Abstract
HIV infection is associated with lower health-related quality of life (HRQoL), which is influenced by immunovirological factors, negative affect, neurocognitive impairment, and functional dependence. Although apathy is a common neuropsychiatric sequela of HIV infection, emerging findings regarding its unique role in lower HRQoL have been mixed. The present study was guided by Wilson and Cleary's (1995) model in examining the association between apathy and physical and mental HRQoL in 80 HIV+ individuals who completed a neuromedical examination, neuropsychological assessment, structured psychiatric interview, and a series of questionnaires including the SF-36. Apathy was measured using a composite of the apathy subscale of the Frontal Systems Behavioral Scale and the vigor-activation subscale of the Profile of Mood States. Independent of major depressive disorder, neurocognitive impairment, functional status, and current CD4 count, apathy was strongly associated with HRQoL. Specifically, apathy and CD4 count were significant predictors of physical HRQoL, whereas apathy and depression were the only predictors of mental HRQoL. All told, these findings suggest that apathy plays a unique role in HRQoL and support the importance of assessing and managing apathy in an effort to maximize health outcomes among individuals with HIV disease.
Keywords: HIV/AIDS, neuropsychiatry, motivation, quality of life, health status
The advances in combination antiretroviral therapy (cART) have improved the longevity of individuals infected with HIV. Yet while cART has improved overall HIV disease outcomes (Kitahata et al., 2009), HIV-infected individuals still face numerous psychosocial (e.g. stigma), medical (e.g. hepatitis C), neuropsychiatric (e.g. depression), and neurological (e.g. neurocognitive impairment) complications. These issues often adversely impact health-related quality of life (HRQoL), which is a multidimensional concept that characterizes an individual's well-being across physical, mental, emotional and social functioning (Wilson & Cleary, 1995). Relative to the general population, HIV-infected individuals consistently report lower levels of HRQoL (e.g. Piette, Wachtel, Mor, & Mayer, 1995). With the reduction in HIV-associated morbidity and mortality in the cART era, improving quality-adjusted survival has become a major goal of HIV disease management (Wu, 2000).
Wilson and Cleary's (1995) model of HRQoL posits that biological factors lead to physical and psychological symptoms, which manifest in functional impairment and consequently lower HRQoL. At the level of individual factors, several studies in HIV+ cohorts provide support for the contribution of demographic (e.g. age; Rodriguez-Penney et al., 2013) and environmental factors (e.g. social support; Oetzel et al., 2014), immunovirological variables (e.g. CD4 counts; Weinfurt, Willke, Glick, Freimuth, & Schulman, 2000), neurocognitive impairment (e.g. Osowiecki et al., 2000) and functional dependence (e.g. Liu et al., 2006) in lower HRQoL in HIV. At the multivariate level, the Wilson and Cleary model was supported in a study of 395 HIV+ persons by Sousa and Kwok (2006) who found that lower current immune functioning (i.e. CD4 count) was related to greater physical symptoms, which was associated with poorer functional status and general health perceptions, which in turn was significantly related to lower overall HRQoL.
Here, we use the Wilson and Clearly model to guide our investigation of the role of neuropsychiatric symptoms in HIV-associated HRQoL. HIV infection is commonly accompanied by various neuropsychiatric complications such as depression, alexithymia, and apathy. To date, depression has received the most empirical attention among the neuropsychiatric aspects of HIV in the context of HRQoL. Depression is reliably associated with multiple aspects of HRQoL among persons living with HIV (Jia, Uphold, Wu, Chen, & Duncan, 2005). Alterations in emotion regulation and cognitive emotional processing, known as alexithymia, are also associated with lower HRQoL in HIV disease (Bogdanova, Díaz-Santos, & Cronin-Golomb, 2010).
