Abstract
Background
Recent animal and human studies reveal distinct cognitive and neurobiological differences between opiate and stimulant addictions; however, our understanding of the common and specific effects of these two classes of drugs remains limited due to the high rates of polysubstance-dependence among drug users.
Methods
The goal of the current study was to identify multivariate substance-specific markers classifying heroin dependence (HD) and amphetamine dependence (AD), by using machine-learning approaches. Participants included 39 amphetamine mono-dependent, 44 heroin mono-dependent, 58 polysubstance dependent, and 81 non-substance dependent individuals. The majority of substance dependent participants were in protracted abstinence. We used demographic, personality (trait impulsivity, trait psychopathy, aggression, sensation seeking), psychiatric (attention deficit hyperactivity disorder, conduct disorder, antisocial personality disorder, psychopathy, anxiety, depression), and neurocognitive impulsivity measures (Delay Discounting, Go/No-Go, Stop Signal, Immediate Memory, Balloon Analogue Risk, Cambridge Gambling, and Iowa Gambling tasks) as predictors in a machine-learning algorithm.
Results
The machine-learning approach revealed substance-specific multivariate profiles that classified HD and AD in new samples with high degree of accuracy. Out of 54 predictors, psychopathy was the only classifier common to both types of addiction. Important dissociations emerged between factors classifying HD and AD, which often showed opposite patterns among individuals with HD and AD.
Conclusions
These results suggest that different mechanisms may underlie HD and AD, challenging the unitary account of drug addiction. This line of work may shed light on the development of standardized and cost-efficient clinical diagnostic tests and facilitate the development of individualized prevention and intervention programs for HD and AD.
Keywords: heroin, amphetamines, addiction, protracted abstinence, impulsivity, machine-learning
1. INTRODUCTION
Drug addiction is typically regarded as a unitary phenomenon (Badiani et al., 2011); however, animal and human studies increasingly suggest that despite their many similarities, different classes of drugs such as opiates and stimulants have distinct mechanisms of action and neurobehavioral correlates (Badiani et al., 2011; George and Koob, 2010; Verdejo-García et al., 2007). Both classes of drugs modulate the dopamine (DA) system but the mechanisms of these modulations differ for opiates and stimulants (Kreek et al., 2012) and there is surprisingly minimal overlap of genes associated with these classes of drugs (Kendler et al., 2003; Tsuang et al., 1998). Moreover, recent studies provide little support for a general liability factor to substance misuse and instead reveal that liability to misuse illicit substances is substance-specific (Clark et al., 2016). There are major differences in the role of the ventromedial prefrontal cortex (vmPFC), which appears to serve fundamentally different roles in opiate and stimulant addictions, acting as a neural OFF switch for cocaine seeking, but an ON switch for heroin seeking (Peters et al., 2013). A growing number of preclinical studies similarly reveal that opiate and stimulant addictions have dissociable effects with opiates producing inhibitory and sedative effects, in contrast to stimulants’ arousing and excitatory effects (Badiani et al., 2011; Stewart et al., 1984). Further, trait impulsivity predicts greater stimulant intake, but not heroin intake (Dalley et al., 2007; McNamara et al., 2010). Also, both clinical and preclinical studies reveal that the differential effects of these drugs depend on the specific environmental context, with the sedative effects of opiates being greater in familiar and non-arousing environments, whereas the rewarding effects of stimulants are enhanced in novel and arousing environments (Caprioli et al., 2008).
However, clinical studies of personality and neurocognitive factors show mixed findings that are inconsistent with preclinical studies. For example, both opiate and stimulant users report increased trait impulsivity (Stanford et al., 2009) and sensation seeking (Ersche et al., 2010). Clinical studies of neurocognitive function also show mixed results: some studies report distinct patterns of neurocognitive performance in opiate and stimulant users (Ornstein et al., 2000; Rogers et al., 1999; Verdejo-García et al., 2007) whereas others report comparable neurocognitive profiles (Kirby and Petry, 2004).
There are three major gaps in the existing clinical literature on opiate and stimulant addictions that we aim to address in this work. First, as previously noted (de Wit, 2008), despite the overwhelming preclinical and clinical evidence supporting impulsivity as a key factor of potential etiological significance for virtually all types of addictions, impulsivity is multidimensional and few studies have concurrently assessed its multiple personality, psychiatric, and neurocognitive dimensions in users of different classes of drugs (c.f., Vassileva et al., 2014). Most previous studies (Ahn et al., 2014b; Kirby and Petry, 2004; Rogers et al., 1999) have typically focused on a single or a limited number of measures (c.f., Whelan et al., 2014); however, evidence suggests that specific dimensions of impulsivity may be differentially related to different aspects of addictive behaviors. For example, urgency (i.e., acting impulsively during negative emotional states) has been associated with substance related problems whereas sensation seeking has been associated with frequency of substance use (Castellanos-Ryan and Conrod, 2011; Cyders et al., 2009; Smith et al., 2007). Further, mounting evidence indicates that some dimensions of impulsivity may be potential endophenotypes for drug addiction (Kreek et al., 2005) and meet endophenotype criteria (Bickel, 2015; MacKillop, 2013); however, the relative predictive utility of these dimensions as putative endophenotypes for drug addiction remains unknown. Given the multidimensional nature of impulsivity, multivariate impulsivity endophenotypes that exploit the relationship between multiple impulsivity phenotypes may increase power to detect common and unique effects of opiate and stimulant addictions. Second, because of the widespread polysubstance use and dependence among substance dependent individuals in the United States and Western Europe (Fernandez-Serrano et al., 2011), it is challenging for clinical studies to dissociate the specific effects of opiate and stimulant addictions. Studies focusing on more homogeneous sub-groups of drug users characterized by relatively “pure” addictions could be more informative in this regard. Third, we have limited understanding about the brain’s recovery of function and whether protracted abstinence, one of the least-well understood stages of the addiction cycle, would reverse some of the residual effects of chronic opiate and stimulant use on brain and behavior. Some evidence suggests that certain manifestations of impulsivity may persist in protracted abstinence and may be implicated in heightened susceptibility to relapse (Ahn et al., 2014b; Stevens et al., 2015a; Vassileva et al., 2014).
Here, we address these challenges by recruiting predominantly mono-substance dependent participants in Bulgaria, where polysubstance dependence is still not as common (Ahn et al., 2014b; Vassileva et al., 2014); focusing on opiate- and stimulant-dependent participants in protracted abstinence; using a wide spectrum of personality, psychiatric, and neurocognitive measures encompassing multiple dimensions of impulsivity; and employing machine-learning approaches, proven to be particularly promising for identifying markers among multiple potential predictors that generalize to new samples (Volkow et al., 2015; Whelan and Garavan, 2014). To identify substance-specific behavioral markers for heroin and amphetamine dependence, we applied a machine-learning approach to demographic, personality, psychiatric, and neurocognitive measures of impulsivity and related constructs from individuals with lifetime mono-dependence on heroin or amphetamine, lifetime polysubstance dependence, and no history of dependence. While conventional univariate methods overlook the relationship among measures and typically compare healthy and psychiatric groups one measure at a time, machine-learning methods characterize multivariate patterns of data that are optimized to predict group membership in new samples. Machine-learning is particularly useful when data are high-dimensional and consideration of multiple comparisons is essential to minimize both Type I and II errors (Hastie et al., 2009). Thus, machine-learning is regarded as the most promising approach to identify predictive markers for psychiatric disorders and classify psychiatric populations with high-dimensional data (Volkow et al., 2015). The main goal of the study was to identify multivariate profiles that will most accurately characterize heroin dependence (HD) and amphetamine dependence (AD) and generalize to new samples.
