Abstract
Norway rats (Rattus norvegicus) living in urban environments are a critical public health and economic problem, particularly in urban slums where residents are at a higher risk for rat borne diseases, yet convenient methods to quantitatively assess population sizes are lacking. We evaluated track plates as a method to determine rat distribution and relative abundance in a complex urban slum environment by correlating the presence and intensity of rat-specific marks on track plates with findings from rat infestation surveys and trapping of rats to population exhaustion. To integrate the zero-inflated track plate data we developed a two-component mixture model with one binary and one censored continuous component. Track plate mark-intensity was highly correlated with signs of rodent infestation (all coefficients between 0.61 and 0.79 and all p-values < 0.05). Moreover, the mean level of pre-trapping rat-mark intensity on plates was significantly associated with the number of rats captured subsequently (Odds ratio1.38; 95% CI 1.19-1.61) and declined significantly following trapping (Odds ratio 0.86; 95% CI 0.78-0.95). Track plates provided robust proxy measurements of rat abundance and distribution and detected rat presence even when populations appeared ‘trapped out’. Tracking plates are relatively easy and inexpensive methods that can be used to intensively sample settings such as urban slums, where traditional trapping or mark-recapture studies are impossible to implement, and therefore the results can inform and assess the impact of targeted urban rodent control campaigns.
Keywords: indirect abundance, Rattus norvegicus, track plates, urban slum, zero-inflated, zoonotic diseases
Introduction
Rodents are a major public health and economic concern in urban environments and are capable of harboring a variety of helminthic, viral, and bacterial zoonotic pathogens (Costa et al. 2014a; Costa et al. 2015; Davis et al. 2005; Himsworth et al. 2013b; Krojgaard et al. 2009; Mills and Childs 1998). The rapid growth of slum settlements globally has created new and expanding habitats for rats, most notably the Norway rat, Rattus norvegicus, and has increased the burden of some rat-borne diseases in these vulnerable areas (Cavia et al. 2009; Himsworth et al. 2013b; Mills and Childs 1998). Norway rats thrive in peri-urban and urban environments and are therefore of particular importance for slum communities. For example, the Norway rat is a major reservoir host for Leptospira spp. that causes leptospirosis in the urban slum setting (Barocchi et al. 2001; Costa et al. 2014a; Costa et al. 2014b; Costa et al. 2015; Ko et al. 2009; Ko et al. 1999). In Brazil alone, this zoonotic disease is responsible for more than 10,000 cases reported annually (Brazil 2005; Maskey et al. 2006) and causes seasonal outbreaks in slum settlements (Flannery et al. 2001; Ko et al. 1999; Sarkar et al. 2002).
Despite the importance of urban rats, little is known about their distribution, abundance and demography, especially in tropical urban settings, and the contribution of these factors to zoonotic disease transmission. Epidemiological studies in Brazil found that leptospirosis is associated with rodent infestation in peri-domicilary areas within slum (favela) communities (Maciel et al. 2008; Reis et al. 2008b; Sarkar et al. 2002). Residents of these areas who reported seeing five or more rodents in their household environment were at significantly higher risk of leptospirosis (Reis et al. 2008a; Sarkar et al. 2002), as were residents residing in un-plastered homes where rat feces and burrows were present (Costa et al. 2014b). Although ecological factors associated with the rat population likely play a major role in pathogen maintenance and prevalence of leptospiral infection (Costa et al. 2014a; Costa et al. 2014b), the dynamics of rat abundance across space and time have not been well-delineated, which in turn has hampered identification effective rodent control strategies in complex urban settings.
A critical barrier to assessing the role of urban rat population dynamics on transmission of leptospires, or any other rat-borne pathogen, is the lack of reliable measures of rodent abundance. Based on rat trapping results, the size of populations can be estimated (Brown 1969; Mills JN 1995; Sheppe 1967); however, trapping of animals to obtain these metrics is time, cost, and labor intensive (Emlen et al. 1949). Absolute rat abundance, the exact enumeration of all individuals in a given area, is rarely possible (Davis 1953; Davis et al. 1948). The few estimates available depend on capture-mark-recapture methods [e.g. (Glass et al. 1988)]. However trap shyness and trap habituation can alter rat behavior and confound the accurate interpretation of abundance obtained from these studies (Emlen et al. 1949; Leslie and Davis 1939). Other indices of relative abundance, such as catch per unit effort or trap success, have frequently been used as indices of rat abundance (Glass et al. 1988; Himsworth et al. 2013a; Lord et al. 1971; Villafane et al. 2013), but results from these studies are subject to the same limitations imposed by variability in rat behavior and can generate inconsistent results (McKelvey and Pearson 2001). Additionally, fine scale sampling at spatial resolutions of <10m between placements of non-kill traps requires a large number of often expensive traps, and trap loss when sampling within high density urban environments is a major concern. As alternatives, a number of proxies of abundance have been used, that can give estimates of relative abundance, that is, estimates that are related to absolute abundance by an unknown multiplier, but can be used for comparative purposes.
Simpler and more easily applied methodologies (such as systematic surveys for signs of rodent infestation and use of track plates) have been recommended for urban areas to evaluate the effectiveness of control programs (CDC 2006). Surveys of signs of rodent infestation, such as rodent runs, feces, and burrows, are widely used (de Masi et al. 2009; Glass et al. 1997; Lambropoulos et al. 1999). However, these require systematic training of personnel, and the quality and consistency of results are subject to variation due to the reliance on independent surveyors and their level of experience (Allen and Engeman 2014; Lord 1983; Whisson et al. 2005). Additionally, attempts to associate rodent-survey results with measures of absolute abundance have failed to obtain conclusive results (Lord 1983). Track plates, where rodent tracks and markings are recorded on plates covered in ink or powder, can provide measures of rodent mark intensity and relative abundance and present an attractive alternative to trapping and surveying techniques (Connors et al. 2005; Lord 1983; Lord et al. 1971; Quy et al. 1994; Sheppe 1965).
