed, mental rotation, working memory, multi-tasking and reasoning (Bopp & Verhaeghen, 2005; Schaie, 1996; Sliwinski & Hall, 1998; Verhaeghen, Cerella, Bopp, & Basak, 2005), but verbal knowledge is relatively spared (Ghisletta & Lindenberger, 2003; Park et al., 2002).
However, there are but a handful of studies that explore this structure-cognition association in aging brains. With regards to executive control functions, better performance on the Wisconsin Card Sorting Test, as measured by fewer perseverative errors, has been associated with larger volumes of prefrontal cortices, because there exists an indirect relationship between volume of prefrontal cortex and age-related increases in perseveration; this relationship accounts for 25% of age-related variance in perseveration (Gunning-Dixon & Raz, 2003). Age-related reduction in brain volume of the putamen and the frontal cortex are moderately correlated with neuropsychological measures of working memory and other executive control functions (Gunning-Dixon & Raz, 2003; Raz, Dixon, Head, Dupuis, & Acker, 1998; Raz & Rodrigue, 2006; Raz, Williamson, Gunning-Dixon, Head, & Acker, 2000). It is possible though that the structure-cognition relationship in older adults could be mediated by other factors, such as, dopamine receptors; that is, age-related decrements in dopamine receptors could contribute to decline in executive control. Post-mortem studies and positron tomography studies of living brains have revealed age-related reductions in the numbers of dopamine D1 and D2 receptors in the frontal cortex, caudate, and putamen (de Keyser, de Backer, Vauquelin, & Ebinger, 1990; Wong et al., 1984), regions of brain where we see greater age-related volumetric decline(Raz et al., 2005). Additionally, age-related decreases in the concentration of dopamine receptors is correlated with various cognitive tasks, such as, Stroop task performance (Volkow et al., 1998), episodic memory and perceptual speed (Bäckman et al., 2000).
A separate group of studies assessed regional brain volume characteristics of groups of subjects who have undergone longitudinal assessments. Participants were subdivided into groups indicative of stable performance or decline in performance, and regional brain volumes were compared. Persson et al. (2006) found significantly smaller left and right hippocampal (HC) volume in the declining group compared to the stable group. Similarly, Tisserand et al. (2004) found that decliners had significantly less grey matter (GM) volume than cognitively stable older adults in the frontal pole, inferior frontal, dorsolateral PFC, hippocampus, inferior temporal gyrus, and posterior parietal cortex. However since volumetric measures were taken after the last time point of the longitudinal assessment, the directional relationship between cognition and structure was not resolvable in this experiment.
Regarding the association between brain structure and skill acquisition in older adults, we know even less. Typically, measures of skill acquisition are accuracy and time taken to perform the task, with the general idea being that the learning process moves from early stage of error-prone and slow conscious processing to a later stage of relatively error – free and fast unconscious processing. A number of theories have been advanced regarding skill acquisition (Anderson, 1982; Delaney, Reder, Staszewski, & Ritter, 1998; Logan, 1988; Rickard, 1997) that have met with success in addressing the learning mechanisms of acquiring new skills. This has been reflected in the studies on brain volume and learning of simple skills in older adults (Raz et al., 2000; Raz & Rodrigue, 2006; Kennedy & Raz, 2005). Larger lateral prefrontal cortex has been associated with better performance in a mirror drawing task and this association was strengthened at later stages of training (Kennedy & Raz, 2005). Volume of the cerebellum was a significant predictor of the acquisition of motor skill in the pursuit rotor task (Raz et al., 2000). A challenge to learning theories has been complex skill acquisition with some frameworks suitable for such learning; for example, Anderson (1982) explained that stages of skill acquisition move from an early stage of declarative knowledge to a later stage of procedural knowledge. Regarding studies on volumetric measures of brain regions predicting complex skill acquisition, there are but a few. Acquisition of executive control skills, measured by the Wisconsin Card Sorting Test (WCST) and Tower of Hanoi (ToH) puzzle, were associated with lateral prefrontal grey matter volume in the early stage of learning; no such association was found at the later stages of skill acquisition (Head, Raz, Gunning-Dixon, Williamson, & Acker, 2002).
Hence, little is known about association of brain structure and acquisition of complex skills in older adults, particularly skills that may not just tap motor abilities but also executive control functions - functions that have been shown to decline rapidly with aging. Moreover, the training time for acquisition of executive control skills by Head et al. (2002) lasted for just a few minutes (two blocks, each with 4 trials, of ToH separated by 45-min interval). Can regional brain volumes that have been observed to decline with age and are related to memory and executive control functions predict capacity for complex skill acquisition when the training lasts for many hours or even days?
1.1. The present study
In the present study we explore the structure-function relationship of the aging human brain by training older adults for more than 20 hours in a complex real-time strategy videogame called Rise of Nations®: Gold Edition developed by Big Huge Games and published by Microsoft Game Studios in 2004. Rise of Nations (RON) is a real-time strategy game that combines both speed of real-time gaming and complexity of turn-based strategy games. In RON, one has to build new cities, improve city infrastructure and expand your national border. An important aspect of this game is that it allows for multiple ways to achieve victory. The player may either win the game by using military strategies of conquering 70 % percent of the world map, or destroying all other civilizations, or by opting for non-military and quasi-military strategies, which involve building a certain number of wonders. In this game, the player has to continually assess his or her available resources, plan and expend those resources, monitor expanding territories and multiple cities, and introduce methods to generate revenues and improve technology.
