Abstract
The influence of light and maternal activity on early infant activity rhythm were studied in 43 healthy, maternal-infant pairs. Aims included description of infant and maternal circadian rhythm of environmental light, assessing relations among of activity and light circadian rhythm parameters, and exploring the influence of light on infant activity independent of maternal activity. Three-day light and activity records were obtained using actigraphy monitors at infant ages 4, 8, and 12 weeks. Circadian rhythm timing, amplitude, 24-hour fit, rhythm center, and regularity were determined using cosinor and nonparametric circadian rhythm analyses (NPCRA). All maternal and infant circadian parameters for light were highly correlated. When maternal activity was controlled, the partial correlations between infant activity and light rhythm timing, amplitude, 24-hour fit, and rhythm center demonstrated significant relation (r = .338 to .662) at infant age 12 weeks, suggesting entrainment. In contrast, when maternal light was controlled there was significant relation between maternal and infant activity rhythm (r = 0.470, 0.500, and 0.638 at 4, 8 and 12 weeks, respectively) suggesting the influence of maternal-infant interaction independent of photo entrainment of cycle timing over the first 12 weeks of life. Both light and maternal activity may offer avenues for shaping infant activity rhythm during early infancy.
Keywords: Circadian rhythm, pediatrics, instrumentation and methodology, sleep hygiene and environment
Introduction
Establishing circadian rhythm is an important infant developmental milestone, facilitating biological synchrony with the mother (1, 2). While multiple aspects of biological rhythm demonstrate maturation, development of typical, diurnal pattern of infant activity rhythm and related sleep-wake pattern is paramount for fit with the home environment and preserving parental sleep. Light is essential in the entrainment of circadian rhythm, but there is a limited body of evidence regarding light effect in early human infant rhythm. Although there is an extensive literature on light entrainment in non-human primates and rodents (3), research in human infants is limited due to methodological considerations. Additionally, though animal research confirms maternal role in early entrainment of newborn rhythm, there is minimal human research addressing possible maternal influence on infant rhythm. The purpose of this research was to examine light and maternal activity effects in early development of infant activity circadian rhythm.
The capacity for human biological rhythm is present by mid-gestation with aspects of circadian rhythm development proceeding throughout infancy and childhood (4, 5). Fetal rhythm is largely shaped by the mother. Though immature, human infants demonstrate early evidence of beginning circadian rhythm. Gross day-night differences in expressed rhythm are present in the first weeks of life (6-8). Ongoing development includes changes in the suprachiasmatic nucleus (SCN), the circadian central clock, as well as input and output pathways, including the retinohypothalamic tract carrying light stimuli from the eye to the SCN, and pituitary melatonin production. Beyond the newborn period, photoentrainment is the most powerful regulator of diurnal rhythm, however the process in the first weeks of life is not well understood. Photoentrainment is part of a larger developmental process in which infant rhythm development is characterized by transition from ultradian to circadian period, increasing amplitude of rhythm excursion, and synchronization of multiple rhythmic functions maturing on differing timelines (6, 9). Rhythm development is progressive and inter-related. Circadian pattern, observed in cortisol at approximately 8 weeks, melatonin at 9 weeks, and temperature at 10 weeks, is associated with the development of consolidated sleep (10).
Rhythm entrainment in adults is broadly researched (11) but a modicum of study is focused on infants. Evidence supports infant responsiveness to the entraining effects of light early in life. Using a baboon model Hao and Rivkees (5) demonstrated response to cycled light pattern at the human equivalent of 24 weeks postconceptual age. Phase response curves have not been evaluated in infants and there is minimal evidence depicting the strength, duration, and timing of light related to entrainment. Melatonin, the key indicator of light entrainment, is present early in life. Melatonin receptors in the SCN are identified by mid-gestation and melatonin production is functional, though immature, in the newborn (12-15). Day-night difference in melatonin level has been shown as early as 4-6 weeks (15) and both response to daily light-dark pattern and seasonal effects in melatonin have been identified at 8 weeks (16).
