Abstract
Pyroglutamate helix B surface peptide (pHBSP) is an 11 amino acid peptide, designed to interact with a novel cell surface receptor, composed of the classical erythropoietin (EPO) receptor disulfide linked to the beta common receptor. pHBSP has the cytoprotective effects of EPO without stimulating erythropoiesis. Effects on early cerebral hemodynamics and neurological outcome at 2 weeks post-injury were compared in a rat model of mild cortical impact injury (3m/sec, 2.5 mm deformation) followed by 50 min of hemorrhagic hypotension (MAP 40 mm Hg for 50 min). Rats were randomly assigned to receive 5000 U/kg of EPO, 30 μg/kg of pHBSP, or an inactive substance every 12 h for 3 days, starting at the end of resuscitation from the hemorrhagic hypotension, which was 110 min post-injury. Both treatments reduced contusion volume at 2 weeks post-injury, from 20.8±2.8 mm3 in the control groups to 7.7±2.0 mm3 in the EPO-treated group and 5.9±1.5 mm3 in the pHBSP-treated group (p=0.001). Both agents improved recovery of cerebral blood flow in the injured brain following resuscitation, and resulted in more rapid recovery of performance on beam balancing and beam walking tests. These studies suggest that pHBSP has neuroprotective effects similar to EPO in this model of combined brain injury and hypotension. pHBSP may be more useful in the clinical situation because there is less risk of thrombotic adverse effects.
Key words: : adult brain injury, head trauma, ischemia, secondary insult, traumatic brain injury
Introduction
Erythropoietin (EPO) is a potentially attractive neuroprotective agent following traumatic brain injury (TBI). In experimental models, EPO has improved outcome after TBI (Brines et al., 2000; Cherian et al., 2007), subarachnoid hemorrhage (Grasso, 2001), stroke (Wang et al., 2004), global ischemia (Catania et al., 2002), and other central nervous system disorders. EPO has been found to improve outcome from spinal cord injury and ischemia in some experimental studies but not in others (Celik et al., 2002; Gorio et al., 2002; Mann et al., 2008; Pinzon et al. 2008).
In experimental TBI studies, EPO has been shown to have neuroprotective effects when given early post-injury, and to have effects that enhance neurological recovery even when given at later times after injury (Lu et al., 2005). The early neuroprotective mechanisms are probably complex, involving anti-inflammatory, anti-apoptotic, and vascular actions (Siren et al., 2001; Villa et al., 2003). The time window for EPO-induced neuroprotection after experimental TBI is at least 6 h post-injury (Brines et al., 2000; Cherian et al., 2007). The late mechanisms of EPO that may enhance neurological recovery may include both neurogenesis and angiogenesis (Lu et al., 2005; Marti et al., 2000; Wang et al., 2004). The time window for these delayed actions of EPO is not clearly defined, but doses given as late as 24 h post-injury significantly enhance neurological recovery in stroke and TBI models (Lu et al. 2005; Wang et al. 2004).
Despite these positive features observed in the laboratory, it has been difficult to translate EPO into the clinical setting for TBI and other neurological disorders, because the doses of EPO required for neuroprotection and enhancement of neurological recovery have some potentially adverse effects in patients. Almost all studies of EPO in critically ill patients and in postoperative patients have shown an increased risk of thrombosis with EPO administration (Corwin et al., 2007; Dicato et al., 2008). The most serious complications associated with EPO occurred in a recent trial, in which the patients given EPO in doses of 40,000 U/day×3 days after stroke, and had a higher rate of intracranial hemorrhage and a higher mortality rate than control patients (Ehrenreich et al., 2009). These complications may have resulted from an interaction with tissue plasminogen activator (t-PA) which was also given in 63% of the patients in the trial, in some cases outside of the therapeutic window for t-PA. Subsequent studies in experimental models have demonstrated that the combination of t-PA and EPO given at 6 h after middle cerebral artery occlusion can exacerbate t-PA-induced brain hemorrhage (Jia et al., 2010). Zechariah and associates found that the combination of EPO and t-PA immediately after reperfusion following 90 min of middle cerebral artery occlusion caused increased vascular permeability and extracellular matrix breakdown (Zechariah et al., 2010).
The dose of 40,000 U, which is approved for human use, is at the lower limit of the doses that have been effective in experimental models. Because of the risk of thrombotic events, it may not be possible to give EPO in sufficient doses to improve neurological outcome without increasing the risk of life-threatening complications. For military and pre-hospital applications, the need to refrigerate EPO is another significant limitation for translation of the therapy.
Leist and associates demonstrated that the hematopoietic and tissue protective effects of EPO can be separated (Leist et al., 2004). A novel cell surface receptor, composed of the classical EPO receptor (EPOR) disulfide linked to the beta common receptor, CD131, may mediate the non-hematopoietic effects of EPO (Brines et al., 2004). Based on these findings, an 11-amino acid synthetic peptide was developed that mimics the 3-D structure of EPO and retains EPO's neuroprotective activities, but not the hematopoietic actions that are responsible for the adverse effects of EPO. Helix B surface peptide (-HBSP) is a linear peptide containing the amino acids from the aqueous face of helix B (amino acids 58–85) of erythropoietin (QEQLERALNSS). A modification of the N-terminal Q to pyroglutamate (U) for stabilization, results in a peptide (UEQLERALNSS) that can be stored for 2 years at 4°C or up to 12 months at 25°C. Therefore, pyroglutamate helix B (pHBSP) may have significant advantages over EPO as a therapeutic agent for use in the pre-hospital setting and on the battlefield.
The purpose of this study was to evaluate the neuroprotective effects of pHBSP compared to EPO in a model of mild TBI complicated by hemorrhagic shock. In addition, the effect of pHBSP and EPO on early cerebral blood flow was studied, as one potential mechanism for neuroprotective effects.
Methods
To accomplish these goals, an acute experiment of early cerebral hemodynamics and a chronic experiment with behavioral and histological endpoints were performed. The model for the two parts of the study was a mild cortical impact injury followed by a period of hemorrhagic hypotension in rats. The experiments were approved by the Baylor Institutional Animal Care and Use Committee.
Experimental model
Long Evans rats weighing an average of 371±8 g were used for the experiments. The animals were anesthetized with isofluorane in oxygen, intubated, and ventilated to maintain normal arterial blood gases during the surgical preparation, the injury production, and acute cerebral hemodynamic monitoring. Surgical preparations included placement of a femoral or tail arterial catheter for monitoring blood pressure and withdrawing blood samples, placement of a femoral venous catheter for administering fluid and/or drugs, and a craniectomy for producing a lateral cortical impact injury and for monitoring intracranial pressure (ICP) with a Codman microsensor catheter (Johnson & Johnson, Raynham, MA) and cerebral blood flow with a Periscan PIM3 laser Doppler imager (Perimed, Stockholm, Sweden). The craniotomy was 10 mm in diameter, centered over the right parietal cortex between bregma and lambda. An impact injury of 3 m/sec, 2.5 mm deformation was induced using a pneumatic impactor device. The tip of the impactor was 8 mm in diameter and rounded. The impact was directed at an angle perpendicular to the surface of the brain.