Training on this videogame has shown transfer to task-switching, working memory, reasoning and mental rotation (Basak, Boot, Voss, & Kramer, 2008), so it is conceivable that learning such a complex videogame, where one is not only learning how to use cognitive strategies but also to employ them as quickly and as efficiently as possible, may be related to the volume of several cortical and subcortical areas. Within the framework of skill acquisition described above, we hypothesize that given such a complex videogame and just 23.5 hours of training, we expect the participants to be in the early stages of learning at the conclusion of the training period. This can be verified with lack of reaching asymptotic performance as indicated by the function best fitting the learning data. RON training encourages the flexible use of strategies and integrating different cognitive abilities; such integration and flexibility might generalize more effectively to other task situations than training paradigms that train specific cognitive abilities (such as, memory, reasoning, or attention; Ball et al., 2002) or do not involve strategic interventions of flexibly shifting task priorities (Boot et al., 2010), possibly contributing to the potential for strategy-based real time video games to improve performance on untrained tasks (Basak et al., 2008). Association cortices are involved in flexibility and integration. They include frontal areas that subserve executive functions and temporoparietal areas that support visuo-spatial processing. It is possible that individual differences in the circuitry of the association cortices are related to videogame learning. Thus, we hypothesize that the volume of brain regions associated with executive control and visuo-spatial processing (e.g., mental rotation) may predict skill acquisition in the real-time strategy game, RON. That is, volumes of lateral frontal cortex and anterior cingulate cortex are expected to predict improvements in game performance, because these regions are associated with engagement of cognitive control (Alexander, Stuss, Picton, Shallice, & Gillingham, 2007; Kerns et al., 2004). Lateral frontal cortex, has previously been found to be associated with skill learning (Kennedy & Raz, 2005). Additionally, in a 2-day rule learning study (Fincham & Anderson, 2006), both left pre frontal cortex and anterior cingulate cortex showed changes in functional activity post-training, which the authors interpret as greater ease of retrieval and increase of attentional control.
Because training on this complex video game improved performance on a mental rotation task, lateral parietal regions might also be predictive of training-related improvements (Harris et al., 2000). In addition to association cortices, learning to play the game entails the increased use of fine-motor skills, such as mouse and computer keys. Thus, we expect volumes of brain regions associated with motor learning, such as cerebellum, premotor (Brodmann area 6) and primary motor (Brodmann area 4) areas (Chouinard & Paus, 2006), may also predict improvements in learning the real-time strategy video game. Volume of basal ganglia could also be predictive of learning of RON, because in a recent study we found that basal ganglia volumes and learning of a different videogame are associated, although this association was sensitive to training strategy (Erickson et al., 2010).
2. Methods
2.1. Participants
Twenty older adults (mean age = 70.1, SD = 4.81; 62 to 75 years of age; mean years of education = 15.55, SD = 3.44; 12 to 22 years of education; 5 males and 15 females) participated in the study. All participants were right-handed, Caucasians, demonstrated normal visual acuity (20/30 corrected) and normal color vision as assessed with the Ishihara color test (1989), did not use psychotic drugs, and were screened for dementia by the modified Mini-Mental Status Examination, M = 55.75, SD = 1.77 (Stern, Sano, Paulsen, & Mayeux, 1987). The self-rated health of the participants was above average on a 5-point Likert scale, M = 3.75, SD = .5, where 5 indicates excellent health. Potential participants were contacted through flyers posted in campus buildings and local businesses, study notices published in local newspapers, and through advertisements posted to online bulletin boards. People responding to these flyers and advertisements completed a survey of their video game habits. All participants selected were novices; they reported playing less than 1 hour per week of computer-based games, with no participant having familiarity with any type of strategy-based videogames, including RON.
2.2. Apparatus
Two Pentium 4 based PCs were used for the game training. These computers were attached to 21-inch monitors, and for all gaming sessions, seating was adjusted so that participants were approximately 57 cm from the monitor.
2.3. Structural MRI Protocol
For all participants, high resolution (1mm × 1 mm × 1 mm) T1-weighted brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 256 contiguous axial slices, collected in ascending fashion parallel to the anterior and posterior commissures, echo time (TE)=1.75 ms, repetition time (TR)=1790 ms, and flip angle=8°. All images were collected on a 3T head-only Siemens Allegra MRI scanner before the participants started the videogame training.
2.4. Videogame Training Schedule
All twenty participants, individually tested, completed fifteen 1.5-hour videogame learning sessions over a period of five (or six) weeks, resulting in a total training time of 23.5 hours. The participants started learning the game by completing a tutorial in their first session. At the end of each session, game progress was saved and participants began the next session at that point. If within a session, the participant completed the game they were playing, the results of the game were saved and the next game was started. All games were set to the easiest level of difficulty, and the game settings and the sequencing of the games were constant across all participants. Game performance was recorded for each participant. A scenario was completed when participants reached one of the game criteria for winning. Criteria included controlling 70% of the land, destroying all other civilizations, or building a majority of the “Wonders of the World”. At the end of each game, Rise of Nations provides a measure of the time taken to complete the game and whether the game was won or lost. For more information about the methodology and the analyses of the game performance, see Basak et al. (2008). Transfer tasks, such as task-switching, memory updating, change detection, Raven's Advanced Progressive Matrices, operation span, stopping task, mental rotation, functional field of view, enumeration and attentional blink, were administered both before and after training (see Basak et al., 2008 for details on these paradigms). An experimenter, who had experience with RON, was present outside the training room during all training sessions to address any queries of the participant.