Few studies have examined the effect of the light environment on the expression of developing circadian rhythm. Lighting in neonatal intensive care units has received attention (17-19) but home environment light is understudied. Infants are completely dependent on caregivers and the passive recipients of light stimuli characterizing the caregiver’s environment. In a previous exploratory study mothers and infants (4-10 weeks postnatal age) experienced low light conditions with approximately 80% of daytime hours at < 50 lux in infants (20). The effect of light on infant sleep-activity rhythm in the home environment has received little attention yet in one study, light in the afternoon particularly 12:01-16:00, led to increased infant sleep at night (21). While the effect of light on infant rhythm expression is recognized, the role of the mother in human infant rhythm entrainment is not well understood. Maternal effects in shaping 24-hour pattern involve both maternal control of the light environment as well as possible maternal activity. Among a number of mammalian species maternal rhythmic signals in the postnatal period in life provide entrainment before photoentrainment is mature(22) and somatosensory, olfactory, auditory, and arousal input are proposed as the agents of entrainment(23). The mother-infant dyadic interaction is complex in that it is bi-directional and, while maternal activity may shape rhythm, the infant’s activity cues maternal caregiving. Our previous work has shown development of infant activity rhythm and increasing synchrony of the timing of maternal and infant peak activity levels (24). The purpose of the analysis reported here is to explore the influence of light and maternal activity on early infant activity rhythm. Aims are to: 1) Describe infant and maternal circadian rhythm of environmental light at infant ages 4, 8, and 12 weeks. 2) Assess relations among activity and light circadian rhythm parameters in infant and maternal dyads. 3) Explore the influence of light on infant activity independent of maternal activity.
Materials and Methods
The study method has been described in detail and is briefly reviewed here (24).
Design and Subjects
Forty-four healthy, biologic maternal-infant pairs, recruited from the community, participated in a longitudinal, cross-sectional study with times of measure at infant age 4, 8, and 12 (± 2 days) weeks. Enrollment criteria included absence of complications prior to or following birth in either mother or infant.
Instruments
Infant and maternal activity and photopic light were monitored continuously at one minute intervals over a consecutive three day period using actigraphy (Respironics Actiwatch-L or Actiwatch Spectrum, Respironics, Bend, OR) and verified with maternally recorded log of both maternal and infant activity. Mothers also recorded periods of external motion (holding, swings, etc.).
Procedure
Infant actigraphy monitors were applied to the ankle and mothers wore the monitors on the non-dominant wrist. Mothers were instructed to avoid covering either of the monitors with clothing or bedding. Previous testing shows adherence to this request and comparability of infant monitor recording with a monitor attached to the infant’s bed (20).
Analysis
Periods of external motion were omitted from analysis of infant data. Aggregate maternal and infant minute-by-minute light were graphed against solar light pattern obtained from radiometric recordings by the campus Atmospheric Science Department (Precision Spectral Pyranometer, Eppley Laboratory, Newport, RI). While not as specific as local measurements of natural outdoor light collected proximal to the participants' homes might have been, these regional sky irradiance measurements provide representative indications of the timing and intensity of outdoor light in the area. Both cosinor analysis (mesor, acrophase, magnitude, R2) and non-parametric circadian rhythm analysis (NPCRA) (M10 midpoint,L5 midpoint, midlevel, amplitude, interdaily stability (IS), intradaily variability (IV), described previously (25) were employed to depict pattern of infant and mother activity and light. Cosinor and NPCRA provide complementary as well as distinctive findings in the study of maternal-infant rhythm timing, amplitude, 24-hour fit, rhythm center, and regularity. The correlations among maternal and infant light and activity were then examined. Partial correlation controlling maternal activity was used to assess the relation of infant light and infant activity independent of maternal activity.