Track plate methods have been used successfully to quantify reductions in Norway rat mark intensity following a trapping campaign on farms (Quy et al. 1994), and to monitor seasonal abundance of rats in one urban area (Promkerd et al. 2008). However, previous studies using track plates reported only rodent paw print marks (Brown 1969; Connors et al. 2005; Glennon et al. 2002; Nams and Gillis 2003; Quy et al. 1994; Quy et al. 1993) and evaluated changes in rat populations at large spatial scale, which limited the applicability of these findings to local population dynamics and rodent control. Methodological limitations in quantification (i.e. accurately identifying rat signs and censoring unspecific signs) and in analysis of the data have precluded track plate applications to monitoring rat population changes at finer geographical scales. Particularly, tailored statistical analyses are necessary not only to accommodate zero-inflated data, a common output of these studies, but also to account for varying rodent mark intensity over short distances (Himsworth et al. 2014).
As a first step towards densely sampling rat abundance throughout an urban slum, we developed a track plate method and statistical framework to predict both the probability of rat presence and rat intensity as a proxy for rat abundance in an urban slum community in Salvador, Brazil. Our model captured the variability of rodent relative abundance throughout an urban slum community and identified hot spots of rodent infestation. The results of this study can be used to improve targeted rodent control interventions and further elucidate Norway rat population dynamics within urban slums at fine spatial resolution.
Methods and Preliminary Results
Results obtained from initial studies designed simply to refine methodologies are reported in the Online Resources and noted in the Methods sections below, rather than being reported in the Results.
Study Area
The city of Salvador, with 2.7 million inhabitants, is located on the northeast coast of Brazil (12° 55′ 34″ southern latitude and 38° 31′ 12″ western longitude) and is the third largest city in the country. Salvador has a subtropical climate and temperatures are relatively constant across the year. During the wet season from April-July heavy rainfall is common (mean 272.2 mm/mo) while during the relatively dry season from September - December rainfall is much reduced (mean 124.2 mm/mo).
This study was performed in the slum community of Pau da Lima, which has been described in detail previously (Reis, 2008). Briefly, the area comprises a series of valleys and has a high human population density. Community members are mainly squatters (88%) with low level of education (66% did not finish primary school) and low income (mean per capita daily household income, US$ 2.60) (Riley LW 2007). Lack of structural planning (Fig. 1a) and the lack of basic sanitation (e.g. open sewers) and trash collection are characteristics of this community (Fig. 1b).
Fig. 1.
The densely populated urban slum dwellers of Pau da Lima in Salvador, Brazil, reside in a heterogeneous landscape demarcated by steep slopes and valleys (panel 1A). Track plates were placed near the valley floor where open sewers (1B), poorly constructed houses and limited sanitation services provide abundant resources for Norway rats (1C). Rat signs, including paw prints, tail slides and scratching are readily visible on a track plate (1D) and were scored for activity/abundance metrics
Assessing the types of rat markings on track plates
Given the lack of previous comparative studies, three types of weather resistant track plate methods were evaluated for use in urban slum environments: lampblack and methyl alcohol (Sherpherd and Greaves 1984), equal parts of black ink and canola oil (Lord 1971), and a graphite mixture (Conor 2005). The lampblack and ink solutions were applied to 0.2m by 0.2m polyvinyl floor tiles using a paint roller; the graphite solution was applied to an acetate sheet. We assessed the time each mixture took to dry and the homogeneity of the paint coverage on the tiles or acetate sheets. We evaluated the mixtures’ ability to resist water by pouring 100mL of water over the dried plates as well as during different levels of rainfall when set outdoors. The lampblack plates performed best in the prevailing conditions. These plates dried more rapidly (< 5min) compared to ink/oil and graphite (15min and 24hs, respectively). Additionally, lampblack application produced a uniform, homogeneous dark surface cover, which generated clearly defined rat-specific marks.
As previous studies documented only rodent paw prints and rats leave additional specific marks, such as tail slides and scratches (CDC 2006), we assessed our ability to detect multiple types of marks left by rats in slum areas. In a single household with known high levels of rat infestation, eight sausage-baited tomahawk traps were left overnight with two track plates placed inside the traps and a single track plate placed at the entrance of each trap. The traps were either ‘exposed’ or ‘shielded’ from rats. Four traps were baited and wired open (exposed), two traps were set to catch rats (exposed), and two traps were baited, but wired closed (shielded). Eighty three percent (10/12) of all track plates in the baited and wired open group showed evidence of rat marks, and plates within the two triggered traps (traps not wired open) were marked and successfully captured Norway rats. The shielded track plates remained unmarked. Based on this trial, we identified three specific types of rat marks: rat paw prints (Fig. 1c and 1d, Online Resource Fig. 1a), tail marks (Fig. 1c and d, Online Resource Fig. 1b) and rat scratches (Online Resource Fig. 1c), and subsequently, all track plates with these marks were considered positive for rat markings.
Scoring track plates and sampling design
Prior to assessing the effects of rat removal on track plate mark intensity, we developed methods to score track plates in field conditions. We used a binary variable (presence/absence of rat marks on a plate) and a continuous variable (the intensity of marks on plates) to score track plates. A total of 100 track plates were placed in 10 houses (10 track plates per house). Five of the houses had high rodent infestation and 5 had low levels of rodent infestation. Track plates were placed for three nights in houses with low rat infestation or four nights in houses with high rat infestation in areas likely to be frequented by rats (along rat runs, against walls, near food or water resources).
Track plates were collected the morning after placement, identified by location site, and photographed. After imposing a 5×5 grid over the photographs, two independent reviewers assessed the presence of marks on the plate and scored each grid for rat-specific marks, providing 25 data points per plate. Initially, training was performed among three reviewers to ensure accurate identification of rat signs and non-specific markings. When discrepancies were found (greater than 3 score difference between reviewers) both track readers reviewed the plates and reached a consensus, which improved the concordance of future scoring. The level of agreement between the results obtained by the two trained reviewers was assessed using the kappa method (Landis and Koch 1977) and agreement was excellent (kappa = 0.90). We additionally assessed the correlation between the two scorers (r2 = 0.75, p < 0.0001) and found very high agreement. Marks other than those of rats (chickens, dogs, opossums and unknown blowing debris) were observed on the plates, Plates were censored when >70% of the area either within the grid or the plate was unreadable for specific rat-marks (ie the lampblack ink was completely wiped off, and no marks were distinguishable)(Online Resource Fig 2).