3. Results
3.1. Game Performance
Before analyzing whether regional brain volumes predicted improvements in game performance, we first examined whether training led to an improvement on the game. Performance on the first game was compared with the last game played. The overall time spent on the game was significantly reduced [t (19) = 2.81, p = 0.01, 250.41 s (SD = 211.09) and 111.86 s (SD = 58.26) pre-and post-training, respectively – a 55% improvement in performance]. Although the games were finished faster with training, it was not at the expense of victory. Victory was measured as whether a game played was won or lost, with the number of wins indicating victory. Victory remained unchanged across the first and last game [t (19) = 1, p = 0.33, 1 (SD = 0) and .95 (SD = 0.22) victories pre-and post-training, respectively]. This demonstrates a significant change in efficiency of gaming, that is, winning in less time. Although the game outputs other measures of performance such as number of wonders built, resources spent on army, economy, and research, change in these measures covary with the change in the amount of time spent on the game (.57 < r < .95). Therefore, we choose to use overall time spent on each game as our measure of learning. Because the overall game time for first and last session were not correlated [r(19) = −.02], meaning that initial level of performance did not predict performance at the last game, we did not covary out initial game time or use a proportional score with initial game time as a denominator. Moreover, a greater decrease in focus-switch cost in the N-back task was related to greater decrease in game time [r(16) = 0.42; p = 0.079], although this correlation was not significant.
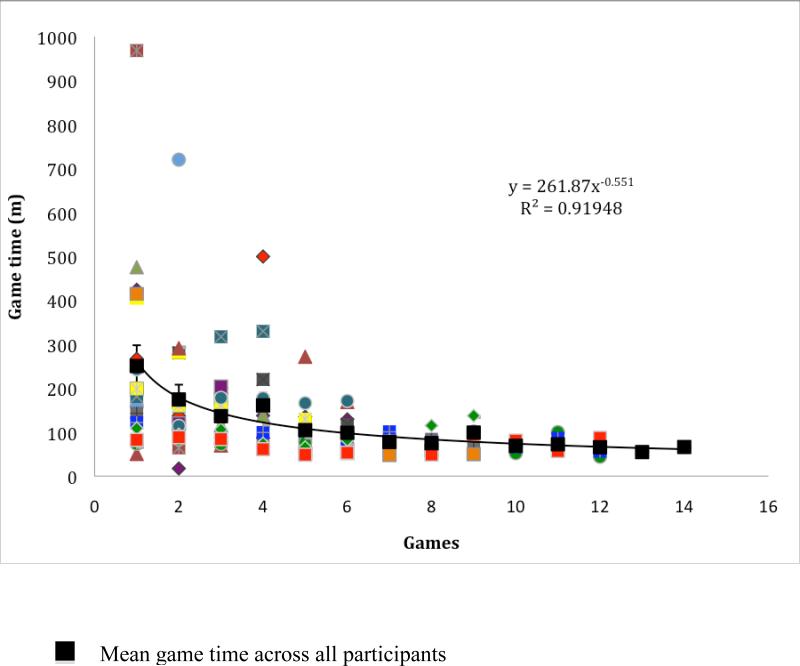
Differences in first and last overall game time discounts data from intermediary games; overall game time for all individuals as a function of games played in the 23.5 hours of training, and the power function fit to the averaged data are depicted in Figure 1. The power function relates the time (y) to perform the game to the amount of practice or number of games (x) as y = a + bx−c, where a is the asymptote, b is the amount of time that decreases with x number of games played, and c is the exponent that specifies the rate of speed up with games played. We fitted exponential function as well, because there exists controversy regarding learning functions, but the power function was a better fit to the data evidenced by a larger r2. Figure 1 shows that, as predicted, on average the participants do not reach an asymptotic performance, although the rate of speed up of learning (−.55), which is characteristically strongly negatively accelerated, improves greatly with practice and is comparable to speed up from previous research (−.55 from Anderson, Fincham & Douglass, 1999).
Figure 1.
Overall game time of all individuals as a function of games played and a power function fit to the averaged data. The individual participant's game time data across the number of games played are represented by various shaded symbols. The averaged data across all participants are represented by black squares, The error bars depict the standard error.
One measure of the game whose change did not covary with change in overall game time is player's speed of moving the mouse and key press (r = .15). Also, change in the task switch cost (post- versus pre-training) was significantly correlated with change in player's speed [r(15) = − 0.68; p = 0.003]; that is, a greater decrease in the task switch cost was associated a greater increase in the game speed. Additionally, following training, the players significantly increased the speed with which game was played [t(19) = 4.28, p < .01; 9.85 and 14.25 pre-and post-training, respectively]. Therefore, we also looked at increase in the speed of the game as an independent variable. We hypothesize that the brain regions associated with increase in the speed measure would be predominantly cerebellum, motor and premotor areas.