Results
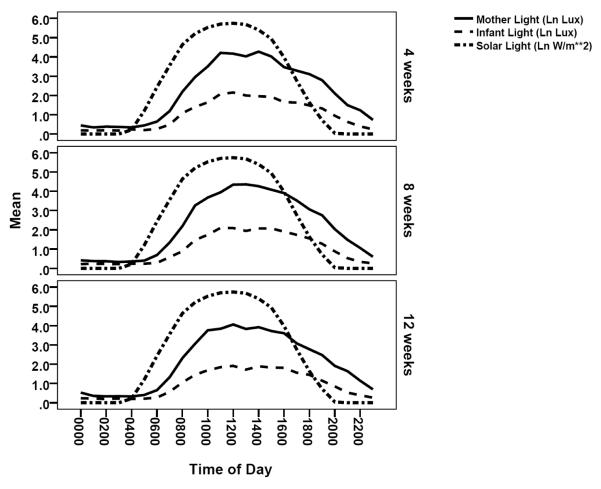
Maternal and infant activity rhythm have been described elsewhere(24, 25). The descriptive values for infant and maternal light rhythm are provided in Table 1. In general infants experienced lower light levels than that of the mother and both mother and infant 24-hour light pattern were not in phase with solar light and involved considerable night time light (Figure 1). As expected, the timing or phase of maternal light and activity rhythm are closely correlated across all three times of measure, as observed in strong correlation of acrophase, M10 midpoint, and L5 midpoint (Table 2). Maternal and infant light pattern are closely associated. The phase of infant light rhythm (acrophase, M10 midpoint), amplitude (or magnitude), 24-hour fit, rhythm center (mesor, midlevel) and IS are strongly correlated with that of the mother, while the L5 midpoint correlation declines over time.
Table 1.
Mother and infant mean (SD) light rhythm parameters and minimum and maximum level.
| Maternal | Infant | |||||
|---|---|---|---|---|---|---|
| 4 wk | 8 wk | 12 wk | 4 wk | 8 wk | 12 wk | |
| Acrophase (dec time) | 14.5(1.15) | 14.3 (1.10) | 14.4 (1.32) | 14.3 (1.06) |
14.3 (1.02) |
14.4 (1.31) |
| M10 midpoint (dec time) |
13.7 (1.23) | 13.4 (1.73) | 13.4 (1.17) | 14.2 (2.54) |
13.6 (2.78) |
13.5 (1.39) |
| L5 midpoint (dec time) |
2.79 (1.277) |
2.44 (1.316) |
2.55 (1.223) |
2.68 (1.667) |
1.96 (2.174) |
2.43 (2.174) |
| Amplitude (Ln lux) | 3.24 (1.190) |
3.42 (1.192) |
3.10 (1.147) |
1.55 (.696) |
1.56 (.918) |
1.44 (.912) |
| Magnitude (Ln lux) | 2.11 (.792) | 2.23 (.827) | 2.01 (.754) | 1.03 (.481) |
1.07 (.652) |
.96 (.620) |
| R2 | .44 (.147) | .46 (.160) | .43 (.144) | .23 (.116) | .23 (.122) | .23 (.135) |
| Mesor (Ln lux) | 2.16 (.655) | 2.23 (.613) | 2.07 (.642) | 1.02 (.357) |
1.03 (.466) |
.96 (.502) |
| M10 value (Ln lux)) | 3.59 (1.159) |
3.75 (1.129) |
3.41 (1.126) |
.952 (.339) |
.98 (.452) | .91 (.469) |
| L5 value (Ln lux)) | .34 (.225) | .30 (.172) | .31 (.194) | .18 (.130) | .20 (.154) | .19 (.141) |
| IS | .56 (.141) | .58 (.152) | .56 (.151) | .36 (.122) | .35 (.131) | .34 (.148) |
| IV | .86 (.324) | .797 (.324) | .88 (.348) | .86 (.283) | .80 (.324) | .88 (.348) |
| Minimum (Ln lux) | .07 (.103) | .06 (.093) | .07 (.101) | .04 (.050) | .06 (.071) | .05 (.052) |
| Maximum (Ln lux)) | 10.58 (1.632) |
10.78 (1.496) |
10.15 (1.795) |
9.21 (1.623) |
9.14 (1.914) |
8.44 (2.021) |
Note: Cosinor analysis – Acrophase, Magnitude, Mesor, R2; NPCRA – M10 midpoint, L5 midpoint, Amplitude, M10 value, L5 value, inter-daily stability (IS), intra-daily variability (IV)
Note: 4 wk, n = 44; 8 and 12 wk, n = 43
Figure 1.