For the “hemorrhage phase”, which lasted 50 min, the anesthesia was isofluorane in room air to mimic the conditions of injury in the field. A blood volume of 2.0 mL/100g body weight, which was usually sufficient to reduce mean arterial pressure (MAP) to ∼40 mm Hg, was withdrawn through the femoral catheter. Half of this volume was withdrawn within 5 min, then 25% was withdrawn over the next 5 min, and the final 25% was withdrawn over the next 5 min. This decelerating rate of blood loss mimics the clinical situation. If this volume of blood did not achieve the desired MAP, an additional amount in 0.5 mL over 5 min was withdrawn. The total time of this injury phase lasted 50 min (i.e., injury+hemorrhage during the first 15 min, followed by 35 min of unresuscitated hypotension).
The second phase of “pre-hospital care”, which consisted of fluid resuscitation, lasted for 30 min. During this time, lactated Ringer's solution was infused in 1 mL boluses to achieve a MAP of at least 50 mm Hg.
The third phase of “definitive hospital care”, which consisted of blood resuscitation and drug treatment, also lasted for 30 min. During this time, the anesthesia was switched to isoflurane in 100% oxygen, and the shed blood was re-infused. At the end of the blood re-infusion, at 110 min post-injury, the assigned drug treatment was administered.
In the chronic experiments, after the animal was fully resuscitated, the isofluorane was discontinued. Escape, righting, head support, corneal, pinna, paw, and tail reflexes were assessed every minute for half an hour once the rats were extubated and breathing spontaneously following termination of anesthetic (Dixon et al., 1991). When fully awake, the animals were returned to their cages and allowed free access to food and water. The animals were given butorphanol tartrate 0.05 mg subcutaneously every 12 h for analgesia, and enrofloxacin 0.05mL/kg subcutaneously every 12 h to prevent postoperative infections for 3 days post-injury.
End point assessments
MAP, ICP, and laser Doppler blood flow (LDF) were monitored throughout the experiment, at 10 min intervals until blood re-infusion and then every 30 min during the 2 h post-treatment monitoring period. At each time point, the LDF measurements were summarized for the contusion core at the impact site, for penumbra brain surrounding the impact site, and for uninjured parietal cortex in the hemisphere contralateral to the impact site. The rectal temperature and brain temperature were kept at 37–37.5°C throughout the experiment.
Tests of motor strength and coordination and tests of memory and learning were performed. These included:
1. Beam balance test. The animal was placed on a narrow wooden beam, and the duration of time that the animal could maintain balance on the beam, up to 60 sec, was recorded. Three trials per day just prior to injury and on days 1–5 post-injury were performed.
2. Beam walking test. Two days prior to the impact injury, the animals was trained to escape a bright light and loud, white noise by traversing a narrow wooden beam (2.5×100 cm) to enter a darkened goal box at the opposite end of the beam. During this training and testing, the animal was placed initially in the goal box for 30 sec, then at the entrance to the goal box at which time the noise and light were turned on and then terminated when the mouse entered the goal box. The animal was placed at one of five distances further away from the goal box until it could successfully run down the beam from the position close to the source of light and noise. Then they learned to run down the beam with four plastic pegs (4.0 cm high) placed in an alternating sequence along the beam to increase the difficulty of the task. Performance was assessed by measuring the time required for the animal to traverse the beam. The animals remained in the goal box for 30 sec between trials. Three trials just prior to injury and on days 1–5 post-injury were performed.
3. Morris Water Maze test. This test measured post-injury learning and memory for the position of a 10 cm circular clear Plexiglas platform hidden 2 cm below the surface of white opaque water in a 5 foot diameter white circular water tank. The platform was placed in the northwest position throughout the test. The animal was placed in the pool facing the wall of the tank at one of four directions: north, south, east, or west according to a different order for each day that was fixed across all animals. If the animal did not find the platform in 120 sec, the animal was placed on the platform for 30 sec. If the animal was not able to swim and keep its head out of the water, it was removed from the tank immediately. If the animal attempted to get off the platform, it was placed back on it. Different visual cues were present on each wall of the room that allowed the animal to learn the position of the platform relative to the cues. Four trials were performed with 4 min for rest between trials on post-injury days 11–15. The animal was kept warm under a heating lamp between trials. For each trial, performance was assessed by measuring the time required for the animal to find the platform, and by the percent of the time spent swimming in the quadrant containing the platform.
On day 15 after the last behavioral test was performed, the animals were deeply anesthetized and perfused via the right ventricle with 0.9% saline, followed by 4% paraformaldehyde. The entire brain was removed and fixed in a buffered 30% sucrose solution overnight. Following sucrose infiltration and immediately prior to subsequent processing, an 18 gauge needle was inserted ventrally on the left side of the brain from rostral-to-caudal position. Brains were frozen and sectioned in the coronal plane on a Hacker Instruments Cryostat at a tissue thickness of 30 μm. Sections were sequentially placed in 48 well-plates in a tris-buffered/polyethylene glycol solution to maintain tissue integrity when stored at −20°C. Every ninth section was collected and immediately mounted, in sequential order, onto gelatin-coated slides and dried on a slide warmer.
Mounted sections were stained with hematoxylin and eosin (H&E), dehydrated, and coverslipped. Slides were then scanned on an Epson Expression 1680 scanner driven by Adobe Photoshop CS3 at a resolution of 1200 dpi. Lesion volumes were determined using ImageJ software. Briefly, after establishing scale, the lesion cavity was outlined and filled, generating the lesion area per section. The lesion volume per animal was subsequently determined as the sum of the area measurement of each section times the thickness of the section (30 μm) times the sampling interval.
Every ninth section was collected and immunolabeled with a monoclonal antibody raised against the pan-neuronal marker NeuN (Millipore) using established immunofluorescent techniques. Briefly, sections were incubated in anti-NeuN antibody overnight at 4°C at a concentration of 0.75 ug/ml. NeuN immunoreactivity was visualized following incubation with goat anti-mouse secondary conjugated to Alexa Fluor® 568 (Invitrogen) for 3 h. Sections were rinsed, mounted and coverslipped using Fluoromount-G (Fisher Scientific), an aqueous mounting medium. Counts of NeuN-immunoreactive (-IR) neurons were performed in the ipsilateral hippocampal CA1 and CA3 regions as follows. Six sections per brain were examined in the coronal plane from rostral-to-caudal extent. Images were generated using a Nikon A1R confocal system. Individual sections were examined by eye at 20×magnification to identify CA1 and CA3 regions of the hippocampus ipsilateral to the lesion site. Once identified, an image stack of 11 optical sections was generated along the z-axis of the field of interest. Laser power, gain, and contrast settings were maintained throughout all stages of acquisition. The sixth, or middle optical section was saved and converted to .TIFF format for subsequent counts of NeuN-IR profiles. Neurons were counted in these images using Nikon's Elements software package. Briefly, an initial threshold value was determined, saved, and applied to all subsequent images. Following the initial application of threshold values, additional segmentation was performed to clearly separate adjacent neuronal cell bodies. Counts/mm2 for each brain section were then generated.