3.2. Structural Brain Analysis
We used an optimized voxel-based morphometry (VBM) technique (Ashburner & Friston, 2000; Good et al., 2001) to assess the extent to which regional brain volume predicts improvements in complex skill acquisition as measured by difference in overall time spent on the videogame (first game compared to the last game) within older adults. VBM provides a means to estimate tissue atrophy in a voxel-wise fashion throughout the brain with reasonably high spatial resolution. This allows regionally specific conclusions about the variables of interest on changes in brain matter. The optimized VBM method improves upon earlier VBM procedures in that a study-specific template, or the average brain of study participants, is generated and used as the reference image for whole head and ROI registrations; this optimized VBM technique reduces registration error that could bias statistical outcomes (Ashburner & Friston, 2000; Good et al., 2001). For all VBM analyses we used the FMRIB Software Library (FSL 3.2) package (http://www.fmrib.ox.ac.uk/fsl/; Smith et al., 2004). For extensive documentation of the VBM procedure used here, see (Erickson et al., 2007; Kennedy et al., 2009).
In short, a two-level approach was taken where in the first-level initial processing of images was conducted and in the second-level, similar steps were re-run with a study-specific template. First-level preprocessing involved a robust brain extraction technique to extract the brain from the skull (BET; Smith, 2002, followed by visual inspection and manual cleaning of any residual non-brain matter. This was followed by segmentation of the skull-stripped images into grey, white and CSF probability density maps via a semiautomated algorithm (FAST; Zhang, Brady, & Smith, 2001), and registration of segmented maps into stereotaxic MNI space using an affine registration with 12-degrees of freedom (FLIRT; Jenkinson, Bannistr, Brady, & Smith, 2002). The study-specific template was created by averaging the registered images from our sample and spatially smoothing the image with a 4-mm HWHM 3-D Gaussian kernel.
Second-level preprocessing involved re-registering the skull-stripped brains to the study-specific template using an affine registration algorithm. Next, we segmented the original images in native space using study-specific grey, white and CSF probability maps obtained from the first level analysis as priors for the segmentation. We then registered the partial volume maps to the study-specific template via a 12- parameter affine transform and spatially smoothed images with a 4-mm HWHM 3-D Gaussian kernel. The images were then modulated based on the Jacobian determinant of the warp field and interrogated for systematic variation in relation to complex skill acquisition using the General Linear Model (see below). Statistical maps associated with the dependent variables were thresholded at Z>3.1 uncorrected for multiple comparisons.
We performed a multiple regression analysis with change in game time from the first to last game as the independent variable to test for association with regional grey matter brain volume. The resulting statistical maps represented where regional grey matter brain volume was associated with greater change in game time from the first to last game (see Table 1). Age and intracranial volume were entered into the regression model so that the effect of volumetric measures derived from structural MRI on cognitive performance was independent of any covariation between age (or intracranial volume) and each game improvement. We performed two additional sets of regression analyses. First, rate of speed up in power function of each individual's game time, as a function of number of games played (Table 1) was used to predict the volumes of brain regions we found in the previous set of analysis, to ascertain that change in game time is an appropriate measure of the complex skill. Second, change in speed measure from the first to last game (Table 2) was an independent variable to test for association with regional grey matter brain volume.
Table 1.
Location and coordinates of voxels that showed the strongest association with improvements in game time for grey matter and mean and standard deviation partial volume estimates (PVEs) from those peak voxels and relation to improvement in game time as well as learning rate assessed from individually fitted power functions, with and without controlling for the effects of age and intracranial volume (ICV).
| Peak location (Brodmann areas) |
Number of voxels |
MNI x ,y ,z | PVE mean±S.D. |
PVE max |
PVE rΔ Game time |
PVE rΔ Game time (age and ICV partialed out) |
PVE rΔ learning rate |
PVE rΔ learning rate (age and ICV partialed out) |
|---|---|---|---|---|---|---|---|---|
| L Medial Frontal Gyrus (6) | 46 | −20, 4, 52 | .33 (.074) | .46 | 0.72 | 0.75 | −.65 | −.68 |
| L Dorsolateral PFC (9) | 36 | −24, 30, 38 | .38 (.062) | .55 | 0.67 | 0.69 | −.63 | −.64 |
| R ACC (24) | 123 | 8, 8, 30 | .25 (.072) | .47 | 0.61 | 0.64 | −.47 | −.47 |
| L Post Central Gyrus (3) | 19 | −22, −34, 54 | .18 (.047) | .24 | 0.68 | 0.71 | −.64 | −.62 |
| Bilateral Cerebellum | 106 | −16, −42, −48 | .29 (.133) | .61 | 0.58 | 0.67 | −.51 | −.65 |
L=Left, R=Right. PFC = Prefrontal Cortex, ACC=Anterior Cingulate Cortex. Bold typeface indicates p≤.05.
Table 2.