Maternal and infant circadian light pattern displayed with solar light.
Table 2.
Correlations among maternal and infant light and activity.
| Maternal Light: Maternal Activity |
Infant Light: Maternal Light |
Infant Activity: Infant Light |
Infant Activity: Infant Light Controlling Maternal Activity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 wk | 8 wk | 12 wk |
4 wk | 8 wk | 12 wk |
4 wk |
8 wk | 12 wk |
4 | 8 | 12 | ||
| Timing | Acroph ase |
.717 ** |
.746 ** |
.688 * |
.422 ** |
.439 ** |
.693 ** |
.38 5* |
.493 ** |
.686 ** |
.17 7 |
− .04 3 |
.491 ** |
| M10 midpoi nt |
.532 ** |
.367 * |
.453 * |
.674 ** |
.611 ** |
.631 ** |
.28 2 |
.243 | .731 ** |
.11 8 |
.01 8 |
.662 ** |
|
| L5 midpoi nt |
.465 ** |
.781 ** |
.651 ** |
.763 ** |
.332 * |
.399 ** |
.25 9 |
.241 | .337 * |
.15 3 |
.17 8 |
.338 * |
|
| Amplit ude |
Amplitu de |
.137 | .279 | .277 | .570 ** |
.723 ** |
.559 ** |
.30 3* |
.256 | .460 ** |
.25 5 |
.24 5 |
.464 ** |
| Magnit ude |
.105 | .240 | .277 | .535 ** |
.664 ** |
.593 ** |
.27 9 |
.205 | .402 ** |
.24 8 |
.19 3 |
.403 ** |
|
| 24 hr fit |
R2 | .186 | .110 | .322 * |
.380 * |
.526 ** |
.516 ** |
.20 7 |
.138 | .377 * |
.23 1 |
.12 3 |
.347 * |
| Center | Mesor | .112 | .275 | .364 * |
.523 ** |
.694 ** |
.502 ** |
.15 3 |
.324 * |
.384 * |
.14 7 |
.32 0* |
.380 * |
| Midleve l |
− .023 |
.044 | .126 | .516 ** |
.686 ** |
.553 ** |
.07 5 |
.342 * |
.322 | .09 5 |
.34 3* |
.296 | |
| M10 Value |
.106 | .181 | .275 | .549 ** |
.728 ** |
.562 ** |
.26 5 |
.399 * |
.458 ** |
.24 7 |
.39 8* |
.448 * |
|
| L5 Value |
.170 | .371 * |
.009 | .406 ** |
.462 ** |
.333 * |
− .22 5 |
.160 | − .082 |
− .24 4 |
.14 6 |
− .073 |
|
| Regular ity |
IS | .160 | .103 | .427 * |
.312 * |
.519 ** |
.483 ** |
.06 6 |
.232 | .386 * |
.16 4 |
.19 9 |
.378 * |
| IV | .245 | .193 | .164 | .211 | .501 ** |
.214 | .21 1 |
.170 | .119 | .25 4 |
.04 8 |
.130 | |
Note: 4 wk, n = 44; 8 and 12 wk, n = 43
Cosinor – Acrophase, Magnitude, Mesor, R2
NPCRA – M10 midpoint, L5 midpoint, Amplitude, Midlevel, M10 value, L5 value, inter-daily stability (IS), intra-daily variability (IV)
p < .05,
p < .01
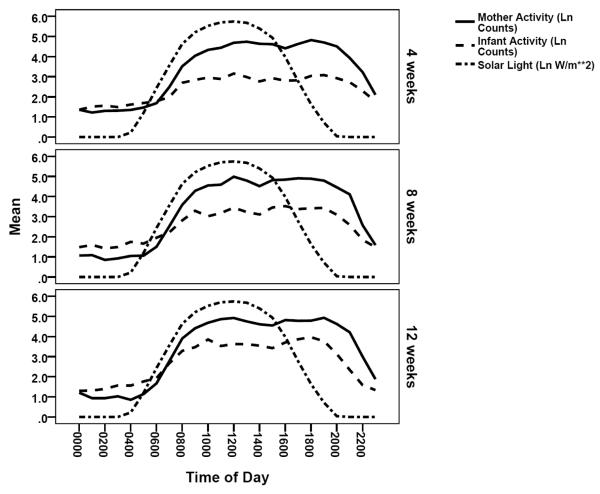
Maternal and infant 24-hour activity are illustrated graphically in Figure 2. The zero order correlations between infant light and activity show significant relations between all parameters, except L5 value and IV, increasing over time, however these correlations must be evaluated with the recognition that mothers are the predominant regulators of light level. Further, infant and maternal activity are interrelated with the possibility that, similar to some mammals, infant rhythm is shaped by the mother before light entrainment is effective. Consequently the relation between infant light and activity was further examined controlling for maternal activity. The partial correlations (Table 2) demonstrate significant correlation between infant light and activity pattern emerging between 8 and 12 weeks of age, suggesting beginning entrainment of infant rhythm. This emergence of light-activity relation between 8 and 12 weeks, with control of maternal activity, is evidenced in timing (acrophase, M10, and L5 midpoint), amplitude (or magnitude), 24-hour fit, and rhythm center (mesor, midlevel).
Figure 2.
Maternal and infant circadian activity pattern displayed with solar light.
