Abstract
As previous studies of obesity in survivors of pediatric acute lymphoblastic leukemia (ALL) have primarily been conducted among non-Hispanic white survivors or children treated on older protocols, our objective was to describe the prevalence and correlates of overweight status among an ethnically diverse population of pediatric ALL survivors, largely treated with more contemporary therapies. We evaluated the overweight/obesity status of pediatric ALL survivors (n=406) followed in the Texas Children's Cancer Center between 2004 and 2014. Survivors were classified as underweight, normal weight, overweight, or obese on the basis of their Body Mass Index (BMI) at their most current follow-up visit. Our results showed that Hispanic ethnicity (39% of subjects) was associated with being overweight (adjusted odds ratio [aOR]=1.88; 95% confidence interval [CI]: 1.13-3.14) or obese (aOR=2.84; 95% CI: 1.59-5.06) at follow-up, even after adjusting for cranial radiotherapy (CRT) exposure. BMI z-score at diagnosis was also associated with overweight/obesity at follow-up. Additionally, there was a statistically significant interaction between younger age at diagnosis and CRT, indicating that younger age at diagnosis was associated with obesity among patients who received CRT. These findings may help identify pediatric ALL patients that are at increased risk of being overweight or obese following treatment.
Keywords: Overweight, Obesity, Pediatrics, Survivors, Precursor Cell Lymphoblastic Leukemia-Lymphoma
Introduction
Acute lymphoblastic leukemia (ALL), the most common pediatric malignancy, accounts for nearly a quarter of all childhood cancers.1 Improved treatment regimens are responsible for increases in survival from pediatric ALL, with current five-year survival rates exceeding 80%.2 Given the improved survival of many pediatric oncology patients, increasing emphasis has been placed on characterizing the long-term, adverse impact of intensive cancer therapy. In fact, according to a recent report from the St. Jude Lifetime Cohort Study, by 45 years of age 95.5% of childhood cancer survivors suffer from at least one chronic health condition,3 including impaired cardiovascular function.
Obesity is a particularly important problem among ALL survivors, which can intensify cardiovascular outcomes and place these individuals at greater risk for other chronic health conditions.4-6 Several patient and treatment-related factors, including age at diagnosis and cranial radiotherapy (CRT), have been studied in association with overweight and obesity among survivors of pediatric ALL.4, 5, 7-33 However, these factors do not account for all of the variation in weight status among pediatric ALL survivors. While younger patients may be particularly sensitive to the adverse effects of ALL treatment, a recent meta-analysis concluded that the existing evidence was inadequate to establish an association between age at diagnosis and obesity.34 Age at diagnosis has been associated with an increased risk of obesity in some studies,18, 20, 25, 35 though other studies did not observe an association.4, 13, 15, 17, 22 Notably, studies using data from the Childhood Cancer Survivor Study cohort have indicated an association between age at diagnosis and the risk of being overweight or obese among female survivors of ALL treated with high doses of CRT,16, 23 suggesting the relationship between age at diagnosis and the development of obesity may be modified by CRT. Once widely used in the treatment of pediatric ALL, CRT is rarely used in contemporary treatment protocols.
There is a need to confirm suspected risk factors and identify novel predictors for obesity among pediatric ALL survivors. Additionally, the current literature is largely based on observations from non-Hispanic white populations and populations treated with older protocols. Therefore, the objectives of this study were to: 1) describe the prevalence and correlates of overweight status among an ethnically diverse population of pediatric ALL survivors; 2) assess whether CRT modifies the association between age at diagnosis and overweight status; and 3) evaluate the robustness of these associations among patients treated with more contemporary protocols.
Methods
The study included pediatric ALL survivors consecutively enrolled and followed at the Texas Children's Cancer Center (Houston, TX) Long-Term Survivor Clinic between 2004 and 2014. Eligible patients were age ≤18 years at diagnosis and at least five years removed from diagnosis. Of the 428 survivors of ALL followed at the Long-Term Survivor Clinic, three patients who were treated for ALL at another institution were excluded from the analysis due to incomplete treatment information. Patients who had relapsed or were undergoing treatment for a second cancer at follow up (n=3), were diagnosed with achondroplastic dwarfism (n=1), were pregnant at follow up (n=1), born with agenesis of corpus callosum (n=1), or Down syndrome (n=13) were further excluded from the analysis due to different growth trajectories, resulting in a final sample size of 406 eligible individuals.36 Written informed consent was obtained from all participants or legal guardians and the study protocol was approved by the Institutional Research Board at Baylor College of Medicine.