2. MATERIALS AND METHODS
2.1. Participants
Study participants included 222 individuals, enrolled in a larger study on impulsivity among drug users in Sofia, Bulgaria. Participants were recruited via flyers placed at substance abuse clinics, nightclubs, bars, and cafes in Sofia as well as by word of mouth. Participants were initially screened via telephone and in-person on their medical and substance use histories.
Participants had to meet the following inclusion criteria: 1) age between 18 and 50 years; 2) estimated IQ>75; 3) minimum of 8th grade education; 4) no history of neurological illness; 5) HIV seronegative status; 6) negative breathalyzer test for alcohol and negative urine toxicology screen for amphetamines, methamphetamines, cocaine, opiates, methadone, cannabis, benzodiazepines, barbiturates, and MDMA. As seen in Table 1, the current study included individuals meeting DSM-IV diagnostic criteria for mono-dependence on heroin (N=44), mono-dependence on amphetamines (N=39), polysubstance dependence (N=58), and no history of substance dependence (N=81). With the exception of caffeine and nicotine, all “pure” mono-dependent substance users had no history of dependence on alcohol or any drug other than stimulants or opiates. At the time of testing, most substance users were in protracted abstinence (i.e., sustained full remission by DSM-IV criteria).
Table 1.
Demographic, clinical, personality, and neurobehavioral characteristics of participants
| HC (N=81) | Heroin (N=44) | Amphetamine (N=39) | Poly (N=58) | Test statistic (F) | Sig. | Post- hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Demographic | |||||||||||
|
| |||||||||||
| Age | 24.27 | 4.42 | 29.32 | 4.86 | 23.44 | 4.04 | 25.95 | 4.86 | 15.03 | 6.31E- 09 | a,d,e,f |
| Gender (%Male) | 72.84 | 75.00 | 66.67 | 84.48 | 4.45 | n.s. | |||||
| Education (yrs) | 13.80 | 2.27 | 12.61 | 2.36 | 13.10 | 2.36 | 12.83 | 1.87 | 3.62 | 0.014 | a,c |
| IQ | 109.94 | 12.85 | 102.50 | 13.35 | 110.85 | 10.78 | 105.84 | 13.21 | 4.49 | 0.004 | a,d |
| # of relatives with alcohol/drug problems | 0.42 | 0.79 | 0.66 | 0.86 | 0.54 | 0.68 | 0.86 | 0.83 | 3.63 | 0.014 | c |
|
| |||||||||||
| Psychiatric | |||||||||||
|
| |||||||||||
| History of Conduct Disorder (%) | 12.34 | 36.36 | 41.03 | 62.07 | 37.54 | 3.53E- 08 | a,b,c,e | ||||
| History of ASPD (%) | 6.17 | 34.09 | 35.90 | 58.62 | 44.95 | 9.50E- 10 | a,b,c,e | ||||
| Yrs of Heroin use | 0.00 | 0.00 | 7.10 | 3.08 | 0.00 | 0.00 | 3.89 | 4.97 | 71.39 | 2.00E- 16 | a,c,d,e,f |
| Yrs of Amphetamine use | 0.69 | 2.42 | 0.16 | 0.94 | 3.98 | 2.42 | 2.90 | 2.98 | 26.25 | 2.01E- 14 | b,c,d,e |
| Yrs of Alcohol use DSM-IV Past dependence | 8.53 | 4.75 | 10.90 | 5.53 | 7.85 | 4.04 | 10.07 | 5.23 | 3.55 | 0.015 | d |
| Alcohol (%) | 0.00 | 0.00 | 0.00 | 22.41 | 44.43 | 1.22E- 09 | c,e,f | ||||
| Sedatives (%) | 0.00 | 0.00 | 0.00 | 6.90 | 12.23 | 0.007 | |||||
| Cannabis (%) | 0.00 | 0.00 | 0.00 | 82.76 | 150.36 | 2.20E- 16 | c,e,f | ||||
| Amphetamine (%) | 0.00 | 0.00 | 100.00 | 67.24 | 156.23 | 2.20E- 16 | b,c,d,e,f | ||||
| Opiates (%) | 0.00 | 100.00 | 0.00 | 44.83 | 155.66 | 2.20E- 16 | a,c,d,e,f | ||||
| Cocaine (%) | 0.00 | 0.00 | 0.00 | 6.90 | 12.87 | 0.005 | |||||
| Hallucinogens (%) | 0.00 | 0.00 | 0.00 | 6.90 | 12.87 | 0.005 | |||||
| Length of Abstinence(yrs) | 1.09 | 2.28 | 1.74 | 2.35 | 0.40 | 0.60 | 0.77 | 0.96 | 4.2 | 0.007 | d,e |
| Fagerstrom Test of nicotine dependence | 1.41 | 2.10 | 4.27 | 2.71 | 2.62 | 2.41 | 4.57 | 2.97 | 22.2 | 1,39E- 12 | a,c,d,f |
| Psychopathy Checklist (PCL:SV) Factor 1 | 2.12 | 2.10 | 4.73 | 2.58 | 3.13 | 2.58 | 4.88 | 2.68 | 18.71 | 7.78E- 11 | a,c,d,f |
| Psychopathy Checklist (PCL:SV) Factor 2 | 2.57 | 2.44 | 7.16 | 2.82 | 5.00 | 2.76 | 7.67 | 2.65 | 52.23 | 2.00E- 16 | a,b,c,d,f |
| Wender Utah Rating Scale (WURS) for ADHD | 21.90 | 11.58 | 30.98 | 16.20 | 26.28 | 13.51 | 32.10 | 15.40 | 7.41 | 9.53E- 05 | a,c |
| Depression (BDI-II) | 5.67 | 5.70 | 8.61 | 5.75 | 6.54 | 4.89 | 8.84 | 7.72 | 3.95 | 0.009 | c |
| Stait Anxiety | 31.86 | 7.12 | 36.32 | 8.51 | 32.15 | 6.57 | 35.76 | 8.28 | 5.20 | 0.002 | a,c |
| Trait Anxiety | 36.30 | 8.42 | 40.25 | 9.53 | 38.87 | 9.31 | 42.29 | 10.01 | 5.04 | 0.002 | c |
| Anxiety Sensitivity | 15.23 | 7.22 | 17.27 | 9.02 | 17.62 | 8.87 | 19.69 | 9.49 | 3.13 | 0.027 | c |
|