Apathy is a related, but dissociable, neuropsychiatric syndrome that might play an important role in HRQoL among persons infected with HIV. Defined as the lack of cognitive, physical, and emotional motivation, apathy is exacerbated in early HIV infection (Kamat et al., in press) and occurs in an estimated 40% of chronically infected HIV+ persons (e.g. Kamat, Woods, Marcotte, Ellis, & Grant, 2012). Apathy is separable from depression (e.g. Paul et al., 2005) and neurocognitive impairment (e.g. Robinson-Papp et al., 2008) in HIV disease and is related to frontostriatal white matter abnormalities (e.g. Kamat et al., 2014). Apathy is also associated with worse functional outcomes in HIV, including poor medication management outcomes (e.g. Barclay et al., 2007) and greater severity of impairments in activities of daily living (Kamat et al., 2012). In the context of Wilson and Cleary's model, this literature suggests that circuit-specific central nervous system changes (i.e. biological factors) lead to apathy (i.e. neuropsychiatric symptom), which has an adverse effect of everyday functioning outcomes (i.e. functional status) in HIV-infected persons. Thus, it stands to reason that apathy may also be a determinant of HRQoL in HIV.
To date, there have only been two studies that have examined the relationship between apathy and HRQoL in HIV. In Rabkin et al. 2000 reported that, after controlling for depression, the Apathy Evaluation Scale (AES) showed medium associations with the Endicott Quality of Life Enjoyment and Satisfaction questionnaire in separate samples of 75 HIV+ men and 58 HIV+ women. In contrast, Tate et al. (2003) found a modest, independent association between the AES and mental health and role disruption scales of the SF-36 in 45 HIV+ persons after controlling for depression. However, the AES was not significantly associated with physical scale of the SF-36 in this study and the authors concluded that apathy might be less important than depression with regard to QoL in HIV disease (Tate et al., 2003). Given these conflicting prior findings, the current study aimed to extend the limited literature by investigating the association between apathy and both mental and physical HRQoL in the context of biologic, neuropsychiatric, and functional factors suggested by the Wilson and Cleary model. Such an approach would serve to characterize the unique relationship between apathy and HRQoL over and above other relevant determinants. It was specifically hypothesized that elevated levels of current apathy (quantified using a composite score rather than a single instrument) would uniquely predict physical and mental HRQoL, independent of HIV disease severity (e.g. current CD4 count), depression, neurocognitive deficits, and functional status in HIV.
Methods
Participants
Study participants included 80 HIV+ individuals recruited from local HIV clinics and community-based organizations. The study was approved by the local Institutional Review Board and all participants provided written, informed consent. Study exclusions included neuromedical conditions (e.g. seizure disorders, closed head injury, and stroke), severe psychiatric disorders (e.g. psychosis), current substance use disorder, and positive Breathalyzer for alcohol or urine toxicology for illicit drugs. HIV serostatus was determined with Medmira rapid tests. The demographic, clinical, neurocognitive, and psychiatric characteristics of the study sample are presented in Table 1.
Table 1.
Study sample demographic and clinical characteristics (N = 80).
| Mean (SD); (range) | |
|---|---|
|
| |
| Demographic variables | |
| Age, years | 46.2 (9.8); (24, 60) |
| Education, years | 13.6 (2.5); (5, 20) |
| Sex (% male) | 89.2 |
| Ethnicity | |
| Caucasian (%) | 57.5 |
| African American (%) | 20.0 |
| Hispanic (%) | 17.5 |
| Other (%) | 5.0 |
| Psychiatric characteristics | |
| Current Major Depressive disorder (%) | 2.5 |
| Lifetime Major Depressive disorder (%) | 60.0 |
| Lifetime Alcohol use disorder (%) | 46.8 |
| Lifetime non-Alcohol use disorder (%) | 21.3 |
| Neurocognitive and Everyday functioning | |
| Global Deficit Score | .84 (.9) |
| Proportion with functional impairment (%) | 50.0 |
| Proportion classified as dependent using PAOFI (%) | 50.0 |
| Proportion classified as dependent using ADLQ (%) | 17.8 |
| Proportion classified as dependent using Karnofsky (%) | 32.9 |
| Proportion unemployed (%) | 52.5 |
| HIV disease characteristics | |
| Nadir CD4 count (median, IQR) | 199.5 (58.2, 307.5) |
| Estimated duration of infection (years; median, IQR) | 10.6 (4.4, 22.1) |
| Proportion on cART (%) | 92.0 |
| Current CD4 count (median, IQR) | 659 (412.0, 854.5) |
| AIDS (%) | 54 |
| HIV RNA log10 (median, IQR) | 1.6 (1.6, 1.6) |
Notes. PAOFI = Patient's Assessment of Own Functioning, with dependence classified as more than 3 cognitive tasks that cause problems at least “often”; ADLQ = Activities of Daily Living Questionnaire, with dependence classified as more than 1 domain in which at least mild dependence was reported; Karnofsky = Karnofsky Performance Scale, clinician-rated functional status. IQR = Interquartile range.
