Abstract
Antisociality is commonly conceptualized as a unitary construct, but there is considerable evidence for multidimensionality. In particular, two partially dissociable symptom clusters – psychopathy and externalizing - have divergent associations to clinical and forensic outcomes and are linked to unique patterns executive dysfunction. Here, we used fMRI in a sample of incarcerated offenders to map these dimensions of antisocial behavior to brain circuits underlying two aspects of inhibitory self-control: interference suppression and response inhibition. We found that psychopathy and externalizing are characterized by unique and task-selective patterns of dysfunction. While higher levels of psychopathy predicted increased activity within a distributed fronto-parietal network for interference suppression, externalizing did not predict brain activity during attentional control. By contrast, each dimension had opposite associations to fronto-parietal activity during response inhibition. These findings provide neurobiological evidence supporting the fractionation of antisocial behavior, and identify dissociable mechanisms through which different facets predispose dysfunction and impairment.
Keywords: Psychopathy, Externalizing, Self-Control, Impulsivity, fMRI
Antisocial behavior is characterized by a persistent pattern of transgressing social, legal, and moral norms, including high levels of criminal offending. Recent estimates suggest that the annual cost of criminal behavior may reach as high as $3.3 trillion per annum in the U.S (Anderson, 1999, converted into 2015 dollars). Despite the significance of antisocial behavior as a driver of costly criminal offending we still know relatively little about its underlying cognitive and neurobiological mechanisms. This is due, in part, to a failure to distinguish between two very important, but distinct, antisocial syndromes. While antisocial behavior is commonly conceptualized in terms of Antisocial Personality Disorder (APD), many have argued that the diagnostic criteria for APD do not account for the rather evident heterogeneity that exists within this clinical population(Edens, Kelley, Lilienfeld, Skeem, & Douglas, 2015; Moffitt, 1993; Poythress et al., 2010; Skeem & Cooke, 2010; Skeem, Polaschek, Patrick, & Lilienfeld, 2011; Venables & Patrick, 2012). In particular, at least two partially dissociable dimensions –externalizing and psychopathy - are thought to be nested within the superordinate construct of antisocial behavior (Edens et al., 2015; Edens, Poythress, Lilienfeld, Patrick, & Test, 2008; Frick & Viding, 2009; Krueger et al., 2002; Krueger, Markon, Patrick, Benning, & Kramer, 2007a; Moffitt, 1993; Poythress et al., 2010; Skeem et al., 2011; Venables & Patrick, 2012).
Externalizing can be conceptualized as a normally distributed latent trait that accounts for the comorbidity among multiple syndromes linked to antisocial behavior, such as attention-deficit hyperactivity disorder (ADHD), conduct disorder (in adolescents), antisocial personality disorder (in adults), and substance abuse(Krueger et al., 2002; Krueger, Markon, Patrick, & Iacono, 2005; Krueger, Markon, Patrick, Benning, & Kramer, 2007a; Patrick et al., 2013). In turn, heritability studies suggest that symptom covariance among these syndromes is driven by a common genetic liability factor, providing further support for the notion that externalizing reflects a symptomatically unified and etiologically coherent dimension that is chiefly characterized by disinhibition (e.g. impulsivity) and negative affect (e.g. reactive aggression) (Krueger et al., 2002; 2005; Krueger, Markon, Patrick, Benning, & Kramer, 2007a; Patrick et al., 2013).
By contrast, psychopathy encompasses aspects of socio-affective function that distinguish it from externalizing. Cleckley’s original characterization of psychopathy centered on three cardinal facets: positive adjustment (low anxiety and neuroticism; superficial charm), behavioral deviance (inadequately motivated antisocial behavior; irresponsibility); and emotional-interpersonal deficits (lack of remorse, empathy and shame; shallow affect) (Cleckley, 1988; Skeem et al., 2011) (Patrick, 2006). Modern conceptualizations of psychopathy have largely retained these features; interpersonal (e.g. manipulation, pathological lying) and affective (e.g. callousness, diminished empathy) deficits are considered central for defining psychopathy, along with lifestyle and antisocial symptoms (but see (Skeem & Cooke, 2010)).
Externalizing and psychopathy are dissociable at multiple levels of analysis. Compared with externalizing, psychopathy is associated with more severe, stable, and violent forms of antisocial behavior in both youth and adults (Blair, 2013; Frick, 2009; Raine, 2002). Distinct patterns of comorbidity have been reported as well: while anxious and depressive symptoms are relatively common concomitants of externalizing, the oft-noted absence of such features in psychopathy has led some to suggest that it acts a protective factor against mood and anxiety psychopathology(Willemsen, Vanheule, & Verhaeghe, 2011). Genetic data provide further evidence for the distinctiveness of these two dimensions. While both externalizing and psychopathy show evidence of moderate-high heritability, heritability magnitude estimates vary according to the presence or absence of the affective-interpersonal personality features (e.g. callous-unemotional traits) that are core to psychopathy (Viding, Jones, Frick, Moffitt, & Plomin, 2008). Differential heritability estimates imply the existence of dissociable genetic architectures for each dimension(Blonigen, Hicks, Krueger, Patrick, & Iacono, 2005) and, in turn, distinct etiological origins for the characteristic symptoms of each.
The clinical and genetic data cited above support the notion that externalizing and psychopathy represent distinct antisocial syndromes, and imply the existence of dimension-specific cognitive and neurobiological mechanisms that predispose a common behavioral endpoint (antisocial behavior). However, the identification of dimension-selective mechanisms has proved challenging. Studies of antisocial behavior commonly rely on one measure, often the Psychopathy Checklist-Revised (PCL-R; Hare 2003). Inferences about dimension-selectivity are gleaned by examining phenotypic associations with the measure’s principal subscales (commonly referred to as “factors”). Factor 1 indexes the emotional and interpersonal symptoms that many consider core to the construct, while Factor 2 captures behaviors that align more with the externalizing dimension noted above, such as impulsivity, irresponsibility and aggression. Despite being labeled as factors, Factor 1 and Factor 2 exhibit a modest positive correlation (typically ~.5–.6)(Hare & Neumann, 2008). Consistent with the notion that PCL-R Factor 2 accesses the externalizing dimension, modest correlations between Factor 2 and scores from the Externalizing Spectrum Inventory (ESI) (Venables & Patrick, 2012) have been reported; further, these correlations are significantly stronger than association between Factor 1 and ESI scores(Patrick et al., 2013; Venables & Patrick, 2012). On the whole, this pattern of covariance suggests that commonly used clinical assessments of externalizing and psychopathy are relatively non-selective. This situation limits the specificity of inference when such measures are used as predictors of cognitive and neurobiological phenotypes, as it is unclear whether significant associations are driven by shared variance between psychopathy and externalizing or due to the unique variance associated with either dimension.