| |||||||||||
| Personality | |||||||||||
|
| |||||||||||
| Levenson’s Self-Report Psychopathy Scale | 35.52 | 8.67 | 37.70 | 9.95 | 37.87 | 6.87 | 39.78 | 7.37 | 3.00 | 0.032 | c |
| BIS Nonplanning | 22.48 | 4.97 | 26.00 | 4.77 | 24.79 | 4.97 | 25.00 | 4.71 | 6.09 | 5.38E- 04 | a,c |
| BIS Motor | 21.35 | 4.04 | 23.34 | 5.76 | 24.79 | 4.41 | 24.10 | 4.80 | 6.41 | 3.50E- 04 | b,c |
| BIS Attention | 14.86 | 3.35 | 15.36 | 3.89 | 16.74 | 3.70 | 16.43 | 3.81 | 3.39 | 0.019 | b |
| Buss-Warren Physical | 15.47 | 5.52 | 18.70 | 6.59 | 18.97 | 6.09 | 20.91 | 6.36 | 9.65 | 5.21E- 06 | a,b,c |
| Buss-Warren Verbal | 14.67 | 3.75 | 15.09 | 3.21 | 15.51 | 3.46 | 15.67 | 3.37 | 1.09 | n.s. | |
| Buss-Warren Anger | 15.00 | 3.69 | 16.91 | 3.92 | 16.46 | 3.15 | 17.64 | 4.32 | 5.87 | 7.12E- 04 | a,c |
| Buss-Warren Hostility | 15.04 | 5.10 | 16.52 | 4.65 | 18.05 | 6.25 | 18.21 | 5.46 | 5.03 | 0.002 | b,c |
| Buss-Warren Indirect | 13.17 | 4.44 | 15.23 | 4.45 | 14.44 | 3.89 | 16.09 | 4.23 | 5.63 | 0.001 | c |
| UPPS Urgency | 21.93 | 6.07 | 27.50 | 6.28 | 24.97 | 5.86 | 28.10 | 6.75 | 13.54 | 3.91E- 08 | a,c |
| UPPS Lack of Premeditation | 24.14 | 5.58 | 26.14 | 6.00 | 26.10 | 5.46 | 26.90 | 6.18 | 2.90 | 0.036 | a |
| UPPS Lack of Perseverance | 19.07 | 4.76 | 21.20 | 4.74 | 20.59 | 5.70 | 21.26 | 5.28 | 2.78 | 0.042 | |
| UPPS Sensation Seeking | 27.21 | 7.04 | 27.00 | 8.23 | 30.33 | 7.93 | 29.84 | 6.90 | 2.84 | 0.039 | |
| SSS: Disinhibition | 4.33 | 2.50 | 4.66 | 2.84 | 6.08 | 2.16 | 5.78 | 2.22 | 6.66 | 2.53E- 04 | b,c,d |
| SSS: Boredom Susceptibility | 3.22 | 1.87 | 3.70 | 2.15 | 4.26 | 2.21 | 4.03 | 2.06 | 2.97 | 0.033 | b |
| SSS: Thrill and Adventure Seeking | 6.15 | 3.08 | 5.73 | 3.03 | 7.00 | 2.89 | 7.43 | 2.11 | 4.03 | 0.008 | c,e |
| SSS: Experience Seeking | 5.37 | 2.00 | 5.34 | 2.07 | 6.49 | 1.59 | 6.34 | 1.83 | 5.5 | 0.001 | b,c,d,e |
|
| |||||||||||
| Neurobehavioral | |||||||||||
|
| |||||||||||
| IGT: ABCD | 4.86 | 26.37 | −0.59 | 25.98 | 2.64 | 28.04 | 3.02 | 25.94 | 0.41 | n.s. | |
| IGT: EFGH | 18.51 | 38.94 | 10.93 | 32.54 | 11.18 | 37.76 | 20.24 | 36.86 | 0.87 | n.s. | |
| SST %inhibition, 50ms | 93.02 | 9.70 | 94.77 | 6.73 | 92.05 | 9.23 | 88.97 | 14.01 | 2.90 | 0.036 | e |
| SST %inhibition, 150ms | 76.67 | 18.17 | 76.14 | 13.46 | 72.44 | 20.68 | 74.57 | 19.27 | 0.54 | n.s. | |
| SST %inhibition, 250ms | 52.53 | 20.83 | 50.34 | 15.94 | 52.31 | 24.06 | 47.59 | 23.49 | 0.69 | n.s. | |
| SST %inhibition, 350ms | 30.37 | 18.60 | 25.80 | 12.80 | 33.33 | 17.07 | 31.72 | 20.87 | 1.41 | n.s. | |
| IMT d′ | 1.22 | 0.50 | 1.03 | 0.46 | 1.17 | 0.43 | 1.04 | 0.58 | 2.18 | n.s. | |
| IMT b | 0.80 | 0.30 | 0.83 | 0.35 | 0.76 | 0.29 | 0.84 | 0.34 | 0.58 | n.s. | |
| IMT Commission Error (%) | 36.19 | 13.24 | 38.95 | 13.10 | 38.75 | 11.50 | 38.62 | 14.91 | 0.64 | n.s. | |
| IMT Omission Error (%) | 22.32 | 12.31 | 26.38 | 15.63 | 20.89 | 10.97 | 26.29 | 15.53 | 2.05 | n.s. | |
| Delay discounting rate (log(k)) | −3.11 | 1.20 | −2.88 | 1.01 | −2.45 | 0.86 | −2.91 | 0.94 | 3.51 | 0.016 | b |
| BART pumps | 40.14 | 10.07 | 39.92 | 13.43 | 40.88 | 13.86 | 40.65 | 15.04 | 0.06 | n.s. | |
| Go/Nogo False Positives | 14.49 | 8.15 | 16.55 | 8.44 | 16.74 | 6.57 | 18.17 | 10.09 | 2.19 | 0.090 | |
| Go/Nogo False Negatives | 16.10 | 18.19 | 18.93 | 15.45 | 17.15 | 13.34 | 18.02 | 16.60 | 0.32 | n.s. | |
| Go/Nogo d′ | 2.30 | 0.77 | 2.07 | 0.83 | 2.06 | 0.64 | 2.01 | 0.78 | 2.02 | n.s. | |
| Go/Nogo b | 0.54 | 0.43 | 0.61 | 0.60 | 0.49 | 0.29 | 0.56 | 0.48 | 0.47 | n.s. | |
| CGT Delay Aversion | 0.32 | 0.19 | 0.42 | 0.22 | 0.31 | 0.16 | 0.34 | 0.20 | 3.29 | 0.022 | a,d |
| CGT Decision Time (msec) | 2314.61 | 650.45 | 2269.23 | 707.88 | 2510.87 | 714.98 | 2415.23 | 748.75 | 1.08 | n.s. | |
| CGT Quality Decision Making | 0.87 | 0.14 | 0.85 | 0.17 | 0.87 | 0.12 | 0.87 | 0.12 | 0.39 | n.s. | |
| CGT Risk Aversion | 0.99 | 0.86 | 0.91 | 0.91 | 1.03 | 0.89 | 0.71 | 0.66 | 1.63 | n.s. | |
| CGT Risk Taking | 0.63 | 0.12 | 0.60 | 0.13 | 0.62 | 0.13 | 0.64 | 0.13 | 0.55 | n.s. | |
Note. ASPD = Antisocial Personality Disorder; BDI = Beck Depression Inventory; BIS = Barratt Impulsiveness Scale; SSS = Sensation-Seeking Scale; IGT = Iowa Gambling Task; SST = Stop Signal Task; IMT = Immediate Memory Task; CGT = Cambridge Gambling Task; n.s = non-significant (p > 0.10).