To evaluate the number of track plates and the number of nights required to accurately capture variability in rat activity between slum households, we varied the number of tracking boards and number of days placed. Boards were placed for 1, 3, 4, 5, and 7 days, and we placed plates in groups of 3, 5, 7, and 10 boards per house. The general aim was to select a combination of number of nights and number of track plates that provided an acceptably low standard deviation across the plates, while taking into consideration the effort for data collection. The methodology and results of this are summarized in Online Resource Figure 3. Based on these results, and to allow field teams to examine fine scale rodent activity per house and still cover large areas in a time- and resource-efficient manner, we opted to place 5 track plates per house for 3 days.
Track plates as a proxy for the number of rats captured and their association with rodent infestation
We examined the relationship between the number of rats captured in a trapping campaign designed to ‘exhaust’ the population (remove the local rat population to estimate population size by the catch per unit method: CPUE (Efford 2004)) and the track plate scores around households before and after that campaign. We used data from previous rat studies to inform our sampling scheme and identify areas where rats were previously captured to calibrate the model (Costa et al. 2014a; Porter et al. 2015). Initially, 40 sites (locations at least 15m apart) within the study area were selected as potential trapping sites by spatial randomization incorporating spatial heterogeneity throughout Pau da Lima, and of these, 10 sites were chosen with the highest rat-capture success from previous sampling. Within each site, three randomly selected households (a triad; total of 30 households) were identified an effective sampling area of 450m.
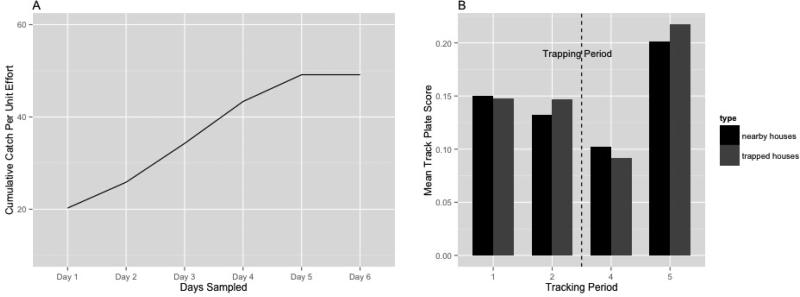
Five plates were placed within15m of each household for three days during each of four tracking board periods: periods 1 and 2 before the trapping period (days 1- 3 and 20 - 22, respectively) and periods 3 and 4 after trapping (days 34-36 and 61-63), giving a potential total of 1200 track-plate nights (Fig. 2). Results were not significantly different between periods 1 and 2, so results were pooled for analyses. Following the pre-trapping period two households of a triad were selected as “trapped households” (n=20), where both track plates and traps were placed. In the third house at each site (n=10) only track plates were set. These “nearby” houses enabled us to observe the impact of the trapping in the immediate neighborhood. Track plates were evaluated each morning and replaced whenever marks were present. Rodent trapping was performed at trapped households by setting two tomahawk traps per house for 6 days (days 27-32, a total of 240 trap-nights; Fig. 2) and the number of rats caught per household was recorded. Each morning, traps containing rats were removed and new traps were set (below). No pre-baiting of the traps was utilized.
Fig. 2.
Timeline of the track plate and trapping experiment conducted in Pau da Lima
The CPUE was computed across the 6 days of trapping to evaluate whether we exhausted the rat population within the study area (CPUE reached an asymptote). The cumulative CPUE was calculated by dividing the cumulative number of captured rats by the cumulative sampling effort. The sampling effort each day was computed as the number of traps containing rats or not sprung plus half the number of traps that were found to be sprung but empty (Borchers et al. 2002).
Simultaneously with tracking period 1, an interview with the head of the household and a visual survey of rodent signs were performed. During the interview, data on the number of rat sightings at night during the last month were collected (Reis et al. 2008b). The visual survey was performed by a trained team from the Zoonosis Control Center (Salvador Municipal Secretary of Health), using a form previously adapted from the Centers for Disease Control for urban settings (CDC 2006), to record peri-domestic rat signs (rat burrows, trails and feces). Details of this form were previously described in Costa et al 2014, Table S1. All individuals who participated in rodent surveys were considered experts in rodenticide campaigns and had extensive experience in performing infestation surveys. Rat burrows were identified as fist sized holes with signs of rodent activity (typically with defined trails leading from the entrances and no rubbish or cobwebs present at entrance). Trails were identified as well worn areas at least 10cm wide, typically leading from burrows or other foraging sites. Feces were identified according to CDC recommendations, and when possible the species were identified as Rattus norvegicus or Rattus rattus (CDC 2006).
We evaluated the association between track plate scores and other signs of rodent infestation using a correlation matrix for a subset of 13 households where trapping and extensive rodent surveys took place. Additionally, since the variables were evaluated on different scales, we performed a Principal Components Analyses (PCA) using the correlation matrix rather than the covariance matrix.
Development of a Statistical Method to Analyze Track Plate Data
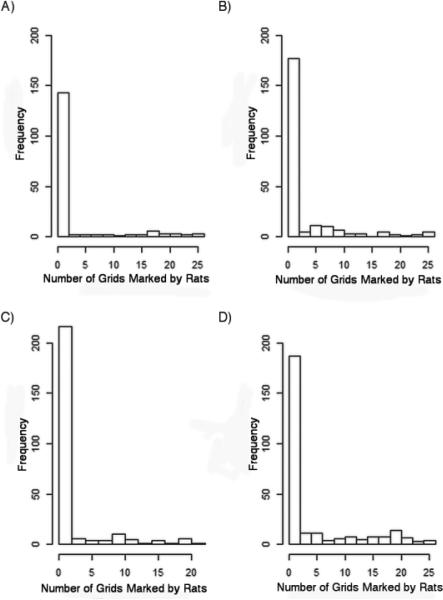
The distribution of grid-score data was zero-inflated and heavily right-skewed (Fig. 3) and we were not able to apply a linear modeling approach incorporating random effects as previously described (Whisson et al. 2005). As previously described methodologies limit the use of spiked zero data to binomial analysis (Welsh et al. 1996), we developed a mixed model that separately assessed the probability of the presence of rats (dichotomous) and intensity levels (continuous) of rat markings (for full details of the model see Online Resource 1). In brief, our model considered the probability of the presence or absence of a rat (θ and 1- θ in Online Resource 1) and a measure of the intensity of rat markings (λ; the proportion of the plate with marked cells) as an interval-censored underlying continuous measure. For example, a plate with 5 out of 25 marked cells (observed proportion 0.20) was assigned to an underlying continuous score binned to the interval of 0.18 to 0.22. The model also weighted observations by the number of censored cells, so that a plate with 25 readable grids would be assigned a higher weight than a plate scored out of only 20 grids where 5 grids were censored. The variation in the underlying continuous score was considered to have a gamma distribution as it proved to be sufficiently flexible to fit our data, but, in principle, any continuous distribution could be used.