Treatment groups
The treatment groups in each experiment consisted of a randomized assignment to either EPO 5000 U/kg i.v. or i.p., pHBSP 30 μg/kg, or control, which consisted of either saline or an inactive peptide 30 μg/kg. The first dose of the assigned drug was given i.v. and the subsequent doses were given i.p. The inactive peptide sequence (ULSEARNQSEL) had the same amino acid components as pHBSP, but in scrambled order. The investigators conducting the injury experiment and the outcome measures were blinded to the treatment assignment. The dose of pHBSP was based on previous studies in other tissues that showed cytoprotection with doses ranging from 1 to 60 μg/kg (Ahmet et al., 2011).
For the acute experiment, 31 animals received a single dose of the assigned treatment (pHBSP 11 animals, EPO 9 animals, and control 11 animals). In the chronic experiment, 56 animals received doses of the assigned drug every 12 h for 3 days after injury (pHBSP 19 animals, EPO 15 animals, control 22 animals).
Statistical analysis
Outcome assessments that were repeated over time, such as the hemodynamic measurements and the behavioral tests, were analyzed using repeated measures ANOVA. Histology assessments were analyzed using one-way ANOVA. A post-hoc test to adjust for multiple comparisons (such as Sidak-Holms) was used when the main effects from the ANOVA test were significant. The summary values in the text and figures are the mean±standard error.
Results
Injury parameters
To assure that all animals received a similar impact injury, the impact parameters that can be varied were monitored in all of the experiments. In both the acute hemodynamic and the chronic behavioral/histology experiments, there were no differences in these impact characteristics among the experimental groups.
Recovery of the righting reflex post-injury is sometimes used as a measure of the initial injury, being analogous to the human loss of consciousness. For the animals in the chronic experiment, reflex recovery time (in minutes) after the post-injury termination of anesthetic did not vary significantly across treatment groups for escape, head support, righting, corneal, pinna, paw, and tail reflexes (p>0.4 for all reflexes). This neurological finding supports the impact velocity data indicating that the initial injury was similar in all treatment groups.
Acute hemodynamic changes
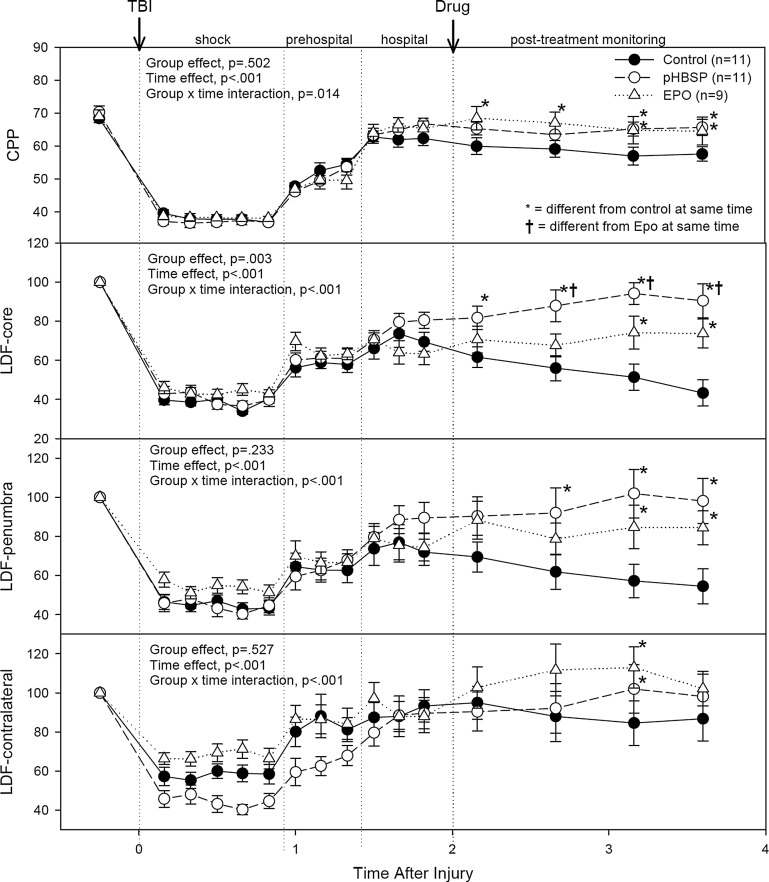
The changes in cerebral perfusion pressure (CPP) and LDF are illustrated in Figure 1. The p values from the repeated measures ANOVA for the effects of treatment group and of time, as well as for the treatment by time interaction, are given in each graph. When the treatment by time interaction was significant, symbols indicate which individual points were significantly different between the treatment groups. Data for ICP and MAP during the different experimental time periods are summarized in Table 1.
FIG. 1.
The cerebral hemodynamic response to the impact injury (first arrow) followed by 50 min of hemorrhagic hypotension (shock), 30 min of fluid resuscitation (pre-hospital resuscitation), 30 min of blood re-infusion and oxygen therapy (hospital resuscitation), administration of the assigned drug treatment (second arrow), and 2 h of post-treatment monitoring. The numbers of animals in each group are given in the figure legend. The results of the repeated measures ANOVA test are summarized in each graph. When the group×time interaction was significant, symbols indicate which treatments were significantly different among the treatment groups by Holm-Sidak post-hoc test. CPP, cerebral perfusion pressure; LDF, laser Doppler flow.
Table 1.
Intracranial Pressure (ICP) and Mean Arterial Pressure (MAP) for the Three Experimental Groups at Key Time Periods
| Time Period | |||||
|---|---|---|---|---|---|
| Baseline | Hypotension | After Fluid Resuscitation | After Blood Re-Infusion | End of Experiment | |
| ICP-controls | 7.5±0.9 | 4.4±0.8 | 6.8±0.9 | 8.6±1.2 | 8.6±1.2 |
| -EPO | 6.7±0.7 | 4.6±0.4 | 8.1±1.1 | 10.8±2.1 | 11.5±1.9 |
| -pHBSP | 9.0±1.1 | 5.1±0.6 | 9.6±1.3 | 11.0±1.4 | 11.4±1.6 |
| MAP-controls | 75.9±1.4 | 41.2±1.3 | 61.3±1.3 | 71.0±2.3 | 65.2±2.0 |
| -EPO | 75.8±1.8 | 42.6±0.6 | 57.7±2.3 | 76.1±2.2 | 76.1±3.0* |
| -pHBSP | 79.3±1.9 | 41.9±0.5 | 63.2±1.8 | 77.8±1.8 | 77.1±1.6* |
An asterisk indicates the values that are significantly different from the control group.
MAP fell from 77±1 mm Hg at baseline to a nadir of 42±1 mm Hg during the hemorrhagic shock period, and then recovered to 61±1 mm Hg with fluid resuscitation and to 75±1 mm Hg with the definite resuscitation that included re-infusion of blood. There were no differences in MAP between treatment groups at baseline, during hemorrhagic shock, or during resuscitation. However, following administration of the drug treatments, the EPO- and pHBSP-treated animals had better preservation of blood pressure than the controls (treatment effect, p=0.002; time effect, p<0.001; time×treatment interaction, p<0.001). At the end of the monitoring period, MAP averaged 77±2 mm Hg in the pHBSP-treated animals, and 76±3 mm Hg in the EPO-treated animals, compared to 65±2 mm Hg in the control animals (p<0.05, Holm-Sidak test).