When maternal activity is controlled, the partial correlation between infant activity and infant light timing (acrophase, M10 midpoint) is attenuated at weeks 4 and 8, compared to the zero order correlation, with significant partial correlation noted at infant age 12 weeks. The difference in zero order vs. partial correlation is greatest for acrophase (4 weeks, r = 0.385 vs. 0.177; 8 weeks, r = 0.493 vs. −0.043; 12 weeks, r = 0.686 vs 0.491). To further explore possible maternal-infant interaction effect, partial correlation of maternal and infant activity acrophase, controlling for maternal and infant light, was computed. The findings (r = 0.470, 0.500, and 0.638 at 4, 8 and 12 weeks respectively, compared with zero order correlations reported earlier(24) r = 0.514, 0.483, and 0.704) suggest the influence of maternal-infant interaction independent of photo entrainment of cycle timing over the first 12 weeks of life. This finding may implicate maternal activity in entraining infant rhythm in early infancy.
Discussion
Results describing the home lighting environment reflect effects of the constructed environment including predominance of interior lighting and lighting at night (26). Mothers clearly establish the light environment experienced by their infants and, similar to the mother, infants experience a light environment that is not in phase with the solar light pattern. The health implications of such deviation from natural light pattern are implied but not well understood (27), particularly in the developing infant. Findings are consistent with a prior report of close correlation between mother and infant lighting and low light levels in the home (20). While infant light levels were lower than that of mothers, evidence suggests the entraining influence of low light exposure. (28)The increase in correlation between maternal activity and light pattern over time (mesor, R2, and IS) suggest mother’s activity and light patterns, altered in early postpartum, assuming a more regular circadian rhythm. The decline in correlation of maternal and infant and light L5 midpoint likely reflects infant sleep-wake pattern development and differing timing of least active night time periods. In other words, with increasing age the infant’s nighttime sleep matches less closely with that of the mother.
With or without control of maternal activity, infants demonstrated developmental increase over time in the correlations of light with activity amplitude, 24 hour fit, rhythm center, and IS. Control of maternal activity decreased the partial correlation between infant light and activity timing (acrophase, M10 midpoint) with significant correlation occurring by 12 weeks of age. Taken together these findings are evidence of infant maturing circadian rhythm and suggest maternal influence early in life. Infant light entrainment, suggested by the study results, is consistent with reviews of infant rhythm development (7, 9, 22), however there is a lack of study directly depicting photo effects in the home environment. Research has however demonstrated 24-hour diurnal patterns in endocrine function, temperature, as well as sleep-wake pattern during early infant life (29-31). In one study infants 6 to 12 weeks of age exposed to increased afternoon light had increased night time sleep (21). The effect of human maternal activity in shaping infant circadian rhythm has received little attention but may be an essential influence early in life when rhythm is immature.