Patient information was collected from the available medical records of consenting patients. Body mass index (BMI) was calculated from height and weight data obtained at the most recent clinical follow-up visit. Each individual was assigned to a BMI category according to standard BMI classifications.37, 38 Specifically, individuals ≥19 years of age were defined as underweight (BMI <18.5 kg/m2), normal weight (BMI ≥18.5 and <25.0 kg/m2), overweight (BMI ≥25.0 kg/m2), or obese (BMI ≥30.0 kg/m2) on the basis of recorded BMI. Similarly, individuals 2-19 years of age at follow up were defined as underweight (BMI <5th percentile), normal weight (BMI ≥5th and <85th percentile), overweight (BMI ≥85th percentile), or obese (BMI ≥95th percentile) using age- and sex-specific growth charts obtained from the Centers for Disease Control and Prevention.39 Additional variables considered in our analysis included: ALL subtype (B-cell, T-cell, or infantile); age at diagnosis (continuous years); BMI (age 2-18 years at diagnosis) and weight-for-length (age < 2 years at diagnosis) z-score at diagnosis; race/ethnicity (non-Hispanic white, Hispanic, non-Hispanic back, or other); gender (female or male); CRT (none, low dose [≤18 Gy], high dose [≥24 Gy]); and age at follow-up visit (continuous years).
All statistical analyses were conducted in Stata v.13.1 (StataCorp, College Station, TX) using a 5% significance level. Descriptive statistics, reported as means and standard deviations for continuous variables or frequencies and proportions for categorical variables, are presented by weight status categories at follow up. A multinomial logistic regression model was constructed to describe the association between baseline patient and treatment characteristics and each weight category at follow up. Associations are reported as adjusted odds ratios (aOR) with corresponding 95% confidence intervals (CI). The joint effects of age at diagnosis and CRT were assessed by including interaction terms in logistic regression models, which combined overweight and obese status at follow up. To evaluate the reproducibility of these models in populations treated under more contemporary protocols, a sensitivity analysis was restricted to patients treated after 1995. This cut point was adopted based on previous assessments indicating that shifts in ALL treatment regimens occurred after 1995, when the use of CRT decreased significantly.40, 41 We constructed an additional model adjusted for BMI and weight-for-length z-score at diagnosis. Finally, restricted cubic splines were used to graphically explore potential non-linear relationships between age at diagnosis and the probability of being overweight at follow up, with knots defined at ages 4, 8, and 12 years at diagnosis.
Results
The mean age at diagnosis for 406 eligible patients was 5.4 years (range: 0.25-17.9) with a mean follow-up of 11.4 years (range: 5.2-37.2) post-diagnosis. Demographic and treatment characteristics are displayed by underweight (2.7%), normal weight (47.5%), overweight (27.6%) and obesity (22.2%) status at follow up in Table 1. Most patients were male (54.2%), non-Hispanic white (49.0%) or Hispanic (38.7%), diagnosed with B-cell lineage ALL (89.9%), age less than five years (64.8%) and normal weight (68.9%) at diagnosis. Only 46 (11.3%) patients received CRT, and 317 (78.1%) patients were treated after 1995.
Table 1.
Treatment and demographic characteristics of survivors of pediatric acute lymphoblastic leukemia at Texas Children's Hospital
| BMI Class at Follow up |
|||||
|---|---|---|---|---|---|
| Overall (n=406) | Underweight (n=11) | Normal (n=193) | Overweight (n=112) | Obese (n=90) | |
| Subtype, n(%)1 | |||||
| B-cell lineage | 27 (6.8) | 9 (90.0) | 169 (90.9) | 96 (87.3) | 82 (91.1) |
| T-cell lineage | 357 (89.9) | 1 (10.0) | 9 (4.8) | 12 (10.9) | 5 (5.6) |
| Infantile | 13 (3.3) | 0 (0.0) | 8 (4.3) | 2 (1.8) | 3 (3.3) |
| Age at Diagnosis, n(%) | |||||
| 0-5 years | 263 (64.8) | 7 (63.6) | 132 (68.4) | 70 (62.5) | 54 (60.0) |
| 6-10 years | 98 (24.1) | 3 (27.3) | 39 (20.2) | 29 (25.9) | 27 (30.0) |
| 11-18 years | 45 (11.1) | 1 (9.1) | 22 (11.4) | 13 (11.6) | 9 (10.0) |
| Mean, year (sd) | 5.44 (3.71) | 5.58 (2.62) | 5.33 (3.87) | 5.53 (3.85) | 5.55 (3.33) |
| Diagnosis BMI, z-score (sd)2 | 0.21 (1.29) | −1.79 (1.31) | −0.23 (1.13) | 0.56 (1.21) | 1.05 (1.01) |
| Race/Ethnicity, n(%) | |||||
| Non-Hispanic white | 199 (49.0) | 8 (72.7) | 110 (57.0) | 52 (46.4) | 29 (32.2) |
| Hispanic | 157 (38.7) | 1 (9.1) | 60 (31.1) | 53 (47.3) | 43 (47.8) |
| Non-Hispanic black | 24 (5.9) | 0 (0.0) | 9 (4.7) | 3 (2.7) | 12 (13.3) |
| Other | 26 (6.4) | 2 (18.2) | 14 (7.2) | 4 (3.6) | 6 (6.7) |
| Gender, n(%) | |||||
| Female | 186 (45.8) | 7 (63.6) | 96 (49.7) | 39 (34.8) | 44 (48.9) |
| Male | 220 (54.2) | 4 (36.4) | 97 (50.3) | 73 (65.2) | 46 (51.1) |
| CRT, n(%)3 | |||||
| None | 360 (89.1) | 9 (81.8) | 180 (93.7) | 94 (84.7) | 77 (85.6) |