ICP followed a similar pattern to the MAP, but the differences between the treatment groups were not significant during any time period, and ICP values in all treatment groups remained within normal values. ICP fell from 7.8±0.3 mm Hg at baseline to a low of 4.2±0.2 mm Hg during the hemorrhagic shock period. ICP increased then to 8.3±0.3 mm Hg with fluid resuscitation, and then to 10.3±0.3 mm Hg following re-infusion of blood.
CPP, calculated from the difference between MAP and ICP, was 69±1 mm Hg at baseline, and fell to a low of 38±1 mm Hg during the period of hemorrhagic hypotension. Following fluid resuscitation, CPP increased to 53±1 mm Hg. With re-infusion of blood, CPP increased further to 65±1 mm Hg. The post-resuscitation and post-treatment CPP was significantly better in the EPO-treated and the pHBSP-treated animals than in the control animals (treatment effect, p=0.502; time effect, p<0.001; time×treatment interaction, p=0.014). At the end of the monitoring period, CPP averaged 66±2 mm Hg in the pHBSP group, 65±2 mm Hg in the EPO group, compared to 56±2 mm Hg in the control animals (p<0.05, Holm-Sidak test).
The LDF values were normalized to the pre-injury levels for each brain site. Treatment effects for LDF were greatest in the core contusion region of the brain. Changes in LDF in the core contusion site fell from 100% to a low value of 39±3% during hemorrhagic shock, and then recovered partially to 60±2% with fluid resuscitation, and to 72±2% following reinfusion of blood. Following infusion of the assigned drug treatment, LDF in the core contusion began to fall in the control group to a low at the end of the monitoring period of 43±4% of the baseline values. In contrast, the LDF in the core contusion continued to improve following the drug treatment in the pHBSP-treated group to 88±4%, and remained constant at 74+4% of the baseline values in the EPO-treated group at the end of the monitoring period. LDF values in the penumbra area surrounding the core contusion followed the same pattern as in the core. LDF values on the contralateral side had less severe reduction in values during the hemorrhagic shock period, and better recovery following resuscitation of blood pressure, but little difference between the treatment groups.
Chronic assessments
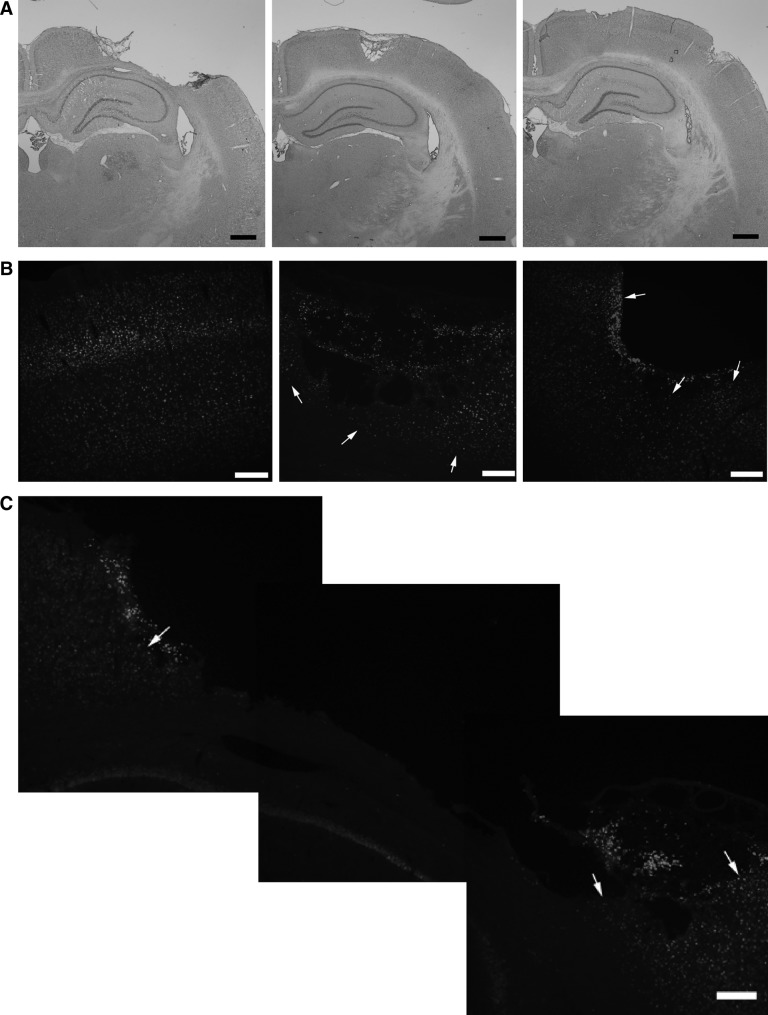
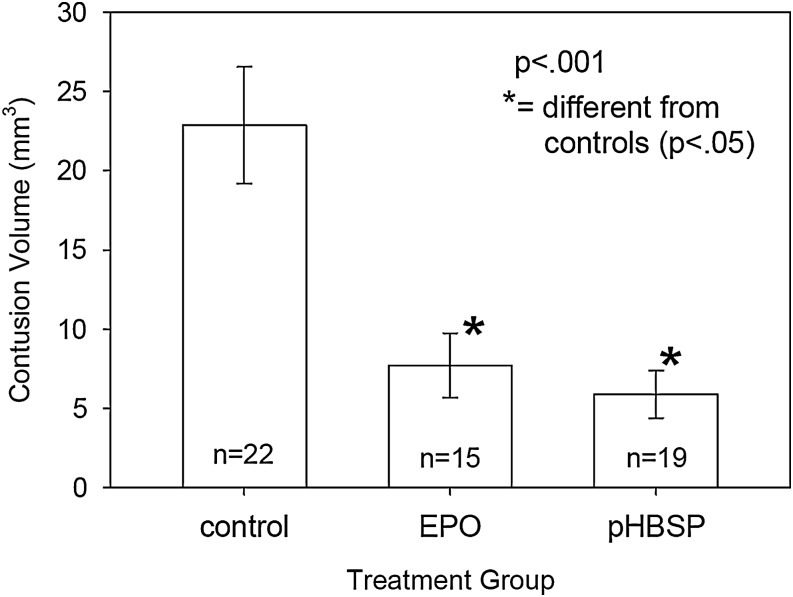
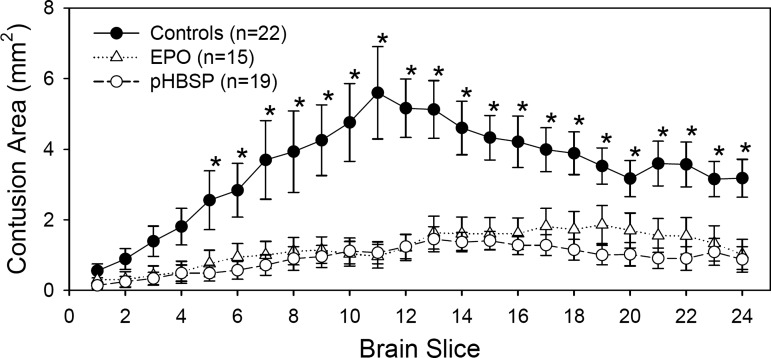
As is illustrated in Figure 2, the injury that resulted from the mild cortical impact injury followed by hemorrhagic hypotension was loss of cortical tissue at the site of the impact. Both drug treatments resulted in a 70% reduction in the volume of contused tissue at 2 weeks post-injury. Contusion volume averaged 20.8±2.8 mm3 in the control groups compared to 7.7±2.0 mm3 in the EPO-treated group and 5.9±1.5 mm3 in the pHBSP-treated group (Fig. 3) (p=0.001). As is shown in Figure 4, the reduction in the injured tissue occurred throughout the brain with EPO and pHBSP, but was greatest in the center of the injury site.