Notwithstanding the methodological confound noted above, relatively consistent evidence for dimension-specific mechanisms can be gleaned from studies of executive function (EF). While executive dysfunction has long been noted in antisocial individuals (Dolan, 2012a; Dolan & Park, 2002b; Morgan & Lilienfeld, 2000), recent work suggests that externalizing and psychopathy are associated with distinct patterns of EF deficits, particularly in the domain of selective attention. In externalizing, research to date suggests that these individuals display broad pattern of EF deficits, encompassing selective attention, interference suppression, and response inhibition. By contrast, many of these EF components appear to be preserved, and in some cases enhanced, in psychopathy. For example, while externalizing predicts larger “attentional blinks” in a rapid serial visual presentation task (Baskin-Sommers, Wolf, Buckholtz, Warren, & Newman, 2012c), the attentional blink is attenuated in psychopathic individuals(Wolf et al., 2012). These findings may reflect fundamental differences in the flexible allocation of selective attention between the two dimensions (See (Baskin-Sommers & Newman, 2013) for review). Consistent with this hypothesis, PCL-R factor 1 (indexing affective-interpersonal dysfunction) and PCL-R factor 2 (thought to preferentially access externalizing) appear to have opposite associations to (self-reported) attentional control, such that the core features of psychopathy are linked to enhanced, and impulsive-antisocial features to diminished, selective attention (Baskin-Sommers et al., 2015; Baskin-Sommers, Zeier, & Newman, 2009).
While such findings might suggest that psychopathic individuals have superior EF overall, this is not consistently found across the entire range of EF subcomponents. For example, while both interference suppression and response inhibition appear to be compromised in externalizing psychopathology(Heritage & Benning, 2013; Sadeh & Verona, 2008; Sellbom & Verona, 2007; Swann, Lijffijt, Lane, Steinberg, & Moeller, 2009a; Zeier, Baskin-Sommers, Hiatt Racer, & Newman, 2012), the evidence that psychopathic individuals are better at inhibiting prepotent motor responses is inconsistent at best(Feilhauer, Cima, Korebrits, & Kunert, 2012; Sadeh & Verona, 2008; Sellbom & Verona, 2007). Moreover, enhanced interference suppression in psychopathy is context dependent, with psychopathic individuals showing reduced interference only in conditions where their attention is cued to the target location(Hiatt, Schmitt, & Newman, 2004; Zeier & Newman, 2013; Zeier, Maxwell, & Newman, 2009). On the whole, neuropsychological work suggests that psychopathic individuals inflexibly allocate limited capacity early attentional resources. This may lead to an attentional “bottleneck” that limits the ability to process information that is motivationally salient but peripheral to their goal-directed task focus(Baskin-Sommers, Curtin, & Newman, 2011; Baskin-Sommers, Curtin, Li, & Newman, 2012a).
Taken together, work to date suggests that externalizing is associated with a broad pattern of EF deficits, encompassing selective attention, interference suppression, and response inhibition. By contrast, these aspects of EF appears to be preserved, and in some cases enhanced, in psychopathy. However, the neural mechanisms underlying these putatively dimension-selective associations with EF remain unknown. The goal of the current study is to map the unique variance associated with externalizing and psychopathy to well-characterized brain circuitry for interference suppression and response inhibition. To that end, we used a multi-method approach that integrates clinical, trait, neuropsychological and neurobiological assessments. Specifically, we scanned a sample of 49 incarcerated offenders while they performed a modified Eriksen flanker task that separately manipulated the requirement for interference suppression (IS) and response inhibition (RI). We predicted that after adjusting for shared variance, psychopathy and externalizing would show an opposing pattern of correlation (psychopathy positive, externalizing negative) with dissociable fronto-parietal networks subserving IS and RI.
METHODS
Participants
Participants were recruited from two medium-security correctional institutions in Wisconsin. A total of 49 right-handed, male participants were enrolled (Age range: 20–45; mean = 31.52 +/− 7.1 years;). Criteria for eligibility were defined as follows: 45 years old or younger, WAIS-III IQ above 70 (Wechsler D, 1993), and not concurrently taking psychotropic medications. Three participants were excluded from analyses due to excessive head movement (2 subjects) or poor fMRI quality assurance metrics (1 subject; see below). Oral and written consent were obtained for all participants, and all methods and procedures were approved by the University of New Mexico, University of Wisconsin-Madison, and Harvard University Institutional Review Boards.
Measures
Participants completed a battery of clinical, and neuropsychological assessments through interview and questionnaire measures.
Psychopathy Checklist-Revised (PCL-R) (Hare, 2003). The PCL-R is a “gold standard” for the forensic evaluation of psychopathy. PCL-R ratings were completed using information from prison files and a semistructured interview that lasted approximately 60 minutes. Based on information gathered from the interview and file review, the 20 items of the PCL-R were rated 0, 1, or 2, reflecting the degree to which a trait was present: significantly (2), moderately (1), or not at all (0). PCL-R assessment was performed by a trained rater and consisted of both a and file review. The reliability and validity of the PCL-R is well established(Hare et al., 1990). In the present study the inter-rater or internal consistency was (interrater reliability=.96 on 30% of the sample with dual ratings).
Addiction Severity Index (ASI)(Leonhard, Mulvey, Gastfriend, & Shwartz, 2000; Rosen, Henson, Finney, & Moos, 2000). The ASI was used to estimate severity of substance misuse. In addition to the original ASI questions, participants were asked to indicate, for each substance they endorsed using, their total years of use. We summed each answer across all drugs to calculate a “cumulative use” score (range = 0–76, mean = 14.89), which was then used as a covariate in subsequent analyses to control for the potentially confounding effects of chronic substance use on brain function. The validity and reliability of the ASI is well established (McLellan et al., 1985). Inter-rater reliability data for the ASI were not obtained for this sample.
Externalizing Spectrum Inventory-100 (ESI)(Krueger, Markon, Patrick, Benning, & Kramer, 2007b). Externalizing was measured using the ESI, a 100-item self-report questionnaire developed to assess a broad range of behavioral (i.e., substance use) and personality characteristics (i.e., alienation, rebelliousness, and impulsivity) associated with the externalizing spectrum of psychopathology. The 100-item version was derived from Krueger et al.’s (2007) 415-item self-report measure and is correlated r .98 with the original measure(Krueger, Markon, Patrick, Benning, & Kramer, 2007c). The total range of scores on the ESI is 100 to 400. The validity and reliability of the ESI-100 is well established(Venables & Patrick, 2012). For this sample the internal consistency (Cronbach’s alpha) was .96.
Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, Kramer, 2001). The D-KEFS was developed to assess components of EF through well-established tests. Contrast measures from the Color-Word Interference Test (inhibition vs. color-naming [scaled], inhibition-switching vs. color-naming [scaled], inhibition errors [percentile rank], inhibition-switching errors [percentile rank], inhibition-switching vs. inhibition [scaled]) were analyzed. The validity and reliability of the D-KEFS is well established (Delis, Kramer, Kaplan, & Holdnack, 2004). We did not assess inter-rater reliability for the D-KEFS in this sample.