| ≤18 Gy | 27 (6.7) | 2 (18.2) | 8 (4.2) | 12 (10.8) | 5 (5.6) |
| ≥24 Gy | 17 (4.2) | 0 (0.0) | 4 (2.1) | 5 (4.5) | 8 (8.8) |
| Year of diagnosis, n(%) | |||||
| 1972-1994 | 89 (21.9) | 1 (9.1) | 40 (20.7) | 25 (22.3) | 23 (25.6) |
| 1995-2008 | 317 (78.1) | 10 (90.9) | 153 (79.3) | 87 (77.7) | 32 (74.4) |
| Age at follow-up, n(%) | |||||
| ≤ 10 years | 42 (10.3) | 0 (0.0) | 23 (11.9) | 23 (20.5) | 16 (17.8) |
| 11-20 years | 260 (64.0) | 9 (81.8) | 132 (68.4) | 64 (57.1) | 51 (56.7) |
| 21-30 years | 92 (22.7) | 1 (9.1) | 34 (17.6) | 22 (19.6) | 19 (21.1) |
| ≥ 31 years | 12 (3.0) | 1 (9.1) | 4 (2.1) | 3 (2.7) | 4 (4.4) |
| Mean, year (sd) | 16.83 (5.94) | 16.35 (6.05) | 16.86 (5.79) | 16.68 (6.13) | 17.04 (6.08) |
| Time fom diagnosis, year (sd) | 11.39 (5.33) | 10.77 (5.17) | 11.53 (4.93) | 11.14 (5.86) | 11.48 (5.54) |
| Treatment duration, year (sd) | 2.84 (1.33) | 3.06 (1.39) | 2.74 (1.25) | 3.02 (1.54) | 2.83 (1.17) |
BMI, Body Mass Index; SD, Standard Deviation; CRT, Cranial Radiation Therapy
Missing subtype information on n=10
Restricted to patients with height and weight recorded at diagnosis (n=292)
Missing radiation dose information on n=2 radiated participants
A multinomial logistic regression model was constructed to assess the association between baseline characteristics and BMI class at follow up, with normal weight as the reference group (Table 2). Receipt of CRT was associated with being overweight (aOR=2.99; 95% CI: 1.33-6.71) or obese (aOR=2.40; 95% CI: 1.00-5.77) at follow up, after adjusting for age at diagnosis, gender, race/ethnicity, and age at follow up. Females were less likely than males to be overweight at follow up (aOR=0.56; 95% CI: 0.34-0.91); however, there was no association observed between female gender and obesity at follow up (aOR=1.01; 95% CI: 0.60-1.70). Overweight and obesity status also differed by race/ethnicity. Compared to non-Hispanic whites, Hispanics were more likely to be overweight (aOR=1.88; 95% CI: 1.13-3.14) and obese (aOR=2.84; 95% CI: 1.59-5.06) at follow up, after adjusting for age at diagnosis, CRT exposure, gender, and age at follow up. Additionally, non-Hispanic blacks were more likely to be obese at follow up when compared to non-Hispanic whites (aOR=4.99; 95% CI: 1.89-13.19).
Table 2.
Multinomial logistic regression1 of pediatric acute lymphoblastic leukemia survivor weight status at Texas Children's Hospital
| BMI Class at Follow up | |||
|---|---|---|---|
| Underweight (n=11) | Overweight (n=112) | Obese (n=90) | |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age at Diagnosis, year | 1.04 (0.86-1.26) | 1.02 (0.95-1.10) | 0.99 (0.92-1.08) |
| Age at Follow up, year | 0.94 (0.82-1.08) | 0.97 (0.93-1.02) | 1.01 (0.60-1.70) |
| CRT | |||
| None | Ref. | Ref. | Ref. |
| Any | 3.92 (0.62-24.55) | 2.99 (1.33-6.71) | 2.40 (1.00-5.77) |
| Gender | |||
| Male | Ref. | Ref. | Ref. |
| Female | 1.86 (0.51-6.78) | 0.56 (0.34-0.91) | 1.01 (0.60-1.70) |
| Race/Ethnicity | |||
| Non-Hispanic white | Ref. | Ref. | Ref. |
| Hispanic | 0.21 (0.03-1.78) | 1.88 (1.13-3.14) | 2.84 (1.59-5.06) |
| Non-Hispanic black | -- | 0.66 (0.17-2.59) | 4.99 (1.89-13.19) |
| Other | 1.76 (0.33-9.38) | 0.58 (0.18-1.88) | 1.59 (0.55-4.54) |
BMI, Body Mass Index; aOR Adjusted Odds Ratio; CI, Confidence Interval; CRT, Cranial Radiation Therapy
Adjusted for other variables included in table, relative to normal weight group (n=193)
Because younger individuals may be increasingly susceptible to the adverse effects of CRT, we evaluated interactions between CRT and age at diagnosis in logistic regression models (Table 3). Specifically, we compared the estimated association between patient characteristics and the odds of overweight and obesity combined relative to normal weight at follow up. Interaction terms between age at diagnosis and CRT status were statistically significant (interaction OR=0.84, 95% CI: 0.71-0.98), and suggested that age at diagnosis was inversely associated with overweight status at follow up among patients who received CRT. Conversely, there was no association or a weak positive association between age at diagnosis and overweight status at follow up among those that did not receive CRT. Other interaction terms between age at diagnosis, CRT, and gender and ethnicity were not statistically significant (results not shown). These findings were further evaluated in a sensitivity analysis restricted to a more contemporary subset of the population (i.e., those treated after 1995). Overall, the results from the entire cohort were similar to those obtained in the more contemporary sample (Model 1, n=307). In both samples overweight status at follow up was significantly associated with CRT and Hispanic ethnicity, though exposure to CRT was infrequent in the contemporary population (n=28). The observed associations persisted after adjusting for BMI and weight-for-length z-scores at diagnosis (Model 2, n=276).