FIG. 2.
(A) Representative images of the injured hemisphere for the three treatment groups, showing sizeable contusion in the control animal (left panel), and much smaller contusions in the EPO-treated (middle panel) and the pHBSP-treated animal (right panel). (B) Representative appearance of cortical neurons around and under the lesion site in EPO-treated (middle panel) and ARA290-treated (right panel) animals, compared to the normal distribution of neurons observed in the contralateral uninjured cortex (left panel). The arrows indicate areas where NeuN-positive neurons were detected. The images were captured using widefield fluorescence imaging. The scale bar indicates 200 μm. (C) Representative appearance of cortical neurons around and under the larger lesion site of saline-treated animals, using widefield fluorescence imaging. The arrows show areas at the edge of the contusion cavity where NeuN- positive neurons were detected. The scale bar indicates 200 μm.
FIG. 3.
Mean contusion volume at 2 weeks post-injury for the three treatment groups. EPO and pHBSP both resulted in a 70% decrease in contusion volume. The p value is the result from the ANOVA test. An asterisk indicates the groups that are significantly different from the control group (p<0.05) after adjusting for multiple comparisons by the Holm-Sidak method.
FIG. 4.
Area of contusion on serial brain slices from anterior to posterior. The brain slices were 30 μm thick and every ninth slice was examined for a total of 24 slices for each animal. Each point in the graph represents the average contusion area of the animals in each treatment group for the individual slice. The contusion areas were analyzed by repeated measures ANOVA with treatment group and brain slice as factors (treatment group effect, p<0.001; treatment group×slice interaction, p<0.001). An asterisk indicates the slices where the EPO- and pHBSP- treated groups were significantly different from the control group by Holm-Sidak post-hoc test.
In contrast to the cortex, the hippocampus underlying the impact site remained well preserved in the control animals, and there was not a significant difference between the treatment groups in the cell counts from the CA1 and CA3 regions of the hippocampus. For the CA3 region, the cell count averaged 50.2±2.3 cells/mm2 for the control animals, compared to 54.2±1.6 cells/mm2 for the EPO-treated animals and 54.3±1.4 cells/mm2 in the pHBSP-treated animals (p=0.226).
Weight of the rats decreased after the injury with the lowest values 8.7% below baseline on post-injury day 1. Thereafter weight gradually increased and surpassed the pre-injury level by day 15. Weight did not vary significantly by treatment group or days (the day of injury, compared to days 1–5 and 11–15 post-injury).
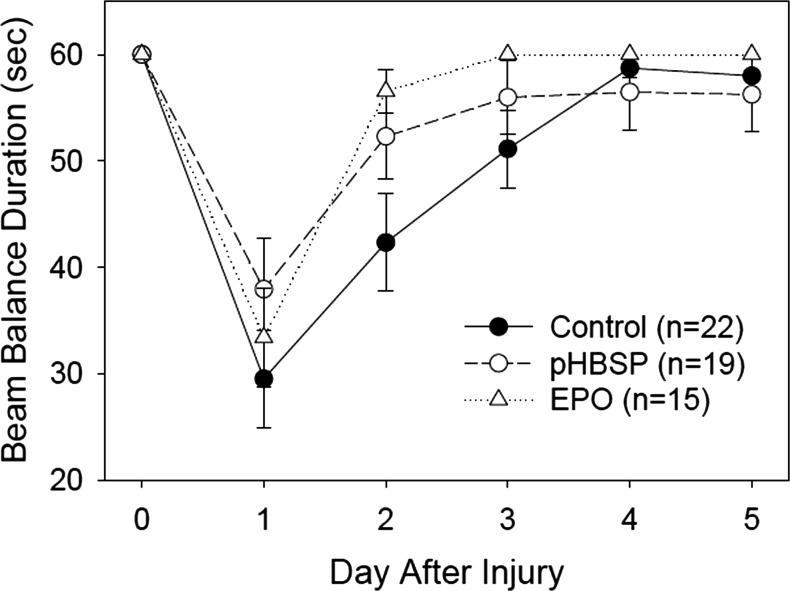
For the beam balance task, all of the rats balanced on the beam for the 60 sec duration of each of the 3 trials immediately prior to the injury; therefore, data prior to the injury were not included in the model used to analyze the data. The best fit model for the beam balancing data included the independent variables “treatment group” and “days post-injury”. Performance on the task improved significantly over days 1–5 post-injury (p<0.001) as might be expected with recovery of function. The pHBSP (p<0.001) and EPO (p<0.001) treatment groups balanced significantly longer on the beam than the control group after taking into account the day effect. The data for each group over days is represented in Figure 5.
FIG. 5.
Results for the beam balance test. The beam balance duration is the time that the animals were able to balance on a narrow beam. All animals were able to balance for 60 sec on Day 0, which is prior to the injury, and all treatment groups improved significantly over time (p<0.001). The animals treated with EPO (p<0.001) and pHBSP (p<0.001) were able to balance for significantly longer post-injury than were the control animals, after adjusting by day.