Our study provides the advantage of longitudinal design depicting developmental changes in infant and maternal light and activity circadian pattern over the first 12 weeks of life in the natural home environment. Additionally we have utilized both cosinor analysis and NPCRA approaches and shown comparable results describing infant and maternal circadian rhythm of light. Several limitation are noted. Photopic light, rather than light in the blue range was measured, however in subjects recorded with the Actiwatch Spectrum monitors we have found very close relation r = .890, mothers; .863, infants) between photopic and blue light. Data collection was not equally distributed throughout a year and seasonal effects were detected, however our focus was the relation between infant light and activity and not seasonality. Our research examines the expressed activity rhythm of mothers and infants and the status of the circadian timing system is inferred from activity, the output rhythm (28). Further the mother and infant form an interactional unit with bidirectional effects. While mothers may predominantly control the light environment, the infant’s activity elicits light and maternal activity as the mother responds to infant care needs.
In summary, infant light and activity rhythm demonstrated correlation increasing between infant age 8 and 12 weeks, suggesting photo entrainment. Mothers predominantly control environmental light. Maternal and infant activity rhythm are closely related and the interaction is bidirectional. Further research is needed examining the lead-lag relationship between mother and infant activity prior to infant photoentrainment. Both light and maternal activity may be used therapeutically to shape infant developing circadian rhythm.
Acknowledgements
Funded by NICHD R21 HD068597-01A and P30 NR011400
Footnotes
Disclosure
The authors have no financial conflict of interest to report.
Contributor Information
Karen A. Thomas, Department of Family and Child Nursing University of Washington Seattle, WA 98195-7262.
Robert L. Burr, Department of Biobehavioral Nursing and Health Systems University of Washington Seattle, WA 98195-7266.
Susan Spieker, Barnard Center for Infant Mental Health & Development Department of Family and Child Nursing University of Washington Seattle, WA 98195-7262.
References
- 1.Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007;48(3-4):329–54. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 2.Feldman R. From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Dev Psychol. 2006;42(1):175–88. doi: 10.1037/0012-1649.42.1.175. [DOI] [PubMed] [Google Scholar]
- 3.Brooks E, Canal MM. Development of circadian rhythms: role of postnatal light environment. Neurosci Biobehav Rev. 2013;37(4):551–60. doi: 10.1016/j.neubiorev.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Seron-Ferre M, Torres C, Parraguez VH, Vergara M, Valladares L, Forcelledo ML, et al. Perinatal neuroendocrine regulation. Development of the circadian time-keeping system. MolCell Endocrinol. 2002;186(2):169–73. doi: 10.1016/s0303-7207(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 5.Hao H, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Neurobiology. 1999;96:2426–9. doi: 10.1073/pnas.96.5.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivkees SA, Hao H. Developing circadian rhythmicity. Semin Perinatol. 2000;24(4):232–42. doi: 10.1053/sper.2000.8598. [DOI] [PubMed] [Google Scholar]
- 7.Rivkees SA. Developing circadian rhythmicity in infants. Pediatrics. 2003;112(2):373–81. doi: 10.1542/peds.112.2.373. [DOI] [PubMed] [Google Scholar]
- 8.Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7(4):321–34. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 9.Seron-Ferre M, Torres-Farfan C, Forcelledo ML, Valenzuela GJ. The development of circadian rhythms in the fetus and neonate. SeminPerinatol. 2001;25(6):363–70. doi: 10.1053/sper.2001.29037. [DOI] [PubMed] [Google Scholar]
- 10.Joseph D, Chong NW, Shanks ME, Rosato E, Taub NA, Petersen SA, et al. Getting rhythm: how do babies do it? Arch Dis Child Fetal Neonatal Ed. 2015;100(1):F50–4. doi: 10.1136/archdischild-2014-306104. [DOI] [PubMed] [Google Scholar]