Table 3.
Logistic regression1 of overweight/obesity among pediatric acute lymphoblastic leukemia survivors at Texas Children's Hospital
| Year of Diagnosis ≥1995 |
|||
|---|---|---|---|
| Complete Data (n=193 normal weight; 202 overweight/obese) | Model 1 (n=153 normal weight; 154 overweight/obese) | Model 22 (n=137 normal weight; 139 overweight/obese) | |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age at Diagnosis, year3 | 1.05 (0.97-1.12) | 1.15 (1.04-1.28) | 1.14 (1.02-1.29) |
| Age at Follow up, year | 0.98 (0.94-1.02) | 0.88 (0.80-0.96) | 0.90 (0.81-1.01) |
| CRT | |||
| None | Ref. | Ref. | Ref. |
| Any | 3.44 (1.53-7.74) | 4.72 (1.32-16.90) | 17.45 (1.14-266.26) |
| Age-by-CRT Interaction | 0.84 (0.71-0.98) | 0.77 (0.61-0.96) | 0.66 (0.45-0.96) |
| Gender | |||
| Male | Ref. | Ref. | Ref. |
| Female | 0.74 (0.49-1.13) | 0.86 (0.54-1.38) | 0.77 (0.45-1.33) |
| Race/Ethnicity | |||
| Non-Hispanic white | Ref. | Ref. | Ref. |
| Hispanic | 2.18 (1.40-3.41) | 1.89 (1.14-3.13) | 2.03 (1.14-3.59) |
| Non-Hispanic black | 2.33 (0.95-5.69) | 2.30 (0.88-5.99) | 1.79 (0.56-5.75) |
| Other | 0.93 (0.38-2.26) | 1.08 (0.39-2.96) | 1.08 (0.30-3.85) |
| Diagnosis BMI, z-score | NA | NA | 2.03 (1.57-2.62) |
aOR Adjusted Odds Ratio; CI, Confidence Interval; CRT, Cranial Radiation Therapy; BMI, Body Mass Index
Adjusted for age at follow up (continuous) and other variables included in table, relative to normal weight, underweight individuals excluded (n=11)
Model 2 restricted to patients with height and weight recorded at diagnosis (n=292)
Age at diagnosis centered at 7 years
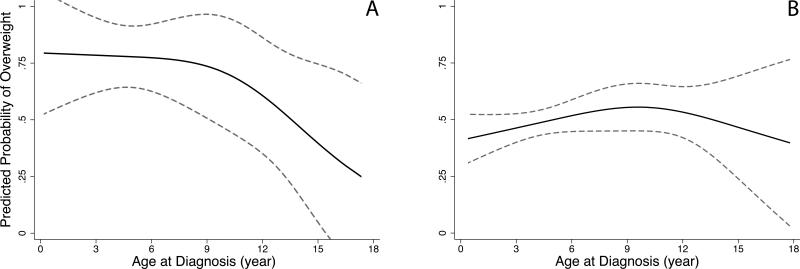
Restricted cubic splines were constructed to graphically depict non-linear dose-response relationships between age at diagnosis and the probability of being overweight at follow up by CRT group (Fig. 1). The predicted probability of being overweight at follow up was approximately 75% among children younger than three years of age at diagnosis who received CRT. The probability of being overweight at follow up declined gradually with increasing age until about age nine, and decreased rapidly after that point. Conversely, the predicted probability of being overweight at follow up was more consistent for children who did not receive CRT, ranging between 40% and 55% across all ages at diagnosis.
Figure 1. Restricted cubic splines for age at diagnosis and predicted probability of overweight/obesity at follow up1.