HC vs. Heroin groups is significantly different.
HC vs. Amphetamine groups is significantly different.
HC vs. Poly-substance groups is significantly different.
Heroin vs. Amphetamine groups is significantly different.
Heroin vs. Poly-substance groups is significantly different.
Amphetamine vs. Poly-substance groups is significantly different.
Given that our goal was to classify heroin and amphetamine dependence, we combined the poly-and mono-dependent groups and classified individuals as “with HD”/“without HD” (Table S11) and “with AD”/“without AD” (Table S22). This approach allowed us to examine HD and AD with larger sample sizes. For the classification of HD, we had 70 individuals with HD (all 44 from the pure heroin-dependence group and 26 from the polysubstance dependence group) and 152 individuals without HD (all 81 healthy controls, all 39 from the pure amphetamine-dependence group, and 32 from the polysubstance dependence group). For the classification of AD, we had 79 individuals with AD (all 39 from the pure amphetamine-dependence group and 40 from the polysubstance dependence group) and 143 individuals without AD (all 81 healthy controls, all 44 from the pure heroin-dependence group, and 18 from the polysubstance dependence group).
2.2. Procedures
The study was approved by the Institutional Review Boards of Virginia Commonwealth University and the Medical University-Sofia on behalf of the Bulgarian Addictions Institute. For more detailed description of the study protocol and screening procedures, see Vassileva et al. (2014). Briefly, after signing an informed consent form participants underwent urine drug screens and a Breathalyzer test for alcohol. Then participants completed two study sessions of approximately 4 hours each, which included assessment of substance use (SCID-SAM; First and Gibbon, 1997), IQ, trait impulsivity, neurocognitive impulsivity, and psychiatric comorbidities (see Measures for details).
2.3. Measures Included in the Machine-learning Analyses
2.3.1. Demographic Measures
Demographic measures included gender, age, years of education, IQ assessed with the Raven’s Progressive Matrices (Raven and Raven, 2000), and family history of substance use problems (number of relatives with alcohol or substance use problems) determined via pedigree. In total, we included 5 demographic measures.
2.3.2. Psychiatric Measures
Psychiatric measures included length of abstinence (time since last used drugs); severity of nicotine dependence, assessed with the Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991); history of conduct disorder (CD) and antisocial personality disorder (ASPD), assessed with the Structured Clinical Interview for DSM-IV; psychopathy, indexed with the interpersonal/affective Factor 1 and the antisocial/lifestyle Factor 2 of the Psychopathy Checklist: Screening Version (PCL:SV; Hart et al., 1995); self-report childhood symptoms of ADHD assessed with the Wender Utah Rating Scale (WURS; Ward et al., 1993); current depression assessed with the Beck Depression Inventory–II (BDI-II; Beck et al., 1996); anxiety assessed with the State-Trait Anxiety Inventory (Spielberger and Gorsuch, 1983); and anxiety sensitivity indexed with the Anxiety Sensitivity Index (Reiss et al., 1986). In total, we included 11 psychiatric measures.
2.3.3. Personality measures
Personality trait measures included three subscales (motor, non-planning, and attentional impulsivity) of the Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995); four subscales (negative urgency, lack of planning, lack of perseverance, and sensation seeking) of the UPPS Impulsive Behavior Scale (Whiteside and Lynam, 2001); five subscales (physical aggression, verbal aggression, hostility, anger, and indirect hostility) of the Buss-Warren Aggression Questionnaire (BWAQ; Buss and Warren, 2000); the Levenson’s Self-Report Psychopathy Scale (LSRP; Levenson et al., 1995); and four subscales (experience-seeking, thrill/adventure-seeking, disinhibition, boredom susceptibility) of the Sensation-Seeking Scale (SSS; Zuckerman et al., 1964). In total, we included 17 personality indices.
2.3.4. Neurocognitive impulsivity (laboratory task) measures
Our battery of neurocognitive impulsivity measures included two versions (ABCD and EFGH) of the Iowa Gambling Task (IGT; Bechara et al., 1994; 2000), the Stop Signal Task (SST; Dougherty et al., 2005), the Immediate Memory Task (IMT; Dougherty et al., 2002), the Monetary Choice Questionnaire indexing delayed reward discounting (DRDT; Kirby et al., 1999), the Balloon Analogue Risk Task (BART; Lejuez et al., 2002), the Go/Nogo Task (GNGT; Lane et al., 2007), and the Cambridge Gambling Task (CGT; Rogers et al., 1999). See Supplementary Material3 for detailed description of the measures. In total, we included 21 neurocognitive indices: 2 from the IGT (net scores on IGT-ABCD and IGT-EFGH), 4 from the SST (response inhibition ratio at 50-msec, 150-msec, 250-msec, and 350-msec intervals), 4 from the IMT (discriminability (d′), response bias (b), commission error rate, and omission error rate), 1 from the DRDT (natural log of k discounting rate), 1 from the BART (the adjusted average number of pumps), 4 from the GNGT (discriminability, response bias, commission error rate, and omission error rate), and 5 from the CGT (risk taking, risk adjustment, quality of decision-making, deliberation time, and delay aversion).
2.4. Statistical Analyses
We first compared the groups using a traditional approach: On each measure (test variable), we used omnibus analysis of variance (ANOVA) across all four groups, then Tukey’s HSD test for post hoc pair-wise group comparisons, the findings of which are summarized in Table 1 (see Tables S1–24 for group comparisons based on participants’ HD or AD dependence status).
Next, we applied a machine-learning method called the elastic net (Zou and Hastie, 2005) to all the data from a total of 54 demographic, psychiatric, personality, and neurocognitive measures to uncover multivariate profiles that contribute to the out-of-sample classification of heroin- and amphetamine-dependence. The elastic net, which is one of penalized regression (supervised learning) methods, enjoys automatic variable selection and the regression coefficients of unimportant variables shrink to zero. Also, highly correlated variables can be selected all together, if they are predictive, because of its grouping effect.