Fig. 3.
Histograms of track plate mark intensity, recorded as a score out of 25 (number of grids potentially marked by rats) for each of the four sampling periods; a) details the distribution of track plate intensity for tracking period 1, b) details the distribution of track plate intensity for tracking period 2, c) details the distribution of the track plate intensity for period 4, and d) details the distribution of the track plate intensity for period 5. The frequencies across the time periods are unequal due to missing data outlined in the results
Lamda (λ) and θ were modeled as logistic and log-linear regressions, respectively. Maximum likelihood methods were used to estimate the effect on each of total trap counts at the house level and observation period. As the number of track plates (and hence the number of track-plate nights of data) varied to some degree, a model was developed taking into account site as a ten-level factor (see above), trap count per house, and an interaction between the trap count and the observation period (a three-level factor: periods 1 and 2 combined, 4, and 5). The presence or absence of rats and levels of rat intensity were assumed to be measures of the same underlying phenomenon, and so the same parameterization was used for both. The analysis was implemented using R: an R package zig is available from the authors to perform parameter estimation using the zero inflated gamma model.
Results
Association between tracking plates, rodent surveys, household rat-sightings, and trapping
Track plate scores were highly correlated with the number of rats captured (0.77, p = 0.002) and visual signs of rodent infestation (correlation 0.83, p < 0.001 with any rat sign) and showed strong associations with independent measures of the presence of feces, burrows, and trails (all coefficients between 0.61 and 0.79 and all p-values < 0.05; see Online Resource 2 Table 1). The highest association between plates and rat signs were the presence of rat trails (r = 0.79, p = 0.001). Track plate scores were less strongly correlated with rat sightings (r = 0.38, p = 0.2). However, rat sightings did not correlate well with any signs of rodent infestation or number of rats captured (r between 0.18 and 0.38 and all p-values > 0.05).
In the PCA that evaluated the association of track plate scores, signs of rodent infestation, and trapping results in period 1 of the experiment, the first principal component explained 60% of the variation. All loadings in this component had the same sign. All variables contributed similarly to the variation (loadings between 0.40 and 0.49) except for the variable of reported rat sightings (loading of 0.23), indicating that track plate scores were positively associated with all other indicators of rodent infestation, but the association was weakest with reported rat sightings (Online Resource 2 Table 2). There were no substantial trends in the remaining components, which accounted for the remaining variation (40%).
Track plates as a proxy for the number of rats captured
By the end of the trapping period, a total of 18 rats were captured in 10 out of the 20 houses sampled. The CPUE approached an asymptote by day 4, indicating that the rat population was being trapped out (Fig. 4a). Overall, we collected information from 88.5% of track plates. The remaining plates were lost or removed by residents. Also, during period 1, the field data collected from the third day were lost for technical reasons. Rat mark intensity assessed by track plates during the experiment is summarized in Fig. 4b.
Fig. 4.
Figures of rats captured at the two-trapped households (both traps and plates set: panel 2A) and the mean track plate scores for the trapped and the nearby households (only plates) households are similar, indicating that the presence of traps did not influence subsequent rat activity/abundance. A) Data for rats trapped during period 3 (excluded for clarity from panel B), after baseline track plate sampling (periods 1 & 2: Figure 2B), show an asymptotic curve based on the Catch Per Unit Effort function, suggesting the population was exhausted. The mean level of rat activity declined significantly immediately after trapping (period 4) but rebounded 4 weeks after the trapping (period 5) for both experimental and control groups.
Prior to rat trapping (Periods 1 & 2; 522 track nights), 38% of the houses with track plates had rat markings and the mean level of rat mark intensity was 0.144. Further, both trapped and nearby houses had similar levels of rat mark intensity (0.142 and 0.147). Indeed, throughout, the track board mark intensity in both trapped and nearby houses followed similar patterns (Fig. 4). Using our modeling approach, we compared the track plate metrics for the tracking periods 3 and 4 to the combined tracking periods 1 and 2 in both trapped and nearby houses (Online resource Table 3). In houses where trapping took place, the mean level of rat mark intensity (0.795 CI 0.627, 1.009) and the presence/absence of rats (0.712 CI 0.478, 1.062) decreased immediately following trapping (Online resource Table 3), as it did for the intensity of rat marks in nearby houses (0.769 CI 0.551- 1.073). However, four weeks after trapping, the presence/absence of rats increased significantly in both trapped (1.783 CI1.229, 2.586) and nearby houses (1.863 CI 1.144, 3.035) (Online resource Table 3).
When assessing the association between rats captured and track plate intensity pre- and post-trapping, for the pre-trapping periods, the mean level of rat mark intensity increased by a factor of 1.38 (95% CI 1.19-1.61), for each rat captured during the subsequent trapping session (Table 1). The relative odds of rats being present on tracking boards for the same time period was 2.54 times higher (95% CI 1.93-3.33) for each unit increase in the number of rats captured. The estimate for the interaction between the number of rats captured and post-trapping rat mark intensity (period 4) was below one (Factor 0.86, CI 0.78-095), indicating that track plate mark intensity in a house decreased after trapping in that house (Table 1). Four weeks after trapping (period 5) we observed a rapid increase in track plate intensity following the exhaustive trapping (see Fig. 4b). At period 5 (4 weeks after trapping) there were no significant interaction between the number of rats captured and the intensity of rat marks or rat presence.
Table 1.