1Predicted probability of overweight and obesity combined (95% Confidence Intervals) among pediatric acute lymphoblastic leukemia survivors at Texas Children's Hospital A) exposed to CRT (n=44) and B) not exposed to CRT (n=351), adjusted for ethnicity, sex, and age at follow up (continuous)
Discussion
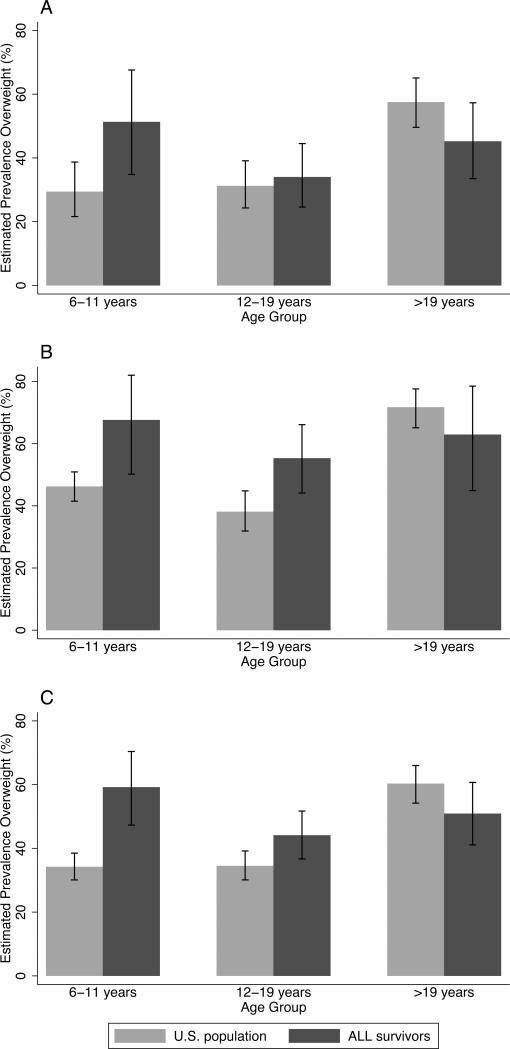
In this assessment of post-therapy weight status among a multiethnic population of pediatric ALL survivors, nearly half were overweight or obese at follow up. Our assessment provides a refined evaluation of the interaction between age at diagnosis and exposure to radiation therapy on the long-term risk of overweight and obesity among pediatric ALL survivors. Additionally, our study adds to the growing literature indicating that Hispanics are at a greater risk of post-treatment overweight and obesity, even after adjusting for CRT exposure.18, 24, 25 In order to compare our findings with the general population, we explored differences between our population and participants in the National Health and Nutrition Examination Survey (NHANES). Specifically, relative to non-Hispanic white and Hispanic participants in NHANES, the prevalence of overweight and obesity in this population of pediatric ALL survivors (Figure 2) was higher among those 6 to 11 years of age (59.2% among survivors vs. 34.2% among general population) and 12 to 19 years of age (44.1% among survivors vs. 34.5% among general population), but slightly lower among those over 19 years of age (50.9% among survivors vs. 60.3% among general population).42 There are several possible explanations for the lower than expected prevalence of overweight and obesity observed among older survivors. First, the obesogenic effects of ALL therapy may not persist into adulthood. Furthermore, many of the ALL survivors included in this analysis were identified at our institutional long-term survivor clinic. It is possible that the population seeking medical care at long-term survivor clinics is comprised of health-conscious survivors and may, therefore, underestimating the true prevalence of obesity in older age groups.
Figure 2. Prevalence of overweight among U.S. general population of pediatric leukemia survivors at Texas Children's Hospital by ethnicity and age group1.
1Prevalence (95% Confidence Interval) of overweight and obesity among n=199 non-Hispanic white (A), n=157 Hispanic (B), and n=356 combined non-Hispanic white and Hispanic survivors of childhood acute lymphoblastic leukemia (C) compared to NHANES U.S. population estimates by age group
The prevalence of overweight and obesity was particularly high among Hispanic survivors less than 19 years of age (61%), exceeding the NHANES estimate of overweight and obesity among Hispanic adolescents less than 19 year of age (38.9%). Notably, Hispanic ethnicity was associated with overweight and obesity in survivors of childhood ALL. Although Hispanics were slightly heavier on average than non-Hispanic whites at diagnosis (mean BMI or weight-for-length z-score: 0.43 vs. 0.08), the increased risk of post-therapy overweight and obesity among Hispanics persisted after adjustment for adiposity at diagnosis. The reason for the observed association between Hispanic ethnicity and post-therapy overweight status is unknown but could be related to non-genetic differences (e.g., adherence to care, socio-economic, cultural, etc.) or differences in underlying genetic susceptibility.43 For instances, genetically-defined ancestry, consistent with admixed Hispanic populations, has been associated with an increased risk of relapse in pediatric ALL.44
Although patient characteristics, including female gender, weight status and age at diagnosis have been previously associated with post-therapy weight status among survivors of ALL,4, 5, 7-33 results have been equivocal across studies. Our findings are consistent with past research which demonstrated an association between pre-treatment overweight status,5, 20, 22 but not associated with female gender.4, 18, 22, 30 Inconsistent findings across studies regarding the role of female gender on obesity risk in pediatric ALL survivors may be due in part to difference in underlying patient characteristics (e.g., race/ethnicity, age at diagnosis) or treatment strategies (e.g., CRT exposure).