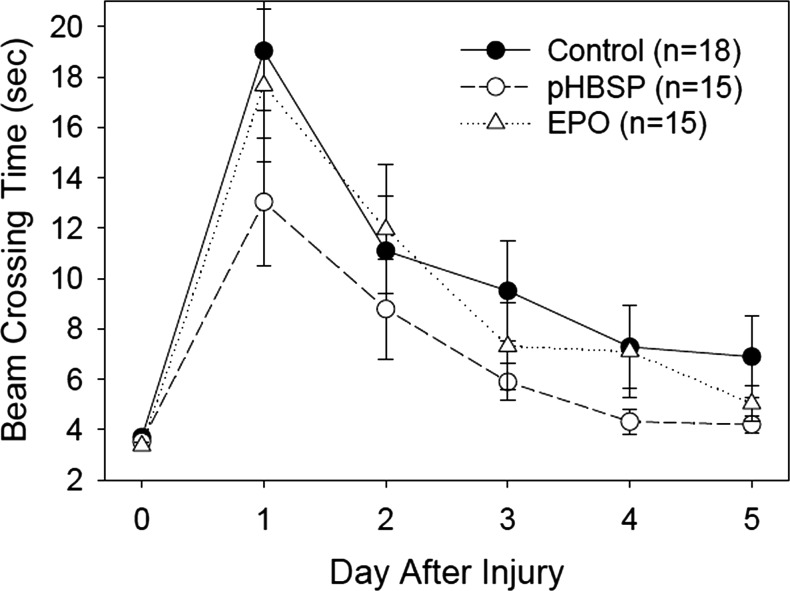
Only rats who met the final pre-injury criterion of walking down the beam in ≤ 5 sec on three consecutive trials were involved in the experiment. This produced very little variability in the beam walking time for the three trials just prior to injury. Only data from performance on the three trials for days 1–5 post-injury were therefore included in the analysis. The best fit model for the beam walking data involved the independent variables “treatment group” and “days post- injury”. Performance on the task improved significantly over days 1–5 post-injury in all groups (p<0.001) with beam walking time decreasing over days. This is to be expected with recovery of function over days. The pHBSP (p<0.001) and EPO (p=0.010) treatment groups walked significantly faster on the beam than the control group after adjusting by day. The data for each group over trials and days is represented in Figure 6.
FIG. 6.
Results for the beam walking test. The beam crossing time is the length of time required to traverse the narrow beam. All animals were able to accomplish this task within 5 sec on Day 0 prior to the injury, and all treatment groups improved significantly over time (p<0.001). The animals treated with EPO (p=0.010) and pHBSP (p<0.001) were significantly faster in crossing the beam post-injury than were the control animals, after adjusting by day.
The model that best fit the Morris Water Maze latency data included weight, treatment group, day, and trial. Weight on days 11–15 proved to be related to latency in finding the platform; therefore, the data were corrected for this covariate. With this correction, there were no significant differences between the treatment groups in their latency to find the platform. Latency decreased significantly over trials (p<0.001) and days (p<0.001), as expected.
When the hidden platform was removed from the tank after the last (fourth) learning and memory trial of day 15, the percentage of time spent swimming in the platform quadrant provided a measure of memory for where the platform should have been. This time was similar for the treatment and control groups. The platform was put back in the tank in its previous position for a visible platform trial. A piece of Plexiglas was added to the top of the platform so as to make it visible above the water. Latency (seconds) to swim to the visible platform was similar across treatment groups indicating no differences in using visual cues to find the platform.
Discussion
Cytoprotection with EPO after trauma
In the early 1990s, the first observations of EPO-induced in vitro trophic effects, and of the presence in central nervous system tissues of EPOR as well as local EPO production following hypoxia, were reported (Digicaylioglu et al., 1995; Konishi et al., 1993; Marti et al., 1996; Masuda et al. 1993). In 2000, Brines and associates demonstrated that systemically administered recombinant human EPO (rhEPO) could cross the blood–brain barrier and reduce the size of injury in animal models of stroke and TBI (Brines et al., 2000). Since that time, many laboratories have shown that EPO possesses tissue protective effects in different tissues and organs, including the heart, kidney, and immune system. Multiple pathways that might have neuroprotective effects are activated in the brain by EPO after a brain injury. EPO decreases neuronal apoptosis, improves blood–brain barrier integrity and reduces brain edema. In addition, EPO has anti-inflammatory, neurotrophic, angiogenic and synaptogenic activities, and has been shown to promote the recruitment of stem cells after injury.
Some clinical studies also suggest that there is cytoprotection with EPO. Zarychanski and associates (2007) published a meta-analysis of nine clinical trials of rhEPO in critically ill patients for the treatment of anemia of critical illness. The total number of patients in these trials was 3314, and the overall odds ratio for mortality was 0.86 (p=0.14). Two trials (EPO-2 and EPO-3) contributed most of the patients to this analysis (Corwin et al., 2002, 2007). Although there was not a clear survival benefit with rhEpo for all patients enrolled in either study, rhEPO did have a significantly improved mortality rate for the subgroup of patients who had traumatic injuries in both studies. For the EPO-2 study, this subgroup analysis was post hoc. For the EPO-3 study, this was a planned subgroup analysis with stratification of treatment by admission diagnosis category (medical, surgical, or trauma) (Napolitano et al., 2008).
Of the total 1423 trauma patients enrolled in the EPO-2 study (n=630) and the EPO-3 study (n=793), most had some degree of brain injury, but 456 (32%) had a severe TBI defined by an admission Glasgow Coma Score (GCS) ≤ 8. Mortality rate in those trauma patients with GCS ≤ 8 was 6.6% (15/229) in the placebo-treated group, and 4% (9/227) in the EPO-treated group (Napolitano et al., 2008).
Despite the improvement in the outcome of the EPO-treated patients, there was a significant increase in the incidence of thrombophlebitis, from 5.7% to 8.7% (p=0.04) (Corwin et al., 2007). The concern about thromboembolic complications with EPO, which are dose-related (Dicato, 2008), has limited the translation of EPO as a neuroprotective agent. Development of a drug that would have the neuroprotective effects of EPO without these hematologic complications would be a significant advance in TBI treatment, both civilian and military. The present studies suggest that pHBSP may have these characteristics.
Cytoprotection with EPO derivatives
The molecular interaction of EPO with the homodimer erythropoietic receptor complex [(EPOR)2] is well studied. Critical regions within the EPO molecule that interact with (EPOR) 2 have been identified, and include portions of helices A and C (site II) as well as helix D and the loop connecting helices A and B (site I) (Elliott et al., 1997).
Chemical or mutational modification of amino acid residues within sites I and II on the EPO molecule eliminates its ability to bind to (EPOR)2. Some of these modified EPO molecules, including carbamylated EPO (CEPO), retain tissue protective properties (Leist et al., 2004). CEPO has been reported to have neuroprotective activities similar to EPO in a variety of experimental injury models including TBI, stroke, and spinal cord injury (Adembri et al., 2008; King et al., 2007; Mahmood et al., 2007; Wang et al., 2007; Xiong et al., 2010).
The receptor mediating the cytoprotective effects of such EPO derivatives is thought to be pharmacologically distinct from (EPOR)2. Brines and associates proposed that the receptor that promotes tissue protection is a heteromer composed of two EPOR monomers and two molecules of CD131, also referred to as the beta common receptor (Brines et al., 2004). Brines and Cerami recently reviewed the characteristics of this proposed tissue protective receptor (Brines and Cerami, 2008). First, the receptor has a low affinity for EPO (1–20 nmol/L). As a result, it does not respond to EPO at the 1–7 pmol/L concentrations normally present in the circulation. It is probable that it is activated only by high levels of locally produced EPO. Additionally, the tissue protective receptor is not usually expressed until after an injury occurs, and only requires a brief exposure to EPO to trigger sustained biological activity. This is in contrast to (EPOR)2, which is normally expressed in hematopoietic cells, and requires sustained levels of EPO to support erythropoiesis.
pHBSP is an 11 amino acid peptide composed of the adjacent amino acids in space on the aqueous face of helix B of EPOR. It was designed to mimic the tertiary structure of helix B (Brines et al., 2008). In previous studies, it has provided neuroprotection in models of sciatic nerve compression, and stroke (Brines et al., 2008). It has also been demonstrated to have cardioprotective effects (Ueba et al., 2010).