Experimental Task
Participants completed a modified version of the Eriksen flanker task that incorporated a go/no go manipulation(Blasi et al., 2006; B. A. Eriksen & Eriksen, 1974). On each trial, participants were instructed to indicate, via button press, the direction of a central target arrow (left vs. right) that was situated between a set of flanking arrows. The flanking arrows either pointed in the same direction as the target (congruent condition) or in the opposite direction (incongruent condition). Additionally, on some trials the central arrow was surrounded by X’s, signifying the need to withhold a response (no-go condition), or by squares (neutral condition; participants were instructed to respond normally). The incongruent condition introduces interference that must be resolved or suppressed to respond appropriately. By contrast, optimal performance in the no-go condition requires participants to inhibit a pre-potent motor response. These conditions were displayed in a pseudorandom order over two runs; stimulus order within a run was fixed across, with run order counterbalanced across participants. Each stimulus was presented for 800ms. This duration was selected to ensure low error rates, as the focus of this study was on interference suppression and response inhibition rather than error-monitoring. Between trials, a fixation cross was presented; duration of the inter-trial interval randomly jittered across trials, according to a laplacian distribution with mean = 3.5 seconds and range = 2–5 seconds. Each run contained 81 trials, including 23 incongruent and 23 congruent trials, 18 nogo trials, and 17 neutral trials.
fMRI Data Acquisition
Participants were scanned using a 1.5 Tesla Siemens Magnetom Avanto mobile MRI machine equipped with a twelve-channel head coil. While lying supine in the scanner, participants were able to view the stimulus via a back-projection system and made responses on an MRI compatible button box. The presentation of the stimulus and performance of the modified flanker task (described above) was synchronized to fMRI volume acquisition. Functional (T2* weighted) images were collected using a gradient-echo EPI pulse sequence (interleaved) using the following parameters: TR 2500 ms, TE 39 ms, flip angle 90º, 33 slices, voxel resolution 3.4×3.4×3.4 mm, FOV 220 mm. High resolution T1-wighted structural MRI scans were also acquired in order to co-register the functional images to a standardized anatomical space (multi-echo MPRAGE; 1×1×1.3mm).
fMRI preprocessing
Prior to analysis, task-related functional images were slice-time corrected using the first slice as a reference, and motion corrected via spatial realignment (2nd-degree B-spline) of all images to a mean image after alignment to the first image of each run. Images were then spatially normalized using unified segmentation and normalization, via the NewSegment routine in SPM, into a standard stereotactic space (Montreal Neurological Institute, MNI template), resampled into 2mm isotropic voxels, and smoothed with a 6mm full-width-half-maximum Gaussian kernel. A high-pass filter (128s cutoff) was applied to remove low-frequency signal drift. Runs were removed if they had a total rotational plus translational displacement of 1mm or a mean BOLD signal > 3 standard deviations from the norm, using the ART (artifact detection) tool in Nipype. Two subjects were excluded from final analysis because due to movement; another was excluded because their mean BOLD signal for each run was > 3 standard deviations above the group mean.
Behavioral Analyses
We used linear mixed model analyses in SPSS 24 to examine the impact of congruency condition on performance (reaction time) and its interaction with psychopathy and externalizing. Fixed effect predictors included condition (congruent vs. incongruent), PCL-R scores, ESI scores, age, and ASI scores, along with condition*PCL-R and condition*ESI interaction terms. Reaction times were not normally distributed (skew = 1.47), and so were log-transformed prior to analysis. Subject was treated as a random effect. PCL-R and ESI scores were included in the same model in order capture unique variance associated with psychopathy and externalizing. Robust regression in Stata (RReg) was used to assess relationships between psychopathy, externalizing, and no-go commission error rates. For these analyses, we created an adjusted psychopathy variable by regressing PCL-R, age, and ASI scores against participants’ ESI scores and saving the residuals; adjusted externalizing values was similarly constructed. These residual values capture unique variance in psychopathy after controlling for externalizing (and vice versa), age, and substance abuse history. In addition, we employed robust regression to measure associations between adjusted ESI and PCL-R scores, brain activity, and behavior. For robust regression analyses, we report unstandardized coefficients and 95% confidence intervals (CIs); in addition, we provide effect size estimates derived from the equivalent Ordinary Least Squares (OLS) regression analysis. Age and ASI scores were included as covariates in all robust regression analyses. Multivariate general linear model (GLM) analyses were used to assess relationships between between adjusted ESI and PCL-R scores, brain activity, and neuropsychological variables. Age and ASI scores were included as covariates.
fMRI Analyses: Task Effects
Trial onsets were modeled using a canonical hemodynamic response function (HRF) with a time derivative. All runs of the task were modeled together. The design matrix for our first-level general linear model (GLM) included trial onset regressors for each condition (incongruent, congruent, no-go, neutral), motion parameters estimated from realignment, a regressor specifying motion outlier time points, and a regressor of onsets for error trials. To reveal activity related to IS, we constructed contrasts of the beta weights for incongruent and congruent trials (incon>con); RI effects were visualized by contrasting brain activity during no-go trials with that during congruent trials (no-go > congruent). The inclusion of predictors for each trial type in the GLM permits assessment of IS, controlling for RI (and vice versa). First-level contrasts were created for each subject; the resulting contrast images were entered into a random-effects one-sample t-test at the second-level (i.e. treating participant as a random effect). To control for Type-1 error due to multiple comparisons, we used a cluster-level false discovery rate (FDR) threshold of p < 0.05 in conjunction with a cluster-forming height threshold of t > 3.
fMRI Analyses: Individual Differences
To identify relationships between psychopathy, externalizing, interference suppression and response inhibition, we created two multiple regression models in SPM8. In the first, PCL-R and ESI scores, along with age and substance abuse values, were modeled as predictors of interference suppression-related activation (incongruent>congruent contrasts). In the second, the same set of variables were modeled as predictors of response inhibition-related activity (no-go contrasts). In each model, PCL-R and ESI predictors were separately weighted with a “1” or “−1” to reveal correlations with psychopathy (controlling for externalizing) and externalizing (controlling for psychopathy). Control over Type-1 error across the whole brain was achieved via cluster-level FDR correction (p < 0.05, with a cluster-forming height threshold of t > 3).
RESULTS
Clinical Measures
The zero-order Pearson product-moment correlation between PCL-R total and ESI total scores was r = 0.64, p < 0.001; correlations between ESI total and PCL-R Factor 1 and Factor 2 scores were r= 0.45, p = 0.002 and r = 0.65, p = < 0.001 respectively. The two PCL-R factors were correlated at r = 0.53, p =< 0.001.