In addition to patient characteristics, overweight status has also been associated with ALL treatment factors, including CRT and corticosteroid dose.4, 8, 15, 16, 23, 32 While we were unable to assess the impact of corticosteroids on overweight status at follow up due to inadequate dose documentation in a subset of medical records, patients who received CRT during treatment for pediatric ALL were significantly more likely to be (aOR=2.99; 95% CI: 1.33-6.71) or obese (aOR=2.40; 95% CI: 1.00-5.77) at follow up. This is consistent with the body of information on the role of CRT on obesity risk in pediatric ALL survivors.34
The mechanism underlying the association between CRT and obesity remains largely unknown, but may involve radiation-induced damage to the pituitary-hypothalamus axis of the brain. Exposure to high doses of cranial radiation may disrupt hormonal signaling pathways involved in satiety, growth, and metabolic homeostasis. Growth hormone deficiency and increased serum leptin levels following CRT have been linked to obesity among childhood cancer survivors.12, 27, 45-48 The detrimental effects of hypothalamic radiation may be exacerbated during critical periods of development. While some research suggests the risk of obesity is more pronounced in young females receiving CRT,16, 23, 49 the association between CRT and overweight and obesity observed in this study was similar between males and females. We did, however, identify a statistically significant interaction between age at diagnosis and CRT, suggesting the association between CRT and overweight status depends on the age of the patient at the time of therapy. Notably, age at diagnosis was not strongly associated with the odds of being overweight or obese at follow up among patients who did not receive CRT. The results of this study indicate younger patients who receive CRT are at the highest risk of being overweight at follow up, and the adverse effects of CRT on overweight and obesity appeared to diminish with increasing age at diagnosis.
Our study must be considered in light of certain limitations. For instance, our population represents a single institution and includes patients seeking long-term follow-up care, which may not represent the experiences of all survivors. It is possible that health-conscious, lean adult survivors are more likely to return for follow-up care, which would bias our estimate of overweight and obesity downward in this population. Other potential limitations include the cross-sectional study design and lack of information pertaining to on-therapy changes in body mass. Additionally, we were not able to account behavioral or social determinants of obesity in this population. Still, the inclusion of a multiethnic population with a mean post-treatment follow-up of over 11 years is an important strength of the current study. This report also adds to the limited evidence suggesting Hispanic and non-Hispanic black ALL survivors are at increased risk of overweight and obesity when compared to non-Hispanic whites.18, 24, 25 Further, as noted, the evolution of ALL treatment protocols over time may contribute to inconsistent findings across studies. Most patients included in this analysis were treated under contemporary protocols (78% treated after 1995), allowing greater generalizability to populations currently undergoing treatment. Restricting our analysis to patients treated since 1995 did not meaningfully change the results; though the proportion of patients receiving CRT was notably lower (20.2% prior to 1995 vs. 8.8% after 1995). Among patients treated since 1995 who did not receive CRT only Hispanic ethnicity (OR=2.21, 95% CI: 1.23-3.97) and BMI or weight-for-length z-score at diagnosis (OR=2.09, 95% CI: 1.61-2.72) were significant predictors of overweight status at follow up.
Many survivors of pediatric ALL experience treatment-related late effects.50 The long-term sequelae of therapy predispose pediatric ALL survivors to cardiovascular disease and stroke.51-53 The potential cardiovascular late effect of ALL therapy are likely compounded in overweight and obese survivors; thus, obesity prevention is likely a critical component of long-term health promotion programs for survivors of pediatric ALL. A better understanding of the risk factors associated with treatment-related obesity could help identify patients who may benefit most from targeted obesity prevention interventions. Unfortunately, few risk factors have consistently been associated with obesity across studies. In this study, BMI or age-for-length z-scores at diagnosis, Hispanic and non-Hispanic black ethnicity were associated with overweight status at follow up. Exposure to CRT was also associated with an increased risk of overweight and obesity in this study, particularly among younger patients. Although treatment options for pediatric ALL have evolved over the past several decades to greatly reduce the use and dosage of CRT, restricting our analysis did not notably alter our results. Even among non-radiated patients treated since 1995, nearly half of patients were identified as overweight or obese (26.0% overweight, 21.5% obese) at follow up. Thus, overweight and obesity appear to be highly prevalent conditions among survivors of pediatric ALL, independent of suspected patient and clinical risk factors.
Conclusions
Healthcare professionals should recognize pediatric ALL survivors are uniquely susceptible late-effects related to ALL therapy, including obesity. As many survivors of childhood cancer will seek long-term medical care from healthcare providers who may be unfamiliar with the challenges facing this population,54, 55 it is critical that these professionals consult published guidelines for providing long-term follow up care to childhood cancer survivors.56
Acknowledgements
The authors would like to acknowledge M. Pierce and C. McGehee for their contributions, including data collection. This work was supported by the National Cancer Institute funded Training Program in Pediatric Cancer Epidemiology and Control (R25CA160078) and the Kurt Groten Family Research Scholars Award.