- 11.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242(4875):78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- 13.Kennaway DJ. Melatonin and development: physiology and pharmacology. Semin Perinatol. 2000;24(4):258–66. doi: 10.1053/sper.2000.8594. [DOI] [PubMed] [Google Scholar]
- 14.Kennaway DJ, Goble FC, Stamp GE. Factors influencing the development of melatonin rhythmicity in humans. J Clin Endocrinol Metab. 1996;81(4):1525–32. doi: 10.1210/jcem.81.4.8636362. [DOI] [PubMed] [Google Scholar]
- 15.Ardura J, Gutierrez R, Andres J, Agapito T. Emergence and evolution of the circadian rhythm of melatonin in children. Horm Res. 2003;59(2):66–72. doi: 10.1159/000068571. [DOI] [PubMed] [Google Scholar]
- 16.Sivan Y, Laudon M, Tauman R, Zisapel N. Melatonin production in healthy infants: evidence for seasonal variations. Pediatr Res. 2001;49(1):63–8. doi: 10.1203/00006450-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Rivkees SA, Mayes L, Jacobs H, Gross I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 2004;113(4):833–9. doi: 10.1542/peds.113.4.833. [DOI] [PubMed] [Google Scholar]
- 18.Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks' gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr. 2002;140(2):192–9. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- 19.Mirmiran M, Ariagno RL. Influence of light in the NICU on the development of circadian rhythms in preterm infants. Semin Perinatol. 2000;24(4):247–57. doi: 10.1053/sper.2000.8593. [DOI] [PubMed] [Google Scholar]
- 20.Tsai SY, Barnard KE, Lentz MJ, Thomas KA. Twenty-four hours light exposure experiences in postpartum women and their 2-10-week-old infants: An intensive within-subject design pilot study. Int J Nurs Stud. 2009:181–8. doi: 10.1016/j.ijnurstu.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Harrison Y. The relationship between daytime exposure to light and night-time sleep in 6-12-week-old infants. J Sleep Res. 2004;13(4):345–52. doi: 10.1111/j.1365-2869.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 22.Christ E, Korf HW, von Gall C. When does it start ticking? Ontogenetic development of the mammalian circadian system. Prog Brain Res. 2012;199:105–18. doi: 10.1016/B978-0-444-59427-3.00006-X. [DOI] [PubMed] [Google Scholar]
- 23.Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc. 2004;79(3):533–56. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- 24.Thomas KA, Burr RL, Spieker S, Lee J, Chen J. Mother-infant circadian rhythm: development of individual patterns and dyadic synchrony. Early Hum Dev. 2014;90(12):885–90. doi: 10.1016/j.earlhumdev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas KA, Burr RL, Spieker S. Maternal and infant activity: Analytic approaches for the study of circadian rhythm. Infant Behav Dev. 2015;41:80–7. doi: 10.1016/j.infbeh.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright KP, Jr., McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23(16):1554–8. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter R, Hardeland R, Rol MA, et al. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014;15(12):23448–500. doi: 10.3390/ijms151223448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffy JF, Wright KP., Jr. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326–38. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 29.McMillen IC, Kok JSM, Adamson TM, Deayton JM, Nowak R. Development of circadian sleep-wake rhythms in preterm and full-term infants. Pediatr Res. 1991;29(4):381–4. doi: 10.1203/00006450-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Zornoza-Moreno M, Fuentes-Hernandez S, Sanchez-Solis M, Rol MA, Larque E, Madrid JA. Assessment of circadian rhythms of both skin temperature and motor activity in infants during the first 6 months of life. Chronobiol Int. 2011;28(4):330–7. doi: 10.3109/07420528.2011.565895. [DOI] [PubMed] [Google Scholar]
- 31.de Weerth C, Zijl RH, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Hum Dev. 2003;73(1-2):39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]