Association between rats captured and track plate intensity pre- and post-trapping. The table describes the two-component model fit summarized by parameter estimates and 95% confidence intervals. The model measures rat activity as a continuous measure (mean level of rat mark intensity) and the presence/absence of rats (odds of rat marks being present) as a binomial measure.
| Time Period | Association Between Number of Rats Captured and Track Plate Metrics | |
|---|---|---|
| Mean Level of Rat Mark Intensity Factor (95% CI) | Presence/Absence of Rat Marks Relative odds (95% CI) | |
| Immediately Before Trapping | 1.38 (1.19, 1.61) | 2.54 (1.93, 3.33) |
| Immediately After Trapping | 0.86 (0.78, 0.95) | 0.96 (0.75, 1.23) |
| Four Weeks After Trapping | 0.98 (0.88, 1.08) | 1.18 (0.91, 1.52) |
Discussion
Previous track plate studies monitoring rodents have primarily been conducted in undisturbed (Brown 1969; Connors et al. 2005; Drennan et al. 1998; Glennon et al. 2002; Nams and Gillis 2003; Sheppe 1965; Sheppe 1967; Taylor and Raphael 1988) or rural environments (Lord 1983; Lord et al. 1971; Quy et al. 1993). Studies in Mexico (Lord 1983) and Lao PDR (Promkerd et al. 2008) at the municipal and village level, respectively, are rare examples using track plates in urban settings. However, no studies have focused on urban slums or evaluated the use of track plates at the household level, though these areas are critical for evaluating an individual resident's risk for acquiring a rat-borne disease, such as leptospirosis, as demonstrated by numerous studies in our Pau de Lima study site in Salvador (Kajdacsi et al. 2013; Ko et al. 1999; Reis et al. 2008b). Track plates can be easily distributed throughout urban habitats in areas where it would be impossible to place traps allowing for broad spatial coverage. Scores were not only strongly correlated with proxies for rodent abundance (rodent signs and trapping) at the household level, but also were sensitive enough to differentiate between relative levels of abundance before and after trapping. Track plates also detected the presence of rats even when the CPUE curve suggested that the rat population had been trapped out. Furthermore, this methodology, although targeted at Norway rat-borne leptospirosis, provides a means for the study of other rat-borne pathogens in rural and urban settings, which include Seoul virus, hantavirus, plague, bartonellosis, and toxoplasmosis present in Norway rats (Childs et al. 1995; Costa et al. 2014a).
As noted above, abundance estimates obtained through trapping do not account for variability in rat behavior, such as neo-phobia and trap avoidance, although our nearby sites suggest that trapping did not influence rat activity at household triads. Both the intensity of rat marks and the binary index of presence/absence of specific rat markings on track plates were associated with the number of rats trapped. These findings are consistent with previous studies performed in other environmental settings (Brown 1969; Dickman 1986; Drennan et al. 1998; Glennon et al. 2002; Lord et al. 1971; Promkerd et al. 2008; Quy et al. 1994; Quy et al. 1993) and support the use of this methodology in urban slums. Furthermore, as noted above, track plates detected the presence of rats even when the rat population appeared to have been trapped out. The finding that rat mark intensity scores rebounded four weeks after trapping to levels comparable or higher than pre-trap values clearly indicates that a considerable rat population remained after apparent ‘exhaustive’ trapping efforts, an effect that is consistent in temperate cities (Lambropoulos et al. 1999; Leslie and Davis 1939).
Track plate methods are appropriate for evaluating the effectiveness of integrated pest-management program and the success of rodent control programs using targeted placement of rodenticides (Engeman and Witmer 2000), even if only presence/absence data are scored. Municipal zoonotic control centers, such as the CCZ in Salvador, implement rodent control for the prevention of leptospirosis and may target interventions at groups of households in and surrounding that residence at which a case of leptospirosis has been identified. These efforts have been associated with reductions in the level of rodent infestation as evaluated by rodent signs (de Masi et al. 2009), but the extent to which rat populations have been controlled and the period of control and rate of population recovery has not been assessed.
In particular, re-infestation or the “boomerang effect”, whereby rodents quickly recolonize intervention areas is not well understood (de Masi et al. 2009; Smith 1963). This effect has been documented in both rural and urban studies and is critical to the timing and implementation of rodent control strategies (de Masi et al. 2009; Smith 1963). Genetic studies have demonstrated that both uncontrolled remnants of the pre-existing rat population and immigration from other locations have a role in population recovery of R. norvegicus in urban areas where rodent control has been implemented (Gardner-Santana et al. 2009; Kajdacsi et al. 2013). Herein, we ascertained that immediately post-trapping there was a decrease in track plate scores, but just four weeks after trapping, the scores had returned to previous levels, suggesting rapid recolonization of the study areas. Track plates may therefore provide an easily applied metric to evaluate the effectiveness of rodent control interventions and recolonization events, particularly in difficult to sample urban environments.
Analyzing data sets with zero-inflated data, such as the track plate data, has been problematic in the fields of ecology (Welsh et al. 1996; Wenger and Freeman 2008), epidemiology (Conceicao et al. 2013) and other areas of research (Lambert 1992). It is not desirable to solve these problems by discarding information from data with a mixed distribution when many resources have been used to collect experimental data. To address this challenge, we developed a model based on the two main features of the data: the spike at zero and the smoothly-varying distribution over positive values. Using our model, we quantified the two phenomena of interest – the presence or absence of rats in the urban slums of Salvador and the level of rat intensity –without forcing the data into an inappropriate parametric framework. This framework enabled us to prioritize the biological features of interest, namely the presence of rats and the intensity of rats within a given area. Rather than using other regression-based techniques, this model, tailored to the biological outcomes of presence and abundance, enabled us to utilize all data generated from the track plates and monitor abundance throughout a complex urban setting.
One concern with track plate studies is their ability to accurately assess the abundance of rodents and not simply activity defined as the movement patterns of an individual rat – a two-fold difference in abundance and a twofold difference in activity with the same abundance would not be distinguishable with track plates. For example, an increase in track plate scores post-trapping may be due to an increase in foraging behavior of un-trapped rodents, owing to the lessening of social constraints. However, the high correlation found between signs of rodent infestation and track plate scores suggests that track plates are measuring the degree of infestation and therefore the relative abundance of rodent populations. Similarly, while track plates are measuring some combination of activity and abundance (Allen and Engeman 2014), the significant association found between track plate scores and number of rats captured indicates that track plates are measuring a metric of abundance and not solely rat activity. This finding is consistent with other studies that found positive associations with rat abundance and track plate scores (Drennan et al. 1998; Glennon et al. 2002; Lord 1983; Quy et al. 1993).