Procedure
Apathy
As there are no single, “gold standard” measures of apathy, we used a composite score approach by using two well-validated questionnaires that assess apathy symptomatology. As shown in Table 2, study participants completed the 14-item apathy subscale of the self-report version of the Frontal Systems Behavior Scale (FrSBe; Grace & Malloy, 2001) and the Vigor-Activation subscale of the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1981). Raw scores from these two subscales were converted to sample-based z-scores and averaged to yield the apathy composite score. The Pearson product moment correlation coefficient for the two scales was large (r = −.55, p < .001) providing evidence of convergent validity and thus supported their use as a composite.
Table 2.
Apathy and quality of life scores for the study sample (N = 80).
| Mean (SD); (range) | |
|---|---|
| Apathy variables | |
| FrSBe Apathy Scale | 29.8 (8.8); (15, 52) |
| Clinically elevated (%) | 32.5 |
| POMS Vigor-Activation Scale | 16.7 (7.5) |
| Clinically elevated (%) | 15.0 |
| Quality of Life (SF-36) variables | |
| Physical health | 47.7 (13.0); (17.4, 71.5) |
| Physical functioning | 79.94 (24.2); (0, 100) |
| Physical role limitation | 68.15 (41.7); (0, 100) |
| Bodily pain | 66.51 (30.5); (0, 100) |
| General health | 60.78 (25.0); (0, 100) |
| Mental health | 45.35 (13.3); (11.4, 64.6) |
| Vitality | 59.52 (20.3); (0, 100) |
| Social functioning | 72.17 (27.1); (0, 100) |
| Emotional role limitation | 65.87 (42.3); (0, 100) |
| Emotional well-being | 73.90 (17.80); (0, 100) |
Note:
FrSBe = Frontal Systems Behavior Scale, T-scores ≥ 65 are considered clinically elevated; POMS = Profile of Mood States, on the Vigor-Activation scale, T-scores < 35 are considered clinically elevated.
SF-36 Subscale scores are norm-based scores. The Physical and Mental Health summary scores are calculated using the norm-based subscale z-scores and the physical and mental factor coefficients.
Health-related quality of life
Participants were also administered the RAND 36-item Short Form Health Survey (SF-36), a well-validated index of HRQoL that is designed to assess physical and mental health well-being (Bing et al., 2000; Lamping, 1994). The Physical and Mental Health subscales are each comprised of four subscales, and range from 0 to 100 where higher scores indicate better HRQoL (see Table 2). The Physical Health summary measure consists of the Physical Functioning, Physical Role Functioning, Bodily Pain, and General Health subscales. The SF-36's Mental Health summary score consists of the Vitality, Social Functioning, Emotional well-being, and Emotional Role Functioning subscales. Continuous Physical and Mental HRQoL summary scores were used for the primary study analyses.
Mood and substance use disorders
Current (i.e. 30-day) and lifetime mood disorders, as well as diagnoses of lifetime alcohol, marijuana, methamphetamine, cocaine, and opioid use disorders were determined with the Composite International Diagnostic Interview (CIDI; Wittchen, 1994).