The core procedures for generating out-of-sample classification (penalized logistic regression) are similar to those used in a previous study (Ahn et al., 2014a), which provide detailed illustration of the method (but also see Ahn et al., under review). The dependent variable was whether an individual has met dependence criteria for heroin or amphetamine. For model fitting and generating out-of-sample classification, we first split the data (N=222) into a training set (67% of the data, N=148) and a test (validation) set (33% of the data, N=74), fitted the elastic net model using the training set, and made classifications on the test and training sets separately. Out-of-sample classification accuracy can be affected by how similar the training and test sets are. Thus, we further checked the generalizability of our findings by randomly dividing the data into training and test sets and checking model performance 1,000 times. We used the area under the curve (AUC) of the receiver operating characteristic (ROC) curve as an index of model performance. See Supplementary Material5 for details.
3. RESULTS
3.1. Participants’ Characteristics
Table 1 shows the demographic, psychiatric, personality, and neurocognitive characteristics of participants. Reflecting the timeline of heroin and amphetamine influx in Bulgaria (Ahn et al., 2014b), groups differed significantly on age: heroin dependent individuals (HDIs) were significantly older than all other groups, and polysubstance dependent individuals (PDIs) were significantly older than amphetamine dependent individuals (ADIs). Healthy control individuals (HCIs) had more years of education than ADIs. HDIs had lower IQ than both HCIs and ADIs. In terms of family history of substance misuse, PDIs had more relatives with alcohol/drug problems than HCIs. There were no group differences in sex or handedness. See Table 1 for more between-group comparison results and Tables S1–26 for the characteristics and between-group comparisons of individuals classified by HD or AD status.
3.2. Elastic Net Results
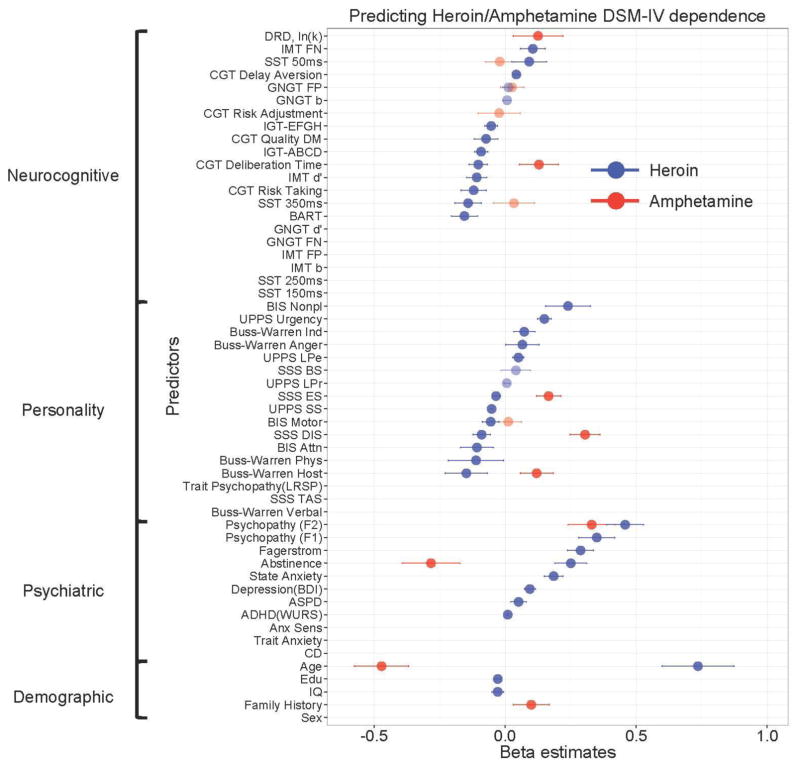
Figure 1 shows the multivariate profiles for HD or AD, revealed by the machine-learning method. HD was classified by older age, lower IQ, lower years of education, and several psychiatric (longer abstinence, more severe nicotine dependence, higher depression, higher state anxiety, higher PCL:SV Factor 1 and Factor 2 psychopathy, and higher ADHD) and personality indices (higher BIS Non-planning Impulsivity; higher BWAQ Indirect Aggression and Anger; higher UPPS Urgency and Lack of Perseverance; lower BIS Attentional and Motor Impulsivity; lower BWAQ Physical Aggression and Hostility; lower UPPS Sensation Seeking; and lower SSS Experience-seeking and Disinhibition). HD was also classified by several neurocognitive measures including impaired decision-making performance on the IGT (ABCD and EFGH), higher IMT omission errors, higher CGT delay aversion, higher SST 50-msec response inhibition ratio (i.e., lower impulsivity), lower IMT discriminability, lower BART risk score, lower CGT deliberation time (i.e., faster responding), lower CGT risk taking, lower CGT quality of decision-making, and lower SST 350-msec response inhibition ratio (i.e., higher impulsivity).
Figure 1.
Multivariate patterns of demographic, psychiatric, personality, and neurocognitive measures classifying individuals with past heroin- or amphetamine-dependence. CD = Conduct Disorder; ASPD = Antisocial Personality Disorder; BDI = Beck Depression Inventory; Anx = Anxiety; Anx Sens = Anxiety Sensitivity; LRSP = Levenson’s Self-Report Psychopathy Scale; PCL = Psychopathy Checklist: Screening Version; WURS = Wender Utah Rating Scale for ADHD; BIS = Barratt Impulsiveness Scale; SSS = Sensation Seeking Scale; IGT = Iowa Gambling Task; SST = Stop Signal Task; IMT = Immediate Memory Task; DRD = Delayed Reward Discounting; BART = Balloon Analogue Risk Task; GNGT = Go/Nogo Task; CGT = Cambridge Gambling Task; DA = Delay Aversion; DT = Decision Time; QDM = Quality Decision-Making; RA = Risk Adjustment; RT = Risk Taking.
In contrast, AD was classified by younger age, family history of substance use problems, shorter abstinence, higher psychopathy PCL:SV Factor 2, higher BWAQ Hostility, higher SSS Disinhibition, higher SSS Experience-seeking, higher delay discounting on the DRDT and higher CGT deliberation time (i.e., slower responding).
Table 2 summarizes the multivariate classification patterns for each category of measures that were common to both classes of drugs, specific to HD or AD, and in opposite directions in HD and AD. Contrary to the unitary account of drug addiction, we found that only one out of 54 indices, namely the antisocial/lifestyle factor of psychopathy (PCL:SV Factor 2) was common to both HD and AD. Several other measures (age, length of abstinence, SSS Experience-seeking, SSS Disinhibition, Buss-Warren Hostility, CGT deliberation time) were associated with both HD and AD, but the association was in opposite direction for HD and AD.
Table 2.