We observed a decrease in rat intensity in nearby households where no trapping occurred (following the same pattern as trapped households). In fact, rat mark intensity in nearby houses followed the same trends as trapped households throughout the study period. In urban environments, rats can move longer distances (Kajdacsi et al. 2013); therefore it is likely that rats caught in trapped households were also circulating in nearby households, causing the reduction in those houses. Track plate data from locations distant to trapped and nearby sites were not used in this experiment, which limited options for a direct control group. In future efforts, such data will be collected. However, our findings were robust across the triads of three houses and indicate that household-based intervention methods, aimed at reducing zoonotic pathogen transmission to humans, can be better informed by this simple methodology that can be used alone, in conjunction with rodent survey methods, and as a complement to traditional rat trapping-abundance measures. Given these characteristics, our methodology will facilitate studies that aim to provide a better understanding of the ecology of urban rodents and the risk of spill-over of zoonotic infection from these reservoir hosts, and inform evidence-based rodent control campaigns within vulnerable urban areas.
Supplementary Material
Acknowledgments
We would like to thank team member from Oswaldo Cruz Foundation, Iohama Paim, Arsinoe Pertile as well as the Zoonosis Control Center Luciano Souza Lima, Lucineide Xavier, and Sheila Cova who participated in the data collection for the study. We would also like to thank Nivison Nery Jr. for their assistance with database processing and management and Steven Belmain for his advice in designing the study. This work could not be accomplished without the joint collaborative effort of the resident associations, community leaders and residents, which constitute the Urban Health Council of Pau da Lima. We would like to thank the Global Leptospirosis Environmental Action Network (GLEAN). This work was supported by the Oswaldo Cruz Foundation and Secretariat of Health Surveillance, Brazilian Ministry of Health, the Yale Institute for Biospheric Studies Doctoral Dissertation Improvement program, the Fulbright Fellowship program, the National Institutes of Health (grants F31 AI114245, R01 AI052473, U01 AI088752, R01 TW009504, and R25 TW009338) and by the Wellcome Trust [102330/Z/13/Z].
References
- Allen LR, Engeman RM. Evaluating and validating abundance monitoring methods in the absence of populations of known size: review and application to a passive tracking index Environmental science and pollution research international. 2014 doi: 10.1007/s11356-014-3567-3. doi:10.1007/s11356-014-3567-3. [DOI] [PubMed] [Google Scholar]
- Barocchi MA, Ko AI, Ferrer SR, Faria MT, Reis MG, Riley LW. Identification of new repetitive element in Leptospira interrogans serovar copenhageni and its application to PCR-based differentiation of Leptospira serogroups. J Clin Microbiol. 2001;39:191–195. doi: 10.1128/JCM.39.1.191-195.2001. doi:Doi 10.1128/Jcm.39.1.191-195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers D, Buckland S, Zucchini W. Estimating Animal Abundance: Closed Populations. London. 2002 [Google Scholar]
- Ministry of Health. Epidemiological Surveilance of Infectious Diseases. 2005 Brazil http://portalsaudegovbr/portal/svs/areacfm?id_area=451.
- Brown LE. Field Experiments on Movements of Apodemus Sylvaticus L Using Trapping and Tracking Techniques Oecologia. 1969;2:198–222. doi: 10.1007/BF00379159. doi:Doi 10.1007/Bf00379159. [DOI] [PubMed] [Google Scholar]
- Cavia R, Cueto GR, Suarez OV. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landscape Urban Plan. 2009;90:11–19. doi:Doi 10.1016/J.Landurbplan.2008.10.017. [Google Scholar]
- CDC. Centers for Disase Control and Prevention . Integrated pest management: conducting urban rodent surveys. Vol. 50. US Department of Health and Human Services; Atlanta: 2006. [Google Scholar]
- Childs JE, Krebs JW, Ksiazek TG, Maupin GO, Gage KL, Rollin PE, Zeitz PS, Sarisky J, Enscore RE, Butler JC, Cheek JE, Glass GE, Peters CJ. A Household-Based, Case-Control Study of Environmental-Factors Associated with Hantavirus Pulmonary Syndrome in the Southwestern United-States. Am J Trop Med Hyg. 1995;52:393–397. doi: 10.4269/ajtmh.1995.52.393. [DOI] [PubMed] [Google Scholar]
- Conceicao KS, Andrade MG, Louzada F. Zero-modified Poisson model: Bayesian approach, influence diagnostics, and an application to a Brazilian leptospirosis notification data. Biometrical J. 2013;55:661–678. doi: 10.1002/bimj.201100175. doi:Doi 10.1002/Bimj.201100175. [DOI] [PubMed] [Google Scholar]
- Connors MJ, Schauber EM, Forbes A, Jones CG, Goodwin BJ, Ostfeld RS. Use of track plates to quantify predation risk at small spatial scales. J Mammal. 2005;86:991–996. doi:Doi 10.1644/1545-1542(2005)86[991:Uotptq]2.0.Co;2. [Google Scholar]