List of Abbreviations
- ALL
Acute Lymphoblastic Leukemia
- CRT
Cranial Radiotherapy
- BMI
Body Mass Index
- aOR
Adjusted Odds Ratio
- CI
Confidence Interval
- NHANES
National Health and Nutrition Examination Survey
Footnotes
Authors’ Contributions
KYK, PJL, MES, and MFO participated in the conception and design of the study. MFO and HED participated in data collection and coordination. ALB, PJL, and HED participated in data analysis and interpretation of results. All authors participated in drafting the manuscript, and have approved the final version of the manuscript.
Conflicts of Interest The authors have no conflicts of interest to declare.
Contributor Information
Austin L. Brown, Section of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA, Austin.Brown@bcm.edu.
Philip J. Lupo, Section of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA, Philip.Lupo@bcm.edu.
Heather E. Danysh, Section of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA, Danysh@bcm.edu.
M. Fatih Okcu, Section of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA, MFOkcu@txch.org.
Michael E. Scheurer, Section of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA, Scheurer@bcm.edu.
Kala Y. Kamdar, Section of Hematology-Oncology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA, 6701 Fannin Street, Suite 1510, Houston, TX 77030.
References
- 1.United States Cancer Statistics . 1999–2006 Incidence and Mortality Web-based Report. US Cancer Statistics Working Group; [Jan 6, 2015]. Available at: www.cdc.gov/uscs. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 5.Esbenshade AJ, Simmons JH, Koyama T, et al. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):372–378. doi: 10.1002/pbc.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang FF, Liu S, Chung M, et al. Growth patterns during and after treatment in patients with pediatric ALL: A meta-analysis. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didi M, Didcock E, Davies HA, et al. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J Pediatr. 1995;127(1):63–67. doi: 10.1016/s0022-3476(95)70258-x. [DOI] [PubMed] [Google Scholar]
- 8.Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, et al. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38(1):86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Davies HA, Didcock E, Didi M, et al. Growth, puberty and obesity after treatment for leukaemia. Acta Paediatr Suppl. 1995;411:45–50. doi: 10.1111/j.1651-2227.1995.tb13862.x. discussion 51. [DOI] [PubMed] [Google Scholar]
- 10.Groot-Loonen JJ, Otten BJ, van't Hof MA, et al. Influence of treatment modalities on body weight in acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;27(2):92–97. doi: 10.1002/(SICI)1096-911X(199608)27:2<92::AID-MPO5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Birkebaek NH, Clausen N. Height and weight pattern up to 20 years after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1998;79(2):161–164. doi: 10.1136/adc.79.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan BM, Rahim A, Blum WF, et al. Hyperleptinaemia in young adults following cranial irradiation in childhood: growth hormone deficiency or leptin insensitivity? Clin Endocrinol (Oxf) 1999;50(2):163–169. doi: 10.1046/j.1365-2265.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 13.Nysom K, Holm K, Michaelsen KF, et al. Degree of fatness after treatment for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 1999;84(12):4591–4596. doi: 10.1210/jcem.84.12.6205. [DOI] [PubMed] [Google Scholar]
- 14.Mayer EI, Reuter M, Dopfer RE, et al. Energy expenditure, energy intake and prevalence of obesity after therapy for acute lymphoblastic leukemia during childhood. Horm Res. 2000;53(4):193–199. doi: 10.1159/000023566. [DOI] [PubMed] [Google Scholar]
- 15.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: role of cranial irradiation. Med Pediatr Oncol. 2000;35(2):91–95. doi: 10.1002/1096-911x(200008)35:2<91::aid-mpo1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 17.Nathan PC, Jovcevska V, Ness KK, et al. The prevalence of overweight and obesity in pediatric survivors of cancer. J Pediatr. 2006;149(4):518–525. doi: 10.1016/j.jpeds.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Baillargeon J, Langevin AM, Lewis M, et al. Demographic correlates of body size changes in children undergoing treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(6):793–796. doi: 10.1002/pbc.21063. [DOI] [PubMed] [Google Scholar]
- 19.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(7):975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 20.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25(10):1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 21.Trimis G, Moschovi M, Papassotiriou I, et al. Early indicators of dysmetabolic syndrome in young survivors of acute lymphoblastic leukemia in childhood as a target for preventing disease. J Pediatr Hematol Oncol. 2007;29(5):309–314. doi: 10.1097/MPH.0b013e318059c249. [DOI] [PubMed] [Google Scholar]
- 22.Asner S, Ammann RA, Ozsahin H, et al. Obesity in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51(1):118–122. doi: 10.1002/pbc.21496. [DOI] [PubMed] [Google Scholar]
- 23.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(28):4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gofman I, Ducore J. Risk factors for the development of obesity in children surviving ALL and NHL. J Pediatr Hematol Oncol. 2009;31(2):101–107. doi: 10.1097/MPH.0b013e31818c0120. [DOI] [PubMed] [Google Scholar]