Location and coordinates of voxels that showed the strongest association with improvements in speed measures for grey matter and mean and standard deviation partial volume estimates (PVEs) from those peak voxels and relation to improvement in speed, with and without controlling for the effects of age and ICV.
| Peak location | Number of voxels | MNI x ,y ,z | PVE mean±S.D. | PVE max | PVE rΔ Game time | PVE rΔ Game time (age and ICV partialed out) |
|---|---|---|---|---|---|---|
| Bilateral Pre Central Gyrus | 57 | 36, −10, 50 | .30 (.058) | .41 | 0.59 | 0.45 |
| R Premotor cortex | 23 | 40, 0, 42 | .24 (.062) | .35 | 0.54 | 0.53 |
R=Right. Bold typeface indicates p ≤ .05
3.2.1. Change in game time
Because it is important to consider how the regional brain volumes are likely to vary as a function of whole brain volume, we calculated two measures, grey matter volume and intracranial volume, to assess whether the global brain volume were different across the two sexes or covaried with age or education. First, we analyzed whether the total grey matter volume (in voxels), measured by the product of the mean grey matter partial volume estimate for the grey matter pve map with the total number of voxels classified as grey matter, differed significantly between males and females as well as correlated with age and education. Although grey matter volume did not significantly differ between males and females, t(18) = .385, p=.71, and did not significantly correlate with education, r = .09, p=.70, it was correlated with age, r = −.57, p<.01. That is, decreases in grey matter volume were related with increase in age. Second, we calculated the total intracranial volume by adding up the volumes of grey matter, white matter and cerebrospinal fluid (CSF); it did not vary significantly between males and females, t(18) = 1.88, p=.08, and it did not correlate with either age (r = .09, p = .71) or education (r = .25, p =.30). Given that age was significantly related to total grey matter volume, and intracranial volume (ICV) could affect the relationships between the regional brain volume and measures of skill acquisition, we included age and ICV in our subsequent analyses.
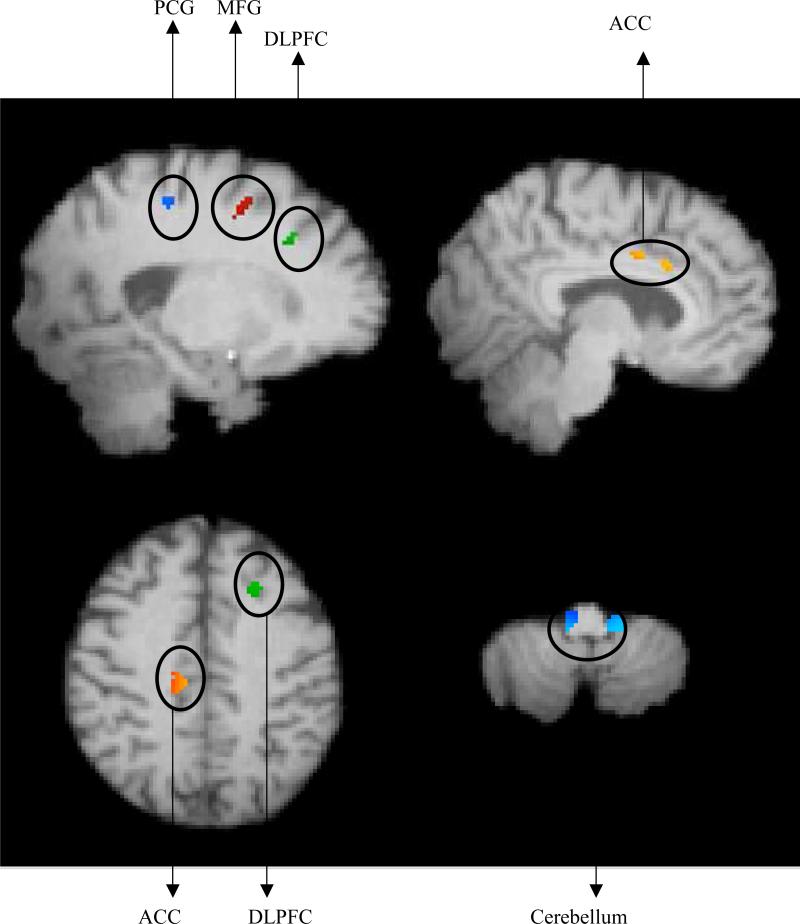
Testing for a significant association between improvement in game time and grey matter resulted in five regions (see Figure 2); these associations remained even after accounting for effects of age and intracranial volume. In the left hemisphere, three clusters of cortical grey matter were related to improvements in game performance, namely, medial frontal gyrus (MFG), post central gyrus (PCG) and dorsolateral prefrontal cortex (DLPFC). In the right hemisphere, volume of the ventral anterior cingulate cortex (ACC) was positively correlated with improvement in game time. Bilateral cerebellar volume was also positively correlated with change in game time. Correlations as well as partial correlations, after accounting for age and intracranial volume, between change in game time and the regional brain volumes are reported in Table 1. Neither age nor ICV was a significant predictor of improvements in the game time (all p > .21).
Figure 2.
Top Left: A significant relationship with improvement in game time was found in left MFG (secondary motor area), left PCG and left DLPFC [MNI coordinates: x = −20, y = 4, z = 52]. Top Right: A significant relationship with improvement in game time was found in Right ACC [MNI coordinates: x = 8, y = 8 ,z = 30]. Bottom Left: Another representation of left DLPFC and Right ACC. Bottom Right: A significant relationship with improvement in game time was found in bilateral cerebellum [MNI coordinates: x = −16 , y = −60 ,z = −48].