Summary of demographic, psychiatric, personality, and neurocognitive measures that uniquely or commonly predict heroin- or amphetamine-depenence
| Predictors | Demographic | Psychiatric | Personality | Neurocognitive |
|---|---|---|---|---|
| Common to heroin & amphetamine | None | PCL:SV Factor 2
|
None | None |
|
| ||||
| Unique to heroin | IQ
|
PCL:SV Factor 1
|
BIS Nonplanning
|
IMT FN
|
SST 50ms
| ||||
Fagerstrom
|
UPPS Urgency
|
CGT DA
|
||
BART
| ||||
BWAQ Ind
|
SST 350ms
|
|||
Education
|
State Anxiety
|
BWAQ Anger
|
CGT RT
|
|
UPPS LPe
|
IMT d′
|
|||
BDI
|
BWAQ Phys
|
CGT DT
|
||
BIS Attention
|
IGT-ABCD
|
|||
ASPD
|
BIS Motor
|
CGT QDM
|
||
WURS
|
UPPS SS
|
IGT-EFGH
|
||
|
| ||||
| Unique to amphetamine | Family history
|
None | None | DRDT, ln(k)
|
|
| ||||
| Opposite in heroin & amphetamine | Age
|
Length of abstinence
|
SSS DIS
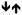 SSS ES 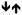 BWAQ Hostility 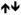
|
CGT DT
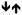
|
Note: PCL:SV = Psychopathy Checklist: Screening Version; BDI = Beck Depression Inventory; ASPD = Antisocial Personality Disorder; WURS = Wender Utah Rating Scale for ADHD; BIS = Barratt Impulsiveness Scale; BWAQ = Buss-Warren Aggression Questionnaire; SSS = Sensation Seeking-Scale; IGT = Iowa Gambling Task; SST = Stop Signal Task; IMT = Immediate Memory Task; DRDT = Delayed Reward Discounting Task; BART = Balloon Analogue Risk Task; CGT = Cambridge Gambling Task; DA = Delay Aversion; DT = Decision Time; QDM = Quality Decision-Making; RA = Risk Adjustment; RT = Risk Taking.
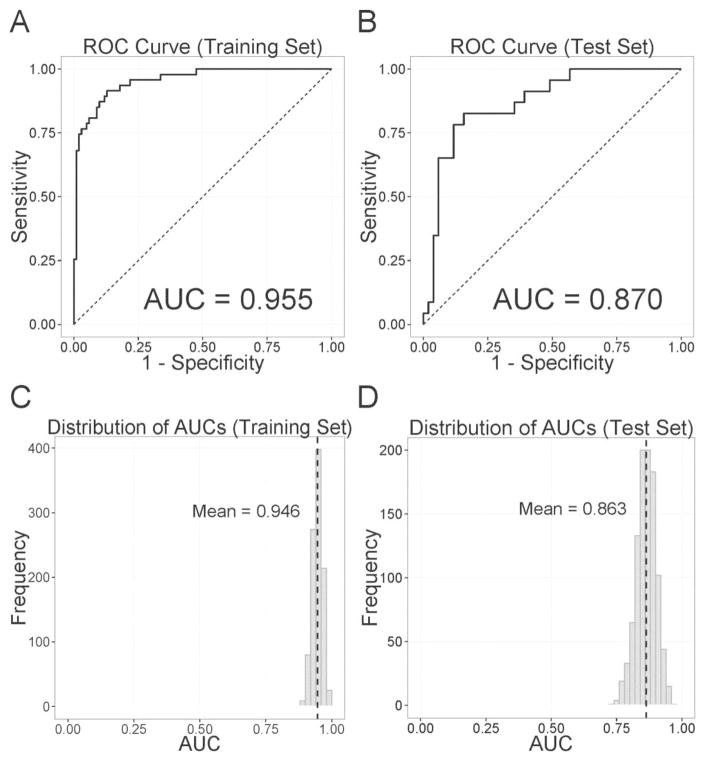
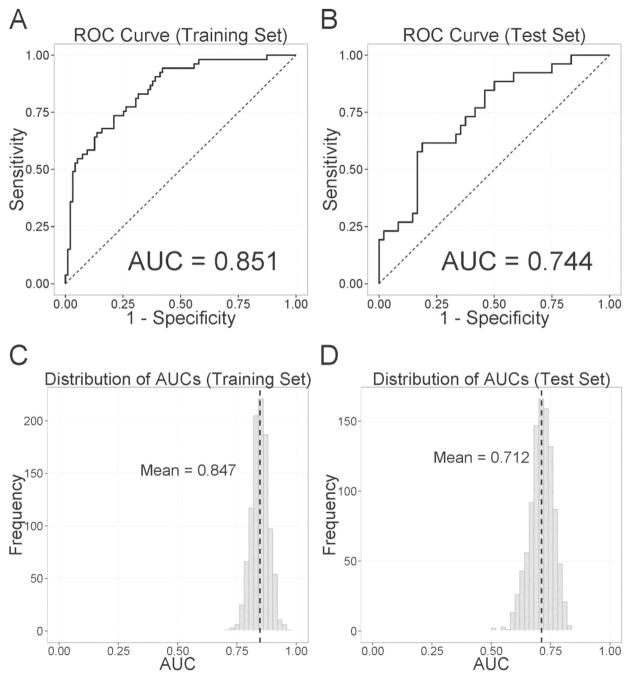
Figures 2 and 3 show the receiver-operating characteristic (ROC) curve and its mean area under the curve (AUC) for the classification of HD and AD, respectively. For HD (Figure 2 A–B), the AUC was 0.955 for the training set and 0.870 for the test set. When we checked model performance on randomly selected 1,000 training and test sets (Figure 2 C–D), the mean AUCs were 0.946 and 0.863 for the training and test sets. For AD, the AUC was 0.851 for the training set and 0.744 for the test set (Figure 3 A–B). The mean AUCs on randomly selected 1,000 training and test sets were 0.847 and 0.712, respectively (Figure 3 C–D).
Figure 2.
Classification accuracy of past heroin dependence as indexed by the Receiver-Operating-Characteristic (ROC) curves and their area under the curve (AUC) of (A) the training set and (B) the test set. Panels C and D represent the histograms of AUCs when we randomly selected training and test sets 1,000 times. Dashed black lines indicate the mean values of histograms.
Figure 3.
Classification accuracy of past amphetamine dependence as indexed by the Receiver-Operating-Characteristic (ROC) curves and their area under the curve (AUC) of (A) the training set and (B) the test set. Panels C and D represent the histograms of AUCs when we randomly selected training and test sets 1,000 times. Dashed black lines indicate the mean values of histograms.
3.3. Elastic Net Results Using Mono-Substance Dependent (Pure) Users
In order to increase the sample size, the results reported above were based on mixed groups of pure users and polysubstance users. However, it was of additional interest to examine the classifiers in pure users to check the generalizability and specificity of the findings. Thus, in separate analyses, we performed direct classifications of pure HDIs (N=44), pure ADIs (N=39), and PDIs (N=58) against drug-naïve HCIs (N=81) using identical machine-learning procedures. Overall, although some differences were noted, most of the important findings remain the same with the pure groups. See Supplementary Material and Figures S1–47 for the results of the analyses.