- 25.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: A report from the Children's Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53(7):1249–1254. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins L, Zarzabal LA, Nayiager T, et al. Growth in children with acute lymphoblastic leukemia during treatment. J Pediatr Hematol Oncol. 2010;32(8):e304–307. doi: 10.1097/MPH.0b013e3181ece2bb. [DOI] [PubMed] [Google Scholar]
- 27.Karaman S, Ercan O, Yildiz I, et al. Late effects of childhood ALL treatment on body mass index and serum leptin levels. J Pediatr Endocrinol Metab. 2010;23(7):669–674. doi: 10.1515/jpem.2010.23.7.669. [DOI] [PubMed] [Google Scholar]
- 28.Pakakasama S, Veerakul G, Sosothikul D, et al. Late effects in survivors of childhood acute lymphoblastic leukemia: a study from Thai Pediatric Oncology Group. Int J Hematol. 2010;91(5):850–854. doi: 10.1007/s12185-010-0594-9. [DOI] [PubMed] [Google Scholar]
- 29.Tylavsky FA, Smith K, Surprise H, et al. Nutritional intake of long-term survivors of childhood acute lymphoblastic leukemia: evidence for bone health interventional opportunities. Pediatr Blood Cancer. 2010;55(7):1362–1369. doi: 10.1002/pbc.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love E, Schneiderman JE, Stephens D, et al. A cross-sectional study of overweight in pediatric survivors of acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2011;57(7):1204–1209. doi: 10.1002/pbc.23010. [DOI] [PubMed] [Google Scholar]
- 31.Green DM, Cox CL, Zhu L, et al. Risk factors for obesity in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30(3):246–255. doi: 10.1200/JCO.2010.34.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veringa SJ, van Dulmen-den Broeder E, Kaspers GJ, et al. Blood pressure and body composition in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(2):278–282. doi: 10.1002/pbc.23251. [DOI] [PubMed] [Google Scholar]
- 33.Chow EJ, Liu W, Srivastava K, et al. Differential effects of radiotherapy on growth and endocrine function among acute leukemia survivors: a childhood cancer survivor study report. Pediatr Blood Cancer. 2013;60(1):110–115. doi: 10.1002/pbc.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang FF, Kelly MJ, Saltzman E, et al. Obesity in pediatric ALL survivors: a meta-analysis. Pediatrics. 2014;133(3):e704–715. doi: 10.1542/peds.2013-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reilly JJ, Ventham JC, Newell J, et al. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24(11):1537–1541. doi: 10.1038/sj.ijo.0801403. [DOI] [PubMed] [Google Scholar]
- 36.Cronk C, Crocker AC, Pueschel SM, et al. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81(1):102–110. [PubMed] [Google Scholar]
- 37.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2000;2002(246):1–190. [PubMed] [Google Scholar]
- 38.Am J Clin Nutr. National Heart, Lung, and Blood Institute; 1998. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. pp. 899–917. 1998/10/15 ed. Am J Clin Nutr. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention [17 July, 2015];2000 CDC Growth Charts for the United States: Methods and Development. Available at: http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf.
- 40.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 41.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104(9):2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim JY, Bhatia S, Robison LL, et al. Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer. 2014;120(7):955–962. doi: 10.1002/cncr.28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nature genetics. 2011;43(3):237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarfelt M, Lannering B, Bosaeus I, et al. Body composition in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol. 2005;153(1):81–89. doi: 10.1530/eje.1.01931. [DOI] [PubMed] [Google Scholar]
- 46.Talvensaari KK, Lanning M, Tapanainen P, et al. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J Clin Endocrinol Metab. 1996;81(8):3051–3055. doi: 10.1210/jcem.81.8.8768873. [DOI] [PubMed] [Google Scholar]
- 47.Birkebaek NH, Fisker S, Clausen N, et al. Growth and endocrinological disorders up to 21 years after treatment for acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 1998;30(6):351–356. doi: 10.1002/(sici)1096-911x(199806)30:6<351::aid-mpo9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 48.Skoczen S, Tomasik PJ, Bik-Multanowski M, et al. Plasma levels of leptin and soluble leptin receptor and polymorphisms of leptin gene -18G > A and leptin receptor genes K109R and Q223R, in survivors of childhood acute lymphoblastic leukemia. J Exp Clin Cancer Res. 2011;30:64. doi: 10.1186/1756-9966-30-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craig F, Leiper AD, Stanhope R, et al. Sexually dimorphic and radiation dose dependent effect of cranial irradiation on body mass index. Arch Dis Child. 1999;81(6):500–504. doi: 10.1136/adc.81.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111(12):5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 52.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 53.Geenen MM, Bakker PJ, Kremer LC, et al. Increased prevalence of risk factors for cardiovascular disease in long-term survivors of acute lymphoblastic leukemia and Wilms tumor treated with radiotherapy. Pediatr Blood Cancer. 2010;55(4):690–697. doi: 10.1002/pbc.22518. [DOI] [PubMed] [Google Scholar]
- 54.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox CL, Hudson MM, Mertens A, et al. Medical screening participation in the childhood cancer survivor study. Arch Intern Med. 2009;169(5):454–462. doi: 10.1001/archinternmed.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Group CsO Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, Version 3.0. Available at: www.survivorshipguidelines.org.