- Costa F, Porter FH, Rodrigues G, Farias H, de Faria MT, Wunder EA, Osikowicz LM, Kosoy MY, Reis MG, Ko AI, Childs JE. Infections by Leptospira interrogans, Seoul Virus, and Bartonella spp. Among Norway Rats (Rattus norvegicus) from the Urban Slum Environment in Brazil Vector-Borne Zoonot. 2014a;14:33–40. doi: 10.1089/vbz.2013.1378. doi:Doi 10.1089/Vbz.2013.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Ribeiro GS, Felzemburgh RDM, Santos N, Reis RB, Santos AC, Fraga DBM, Araujo WN, Santana C, Childs JE, Reis MG, Ko AI. Influence of Household Rat Infestation on Leptospira Transmission in the Urban Slum Environment. Plos Neglect Trop D. 2014b;8 doi: 10.1371/journal.pntd.0003338. doi:ARTN e3338 DOI 10.1371/journal.pntd.0003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Wunder EA, Jr., De Oliveira D, Bisht V, Rodrigues G, Reis MG, Ko AI, Begon M, Childs JE. Patterns in Leptospira Shedding in Norway Rats (Rattus norvegicus) from Brazilian Slum Communities at High Risk of Disease Transmission. PLoS Negl Trop Dis. 2015;9:e0003819. doi: 10.1371/journal.pntd.0003819. doi:10.1371/journal.pntd.0003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DE. The Characteristics of Rat Populations. The Quarterly Review of Biology. 1953;28:373–399. doi: 10.1086/399860. [DOI] [PubMed] [Google Scholar]
- Davis DE, Emlen JT, Stokes AW. Studies on Home Range in the Brown Rat. J Mammal. 1948;29:207–225. doi:Doi 10.2307/1375387. [Google Scholar]
- Davis S, Calvet E, Leirs H. Fluctuating rodent populations and risk to humans from rodent-borne zoonoses Vector-Borne Zoonot. 2005;5:305–314. doi: 10.1089/vbz.2005.5.305. doi:Doi 10.1089/Vbz.2005.5.305. [DOI] [PubMed] [Google Scholar]
- de Masi E, Vilaca PJ, Razzolini MT. Evaluation on the effectiveness of actions for controlling infestation by rodents in Campo Limpo region, Sao Paulo Municipality. Brazil International journal of environmental health research. 2009;19:291–304. doi: 10.1080/09603120802592723. doi:10.1080/09603120802592723. [DOI] [PubMed] [Google Scholar]
- Dickman CR. A Method for Censusing Small Mammals in Urban Habitats. J Zool. 1986;210:631–636. [Google Scholar]
- Drennan JE, Beier P, Dodd NL. Use of track stations to index abundance of sciurids. J Mammal. 1998;79:352–359. doi:Doi 10.2307/1382872. [Google Scholar]
- Efford M. Density estimation in live-trapping studies. Oikos. 2004;106:598–610. doi:Doi 10.1111/J.0030-1299.2004.13043.X. [Google Scholar]
- Emlen JT, Stokes AW, Davis DE. Methods for Estimating Populations of Brown Rats in Urban Habitats. Ecology. 1949;30:430–442. doi:Doi 10.2307/1932446. [Google Scholar]
- Engeman RM, Witmer GW. IPM strategies: Indexing difficult to monitor populations of pest species. Proc Vertebr Pest. 2000;C:183–189. [Google Scholar]
- Flannery B, Pereira MM, Velloso LD, Carvalho CD, De Codes LG, Orrico GD, Dourado CMR, Riley LW, Reis MG, Ko AI. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001;65:657–663. doi: 10.4269/ajtmh.2001.65.657. [DOI] [PubMed] [Google Scholar]
- Gardner-Santana LC, Norris DE, Fornadel CM, Hinson ER, Klein SL, Glass GE. Commensal ecology, urban landscapes, and their influence on the genetic characteristics of city-dwelling Norway rats (Rattus norvegicus) Molecular ecology. 2009;18:2766–2778. doi: 10.1111/j.1365-294X.2009.04232.x. doi:DOI 10.1111/j.1365-294X.2009.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass GE, Johnson JS, Hodenbach GA, Disalvo CLJ, Peters CJ, Childs JE, Mills JN. Experimental evaluation of rodent exclusion methods to reduce hantavirus transmission to humans in rural housing. Am J Trop Med Hyg. 1997;56:359–364. doi: 10.4269/ajtmh.1997.56.359. [DOI] [PubMed] [Google Scholar]
- Glass GE, Korch GW, Childs JE. Seasonal and Habitat Differences in Growth-Rates of Wild Rattus-Norvegicus. J Mammal. 1988;69:587–592. doi:Doi 10.2307/1381350. [Google Scholar]
- Glennon MJ, Porter WF, Demers CL. An alternative field technique for estimating diversity of small-mammal populations. J Mammal. 2002;83:734–742. doi:Doi 10.1644/1545-1542(2002)083<0734:Aaftfe>2.0.Co;2. [Google Scholar]
- Himsworth CG, Bidulka J, Parsons KL, Feng AY, Tang P, Jardine CM, Kerr T, Mak S, Robinson J, Patrick DM. Ecology of Leptospira interrogans in Norway Rats (Rattus norvegicus) in an Inner-City Neighborhood of Vancouver. Canada PLoS Negl Trop Dis. 2013a;7:e2270. doi: 10.1371/journal.pntd.0002270. doi:10.1371/journal.pntd.0002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himsworth CG, Jardine CM, Parsons KL, Feng AYT, Patrick DM. The Characteristics of Wild Rat (Rattus spp.) Populations from an Inner-City Neighborhood with a Focus on Factors Critical to the Understanding of Rat-Associated Zoonoses. Plos One. 2014;9:e91654. doi: 10.1371/journal.pone.0091654. doi:ARTN e91654 DOI 10.1371/journal.pone.0091654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himsworth CG, Parsons KL, Jardine C, Patrick DM. Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers Vector Borne. Zoonotic Dis. 2013b;13:349–359. doi: 10.1089/vbz.2012.1195. doi:10.1089/vbz.2012.1195. [DOI] [PubMed] [Google Scholar]
- Kajdacsi B, Costa F, Hyseni C, Porter F, Brown J, Rodrigues G, Farias H, Reis MG, Childs JE, Ko AI, Caccone A. Urban population genetics of slum-dwelling rats (Rattus norvegicus) in Salvador. Brazil Mol Ecol. 2013;22:5056–5070. doi: 10.1111/mec.12455. doi:10.1111/mec.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736–747. doi: 10.1038/nrmicro2208. doi:10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AI, Reis MG, Dourado CMR, Johnson WD, Riley LW, Grp SLS. Urban epidemic of severe leptospirosis in Brazil. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Krojgaard LH, Villumsen S, Markussen MDK, Jensen JS, Leirs H, Heiberg AC. High prevalence of Leptospira spp. in sewer rats (Rattus norvegicus) Epidemiol Infect. 2009;137:1586–1592. doi: 10.1017/S0950268809002647. doi:Doi 10.1017/S0950268809002647. [DOI] [PubMed] [Google Scholar]
- Lambert D. Zero-Inflated Poisson Regression, with an Application to Defects in Manufacturing Technometrics. 1992;34:1–14. doi:Doi 10.2307/1269547. [Google Scholar]
