Synopsis
Psychotic disorders, as defined by clinical features alone, overlap considerably in terms of symptoms, familial patterns, risk genes, outcome and treatment response.
As a result, numerous neurobiological measurements also fail to distinguish patients with the most prevalent classic psychotic syndromes (schizophrenia, schizo-affective and bipolar with psychosis).
Statistical methods applied to such biological measurements in large numbers of psychosis patients yield novel categories, that cut across traditional diagnostic boundaries, called “Biotypes”, i.e. biologically-defined presumptive disease entities.
Such new classification approaches within psychosis hopefully represent an opportunity to transcend clinical phenomenologically-defined syndromes in psychiatry with neurobiologically-defined diseases that can advance drug discovery and support precision medicine approaches in psychiatry.
Keywords: Psychosis, Biotype, Neurobiology, Reclassification, Schizophrenia, Schizo-affective, bipolar
Introduction
The vast majority of clinical psychiatrists are undoubtedly confident in their ability to diagnose patients with schizophrenia correctly, and to distinguish them straightforwardly from individuals with other disorders manifesting similar symptoms. In so doing, they would likely mention DSM criteria, say something about a presumed unique underlying neurobiology and invoke the name of Emil Kraepelin as having settled these distinctions over a century ago. Because questioning our assumptions is always a useful exercise, this initial chapter is designed both to accomplish that aim by challenging these assumptions, as well as to provide a general conceptual lens through which some of the other articles in this volume can be viewed.
An historical perspective
Given that much of our current clinical classification within psychosis begins with Kraepelin, it is appropriate to start with a brief discussion of the great diagnostic divide that he promulgated in the late 19th century, a delineation that survives and is seldom challenged by clinicians today. Kraepelin made a fundamental diagnostic distinction within serious mental illnesses between those conditions that are clearly recurrent and episodic with between-episode recovery (“manic-depressive insanity”) and another syndrome characterized by lack of recovery plus longitudinal deterioration of personality and intellect (“dementia precox”)1, subsequently termed “schizophrenia” by Bleuler2. Most aspects of this classification are still present in our diagnostic manuals, although Kraepelin’s schema has been altered in subtle ways over time3. For example, major depressive disorder, because it was recurrent, was certainly included within his purview of manic-depressive insanity; single episodes of mania, because they were not repeated, were not within the definition3. Kraepelin provided many detailed case examples of manic-depressive insanity where patients clearly manifested psychotic symptoms, so that hallucinations, formal thought disorder and delusions, the defining symptoms of psychosis, were certainly not limited to cases of schizophrenia; the predominant emphasis was on longitudinal course rather than cross-sectional symptoms. Although (as we will soon discuss) there are troubling problems and inconsistencies with Kraepelin’s delineation, it has persisted for over 100 years because no better diagnostic categorization system arose to replace it.
Problems with Kraepelin’s distinction
First, within much of clinical medicine there are obvious diagnostic boundaries, or “points of rarity” between distinct disorders. However, for schizophrenia and bipolar disorder, there are often areas of symptomatic overlap and substantial numbers of patients are not prototypical, with many left in a diagnostic muddle. This is due both to heterogeneity within these diagnoses and overlap between them, or as has been said, “patients don’t read DSM”. For example, in the realm of long-term outcome, some otherwise typical bipolar patients have clearly progressive chronic courses4, while it was recognized early that some otherwise clinically typical schizophrenia patients show solid clinical recovery5 and/or manifest prominent affective symptoms. These and other observations led Kasanin to propose a third diagnostic entity of “schizo-affective disorder” in 19336, that many clinicians believe has served only to complicate issues, is a diagnostic evasion, and was only necessitated by a lack of clear diagnostic demarcation between many cases of schizophrenia and bipolar illness. Similar findings have been demonstrated recently7.
Moreover, with regard to clinical symptomatology, up to 50% of otherwise typical bipolar patients have clear-cut psychotic symptoms (hallucinations, delusions, formal thought disorder) during episodes,8,9,10,11, as well as sharing some of the cognitive abnormalities that were thought originally only to characterize schizophrenia patients12. One consequence of this is that many cases of psychosis are hard to classify, and are thereby omitted from both clinical trials and genetic analyses, inevitably skewing study outcomes. Furthermore, there is also diagnostic crossover, in that a third of schizophrenia patients meet criteria for major depressive disorder (MDD) if the DSM exclusionary rule (a diagnosis of schizophrenia trumps one of MDD) is set aside13. The Kraepelinian dichotomy also has been challenged for other reasons. Ideally, as implied by early proponents of “external” validations of disease entities (Robins and Guze 1970) we would prefer distinct diseases with substantial genetic origin to “breed true” within families, to show distinct high heritability translating into the discovery of unique sets of risk genes, and, with regard to treatment, different, appropriate non-overlapping therapies for each disorder. Last, we require diagnostic systems that differentiate patients in ways that guide illness-specific, successful and incrementally improving treatments. However, many psychotic patients receive polypharmacy, tacitly acknowledging overlap in efficacy of “mood stabilizers” and “antipsychotics”.