Neurocognitive assessment
Participants completed the CogState (www.cogstate.com), which has been validated for use in HIV (e.g. Cysique, Maruf, Darby, & Brew, 2006) and consists of tasks that assess psychomotor function (simple detection task), visual attention (identification, simple matching and monitoring tasks), executive functions (working memory and complex matching tasks) and learning. Raw scores were converted to demographically-adjusted T-scores, which were then used to generate a Global Deficit Score (see Carey et al., 2004).
Real-world functional assessment
Consistent with previous studies (e.g. Doyle et al., 2013), a composite variable was constructed to measure functional impairment. We assessed five functional domains (i.e. cognitive symptoms in daily life, basic and instrumental activities of daily living, clinician-rated global functioning, and employment). Within each of these domains, participants were dichotomized as “impaired” or “normal” according to established procedures (for details, see Doyle et al., 2013). Individuals with impairment in two or more domains were classified as globally functionally impaired.
Statistical analyses
We first conducted simple univariate Pearson correlations between apathy and the SF-36. Our primary study hypotheses were tested using a series of multiple regressions in which the SF-36 QoL variables were the criteria and the apathy composite was our primary predictor of interest. A linear regression model allowed us to examine the unique relationship between apathy and HRQoL while adjusting for multiple covariates. Guided by the Wilson & Cleary model, we selected key biological (i.e. CD4 count), neuropsychiatric (i.e. major depression diagnosis), neurological (i.e. global neurocognitive deficit score), and functional (i.e. dependence status) covariates on an a priori basis. Of course, there are other variables that might also be considered important on an a priori bases; for example certain demographics like age or gender. We examined whether age and gender were associated with apathy and HRQoL; as they were not confounding variables (i.e. not significantly correlated with both apathy and HRQoL), they were not added as covariates in the model in order to not overfit the models. Also of note, major depressive disorder (MDD) diagnosis was entered in the model rather than current depressive symptoms as this allowed us to take a more stringent approach in accounting for apathy that may be related to a prior episode of depression. The critical alpha was set at .05.
Results
Figures 1 and 2 show that there was a robust negative correlation between apathy and the Physical and Mental Health summary scores of the SF-36 (ps < .001).
Figure 1.
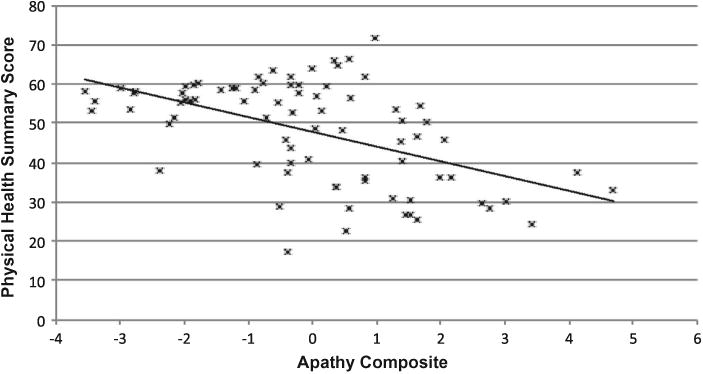
Relationship between the SF-36 Physical Health summary score and apathy composite among 80 participants with HIV disease. Pearson's r =−.51.
Figure 2.
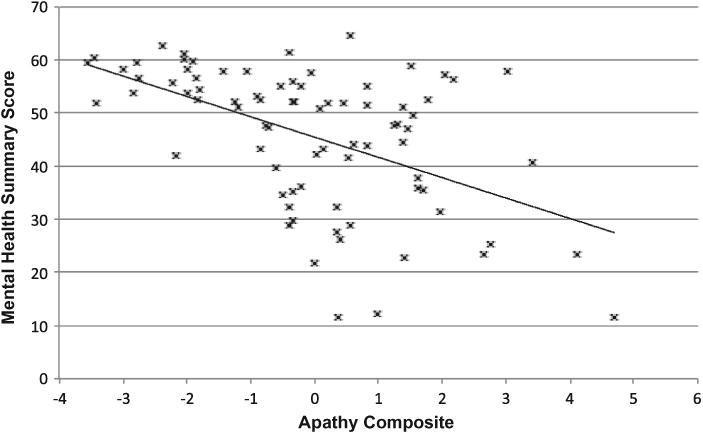
Relationship between the SF-36 Mental Health summary score and apathy composite among 80 participants with HIV disease. Pearson's r = −.51.