When all five regions were entered in a step-wise regression model to predict changes in game time, after accounting for effects of age and ICV, the shared variance amongst the five regions is quite large (adjusted r-square = 0.62).
Neither the total grey matter volume (r = −0.26, p = 0.38) nor the ICV (r = −0.26, p = 0.37) was significantly correlated with the change in game time from the first to the last game, even after controlling for effects of age, education and sex.
3.2.2. Rate of speed up of learning with practice
Learning rate, or rate of speed up in the power function (parameter c) of each individual's game time as a function of number of games played (Table 1) was first computed. Volumes of all of the five regions mentioned above were significantly correlated with the learning rate; faster decelerations in skill acquisition, which can be interpreted as faster learning rate, were associated with greater increase in the brain volume in these five regions of interest. These correlations were significant even after accounting for age and ICV effects. Similar to results from changes in overall game time, neither age nor ICV was a significant predictor of learning rate (all p > .14).
3.2.3. Change in speed measure of the game
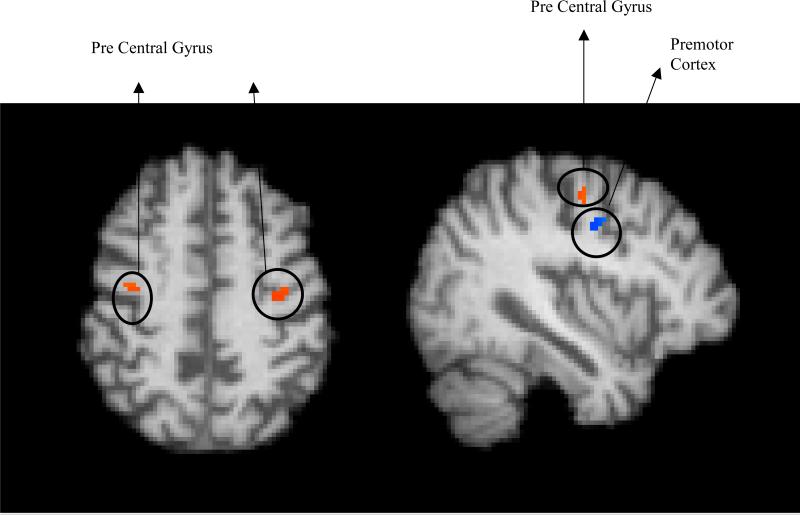
Testing for a significant association between change in speed measure of the game and grey matter, even after controlling for age and ICV, resulted in two regions - bilateral precentral gyrus or motor area and right premotor area (see Table 2, Figure 3). Step-wise regression, after accounting for age and ICV, resulted that the improvement in the speed score predicted the volumes of the bilateral motor area (β = .59, p = .01) and the right premotor volume (β = .54, p = 0.02). Neither age nor ICV was a significant predictor of change in speed measure (all p > .26).
Figure 3.
Left: A significant relationship with increase in game speed was found in left and right Pre Central Gyrus [MNI coordinates: x = 38, y = −8, z = 50]. Right: A significant relationship with increase in game speed was found in left premotor cortex [MNI coordinates: x = 38, y = −8, z = 50].
4. Discussion
In the current study we explored whether regional grey matter volume could predict the rate of acquisition of a complex strategy-based videogame called Rise of Nations. We hypothesized that since learning to play Rise of Nations involves flexible allocation and integration of cognitive skills, individual differences in circuitry of association cortices may be related to the rate of learning. Moreover, training on Rise of Nations transfers to executive control functions, particularly task switching and memory updating (as assessed by focus switch cost in N-back task), as well as problem solving assessed by Raven's Advanced Progressive Matrices, change detection task and mental rotation (Basak et al., 2008). Therefore, the brain regions underlying higher cognitive functions may predict the learning rate of this complex videogame. We found that grey matter volumes of five regions were correlated with complex skill acquisition, as measured by improvements in time spent to successfully play the videogame. The strongest correlation of change in game time was with the medial prefrontal cortex (Brodmann area 6). Brodmann area 6, which consists of supplementary motor area as well as premotor areas associated with use of the right hand, is found to be active during motor control in cognitive operations typically associated with motor functions (Chouinard, P. A. & Paus, T., 2006). It is also associated with executive control tasks involving response inhibition and response conflict (Alexander et al., 2007; Derfuss, Brass, Neumann, & von Cramon, 2005; Picton et al., 2007). Additionally, as hypothesized, cerebellar volume also predicted improvements in game time; this result is in line with that of Raz et al. (2000) where cerebellum was a significant predictor of the acquisition of simple motor skill. This suggests that cerebellar volume is a predictor of not merely simple motor tasks, but also of complex skill acquisition that involves simple motor components, such as use of mouse.
The second strongest correlation of improvement in game performance was with the grey matter volume of PCG, or somatosensory area. Although speculative, we believe that this region may play a role in a feedback mechanism between the prefrontal and motor regions. Feedback between the somatosensory cortex and motor cortex is critical to control the location and movements of the hands and fingers on a computer mouse to play the game. Future research investigating this hypothesis with techniques that can measure white matter integrity between somatosensory regions and prefrontal regions is warranted.