Behavior
We found a main effect of congruency on reaction time (F1,45 = 108.06, p < 0.001, ηp2 = 0.71) such that responses were significantly faster for congruent trials (.595s ± .105) than incongruent trials (M = 650s ± .113). We did not find significant congruency*ESI (F1,43 = 3.45, p = 0.07; ηp2 = 0.07) or congruency*PCLR interactions (F1,43 = 0.63, p = 0.43; ηp2 = 0.01), indicating that neither psychopathy or externalizing-unique variance moderated the effect of congruency on response times during the task. Likewise, we did not observe significant congruency*ESI or congruency*PCLR interactions when ESI and PCLR were considered on their own (i.e. in separate models; p’s > 0.08). However, main effects for psychopathy were evident: adjusted PCL-R scores were associated with slower response times overall (t41 = 2.59, p = 0.01, ηp2 = 0.14), while adjusted ESI scores predicted faster response times irrespective of congruency condition (t = −2.17, p = 0.04, ηp2 = 0.1). The association between adjusted externalizing scores and no-go error rates was not significant (B = 0.008, −0.0009 – 0.012, p = 0.07, ηp2 = 0.08), nor was the association between adjusted psychopathy scores and no-go error rates (B = −0.05, −0.12 – 0.02, p = 0.17, ηp2 = 0.05).
fMRI: Task Effects
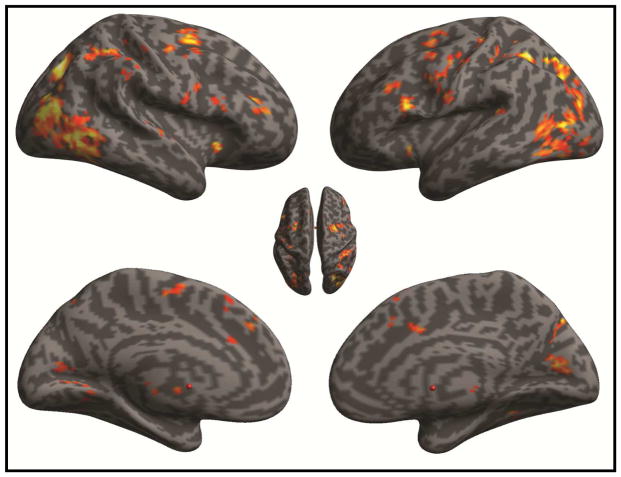
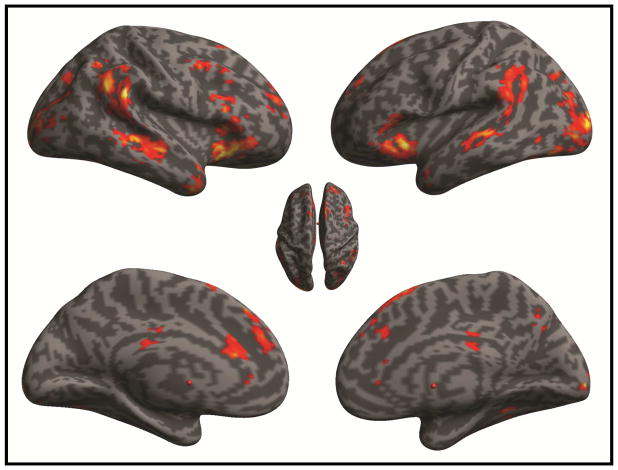
Consistent with prior reports (Blasi et al., 2006) interference suppression (incongruent > congruent) engaged a distributed fronto-parietal network with prominent foci in the supplementary motor area, frontal eye fields, inferior frontal gyrus (pars opercularis; IFGOPR) and inferior parietal cortex (See Table S1; Fig 1). By contrast, activity during response inhibition (No-Go > congruent) was strongest in the inferior frontal gyrus (encompassing pars orbitalis and pars triangularis; IFGORB, IFGTRI), the temporo-parietal junction, anterior cingulate cortex (ACC; Brodmann Area 24/32), dorsolateral prefrontal cortex (DLPFC; Brodmann Area 9) and anterior prefrontal cortex (Brodmann area 10) (See Table S2; Fig 2).
Figure 1.
Brain Activation During Interference Suppression. Statistical parametric map (SPM) displays significant foci revealed by the the incongruent > congruent contrast. SPM is thresholded at pCluster-FDR < 0.05, using a cluster defining height threshold of t >3.
Figure 2.
Brain Activation During Response Inhibition. SPM displays significant foci revealed by the no-go > congruent contrast. SPM is thresholded at pCluster-FDR < 0.05, using a cluster defining height threshold of t >3.
fMRI: Individual Differences
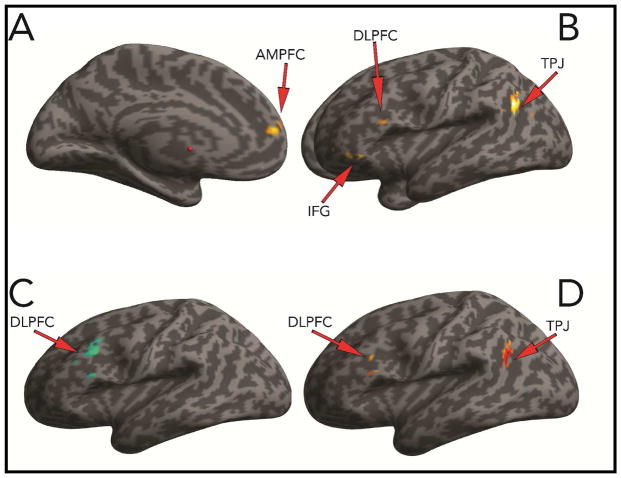
We did not observe any significant correlations with adjusted ESI scores and brain activity during interference suppression. By contrast, significant positive relationships between adjusted PCL-R scores and interference suppression-related BOLD signal were found in left IFGORB (BA 47; −50, 30, 20 [MNI]; k = 95, peak Z = 3.81), left dorsolateral prefrontal cortex (BA 46; −48, 36, −16 [MNI]; k = 84, peak Z = 3.75) anterior medial prefrontal cortex (amPFC; BA 10/32; −2, 64, 22 [MNI]; k = 203, peak Z = 3.69) and left temporo-parietal junction (TPJ; −52, −56, 30 [MNI]; k = 158, peak Z = 4.86) (Fig 3A–3B). During response inhibition, externalizing and psychopathy showed opposite patterns of association to dorsolateral prefrontal cortex activity: higher adjusted ESI scores predicted lower left DLPFC activation during response inhibition (−50, 12, 40 [MNI]; k = 263, peak Z = 4.22, while adjusted PCL-R scores were positively correlated with inhibition-related activity within left DLPFC (−50, 28, 24 [MNI]; k = 100, peak Z = 4.31) and left temporo-parietal junction (−54, −58, 30 [MNI]; k = 105, peak Z = 4.06) (Fig 3C–3D). In sum, these results show that psychopathy-specific variance is associated with heightened fronto-parietal activity during both interference suppression and response inhibition. Externalizing-specific variance, on the other hand, was linked to decreased prefrontal BOLD signal during response inhibition and showed no association to interference suppression-related activity.
Figure 3.
Differential Effects of Psychopathy and Externalizing on Fronto-Parietal Circuit Function During Inhibititory Self-Control. Panels A–B depict regions where adjusted PCL-R scores are significantly positively correlated with brain activity during interference suppression (incongruent > congruent contrast). Panel C shows the significant negative correlation with adjusted EXT scores and DLPFC function during response inhibition (No-Go > Congruent). Panel D displays the significant positive correlation between adjusted PCL-R scores and response inhibition-related activity within DLPFC and the TPJ. SPMs are thresholded at pCluster-FDR < 0.05, using a cluster defining height threshold of t >3.