SF-36 Physical Health
As shown in Table 3, a significant regression model was observed for the SF-36 Physical Health scale (p < .001), with apathy and current CD4 count being significant predictors (ps < .05). The remaining covariates were not significantly associated with the overall Physical Health scale (ps > .10). When age and gender were added to the model as covariates, the pattern of results was unchanged. Similar associations between apathy and HRQoL were observed across the individual subscales that make up the Physical Health domain, including Physical Functioning, Physical Role Limitations, Bodily Pain, and General Health (ps < .05).
Table 3.
Multiple Regression of SF-36 Scores in a Sample of 80 Individuals Infected with HIV.
| Variable | Model | B | B 95% CI | Parameter (β) | p-value |
|---|---|---|---|---|---|
| SF-36 Physical Health Score | |||||
|
| |||||
| Adjusted R2 | .30 | ||||
| F | 7.86 | <.0001 | |||
| Apathy | −3.34 | −4.82 −1.86 | −.46 | <.0001 | |
| Major Depression | 1.36 | −1.19 – 3.09 | .10 | .29 | |
| Neurocognitive impairment | 1.28 | −1.19 – 3.78 | .10 | .31 | |
| Functional status | −1.77 | −4.30 – .74 | −.14 | .16 | |
| Current CD4 count | .01 | 0.0 – .02 | .25 | .01 | |
| SF-36 Mental Health Score | |||||
|
| |||||
| Variable | Model | B | B 95% CI | Parameter (β) | p-value |
| Adjusted R2 | .28 | ||||
| F | 7.22 | <.0001 | |||
| Apathy | −3.52 | −5.06 – −1.99 | −.47 | <.0001 | |
| Major Depression | 2.64 | 0.0 – 5.29 | .20 | .05 | |
| Neurocognitive impairment | .32 | −2.89 – 2.25 | −.02 | .80 | |
| Functional status | .66 | −1.96 – 3.28 | .05 | .62 | |
| Current CD4 count | −.006 | −.01 – .002 | −.14 | .15 | |
SF-36 Mental Health
The multiple regression model for the SF-36 Mental Health was also significant (p < .001). Consistent with the Physical Health regression described above, apathy was a significant predictor of Mental Health (p < .001). Major depression was only marginally related to Mental Health (p = .05), while the other covariates were not significant in this model (see Table 3). The inclusion of age and gender as covariates in this model did not change the pattern of findings.
With regard to the subscales of the Mental Health scale, the models predicting ratings of Vitality, Social Functioning, Emotional Role Functioning, and Emotional Well-being were all significant (ps < .01) and showed significant, notable contributions of apathy in each case (ps < .05).
Discussion
There is growing evidence to suggest that apathy may develop secondary to HIV-associated neuropathologic changes in frontostriatal systems and increase the risk of functional dependence. However, the extent to which apathy plays a key role in lower HRQoL in HIV disease remains undetermined, as the only two existing studies on this topic have produced mixed results (Rabkin et al., 2000; Tate et al., 2003). Results from our study suggest that apathy is strongly and uniquely associated with lower physical and mental HRQoL in persons infected with HIV. The association between apathy and HRQoL was independent of current immune function, depression, neurocognitive impairment, and dependence in everyday functioning. Our findings converge with Rabkin et al. (2000), but differ from Tate et al. (2003) who posited that depression may be more important than apathy in QoL among individuals with HIV. In addition to using a composite measure of apathy and having a larger sample, our study differed from Tate et al. in that less than 10 % of our cohort met criteria for current depression and 31% reported clinically significant current depressive symptoms (vs. 80% with clinically significant mood symptoms in the Tate et al. cohort). Of note, our rates are consistent with the 11 % rate of current major depression or dysthymia reported by Rabkin et al. as well as the national epidemiological estimates of major depressive disorder in HIV (e.g. Heaton et al., 2010). It is possible that the markedly high level of current depression in the Tate et al. study may have led to the over-estimation of the effect of depression on QoL. Nonetheless, the disparity between these findings warrants reconsideration of the possibly unique relationship between apathy and HRQoL in HIV disease.