Volume of right ventral ACC, or Brodmann area 24, was also positively correlated with the acquisition of skill on the complex real-time strategy game. ACC is connected with prefrontal and parietal cortices as well as motor system (Posner & DiGirolamo, 1998) and has been typically associated with monitoring conflict, detecting errors and self-regulation (Bush, Luu, & Posner, 2000; Carter et al., 1998; Kerns et al., 2004; Posner, Rothbart, Sheese, & Tang, 2007). It is an important region that is activated during tasks of cognitive control (for e.g., Flanker task, Stroop task) and hence, could predict improvement in a game that enhances executive control processes in older adults (Basak et al., 2008).
Unlike the ACC which is invoked to detect conflict, the DLPFC is engaged in the resolution of conflict through attentional control and working memory operations (Carter & van Veen, 2007). We found that this region was correlated with improvements in videogame performance over time. DLPFC is also associated with higher cognitive functions, such as, executive control functions (planning, dual tasking) and working memory (Erickson et al., 2007). Not only has it been found to be associated with motor skill acquisition (Kennedy & Raz, 2005), but it has also been found to be active for most complex cognitive tasks; therefore, our results support the conclusions from prior studies and extend them by arguing that the volume of the DLPFC is also a predictor of complex skill acquisition.
One could argue that pre and post difference in game time is not as sophisticated measure of skill acquisition as accounting for data from all sessions. To address this issue, we fitted power functions to each individual's game time across number of games played, and calculated the rate of speed up of learning from the fitted function. This learning rate was also significantly correlated with the above mentioned brain volumes, and the regression analyses showed similar patterns. Thus, we find converging evidence from the two measures used to assess strategy-based video game skill acquisition.
Improvements in the speed measure, a game output that assesses the speed of mouse clicks, predicted increases in motor and premotor volumes, but it did not predict any other fronto-parietal brain regions that are typically associated with higher level cognition, such as ACC, DLPFC or PCG. Thus, improvements in game time reflect on increases in regional grey matter volumes that subserve cognitive control, in addition to the areas that subserve motor control.
To test whether these effects were influenced by age- and ICV-related differences, we reran the analyses with age and ICV as additional covariates. All results described above remained unchanged even after these two variables were included in the model. These results suggest that larger pre-existing volumes of the motor, supplementary motor areas and cerebellum, along with dorsolateral prefrontal cortex, somatosensory area and anterior cingulate cortex are associated with faster overall rates of strategy-based video game skill acquisition.
Thus, the results of our study suggest that regional differences in brain volume among older adults can be used to not only predict performance and the amount of learning and level of mastery achieved on laboratory tasks designed to examine specific perceptual, cognitive and motor function (Head et al., 2002; Kennedy & Raz, 2005; Persson et al., 2006; Raz, 2000; Raz et al., 2000; Raz et al., 1998; Tisserand et al., 2004) but can also be used to predict learning of complex tasks that require the development of a variety of different cognitive and motor skills. Given these findings it will be important in future studies to examine both the impact of lifestyle choices (e.g. diet, exercise, social and intellectual engagement) on brain volume, and the association of these choices with learning of new complex skills as well as the maintenance of such skills over time. This is a preliminary investigation towards understanding of association between brain volumes and complex skill acquisition, and thus suffers from some limitations, such as sample size, and unequal sex distributions. Future research should also extend the training period, because 23.4 hours was not sufficient for participants to reach asymptotic performance in such a complex game. Given limitations of VBM, it should be used as an exploratory tool (Kennedy, et al., 2009); in future, we should take into account a priori regions of interest to understand structure-complex skill learning relationship. Yet these are all interesting future extensions of the current novel research. One such extension could examine whether changes in lifestyle enhance brain volume in particular regions and also predict more rapid acquisition of skills that engage such brain regions. Another could explore age-related differences in this brain structure – complex skill learning relationship in the current areas of brain.
Grey matter volume predicts real-time strategy-based video game learning.
Power functions are fitted to game time across numbers of games played.
The associated regions subserve executive control and motor functions.
They were medial frontal gyrus, postcentral gyrus, DLPFC, ACC and cerebellum.
Acknowledgments
This research was supported by grants from the National Institute on Aging (RO1 AG25667 and RO1 AG25032) to AFK and Beckman Institute Post-doctoral Fellowship to CB. All appropriate university guidelines were followed in the treatment of human participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement. There are no potential conflicts of interest.