Brain-Behavior Relationships
fMRI Task Performance
To determine the relevance of psychopathy and externalizing-linked differences in brain activation to task performance, we extracted BOLD signal from 8mm spheres centered on the peak coordinates of activation foci identified from the adjusted ESI and PCL-R correlation contrasts for interference suppression and response inhibition maps. For interference suppression, we subtracted reaction times in the congruent condition from those in the incongruent condition to create an index of susceptibility to interference (RTDiff). RTDiff values were negatively associated with interference suppression-related activation in IFG (B = −0.004, −0.006 – −0.008, p = 0.01, ηp2 = 0.01). This result showed that individuals with higher IFG activation during IS exhibited decreased distractor susceptibility in the flanker task. Associations between RTDiff and activity within DLPFC, amPFC and TPJ were not significant (p-value range: 0.33 – 0.72).
A similar analysis was performed for RI trials, revealing a negative relationship between commission error rate and DLPFC activation during the task (B = −2.28, −0.51 – −0.06, p = 0.01, ηp2 = 0.16, activation focus from EXT SPM; B = −0.25, −0.44 – −0.06, p = 0.01, ηp2 = 0.19, activation focus from PCL-R SPM). This indicates that individuals with lower DLPFC activity during RI were more prone to impulsive responding. Thus, the pattern of activation linked to unique variance in psychopathy (higher IFG activity during interference suppression and high DLPFC activity during response inhibition) was associated with decreased distractor susceptibility and reduced motor impulsivity. By contrast, the activation pattern that tracked unique variance in externalizing (lower DLPFC activity during response inhibition) was linked to increased motor impulsivity.
Color-Word Interference Test Performance
As a test of convergence, we ran a multivariate general linear model (GLM) analysis to assess relationships between externalizing and psychopathy and measures of inhibitory control and attentional flexibility derived from the DKEFS battery. We found that unique variance in psychopathy negatively predicted inhibition/switching performance (B = −0.21, −0.36 – −0.05, p = 0.01, ηp2 = 0.16, scaled inhibition-switch vs. color contrast; B = −0.15, −0.31 – 0.002, p = 0.05, ηp2 = 0.1, inhibition-switch time). Next, we constructed two multivariate GLM analyses in which IS and RI-related activity were separately considered as predictors of DKEFS inhibitory control and attentional flexibility measures. For the IS analyses, we used signal from each of the four foci identified in the whole-brain individual difference analyses (i.e. DLPFC, IFG, TPJ, and amPFC). We found that IS-related BOLD signal within IFG predicted poorer inhibition/switching performance (B = −1.12, p = 0.02, −2.02 – −0.22, ηp2 = 0.16, scaled inhibition-switch vs. color contrast; B = −1.36, −2.42 – −0.29, p = 0.01, ηp2 = 0.16, scaled inhibition-switch vs. inhibition contrast). Robust regression analyses corroborated this finding (p < 0.001 and p = 0.02, respectively). For the RI analysis, we used signal from each of the three foci identified from the whole-brain correlations with adjusted ESI and PCL-R scores (DLPFC, TPJ). This analysis did not reveal any significant associations between RI-related BOLD signal and DKEFS measures of inhibitory or attentional control. On the whole, these findings suggest that psychopathy, and psychopathy-linked heightened fronto-parietal BOLD signal during interference suppression, is associated with diminished attentional flexibility during a stroop-like color-word interference test.
Discussion
Here, we employed a multi-level and multi-measure approach to map externalizing and psychopathy to brain circuitry supporting two executive capacities for inhibitory self-control: interference suppression and response inhibition. A modified Eriksen flanker task permitted selective evaluation of IS and RI. The unique variance attributable to psychopathy was positively associated with fronto-parietal activation during both IS and RI. By contrast, the unique variance attributable to externalizing was negatively associated with DLPFC activity during RI; no relationship to IS-related brain activity emerged. These results provide a neurobiological dissociation of externalizing and psychopathy; the former is linked to relatively weaker prefrontal activity during response inhibition, while the latter is characterized by relatively stronger recruitment of fronto-parietal networks during both response inhibition and interference suppression.
On the whole, these findings accord well with prior work showing reduced cortical thickness (Yang & Raine, 2009a) and poor performance on RI tasks (Dolan, 2012a; Dolan & Park, 2002b)in participants with high levels of externalizing. Our analyses suggest that externalizing is associated with reduced DLPFC activation during RI. While the correlation between adjusted ESI scores and commission errors was not significant, the strong negative relationship between RI-related DLPFC BOLD signal and commission errors implies that diminished DLPFC engagement in externalizing individuals is dysfunctional.
A significant open question pertains to the relevance of inhibitory control deficits for “real-world” self-control failure (e.g. substance abuse, aggression, and criminal behavior) in externalizing individuals. Prevailing models assume that antisocial behavior in externalizing individuals results from a deficit in the capacity to actively inhibit the execution of prepotent responses to threat and/or reward associated stimuli(Dolan, 2012a; Dolan & Park, 2002a; Herpertz et al., 2008; Hobson, Scott, & Rubia, 2011; Kirisci, Tarter, Mezzich, & Vanyukov, 2007; Patrick, Durbin, & Moser, 2012; Raine & Yang, 2006; Swann, Lijffijt, Lane, Steinberg, & Moeller, 2009b). The current results would appear to support this model, and are consistent with other brain imaging studies in antisocial offenders that have reported reductions in DLPFC gray matter volume and cortical thickness DLPFC(Dolan, 2012b; Montigny et al., 2013; Sarkar et al., 2014; Wallace et al., 2012; Weiland et al., 2014; Yang & Raine, 2009b; Yang, Raine, Colletti, Toga, & Narr, 2010), as well as reduced DLPFC activation during classic neuropsychological indices of inhibitory control(S. J. Moeller et al., 2014; Vollm et al., 2004; Yang & Raine, 2009c; Ziermans et al., 2012). By contrast, antisocial individuals appear to have relatively exaggerated responses to threat stimuli (within the amygdala) and reward cues (within the striatum) (Bjork, Chen, & Hommer, 2012; Buckholtz, Treadway, Cowan, Woodward, Benning, et al., 2010a; Buckholtz, Treadway, Cowan, Woodward, Li, et al., 2010b; Carré, Hyde, Neumann, Viding, & Hariri, 2013; Coccaro, McCloskey, Fitzgerald, & Phan, 2007; Coccaro, Sripada, Yanowitch, & Phan, 2011; Hyde, Byrd, Votruba-Drzal, Hariri, & Manuck, 2014; Pujara, Motzkin, Newman, Kiehl, & Koenigs, 2014). Together, such findings are often construed as evidence that the impulsive-reactive antisocial behavior characteristic of externalizing occurs when bottom up “affective” signals activate or generate a prepotent behavioral response that is inadequately inhibited by top down “cognitive” resources due to poor prefrontal control. However, we (Buckholtz 2015) have speculated that the relevance of EF deficits for antisocial behavior in externalizing individuals may be more apparent than real. Central to this argument is the role of DLPFC; in contrast to “inhibition-centric” models of antisocial behavior, we have focused on the role of prefrontal cortex in value-based decision-making(Buckholtz, 2015; Buckholtz & Faigman, 2014). A wealth of data indicate that prefrontal cortex can optimize decision-making by reweighting striatal action value signals according to prospective simulations that incorporate information about goals, costs, consequences, and context, rather than by inhibiting the execution of an action program after valuation and selection have already occurred. Prefrontal dysfunction, therefore, may predispose impulsive antisocial behavior by preventing these prospective calculations from appropriately modulating “downstream” action value signals, rather than through a failure to actively inhibit a maladaptive motor program that has already been selected for execution. If this is true, associations between inhibitory control-related brain activity and antisocial behavior link may not reflect a direct causal relationship, but rather may arise epiphenomenally from the fact that DLPFC is important for both EF and value-based decision-making. In other words, EF deficits may be a “third variable” marker of compromised prefrontal value modulation. Future work should test this hypothesis by measuring prefrontal function during both RI and value-based decision-making tasks, and determining whether associations between externalizing and RI-related brain activity remain after controlling for brain activity linked to value-based decision-making. Likewise, prospective designs could determine whether EF and value-based decision-making each uniquely predict future antisocial behavior in externalizing individuals (and if so, which of the two has the strongest predictive power).