The analyses of the correlates of physical and mental health related HRQoL revealed a distinct pattern of predictors. Apathy and current CD4 count were associated with physical HRQoL. Within the context of the Wilson and Cleary (1995) model, this is consistent with the theorized role of neurobehavioral impairment and biological factors in disrupting HRQoL. The effects of biological factors (in this case CD4 count) on health and QoL may be mediated by changes in the organ system (e.g. systemic inflammation and central nervous system abnormalities). The resultant symptoms, whether physical or neuropsychiatric, influence an individual's subjective sense of physical well-being (Wilson & Cleary, 1995). We did not examine the causal relationships posited by the Wilson and Cleary (1995) model; future studies may benefit from further characterizing the association between apathy, changes in specific physical abilities, and functional status. These efforts may serve to guide rehabilitation efforts for individuals experiencing impairments in everyday functioning secondary to neuropsychiatric disturbances.
Mental HRQoL, on the other hand, was independently associated with both apathy and depression. Features of apathy such as impaired processing of emotions and affect as well as reduced goal-directed behavior (e.g. Levy & Dubois, 2006) may result in the withdrawal from social activities, occupations, and relationships, and consequently decrease HRQoL within the mental health domain (Leroi et al., 2011). In fact, our data provide some support for this; apathy ratings were independently associated with poor self-reported vitality, extent and duration of social interactions, and emotional well-being. Interestingly, apathy was the only significant predictor of impaired role functioning secondary to emotional problems. This suggests that HIV-associated disruption in emotional-affective processing, compared to depression, may have a greater bearing on psychologically driven problems in activities of daily living. The effect of depression on poor HRQoL has been well documented in various disease groups (e.g. Gaynes, Burns, Tweed, & Erickson, 2002), including HIV-infected individuals (e.g. Tate et al., 2003). It has been proposed that depression may be related to poor perceived control over one's illness and/or the perceived severity of one's disease, thereby impacting mental health related quality of life (e.g. Paschalides et al., 2004). Future longitudinal research is needed to tease apart the mechanisms through which apathy and depression independently impact various domains of mental QoL.
This study has limitations that warrant consideration. The first is the cross-sectional study design, which does not permit examination of the trajectories of apathy, depression, and the consequent changes in HRQoL over the course of the disease. Second, we used self-report measures of apathy and everyday functioning outcomes, whose accuracy may be affected by bias, depression, and/or mild anosognosia (Blackstone et al., 2012). Nonetheless, there is strong evidence of the construct validity of self-report apathy measures, including associations with neuroanatomical abnormalities and impaired everyday functioning. Future studies may benefit from using objective measures of everyday functioning (e.g. electronic medication adherence tracking) and clinical interview (e.g. Neuropsychiatric Inventory; Cummings et al., 1994) or informant report to assess neuropsychiatric disturbance. Future investigations may also elucidate the relative benefit of using apathy composites versus other measures (e.g. Marin Apathy Evaluation scale) that have been commonly used in assessing this construct in HIV.
The high prevalence of apathy in HIV infection and its association with poor HRQoL has implications for the management of this disease in early as well as chronic stages. Our data indicate that HIV treatment providers should assess symptoms of apathy, not only depression, as it has a clinically meaningful impact on physical and mental well-being in this population. There is some evidence supporting pharmacological and behavioral interventions for apathy in non-HIV populations (e.g. Roth, Flashman, & McAllister, 2007), however it remains to be seen whether such therapies are effective in HIV+ persons and can produce downstream improvements in HRQoL.