Experimental TBI model
The model used for these experiments consists of a mild TBI and a period of hypotension induced by withdrawal of blood. This mild cortical impact injury alone causes minimal if any histological consequences (Cherian et al., 1999). However, when the mild cortical impact injury is followed by a period of hemorrhagic hypotension, the resulting cortical contusion is equivalent to a severe cortical impact injury. This injury scenario models the polytrauma patient who has also sustained a mild TBI.
Previous studies have suggested that this magnification of brain injury in the mild cortical impact injury model followed by a secondary insult is caused by a severe reduction in blood flow at the impact site, which does not occur in sham-injured animals with the same secondary insult (Giri et al., 2000). Similar findings have been observed with secondary insults following fluid percussion injury (Matsushita et al., 2001). For this reason, the cerebral hemodynamic effects of the drug treatment were especially pertinent.
Both EPO and pHBSP provided a similar preservation of blood flow in the core of the contusion but also in penumbra tissue surrounding the core following resuscitation. Studies in subarachnoid hemorrhage have suggested that EPO improves cerebral pressure autoregulation (Springborg et al., 2002). This characteristic of EPO might provide better blood flow during periods of hypotension. As the drug treatment in the present study was not given until after resuscitation was complete, the beneficial effects did not alter the actual severity of ischemia that occurred during the period of hemorrhagic hypotension, but instead must have modified consequences of reperfusion injury. For this reason, it cannot be concluded whether the observed hemodynamic effects contributed to the improved outcome or if these findings simply reflected better preservation of brain tissue via some other cytoprotective action. Further studies will be needed to clarify this.
EPO and pHBSP also resulted in better recovery of blood pressure following resuscitation. It is possible that this contributed to the improved perfusion that was observed following resuscitation; however, the actual increases in cerebral perfusion pressure were quite modest, as ICP was also slightly higher.
The plasma half-life of pHBSP in rats is ∼ 2 min (Brines et al., 2008). Despite this short half-life in the circulation, it is equally as cytoprotective on a molar basis as EPO. This characteristic implies that pHBSP has a rapid effect on gene expression. In vitro models demonstrate that a brief exposure to EPO for as little as 5 min is sufficient to initiate neuroprotective activities (Morishita et al., 1997). Another derivative of EPO (asialo EPO) has a similar in vivo neuroprotective effect to EPO even though it has a very short plasma half-life of a few minutes (Erbayraktar et al., 2003). An important consequence of a short half-life, however, might be that the time of administration could be more critical than with an agent having a longer half-life. In the current study, the treatment with pHBSP was started after resuscitation from hemorrhagic hypotension. This time was chosen because it was thought that reperfusion injury was probably a major component of the brain injury in this model. Further studies would be needed to determine if treating earlier or later would have the same effect.
Choice of anesthetic might have played a role in the outcome of the study. Isofluorane was used as the anesthetic throughout the injury–hypotension paradigm, and baseline and resuscitation blood pressures were somewhat lower than normal as a result. The findings might have been different if another anesthesia had been used.
Conclusion
Administration of pHBSP and EPO had similar neuroprotective effects, reducing contusion volume and improving some aspects of neurobehavioral outcome, in this model of mild TBI complicated by hemorrhagic hypotension. As pHBSP has not been observed to have the adverse thrombogenic effects of EPO, it may prove to be a better neuroprotective agent for patients with TBI.
Acknowledgments
`This work was funded by U.S. Army Award numbers W81XWH-08-2-0132 (C.S.R.) and W81XWH-08-2-0150 (R.J.G.). Araim Pharmaceuticals, Ossining, NY, USA supplied the pHBSP for the study.
Author Disclosure Statement
No competing financial interests exist.
References
- Adembri C. Massagrande A. Tani A. Miranda M. Margheri M. De G.R. Pellegrini-Giampietro D.E. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit. Care Med. 2008;36:975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- Ahmet I. Tae H.-J. Juhaszova M. Riordon D.R. Goheler K.R. Sollott S.J. Brines M. Cerami A. Lakatta E.G. Talan M.I. A small nonerythropoietic Helix B surface peptid based on erythropoietin structure is cardioprotective against myocardial damage. Mol. Med. 2011;17:194–200. doi: 10.2119/molmed.2010.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M. Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J. Intern. Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Brines M. Grasso G. Sfacteria A. Ghezzi P. Fratelli M. Latini R. Xie Q.W. Smart J. Su–Rick C. Pobre E. Diaz D. Gomez D. Hand C. Coleman T. Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and beta-common subunit heteroreceptor. Proc. Natl. Acad. Sci. U.S.A. 2004;101(14):907–14. doi: 10.1073/pnas.0406491101. ,912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M. Patel N.S. Villa P. Brines C. Mennini T. De P.M. Erbayraktar Z. Erbayraktar S. Sepodes B. Thiemermann C. Ghezzi P. Yamin M. Hand C.C. Xie Q.W. Coleman T. Cerami A. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. U.S.A. 2008;105(10):925–10. doi: 10.1073/pnas.0805594105. ,930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M.L. Ghezzi P. Keenan S. Agnello D. de Lanerolle N.C. Cerami C. Itri L.M. Cerami A. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. U.S.A. 2000;97(10):526–10. doi: 10.1073/pnas.97.19.10526. ,531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania M.A. Marciano M.C. Parisi A. Sturiale A. Buemi M. Grasso G. Squadrito F. Caputi A.P. Calapai G. Erythropoietin prevents cognition impairment induced by transient brain ischemia in gerbils. Eur. J. Pharmacol. 2002;437:147–150. doi: 10.1016/s0014-2999(02)01292-x. [DOI] [PubMed] [Google Scholar]
- Celik M. Gokmen N. Erbayraktar S. Akhisaroglu M. Konakc S. Ulukus C. Genc S. Genc K. Sagiroglu E. Cerami A. Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L. Goodman J.C. Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J. Pharmacol. Exp. Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Cherian L. Robertson C.S. Goodman J.C. Secondary insults increase injury after cortical impact injury in rats. J. Neurotrauma. 1999;13:371–383. doi: 10.1089/neu.1996.13.371. [DOI] [PubMed] [Google Scholar]
- Corwin H.L. Gettinger A. Fabian T.C. May A. Pearl R.G. Heard S. An R. Bowers P.J. Burton P. Klausner M.A. Corwin M.J. Efficacy and safety of epoetin alfa in critically ill patients. N. Engl. J. Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- Corwin H.L. Gettinger A. Pearl R.G. Fink M.P. Levy M.M. Shapiro M.J. Corwin M.J. Colton T. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. J.A.M.A. 2002;288:2827–2835. doi: 10.1001/jama.288.22.2827. [DOI] [PubMed] [Google Scholar]