References
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S. Regional frontal injuries cause distinct impairments in cognitive control. Neurology. 2007;68:1515–1523. doi: 10.1212/01.wnl.0000261482.99569.fb. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Acquisition of cognitive skill. Psychological Review. 1982;89:369–406. [Google Scholar]
- Anderson JR, Fincham JM, Douglass S. Practice and retention: A unifying analysis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:1120–1136. doi: 10.1037//0278-7393.25.5.1120. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Robins Wahlin T-B, Whalin A, Halldin C, et al. Age-related cognitive deficits mediated by changes in the straital dopamine system. American Journal of Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized control trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy videogame attenuate cognitive decline in older adults? Psychology and Aging: Special Section on Plasticity. 2008;23:765–777. doi: 10.1037/a0013494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory spans: A meta-analyses. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:223–233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Boot WR, Basak C, Erickson KI, Neider M, Simons DJ, Fabiani M, Gratton G, Voss MW, Prackash R, Lee H, Low KA, Kramer AF. Transfer of skill engendered by complex task training under conditions of variable priority. Acta Psychologica. 2010;135:349–357. doi: 10.1016/j.actpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulated cortex. Trends in Cognitive Neuroscience. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick M, Noll D, Cohen JD. Anterior cingulate cortex, error detection and on-line monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulated cortex and conflict detection: an update of theory and data. Cognitive, Affective & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The Primary Motor and Premotor Areas of the Human Cerebral Cortex. Neuroscientist. 2006;12(2):143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12(2):143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- de Keyser MJ, de Backer J-P, Vauquelin G, Ebinger G. The effect of aging on the D1 dopamine receptors in human frontal cortex. Brain Research. 1990;528:309–310. doi: 10.1016/0006-8993(90)91672-4. [DOI] [PubMed] [Google Scholar]
- Delaney PF, Reder LM, Staszewski JJ, Ritter FE. The strategy-specific nature of improvement: The power law applies by strategy within task. Psychological Science. 1998;9:1–7. [Google Scholar]
- Derfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Human Brain Mapping. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Boot WR, Basak C, Neider M, Prakash RS, Voss MW, et al. Is bigger better? Striatum volume predicts the level of video game skill acquisition. Cerebral Cortex. 2010;20:2522–2530. doi: 10.1093/cercor/bhp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Colcombe S, Elavsky S, McAuley E, Korol D, Scalf P, et al. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiology of Aging. 2007;28(2):179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Anderson JR. Distinct roles of the anterior cingulate and prefrontal cortex in the acquisition and performance of a cognitive skill. Proceedings of the National Academy of Sciences USA. 2006;103:12941–12946. doi: 10.1073/pnas.0605493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Age-based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: Direct evidence for ability dedifferentiation in old age. Psychology & Aging. 2003;18:696–713. doi: 10.1037/0882-7974.18.4.696. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Harris IM, Egan GF, Sonkkila C, Tochon-Danguy HJ, Paxinos G, Watson JDG. Selective right parietal lobe activation during mental rotation: A parametric PET study. Brain. 2000;123(1):65–73. doi: 10.1093/brain/123.1.65. [DOI] [PubMed] [Google Scholar]
- Head DP, Raz N, Gunning-Dixon FM, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: the role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannistr P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Moss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiology of Aging. 2009;20(10):1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Age, sex, and regional brain volumes predict perceptual-motor skill acquisition. Cortex. 2005;41:560–569. doi: 10.1016/s0010-9452(08)70196-5. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, McDonald III AW, Cho RY, Stenger A, Carter CS. Anterior cingulated conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Anderson JR. Does learning a complex task have to be complex? A study in learning decomposition. Cognitive Psychology. 2001;42:267–316. doi: 10.1006/cogp.2000.0747. [DOI] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychological Review. 1988;95:492–527. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuo- spatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson L-G, Ingvar M, et al. Structure–Function Correlates of Cognitive Decline in Aging. Cerebral Cortex. 2006;16(7):907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17(4):826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. MIT Press; Cambridge, MA: 1998. pp. 401–423. [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulated gyrus and the mechanism of self-regulation. Cognitive, Affective & Behavioral Neuroscience. 2007;7(4):391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Lawrence Erlbaum Associations; Mahwah, NJ: 2000. pp. 1–90. [Google Scholar]
- Raz N, Dixon FM, Head DP, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural MRI. Neuropsychology. 1998;12:95–106. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microscopy Research and Technique. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rickard TC. Bending the power law: A CMPL theory of strategy shifts and the automatization of cognitive skills. Journal of Experimental Psychology: General. 1997;126:288–311. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Intellectual development in adulthood: The Seattle Longitudinal Study. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- Sliwinski M, Hall C. Constraints on general slowing: A meta-analysis using hierarchical linear models with random coefficients. Psychology and Aging. 1998;13:164–175. doi: 10.1037//0882-7974.13.1.164. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast automated robust brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in structural and functional MR image analysis and implementations as FSL. Neuroimage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulsen J, Mayeux R. Modified mini-mental state examination: validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- Tisserand DJ, van Boxtel MPJ, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in grey matter density associated with age and cognitive change over time. Cerebral Cortex. 2004;2004:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J, Bopp KL, Basak C. Aging and Varieties of cognitive control: A review of meta-analyses on resistance to interference, coordination and task switching, and an experimental exploration of age-sensitivity in a newly identifies process of focus switching. In: Engle GSRW, Von hecker U, McIntosh DN, editors. Cognitive limits in aging and psychopathology: Attention, working memory, and executive functions. Cambridge University Press; New York: 2005. pp. 160–189. [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Pappas N, Logan J, MacGregor R, et al. Association between decline of brain dopamine activity with age and cognitive and motor impairment of healthy individuals. American Journal of Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wong D, Wagner H, Jr, Dannals R, Links J, Frost J, Ravert H, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–1396. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]