Acknowledgments
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, PhD, Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., PhD, and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, PhD; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., PhD (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, PhD, Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, PhD (P.I.), J. Hampton Atkinson, M.D., Thomas D. Marcotte, PhD, Mariana Cherner, PhD, David J. Moore, PhD, Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, PhD (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, PhD, Richard Buxton, PhD, Anders Dale, PhD, Thomas Liu, PhD; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., PhD; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, PhD; Developmental Component: Cristian Achim, M.D., PhD; (P.I.), Stuart Lipton, M.D., PhD; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, PhD (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, PhD (P.I.), Florin Vaida, PhD (Co-PI), Reena Deutsch, PhD, Anya Umlauf, M.S. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Funding: This research was specifically supported by National Institute of Mental Health [grant number R21-MH098607]. Dr. Kamat is supported by R25-MH081482 and Dr. Iudicello is supported by K23-DA037793.
Footnotes
Disclosure statement: No potential conflict of interest was reported by the authors.
References
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, et al. Levine AJ. Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Hays RD, Jacobson LP, Chen B, Gange SJ, Kass NE, et al. Chmiel JS. Health-related quality of life among people with HIV disease: Results from the multicenter aids cohort study. Quality of Life Research. 2000;9:55–63. doi: 10.1023/A:1008919227665. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Cliford DB, et al. Collier AC. Diagnosing symptomatic HIV-associated neurocognitive disorders: Self-Report versus performance-based assessment of everyday functioning. Journal of the International Neuropsychological Society. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova Y, Díaz-Santos M, Cronin-Golomb A. Neurocognitive correlates of alexithymia in asymptomatic individuals with HIV. Neuropsychologia. 2010;48:1295–1304. doi: 10.1016/jneuropsychologia.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK, et al. the HIV Neurobehavioral Research Center (HNRC) Group. Predictive validity of global deficit scores in detecting neuropsychological impairment in hiv infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2308. doi: 10.1212/WNL.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruf P, Darby D, Brew BJ. The assessment of cognitive function in advanced HIV-1 infection and AIDS dementia complex using a new computerised cognitive test battery. Archives of Clinical Neuropsychology. 2006;21:185–194. doi: 10.1016/j.acn.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Morgan EE, Morris S, Smith DM, Little S, Iudicello JE, Blackstone Kaitlin, et al. the Translational Methamphetamine and AIDS Research Center (TMARC) Group. Real-world impact of neurocognitive deficits in acute and early HIV infection. Journal of NeuroVirology. 2013;19:565–573. doi: 10.1007/s13365-013-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Burns BJ, Tweed DL, Erickson P. Depression and health-related quality of life. The Journal of Nervous and Mental Disease. 2002;190:799–806. doi: 10.1097/00005053-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy PF. Frontal systems behavior scale: professional manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Heaton RK, Cliford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Uphold CR, Wu S, Chen GJ, Duncan PW. Predictors of changes in health-related quality of life among men with HIV infection in the HAART era. AIDS Patient Care & STDs. 2005;19(6):395–405. doi: 10.1089/apc.2005.19.395. [DOI] [PubMed] [Google Scholar]
- Kamat R, Brown GG, Bolden K, Fennema-Notestein C, Archibald S, Marcotte TD, et al. Letendre Scott L. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2014;36:854–866. doi: 10.1080/13803395.2014.950636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Doyle KL, Iudicello JE, Morgan EE, Morris S, Smith D, Woods SP the Translational Methamphetamine and AIDS Research Center (TMARC) Group. (in press). Neurobehavioral disturbances in acute and early HIV infection. Cognitive and Behavioral Neurology. doi: 10.1097/WNN.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Archives of Clinical Neuropsychology. 2012;27:520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, Hogg RD. Effect of early versus deferred antiretroviral therapy for HIV on survival. New England Journal of Medicine. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamping DL. Methods for measuring outcomes to evaluate interventions to improve health-related quality of life in HIV infection. Psychology and Health. 1994;9:31–49. doi: 10.1080/08870449408407458. [DOI] [Google Scholar]