- Dicato M. Venous thromboembolic events and erythropoiesis-stimulating agents: an update. Oncologist 13, Suppl. 2008;3:11–15. doi: 10.1634/theoncologist.13-S3-11. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M. Bichet S. Marti H.H. Wenger R.H. Rivas L.A. Bauer C. Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H. Weissenborn K. Prange H. Schneider D. Weimar C. Wartenberg K. Schellinger P.D. Bohn M. Becker H. Wegrzyn M. Jahnig P. Herrmann M. Knauth M. Bahr M. Heide W. Wagner A. Schwab S. Reichmann H. Schwendemann G. Dengler R. Kastrup A. Bartels C. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- Elliott S. Lorenzini T. Chang D. Barzilay J. Delorme E. Mapping of the active site of recombinant human erythropoietin. Blood. 1997;89:493–502. [PubMed] [Google Scholar]
- Erbayraktar S. Grasso G. Sfacteria A. Xie Q.W. Coleman T. Kreilgaard M. Torup L. Sager T. Erbayraktar Z. Gokmen N. Yilmaz O. Ghezzi P. Villa P. Fratelli M. Casagrande S. Leist M. Helboe L. Gerwein J. Christensen S. Geist M.A. Pedersen L.O. Cerami–Hand C. Wuerth J.P. Cerami A. Brines M. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6741–6746. doi: 10.1073/pnas.1031753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri B. Krishnappi I.K. Bryan R.M., Jr. Robertson C.S. Regional cerebral blood flow after cortical impact injury complicated by a secondary insult. Stroke. 2000;31:961–967. doi: 10.1161/01.str.31.4.961. [DOI] [PubMed] [Google Scholar]
- Gorio A. Gokmen N. Erbayraktar S. Yilmaz O. Madaschi L. Cichetti C. Di Giulio A.M. Vardar E. Cerami A. Brines M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G. Neuroprotective effect of recombinant human erythropoietin in experimental subarachnoid hemorrhage. J. Neurosurg. Sci. 2001;45:7–14. [PubMed] [Google Scholar]
- Jia L. Chopp M. Zhang L. Lu M. Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbats brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41:2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V.R. Averill S.A. Hewazy D. Priestley J.V. Torup L. Michael–Titus A.T. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur. J. Neurosci. 2007;26:90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- Konishi Y. Chui D.H. Hirose H. Kunishita T. Tabira T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 1993;609:29–35. doi: 10.1016/0006-8993(93)90850-m. [DOI] [PubMed] [Google Scholar]
- Leist M. Ghezzi P. Grasso G. Bianchi R. Villa P. Fratelli M. Savino C. Bianchi M. Nielsen J. Gerwien J. Kallunki P. Larsen A.K. Helboe L. Christensen S. Pedersen L.O. Nielsen M. Torup L. Sager T. Sfacteria A. Erbayraktar S. Erbayraktar Z. Gokmen N. Yilmaz O. Cerami–Hand C. Xie Q.W. Coleman T. Cerami A. Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Changsheng Q. Goussev A. Schallert T. Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J. Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Zhang Z.G. Lu C. Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J. Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- Mann C. Lee J.H. Liu J. Stammers A.M. Sohn H.M. Tetzlaff W. Kwon B.K. Delayed treatment of spinal cord injury with erythropoietin or darbepoetin—a lack of neuroprotective efficacy in a contusion model of cord injury. Exp. Neurol. 2008;211:34–40. doi: 10.1016/j.expneurol.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Marti H.H. Bernaudin M. Petit E. Bauer C. Neuroprotection and angiogenesis: dual role of erythropoietin in brain ischemia. News Physiol. Sci. 2000;15:225–229. doi: 10.1152/physiologyonline.2000.15.5.225. [DOI] [PubMed] [Google Scholar]
- Marti H.H. Wenger R.H. Rivas L.A. Straumann U. Digicaylioglu M. Henn V. Yonekawa Y. Bauer C. Gassmann M. Erythropoietin gene expression in human, monkey and murine brain. Eur. J. Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Masuda S. Nagao M. Takahata K. Konishi Y. Gallyas F., Jr. Tabira T. Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993;268(11):208–11. ,216. [PubMed] [Google Scholar]
- Matsushita Y. Bramlett H.M. Kuluz J.W. Alonso O. Dietrich W.D. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathological consequences of traumatic brain injury in rats. J. Cereb. Blood Flow Metabol. 2001;21:847–856. doi: 10.1097/00004647-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Morishita E. Masuda S. Nagao M. Yasuda Y. Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- Napolitano L.M. Fabian T.C. Kelly K.M. Bailey J.A. Block E.F. Langholff W. Enny C. Corwin H.L. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J. Trauma. 2008;65:285–297. doi: 10.1097/TA.0b013e31817f2c6e. [DOI] [PubMed] [Google Scholar]
- Pinzon A. Marcillo A. Pabon D. Bramlett H.M. Dietrich W.D. A re-assessment of erythropoietin as a neuroprotective agent following rat spinal cord compression or contusion injury. Exp. Neurol. 2008;213:129–136. doi: 10.1016/j.expneurol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren A.L. Fratelli M. Brines M. Goemans C. Casagrande S. Lewczuk P. Keenan S. Gleiter C. Pasquali C. Capobianco A. Mennini T. Heumann R. Cerami A. Ehrenreich H. Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springborg J.B. Ma X. Rochat P. Knudsen G.M. Amtorp O. Paulson O.B. Juhler M. Olsen N.V. A single subcutaneous bolus of erythropoietin normalizes cerebral blood flow autoregulation after subarachnoid haemorrhage in rats. Br. J. Pharmacol. 2002;135:823–829. doi: 10.1038/sj.bjp.0704521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueba H. Brines M. Yamin M. Umemoto T. Ako J. Momomura S. Cerami A. Kawakami M. Cardioprotection by a nonerythropoietic, tissue-protective peptide mimicking the 3D structure of erythropoietin. Proc. Natl. Acad. Sci. U.S. A. 2010;107:14.357–14.362. doi: 10.1073/pnas.1003019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P. Bigini P. Mennini T. Agnello D. Laragione T. Cagnotto A. Viviani B. Marinovich M. Cerami A. Coleman T.R. Brines M. Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Zhang Z. Wang Y. Zhang R. Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang Y. Zhang Z.G. Rhodes K. Renzi M. Zhang R.L. Kapke A. Lu M. Pool C. Heavner G. Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br. J. Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. Mahmood A. Zhang Y. Meng Y. Zhang Z.G. Qu C. Sager T.N. Chopp M. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J. Neurosurg. 2010;2010;12 doi: 10.3171/2010.10.JNS10925. e-pub ahead of print November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarychanski R. Turgeon A.F. McIntyre L. Fergusson D.A. Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials. Can. Med. J. Assoc. 2007;177:725–734. doi: 10.1503/cmaj.071055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechariah A. ElAli A. Hermann D.M. Combination of tissue-plasminogen activator with erythropoietin induces blood–brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke. 2010;41:1008–1012. doi: 10.1161/STROKEAHA.109.574418. [DOI] [PubMed] [Google Scholar]