4. DISCUSSION
This study demonstrates that HD and AD are characterized by distinct multivariate patterns of demographic, psychiatric, personality, and neurocognitive indices, which challenges the unitary account of drug addiction and validates some preclinical findings. By using machine-learning approaches and by recruiting individuals with past mono-dependence on heroin or amphetamines who were currently in protracted abstinence, this work addressed three major gaps in the existing literature. Specifically, we (1) Concurrently assessed multiple dimensions of impulsivity within the same subject population, as often recommended but rarely done with substance dependent individuals; (2) Identified substance-specific multivariate patterns that classified HD or AD in new samples; and (3) Provided evidence that these patterns are observable in protracted abstinence, i.e., are state independent, which suggests their potential utility as endophenotypes for HD and AD.
Regardless of whether or not polysubstance users were included in the model, the only common classifier of both HD and AD was the antisocial/lifestyle factor of psychopathy (PCL:SV Factor 2), which is strongly related to the externalizing spectrum disorders and is also associated with impulsive violence and criminal versatility (Vassileva et al., 2005). Our finding that the PCL:SV Factor 2 is related to both HD and AD is consistent with previous studies identifying a factor common to all externalizing disorders including ADHD, conduct disorder, and substance misuse (Castellanos-Ryan et al., 2014). Combined, these results suggest that such a broad lifestyle trait-like factor could underlie the development of drug addiction and its comorbidity with other externalizing disorders. Future studies need to replicate the role of PCL:SV Factor 2 in addiction to different classes of drugs and its relationship to the general externalizing factor identified with structural equation modeling (Castellanos-Ryan et al., 2014).
This study also supports previous findings that personality measures are some of the strongest predictors of substance misuse (Castellanos-Ryan et al., 2014; Vassileva et al., 2014; Whelan et al., 2014) and indicate that there are both common and substance-specific personality profiles classifying HD and AD. The interpersonal/affective factor of psychopathy (PCL:SV Factor 1) was uniquely associated with HD and was the strongest classifier of HD (excluding age), in line with previous studies (Vassileva et al., 2014; 2011). In contrast, consistent with preclinical findings, AD was uniquely characterized by sensation seeking (SSS Disinhibition and SSS Experience Seeking). A notable finding was that the heroin users in our study were not particularly impulsive, which could have contributed to their successful maintenance of long-term abstinence without being on opiate maintenance therapy. Specifically, individuals with HD were low sensation seekers (SSS Disinhibition, SSS Experience Seeking, UPPS Sensation Seeking), had low motor and attentional impulsivity (BIS-11) and low hostility. On the other hand, they were characterized by higher non-planning impulsivity (BIS-11) and higher impulsivity under negative affective states (UPPS Negative Urgency). Future studies should determine whether these characteristics have classification utility only for the protracted abstinence stage of HD or whether they would also apply to current HD. Overall, these findings suggest that the efficacy of treatment interventions could be increased if they are tailored to the specific personality characteristics of HDI and ADI (Conrod et al., 2010).
Neurocognitive impulsivity measures revealed additional substance-specific aspects of HD and AD although classifiers revealed by machine-learning differed somewhat depending on whether the model included PDIs for the classification of HD or AD. HD was classified by performance on several tasks including impaired decision-making on the IGT and CGT, reduced risk taking on the CGT (c.f., not in Figure S48 based only on pure HDIs) and fewer impulsive choices on the BART, which further supports the personality trait findings that impulsivity is not strongly related to HD. Instead, HD was associated with attentional problems as indicated by more errors of omission (rather than commission) and low discriminability on the IMT. HDI’s impaired decision-making performance on the IGT is consistent with previous studies (Ahn et al., 2014b), especially among individuals with comorbid HD and psychopathy (Vassileva et al., 2011; 2007). HD was also predicted by higher delay aversion on the CGT, but not by higher delay discounting on the DRDT, which is not entirely consistent with the literature and may be specific to the protracted abstinence stage of HD. In contrast, AD was predicted by higher delay discounting on the DRDT and longer decision time on the CGT (c.f., not in Figure S49 when only using pure ADIs). In a parallel study in USA where we also used a machine-learning approach, we similarly found that higher delay discounting was a robust classifier of current stimulant (cocaine) dependence (Ahn et al., 2015). Together, these results indicate that delay discounting may be uniquely related to stimulant dependence in particular, given the convergence of findings across two different samples (active and former users), in two different countries (USA and Bulgaria), and with two different types of stimulants (cocaine and amphetamines). This suggests that delay discounting may be a viable endophenotype for stimulant (but not opiate) addiction (Bickel, 2015; MacKillop, 2013).
Taken together, our results demonstrate the important role of impulsivity in the etiology of HD and AD and the importance of concurrently considering its multiple dimensions. Recently, impulsivity has gained grounds as one of the strongest putative endophenotypes for addictive disorders. Our findings add to the mounting literature (Kreek et al., 2005) indicating that some measures of impulsivity may meet consensus endophenotype criteria (Gottesman and Gould, 2003). Our current and previous studies (Ahn et al., 2014b; Vassileva et al., 2014) show that deficits in impulsivity are observable regardless of the state of the addiction and persist even after protracted abstinence from drug use. Other cross-sectional and longitudinal studies indicate that children or biological siblings of drug users (Ersche et al., 2012) display elevated impulsivity prior to any drug use and that impulse control deficits are reliable predictors of later drug initiation and problems (Verdejo-García et al., 2008). The heritability of trait impulsivity has been confirmed by numerous genetic studies and there is also growing evidence for the heritability of neurocognitive measures of impulsivity (MacKillop, 2013). One of the most intriguing aspects of the current findings is the possibility that some putative endophenotypes may be substance-specific.
Future studies are needed to address some limitations of the current cross-sectional study and explore the biological basis of HD and AD with novel computational approaches. The current work used only behavioral measures, suggesting that such time- and cost-effective measures can be highly predictive of substance misuse. For example, the behavioral process of delay discounting has been proposed to be candidate behavioral marker of addiction (Bickel et al., 2014) with significant predictive utility for motivation for treatment and treatment retention (Stevens et al., 2015b). Similarly, neurobehavioral indices of various components of impulsivity were put forward as biomarkers or surrogate markers of biological processes (Volkow et al., 2015), given their high correlations with substance use disorders and the growing understanding of their neural substrates brought by neuroimaging, which may further improve their classification and prediction accuracy. We believe this study represents an important step forward for translating research findings into clinical settings. However, until replicated by future studies, the evidence for the differences in classification profiles between HD and AD might remain limited to the measures included in the current study. Finally, the small gap in classification accuracy between training and test sets, suggests that there might be some over-fitting of the model and some variables might not generalize well to unseen new samples. We are currently recruiting a larger group of participants and testing other machine-learning algorithms to address this issue.
In summary, we identified multivariate substance-specific behavioral markers classifying heroin and amphetamine dependence using machine-learning approaches. Results revealed important dissociations between factors classifying opiate and stimulant dependence, which often showed opposite patterns among individuals with heroin and amphetamine users.
Supplementary Material
Highlights.