- Leroi I, Ahearn DJ, Andrews M, McDonald KR, Byrne EJ, Burns A. Behavioural disorders, disability and quality of life in Parkinson's disease. Age and Ageing. 2011;40:614–621. doi: 10.1093/ageing/afr078. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Liu C, Johnson L, Ostrow D, Silvestre A, Visscher B, Jacobson LP. Predictors for lower quality of life in the haart era among HIV-infected men. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;42:470–477. doi: 10.1097/01.qai.0000225730.79610.61. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Oetzel J, Wilcox B, Archiopoli A, Avila M, Hell C, Hill R, Muhammad M. Social support and social undermining as explanatory factors for health-related quality of life in people living with HIV/AIDS. Journal of Health Communication. 2014;19:660–675. doi: 10.1080/10810730.2013.837555. [DOI] [PubMed] [Google Scholar]
- Osowiecki DM, Cohen RA, Morrow KM, Paul RH, Carpenter CC, Flanigan T, Boland RJ. Neurocognitive and psychological contributions to quality of life in HIV-1-infected women. AIDS. 2000;14:1327–1332. doi: 10.1097/00002030-200007070-00004. [DOI] [PubMed] [Google Scholar]
- Paschalides C, Wearden AJ, Dunkerley R, Bundy C, Davies R, Dickens CM. The associations of anxiety, depression and personal illness representations with glycaemic control and health-related quality of life in patients with type 2 diabetes mellitus. Journal of Psychosomatic Research. 2004;57:557–564. doi: 10.1016/j.jpsychores.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Paul R, Flanigan TP, Tashima K, Cohen R, Lawrence J, Alt E, et al. Hinkin C. Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. The Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:114–118. doi: 10.1176/appi.neuropsych.17.1.114. [DOI] [PubMed] [Google Scholar]
- Piette J, Wachtel TJ, Mor V, Mayer K. The impact of age on the quality of life in persons with HIV infection. Journal of Aging and Health. 1995;7:163–178. doi: 10.1177/089826439500700201. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Ferrando SJ, van Gorp W, Rieppi R, McElhiney M, Sewell M. Relationships among apathy, depression, and cognitive impairment in HIV/AIDS. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:451–457. doi: 10.1176/appi.neuropsych.12.4.451. [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Byrd D, Mindt MR, Oden NL, Simpson DM, Morgello S. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Archives of Neurology. 2008;65:1096–1101. doi: 10.1001/archneur.65.8.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, Grant Igor the HIV Neurobehavioral Research Program (HNRP) Group. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care and STDs. 2013;27:5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Flashman LA, McAllister TW. Apathy and its treatment. Current Treatment Options in Neurology. 2007;9:363–370. doi: 10.1007/s11940-007-0022-5. [DOI] [PubMed] [Google Scholar]
- Sousa KH, Kwok OM. Putting wilson and cleary to the test: Analysis of a HRQOL conceptual model using structural equation modeling. Quality of Life Research. 2006;15:725–737. doi: 10.1007/s11136-005-3975-4. [DOI] [PubMed] [Google Scholar]
- Tate D, Paul RH, Flanigan TP, Tashima K, Nash J, Adair C, et al. Boland RA. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care and STDs. 2003;17:115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Weinfurt KP, Willke RJ, Glick HA, Freimuth WW, et al. Schulman KA. Relationship between cd4 count, viral burden, and quality of life over time in HIV-1-infected patients. Medical Care. 2000;38:404–410. doi: 10.1097/00005650-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. JAMA. 1995;273:59–65. doi: 10.1001/jama.1995.03520250075037. [DOI] [PubMed] [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): A critical review. Journal of Psychiatric Research. 1994;28(94):57–84. 90036–1. doi: 10.1016/0022-3956. [DOI] [PubMed] [Google Scholar]
- Wu AW. Quality of life assessment comes of age in the era of highly active antiretroviral therapy. AIDS. 2000;14:1449–1451. doi: 10.1097/00002030-200007070-00019. [DOI] [PubMed] [Google Scholar]