Our finding that psychopathic individuals have increased frontoparietal engagement during interference suppression accords well with reports that these individuals exhibit superior selective attention relative to individuals low on psychopathy (Sadeh & Verona, 2008; Sellbom & Verona, 2007) (Baskin-Sommers et al., 2009; 2015). Moreover, enhanced prefrontal activity during IS trials predicted less susceptibility to distractors. However, some caution is warranted in interpreting the present data as evidence for superior executive function in psychopathic individuals. In particular, the observed correlations between psychopathy-linked fronto-parietal activity and inhibition/switching performance on the Color-Word Interference Test implies that attentional flexibility is compromised in psychopathy. On the whole, the combination of decreased distractor susceptibility and poorer attentional flexibility is consistent with the suggestion that psychopathic individuals have a deficit in early attentional selection mechanisms, leading to an attentional bottleneck phenomenon (Baskin-Sommers, Curtin, Li, & Newman, 2012b; Hamilton, Baskin-Sommers, & Newman, 2014). Future imaging studies with IS tasks that manipulate these early attentional selection mechanisms will be necessary to clarify and extend the present findings.
Taken together, these findings provide neurobiological evidence supporting the existence of two distinct dimensions of antisocial behavior. In addition, they shed light on dimension-specific systems-level pathomechanisms. However, several issues merit consideration. First, we did not observe any significant relationships between adjusted EXT or PCL-R scores and task performance. This may be due to our task design, which was optimized for imaging and resulted in most participants performing near ceiling. While this was done in order to reduce errors (and potentially confounding error-related activity), by minimizing individual variation in performance we may have reduced the likelihood of detecting associations between our assessment measures and task behavior. Future imaging work in this area would benefit from the use of a task design that induces more variable performance, and which includes enough trials to enable an appropriately powered investigation of error-related activity(Aharoni et al., 2013). Second, the associations reported here are modest in size. This is consistent with a multifactorial model of antisociality, wherein relative deficits in multiple cognitive, affective, social and motivational processes contribute to the expression of antisocial behavior(Buckholtz & Meyer-Lindenberg, 2012). Less clear, however, are the specific processes at issue. For example, in the current work we limited our investigation of EF only to only two processes - interference suppression and response inhibition- because of practical considerations. Within the domain of “cognition” alone, this leaves many other candidate processes – such as response selection, action cancellation, and error detection – unexamined. Future work in this area should endeavor to develop a more precise and comprehensive mapping of cognitive, affective, social and motivational processes to common and unique variance associated with externalizing and psychopathy.
Supplementary Material
Footnotes
Author Contributions:
Conceptualization: JWB; Methodology: JWB, EK, ABS; Investigation: AMR, HMD, EK; Formal Analysis: AMR, HMD, EK, JWB; Writing-Original Draft: AMR, JWB; Writing-Reviewing and Editing: JWB, ABS, JPN, KK; Supervision: JWB, JPN, ABS; Project Administration: JWB, ABS, JPN, KK; Resources: KK.
References
- Aharoni E, Vincent GM, Harenski CL, Calhoun VD, Sinnott-Armstrong W, Gazzaniga MS, Kiehl KA. Neuroprediction of future rearrest. Proceedings of the National Academy of Sciences. 2013;110(15):6223–6228. doi: 10.1073/pnas.1219302110. http://doi.org/10.1073/pnas.1219302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DA. The Aggregate Burden of Crime*. The Journal of Law and Economics. 1999;42(2):611–642. http://doi.org/10.1086/467436. [Google Scholar]
- Baskin-Sommers AR, Newman JP. Differentiating the Cognition-Emotion Interactions that Characterize Psychopathy versus Externalizing. In: Dharmon-jones Mrobinson E, Ewatkins, editors. Cognition and Emotion. 2013. [Google Scholar]
- Baskin-Sommers AR, Brazil IA, Ryan J, Kohlenberg NJ, Neumann CS, Newman JP. Mapping the Association of Global Executive Functioning Onto Diverse Measures of Psychopathic Traits. Personality Disorders: Theory, Research, and Treatment. 2015:1–12. doi: 10.1037/per0000125. http://doi.org/10.1037/per0000125. [DOI] [PMC free article] [PubMed]
- Baskin-Sommers AR, Curtin JJ, Newman JP. Specifying the attentional selection that moderates the fearlessness of psychopathic offenders. Psychological Science. 2011;22(2):226–234. doi: 10.1177/0956797610396227. http://doi.org/10.1177/0956797610396227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Zeier JD, Newman JP. Self-reported attentional control differentiates the major factors of psychopathy. Personality and Individual Differences. 2009;47(6):626–630. doi: 10.1016/j.paid.2009.05.027. http://doi.org/10.1016/j.paid.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers A, Curtin JJ, Li W, Newman JP. Psychopathy-related differences in selective attention are captured by an early event-related potential. Personality Disorders. 2012a;3(4):370–378. doi: 10.1037/a0025593. http://doi.org/10.1037/a0025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers A, Curtin JJ, Li W, Newman JP. Psychopathy-related differences in selective attention are captured by an early event-related potential. Personality Disorders. 2012b;3(4):370–378. doi: 10.1037/a0025593. http://doi.org/10.1037/a0025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers A, Wolf R, Buckholtz J, Warren C, Newman J. Exaggerated Attention Blink Response in Prisoners with Externalizing. Journal of Research in Personality. 2012c;46(6):688–693. doi: 10.1016/j.jrp.2012.08.003. http://doi.org/10.1016/j.jrp.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Hommer DW. Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biological Psychology. 2012;89(2):408–415. doi: 10.1016/j.biopsycho.2011.12.003. http://doi.org/10.1016/j.biopsycho.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience. 2013;14(11):786–799. doi: 10.1038/nrn3577. http://doi.org/10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, et al. Brain regions underlying response inhibition and interference monitoring and suppression. European Journal of Neuroscience. 2006;23(6):1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. http://doi.org/10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35(5):637–648. doi: 10.1017/S0033291704004180. http://doi.org/10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW. Social norms, self-control, and the value of antisocial behavior. Current Opinion in Behavioral Sciences. 2015;3:122–129. http://doi.org/10.1016/j.cobeha.2015.03.004. [Google Scholar]