Polysubstance use impedes the study of the unique effects of different drug classes
We tested “pure” heroin dependent (HD) and amphetamine dependent (AD) users
Machine learning revealed substance-specific behavioral markers of HD and AD
Psychopathy was the only classifier common to both HD and AD
Results challenge the unitary account of drug addiction
Acknowledgments
Role of Funding Source
Research reported in this publication was supported by the Fogarty International Center and the National Institute on Drug Abuse under award number R01DA021421 (J.V.).
The authors would like to thank all volunteers for their participation in this study. We thank Georgi Vasilev, Kiril Bozgunov, Rada Naslednikova, Ivaylo Raynov, Elena Psederska, Emiliya Peneva, and Dimitar Nedelchev for assistance with recruitment and testing of study participants. We are grateful to F. Gerard Moeller and Steven Grant for their helpful advice and insightful comments on the manuscript.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Conflicts of Interest
No conflict declared.
Contributors
W.-Y.A. analyzed the data, interpreted the results, and wrote the paper. J.V. designed the study, interpreted the results and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn WY, Kishida KT, Gu X, Lohrenz T, Harvey A, Alford JR, Smith KB, Yaffe G, Hibbing JR, Dayan P, Montague PR. Nonpolitical images evoke neural predictors of political ideology. Curr Biol. 2014a;24:2693–2699. doi: 10.1016/j.cub.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn W-Y, Ramesh D, Moeller FG, Vassileva J. Utility of machine-learning approaches to identify behavioral markers for substance use disorders: impulsivity dimensions as predictors of current cocaine dependence. 2015 doi: 10.3389/fpsyt.2016.00034. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn W-Y, Vasilev G, Lee S-H, Busemeyer JR, Kruschke JK, Bechara A, Vassileva J. Decision-making in stimulant and opiate addicts in protracted abstinence: evidence from computational modeling with pure users. Front Decision Neurosci. 2014b;5 doi: 10.3389/fpsyg.2014.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123( Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, et al. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bickel WK. Discounting of delayed rewards as an endophenotype. Biol Psychiatry. 2015;77:846–847. doi: 10.1016/j.biopsych.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Warren WL. Manual. Western Psychological Services; Los Angeles: 2000. Aggression Questionnaire:(AQ) [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P, Badiani A. Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology (Berl) 2008;198:395–404. doi: 10.1007/s00213-008-1154-3. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Conrod PJ. Personality correlates of the common and unique variance across conduct disorder and substance misuse symptoms in adolescence. J Abnorm Child Psychol. 2011;39:563–576. doi: 10.1007/s10802-010-9481-3. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Büchel C, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gowland P, Heinz A, Lawrence C, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Schumann G, Garavan H, Conrod PJ Consortium TI. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry. 2014;171:1310–1319. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- Clark SL, Gillespie NA, Adkins DE, Kendler KS, Neale MC. Psychometric modeling of abuse and dependence symptoms across six illicit substances indicates novel dimensions of misuse. Addict Behavi. 2016;53:132–140. doi: 10.1016/j.addbeh.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Castellanos-Ryan N, Strang J. Brief, personality-targeted coping skills interventions and survival as a non–drug user over a 2-year period during adolescence. Arch Gen Psychiatry. 2010;67:85–93. doi: 10.1001/archgenpsychiatry.2009.173. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The Role of Personality Dispositions to risky behavior in predicting first year college drinking. Addiction (Abingdon, England) 2009;104:193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2008;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: A computerized behavioral measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comput. 2002;34:391–398. doi: 10.3758/BF03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav Res Methods. 2005;37:82–90. doi: 10.3758/BF03206401. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Pérez-García M, Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. 2011;35:377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. User’s Guide for the Structured Clinical Interview for Dsm-IV Axis I Disorders. American Psychiatric Association; Washington, DC: 1997. [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hart SD, Cox DN, Hare RD. The Hare Psychopathy Checklist: Screening Version. Multi-Health Systems; Toronto, Canada: 1995. [Google Scholar]
- Hastie TJ, Tibshirani RJ, Friedman JJH. The Elements Of Statistical Learning. Springer; New York: 2009. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am J Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. J Person Soc Psychol. 1995;68:151–158. doi: 10.1037/0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- MacKillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology. 2010;212:453–464. doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34:689–695. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven J. The raven’s progressive matrices: change and stability over culture and time. Cogn Psychol. 2000;41:1–48. doi: 10.1006/cogp.1999.0735. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL. Manual for the State-trait anxiety inventory (form Y) (“self-evaluation questionnaire”) Consulting Psychologists Press; New York: 1983. [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Individ Dif. 2009;47:385–395. doi: 10.1016/j.paid.2009.04.008. [DOI] [Google Scholar]
- Stevens L, Goudriaan AE, Verdejo-García A, Dom G, Roeyers H, Vanderplasschen W. Impulsive choice predicts short-term relapse in substance-dependent individuals attending an in-patient detoxification programme. Psychol Med. 2015a;45:1–11. doi: 10.1017/S003329171500001X. [DOI] [PubMed] [Google Scholar]
- Stevens L, Verdejo-García A, Roeyers H, Goudriaan AE, Vanderplasschen W. Delay discounting, treatment motivation and treatment retention among substance-dependent individuals attending an in inpatient detoxification program. J Subst Abuse Treat. 2015b;49:58–64. doi: 10.1016/j.jsat.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Stewart J, De Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251. doi: 10.1037/0033-295X.91.2.251. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Vassileva J, Georgiev S, Martin E, Gonzalez R, Segala L. Psychopathic heroin addicts are not uniformly impaired across neurocognitive domains of impulsivity. Drug Alcohol Depend. 2011;114:194–200. doi: 10.1016/j.drugalcdep.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Kosson DS, Abramowitz C, Conrod P. Psychopathy versus psychopathies in classifying criminal offenders. Legal Criminol Psychol. 2005;10:27–43. doi: 10.1348/135532504X15376/pdf. [DOI] [Google Scholar]
- Vassileva J, Paxton J, Moeller FG, Wilson MJ, Bozgunov K, Martin EM, Gonzalez R, Vasilev G. Heroin and amphetamine users display opposite relationships between trait and neurobehavioral dimensions of impulsivity. Addict Behav. 2014;39:652–659. doi: 10.1016/j.addbeh.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Petkova P, Georgiev S, Martin EM, Tersiyski R, Raycheva M, Velinov V, Marinov P. Impaired decision-making in psychopathic heroin addicts. Drug Alcohol Depend. 2007;86:287–289. doi: 10.1016/j.drugalcdep.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob G, Baler R. Biomarkers in substance use disorders. ACS Chem Neurosci. 2015;6:522–525. doi: 10.1021/acschemneuro.5b00067. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating-Scale - an aid in the retrospective diagnosis of childhood attention-deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2014;75:746–748. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillère-Martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Ströhle A, Schumann G, Garavan H IMAGEN Consortium. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B. 2005;67:301–320. [Google Scholar]
- Zuckerman M, Kolin EA, Price L, Zoob I. Development of a sensation-seeking scale. J Consult Psychol. 1964;28:477–482. doi: 10.1037/h0040995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.