- Lambropoulos AS, Fine JB, Perbeck A, Torres D, Glass GE, McHugh P, Dorsey EA. Rodent control in urban areas - An interdisciplinary approach. J Environ Health. 1999;61:12–17. [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Leslie PH, Davis DHS. An Attempt to Determine the Absolute Number of Rats on a Given Area. Journal of Animal Ecology. 1939;8:94–113. [Google Scholar]
- Lord RD. Rodent control programs: use of the inked tracking board method in Mexico Bulletin of the Pan American Health Organization. 1983;17:259–268. [PubMed] [Google Scholar]
- Lord RD, Vilches AM, Maiztegu Ji, Hall EC, Soldini CA. Frequency of Rodents in Habitats near Pergamino, Argentina, as Related to Junin Virus. Am J Trop Med Hyg. 1971;20:338–342. doi: 10.4269/ajtmh.1971.20.338. [DOI] [PubMed] [Google Scholar]
- Maciel EAP, de Carvalho ALF, Nascimento SF, de Matos RB, Gouveia EL, Reis MG, Ko AI. Household Transmission of Leptospira Infection in Urban Slum Communities. Plos Neglect Trop D. 2008;2 doi: 10.1371/journal.pntd.0000154. doi:ARTN e154 DOI 10.1371/journal.pntd.0000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskey M, Shastri JS, Saraswathi K, Surpam R, Vaidya N. Leptospirosis in Mumbai: post-deluge outbreak 2005. Indian journal of medical microbiology. 2006;24:337–338. doi: 10.4103/0255-0857.29413. [DOI] [PubMed] [Google Scholar]
- McKelvey KS, Pearson DE. Population estimation with sparse data: the role of estimators versus indices revisited. Can J Zool. 2001;79:1754–1765. doi:Doi 10.1139/Cjz-79-10-1754. [Google Scholar]
- Mills JN bJ, Ksiazek TG, Peters CJ, Velleca WM. Methods for trapping and sampling small mammals for virologic testing. U.S. Dept. of Health & Human Services; Atlanta: 1995. [Google Scholar]
- Mills JN, Childs JE. Ecologic studies of rodent reservoirs: Their relevance for human health. Emerg Infect Dis. 1998;4:529–537. doi: 10.3201/eid0404.980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nams VO, Gillis EA. Changes in tracking tube use by small mammals over time. J Mammal. 2003;84:1374–1380. doi:Doi 10.1644/Beh-001. [Google Scholar]
- Porter F, Costa F, Rodrigues G, Farias H, Cunha M, Glass G, Reis M, Ko A, Childs J. Morphometric and demographic differences between tropical and temperate Norway rats (Rattus norvegicus). J Mammalogy. 2015;96:317–323. [Google Scholar]
- Promkerd P, Khoprasert Y, Virathavone P, Thoummabouth M, Sirisak O, Jakel T. Factors explaining the abundance of rodents in the city of Luang Prabang, Lao PDR, as revealed by field and household surveys. Integr Zool. 2008;3:11–20. doi: 10.1111/j.1749-4877.2008.00069.x. doi:Doi 10.1111/J.1749-4877.2008.00069.X. [DOI] [PubMed] [Google Scholar]
- Quy RJ, Cowan DP, Haynes P, Inglis IR, Swinney T. Predicting the Outcome of Rodenticide Trials against Norway Rats Living on Farms Sixteenth Vertebrate Pest Conference. 1994:133–137. [Google Scholar]
- Quy RJ, Cowan DP, Swinney T. Tracking as an Activity Index to Measure Gross Changes in Norway Rat-Populations Wildlife Soc B. 1993;21:122–127. [Google Scholar]
- Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. Impact of Environment and Social Gradient on Leptospira Infection in Urban Slums. Plos Neglect Trop D. 2008a;2 doi: 10.1371/journal.pntd.0000228. doi:ARTN e228 DOI 10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. Impact of Environment and Social Gradient on Leptospira Infection in Urban Slums. Plos Neglect Trop D. 2008b;2:e228. doi: 10.1371/journal.pntd.0000228. doi:ARTN e228 DOI 10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley LW KA, Unger A, Reis MG. Slum health: diseases of neglected populations BMC international health and human rights. 2007;7 doi: 10.1186/1472-698X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar U, Nascimento SF, Barbosa R, Martins R, Nuevo H, Kalafanos I, Grunstein I, Flannery B, Dias J, Riley LW, Reis MG, Ko AI. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am J Trop Med Hyg. 2002;66:605–610. doi: 10.4269/ajtmh.2002.66.605. [DOI] [PubMed] [Google Scholar]
- Sheppe W. Characteristics and Uses of Peromyscus Tracking Data Ecology. 1965;46:630–634. doi:Doi 10.2307/1935002. [Google Scholar]
- Sheppe W. Effect of Livetrapping on Movements of Peromyscus. Am Midl Nat. 1967;78:471–480. doi:Doi 10.2307/2485244. [Google Scholar]
- Sherpherd DS, Greaves JH. A Weather-Resistant Tracking Board In: Vertebrate Pest Conference. University of Nebraska - Lincoln; 1984. [Google Scholar]
- Smith L. An Experimental Rat Eradication Program in an Urban Area. Public Health Rep. 1963;78:807–811. doi:Doi 10.2307/4591943. [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Raphael MG. Identification of Mammal Tracks from Sooted Track Stations in the Pacific Northwest Calif Fish Game. 1988;74:4–15. [Google Scholar]
- Villafane IEG, Cavia R, Vadell MV, Suarez OV, Busch M. Differences in population parameters of Rattus norvegicus in urban and rural habitats of central Argentina Mammalia. 2013;77:187–193. doi:Doi 10.1515/Mammalia-2012-0075. [Google Scholar]
- Welsh AH, Cunningham RB, Donnelly CF, Lindenmayer DB. Modelling the abundance of rare species: Statistical models for counts with extra zeros. Ecol Model. 1996;88:297–308. doi:Doi 10.1016/0304-3800(95)00113-1. [Google Scholar]
- Wenger SJ, Freeman MC. Estimating Species Occurrence, Abundance, and Detection Probability Using Zero-Inflated Distributions. Ecology. 2008;89:2953–2959. doi: 10.1890/07-1127.1. doi:Doi 10.1890/07-1127.1. [DOI] [PubMed] [Google Scholar]
- Whisson DA, Engeman RM, Collins K. Developing relative abundance techniques (RATs) for monitoring rodent populations. Wildlife Res. 2005;32:239–244. doi:Doi 10.1071/Wr03128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.