- Buckholtz JW, Faigman DL. Promises, promises for neuroscience and law. Current Biology : CB. 2014;24(18):R861–7. doi: 10.1016/j.cub.2014.07.057. http://doi.org/10.1016/j.cub.2014.07.057. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74(6):990–1004. doi: 10.1016/j.neuron.2012.06.002. http://doi.org/10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010a;13(4):419–421. doi: 10.1038/nn.2510. http://doi.org/10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic Network Differences in Human Impulsivity. Science. 2010b;329(5991):532–532. doi: 10.1126/science.1185778. http://doi.org/10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Social Neuroscience. 2013;8(2):122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity. 5. Augusta, GA: Book; 1988. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and Orbitofrontal Reactivity to Social Threat in Individuals with Impulsive Aggression. Biological Psychiatry. 2007;62(2):168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic Function in Impulsive Aggressive Behavior. Biological Psychiatry. 2011;69(12):1153–1159. doi: 10.1016/j.biopsych.2011.02.032. http://doi.org/10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. Journal of the International Neuropsychological Society : JINS. 2004;10(2):301–303. doi: 10.1017/S1355617704102191. http://doi.org/10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Dolan M. The neuropsychology of prefrontal function in antisocial personality disordered offenders with varying degrees of psychopathy. Psychological Medicine. 2012a;42(8):1715–1725. doi: 10.1017/S0033291711002686. http://doi.org/10.1017/S0033291711002686. [DOI] [PubMed] [Google Scholar]
- Dolan M. The neuropsychology of prefrontal function in antisocial personality disordered offenders with varying degrees of psychopathy. Psychological Medicine. 2012b;42(8):1715–1725. doi: 10.1017/S0033291711002686. [DOI] [PubMed] [Google Scholar]
- Dolan M, Park I. The neuropsychology of antisocial personality disorder. Psychological Medicine. 2002a;32(3):417–427. doi: 10.1017/s0033291702005378. [DOI] [PubMed] [Google Scholar]
- Dolan M, Park I. The neuropsychology of antisocial personality disorder. Psychological Medicine. 2002b;32(3):417–427. doi: 10.1017/s0033291702005378. [DOI] [PubMed] [Google Scholar]
- Edens JF, Kelley SE, Lilienfeld SO, Skeem JL, Douglas KS. DSM-5 antisocial personality disorder: predictive validity in a prison sample. Law and Human Behavior. 2015;39(2):123–129. doi: 10.1037/lhb0000105. http://doi.org/10.1037/lhb0000105. [DOI] [PubMed] [Google Scholar]
- Edens JF, Poythress NG, Lilienfeld SO, Patrick CJ, Test A. Further evidence of the divergent correlates of the Psychopathic Personality Inventory factors: prediction of institutional misconduct among male prisoners. Psychological Assessment. 2008;20(1):86–91. doi: 10.1037/1040-3590.20.1.86. http://doi.org/10.1037/1040-3590.20.1.86. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a non- search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Feilhauer J, Cima M, Korebrits A, Kunert HJ. Differential associations between psychopathy dimensions, types of aggression, and response inhibition. Aggressive Behavior. 2012;38(1):77–88. doi: 10.1002/ab.20415. http://doi.org/10.1002/ab.20415. [DOI] [PubMed] [Google Scholar]
- Frick PJ. Extending the construct of psychopathy to youth: implications for understanding, diagnosing, and treating antisocial children and adolescents. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie. 2009;54(12):803–812. doi: 10.1177/070674370905401203. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Viding E. Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology. 2009;21(04):1111–21. doi: 10.1017/S0954579409990071. http://doi.org/10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- Hamilton RKB, Baskin-Sommers AR, Newman JP. Relation of frontal N100 to psychopathy-related differences in selective attention. Biological Psychology. 2014;103:107–116. doi: 10.1016/j.biopsycho.2014.08.012. http://doi.org/10.1016/j.biopsycho.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annual Review of Clinical Psychology. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. http://doi.org/10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Hare RD, Harpur TJ, Hakstian AR, Forth AE, Hart SD, Newman JP. The revised Psychopathy Checklist: Reliability and factor structure. Psychological Assessment. 1990;2(3):338–341. http://doi.org/10.1037/1040-3590.2.3.338. [Google Scholar]
- Heritage AJ, Benning SD. Impulsivity and response modulation deficits in psychopathy: evidence from the ERN and N1. Journal of Abnormal Psychology. 2013;122(1):215–222. doi: 10.1037/a0030039. http://doi.org/10.1037/a0030039. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry. 2008;49(7):781–791. doi: 10.1111/j.1469-7610.2008.01905.x. http://doi.org/10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Hiatt KD, Schmitt WA, Newman JP. Stroop tasks reveal abnormal selective attention among psychopathic offenders. Neuropsychology. 2004;18(1):50–59. doi: 10.1037/0894-4105.18.1.50. http://doi.org/10.1037/0894-4105.18.1.50. [DOI] [PubMed] [Google Scholar]
- Hobson CW, Scott S, Rubia K. Investigation of cool and hot executive function in ODD/CD independently of ADHD. Journal of Child Psychology and Psychiatry. 2011;52(10):1035–1043. doi: 10.1111/j.1469-7610.2011.02454.x. http://doi.org/10.1111/j.1469-7610.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Byrd AL, Votruba-Drzal E, Hariri AR, Manuck SB. Amygdala reactivity and negative emotionality: divergent correlates of antisocial personality and psychopathy traits in a community sample. Journal of Abnormal Psychology. 2014;123(1):214–224. doi: 10.1037/a0035467. http://doi.org/10.1037/a0035467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Mezzich A, Vanyukov M. Developmental trajectory classes in substance use disorder etiology. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors. 2007;21(3):287–296. doi: 10.1037/0893-164X.21.3.287. http://doi.org/10.1037/0893-164X.21.3.287. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. http://doi.org/10.1037//0021-843X.111.3.411. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114(4):537–550. doi: 10.1037/0021-843X.114.4.537. http://doi.org/10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007a;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. http://doi.org/10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007b;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. http://doi.org/10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007c;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. http://doi.org/10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The Addiction Severity Index: a field study of internal consistency and validity. Journal of Substance Abuse Treatment. 2000;18(2):129–135. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Froböse MI, Konova AB, Misyrlis M, Parvaz MA, Goldstein RZ, Alia-Klein N. Common and distinct neural correlates of inhibitory dysregulation: stroop fMRI study of cocaine addiction and intermittent explosive disorder. Journal of Psychiatric Research. 2014;58:55–62. doi: 10.1016/j.jpsychires.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Montigny C, Castellanos-Ryan N, Whelan R, Banaschewski T, Barker GJ, Büchel C, et al. A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS One. 2013;8(11):e80151. doi: 10.1371/journal.pone.0080151. http://doi.org/10.1371/journal.pone.0080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20(1):113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Back to the Future: Cleckley as a Guide to the Next Generation of Psychopathy Research. Guilford Press; 2006. [Google Scholar]
- Patrick CJ, Durbin CE, Moser JS. Reconceptualizing antisocial deviance in neurobehavioral terms. Development and Psychopathology. 2012;24(3):1047–1071. doi: 10.1017/S0954579412000533. http://doi.org/10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122(3):902–916. doi: 10.1037/a0032807. http://doi.org/10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poythress NG, Edens JF, Skeem JL, Lilienfeld SO, Douglas KS, Frick PJ, et al. Identifying subtypes among offenders with antisocial personality disorder: a cluster-analytic study. Journal of Abnormal Psychology. 2010;119(2):389–400. doi: 10.1037/a0018611. http://doi.org/10.1037/a0018611. [DOI] [PubMed] [Google Scholar]
- Pujara M, Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Neural correlates of reward and loss sensitivity in psychopathy. Social Cognitive and Affective Neuroscience. 2014;9(6):794–801. doi: 10.1093/scan/nst054. http://doi.org/10.1093/scan/nst054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30(4):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience. 2006;1(3):203–213. doi: 10.1093/scan/nsl033. http://doi.org/10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CS, Henson BR, Finney JW, Moos RH. Consistency of self-administered and interview-based Addiction Severity Index composite scores. Addiction. 2000;95(3):419–425. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Verona E. Psychopathic personality traits associated with abnormal selective attention and impaired cognitive control. Neuropsychology. 2008;22(5):669–680. doi: 10.1037/a0012692. http://doi.org/10.1037/a0012692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Daly E, Feng Y, Ecker C, Craig MC, Harding D, et al. Reduced cortical surface area in adolescents with conduct disorder. European Child & Adolescent Psychiatry. 2014:1–9. doi: 10.1007/s00787-014-0639-3. [DOI] [PubMed] [Google Scholar]
- Sellbom M, Verona E. Neuropsychological correlates of psychopathic traits in a non-incarcerated sample. Journal of Research in Personality. 2007;41(2):276–294. http://doi.org/10.1016/j.jrp.2006.04.001. [Google Scholar]
- Skeem JL, Cooke DJ. Is criminal behavior a central component of psychopathy? Conceptual directions for resolving the debate. Psychological Assessment. 2010;22(2):433–445. doi: 10.1037/a0008512. http://doi.org/10.1037/a0008512. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Polaschek DLL, Patrick CJ, Lilienfeld SO. Psychopathic Personality: Bridging the Gap Between Scientific Evidence and Public Policy. Psychological Science in the Public Interest. 2011;12(3):95–162. doi: 10.1177/1529100611426706. http://doi.org/10.1177/1529100611426706. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Trait impulsivity and response inhibition in antisocial personality disorder. Journal of Psychiatric Research. 2009a;43(12):1057–1063. doi: 10.1016/j.jpsychires.2009.03.003. http://doi.org/10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann A, Lijffijt M, Lane S, Steinberg J, Moeller F. Trait impulsivity and response inhibition in antisocial personality disorder. Journal of Psychiatric Research. 2009b doi: 10.1016/j.jpsychires.2009.03.003. http://doi.org/10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed]
- Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: Relations with disinhibitory psychopathology, personality, and psychopathic features. Psychological Assessment. 2012;24(1):88–100. doi: 10.1037/a0024703. http://doi.org/10.1037/a0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Jones AP, Frick PJ, Moffitt TE, Plomin R. Heritability of antisocial behaviour at 9: do callous-unemotional traits matter? Developmental Science. 2008;11(1):17–22. doi: 10.1111/j.1467-7687.2007.00648.x. http://doi.org/10.1111/j.1467-7687.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, Stirling J, Elliott R, Dolan M, Chaudhry I, et al. Neurobiological substrates of antisocial and borderline personality disorder: preliminary results of a functional fMRI study. Criminal Behaviour and Mental Health : CBMH. 2004;14(1):39–54. doi: 10.1002/cbm.559. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Shaw P, Lee NR, Clasen LS, Raznahan A, Lenroot RK, et al. Distinct Cortical Correlates of Autistic versus Antisocial Traits in a Longitudinal Sample of Typically Developing Youth. Journal of Neuroscience. 2012;32(14):4856–4860. doi: 10.1523/JNEUROSCI.6214-11.2012. http://doi.org/10.1523/JNEUROSCI.6214-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Korycinski ST, Soules M, Zubieta J-K, Zucker RA, Heitzeg MM. Substance abuse risk in emerging adults associated with smaller frontal gray matter volumes and higher externalizing behaviors. 137 Drug and alcohol dependence. 2014:68–75. doi: 10.1016/j.drugalcdep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen J, Vanheule S, Verhaeghe P. Psychopathy and lifetime experiences of depression. Criminal Behaviour and Mental Health : CBMH. 2011;21(4):279–294. doi: 10.1002/cbm.812. http://doi.org/10.1002/cbm.812. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Carpenter RW, Warren CM, Zeier JD, Baskin-Sommers AR, Newman JP. Reduced susceptibility to the attentional blink in psychopathic offenders: Implications for the attention bottleneck hypothesis. Neuropsychology. 2012;26(1):102–109. doi: 10.1037/a0026000. http://doi.org/10.1037/a0026000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Research: Neuroimaging. 2009a;174(2):81–88. doi: 10.1016/j.pscychresns.2009.03.012. http://doi.org/10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Research: Neuroimaging. 2009b;174(2):81–88. doi: 10.1016/j.pscychresns.2009.03.012. http://doi.org/10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Research: Neuroimaging. 2009c;174(2):81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. Journal of Abnormal Psychology. 2010;119(3):546–554. doi: 10.1037/a0019611. http://doi.org/10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Zeier JD, Newman JP. Feature-based attention and conflict monitoring in criminal offenders: Interactive relations of psychopathy with anxiety and externalizing. Journal of Abnormal Psychology. 2013;122(3):797–806. doi: 10.1037/a0033873. http://doi.org/10.1037/a0033873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier JD, Baskin-Sommers AR, Hiatt Racer KD, Newman JP. Cognitive control deficits associated with antisocial personality disorder and psychopathy. Personality Disorders. 2012;3(3):283–293. doi: 10.1037/a0023137. http://doi.org/10.1037/a0023137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier JD, Maxwell JS, Newman JP. Attention moderates the processing of inhibitory information in primary psychopathy. Journal of Abnormal Psychology. 2009;118(3):554–563. doi: 10.1037/a0016480. http://doi.org/10.1037/a0016480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans T, Dumontheil I, Roggeman C, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Working memory brain activity and capacity link MAOA polymorphism to aggressive behavior during development. 2 Translational Psychiatry. 2012;e85 doi: 10.1038/tp.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.