Unfortunately, none of these criteria hold up satisfactorily for bipolar illness and schizophrenia. Familial expression of illness crosses over diagnoses so that these illnesses fail to “breed true”14. While both schizophrenia and bipolar disorder are highly heritable, there is substantial overlap in the risk genes discovered to date15 and treatment modalities converge substantially; for example, the routine employment of second-generation antipsychotics for bipolar illness, whether or not it is characterized by psychotic symptoms. These observations have led some clinicians to posit that psychosis lies on a spectrum without clear boundaries of demarcation, so that it is not possible to “carve nature at its joints”16.
Syndromes versus diseases
Kraepelin was confident that neurobiological evidence would demonstrate conclusively that the syndromes he had identified were distinct entities. He felt both that the cortical neuropathology of schizophrenia would soon be revealed, showing it to be a neurodegenerative illness and noted the strong heritability of manic-depressive illness17. He had cause for such confidence because his colleagues and contemporaries had recently identified microscopic brain changes associated with neurosyphilis and Alzheimer’s disease. Nevertheless, one hundred years later we are still waiting for such conclusive neurobiological evidence to emerge; as Robins and Guze18 stated 45 years ago; “….in the absence of laboratory tests, or a solid understanding of pathogenesis, the criteria available to psychiatry for validating those logical categories have been restricted to clinical features, outcome and family history”. The consequence of this situation (as well as the lack of progress over an almost 50-year time period) cannot be too strongly emphasized. If we lack information on the basic causes and pathologic changes associated with psychosis, then we are operating in a knowledge-space defined by merely describing clinical syndromes, analogous to the fever, dropsy, seizures or cough familiar to early medical practitioners. Stated another way, in the absence of biological tests, phenomenology as a clinical exercise describes heterogeneous syndromes in a reliable manner, but cannot identify true disease entities. Recent versions of DSM19 represent examples of phenomenology writ large. Thus, it is no coincidence that the majority of medications in our psychiatric armamentarium were initially identified based on chance discoveries rather than being designed as a consequence of any rational knowledge of the underlying pathology of the disorders they are intended to treat. As long as the etiology and pathogenesis of schizophrenia and other psychotic illnesses remain elusive, it is unsurprising that clinical diagnosis is not a particularly good guide to treatment response, as might be expected for heterogeneous, catchall diagnostic categories.
Can biological measures help clarify the situation?
Given this rather dismal catalog of facts, several groups have begun to explore conceptual routes to help clarify the situation by re-examining some of our conceptual assumptions, and once again turning to biological classification as a solution to our conundrum. Gathering sufficient information for the resulting data to be sufficiently powered statistically to yield meaningful and generalizable conclusions necessitates multi-site efforts and research consortia. One such research group is the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), an NIH-funded, multi-site consortium of investigators who constructed a multi-measure approach to study stable patients with any one of three psychotic disorders (schizophrenia, schizo-affective disorder or bipolar disorder with psychosis), plus at least one of their first-degree relatives and demographically matched healthy control subjects. I am familiar with this effort, as the director of one of the five currently participating sites, along with Carol Tamminga/Dallas TX, Matcheri Keshavan/Boston MA, Brett Clementz/Athens GA and Elliot Gershon/Chicago IL, plus Gunvant Thaker and John Sweeney.
The total number of subjects assessed in the first wave of the study (B-SNIP1) is approximately 2500. Volunteers were gathered from diverse clinical settings and across multiple geographic regions to allow generalizability regarding resulting conclusions. All probands had been on stable medications for four or more weeks, and none was acutely ill at the time of assessment, although many were symptomatic to varying degrees. As well as carefully documenting symptoms and recording medications, basic study data consisted of standardized and reliable biological assessments conducted on the same models of equipment across the several geographically separated collection sites.
The almost 50 biological measures employed were chosen based on straightforward criteria. These characteristics were that they were reliable, well-studied (defined as being found previously in multiple studies to be abnormal in association with psychotic illnesses), fully quantifiable, not strongly associated with medication status or state/stage of illness, having evidence of heritability, and having been reported to be also abnormal in unaffected close relatives of probands with the illness. This latter criterion qualified them as being endophenotypes (i.e. markers of illness risk)20, rather than merely biomarkers (indices of the presence of manifest illness). It should be emphasized that none of the measures gathered are likely specifically abnormal in schizophrenia subjects (and, by definition, their relatives); many also have been shown to be abnormal in bipolar subjects (probands and relatives) and in some cases in patients with other major mental disorders.
Examples of B-SNIP measures included those in the realms of eye movement/tracking (smooth pursuit, saccades, anti-saccades), electrophysiology (resting measures and various auditory evoked potentials), MRI (structural, diffusion tensor imaging and resting state functional scans), psychophysiological measures (such as pre-pulse inhibition) and assessment of multiple cognitive domains. All subjects were genotyped. A parallel study examining non-psychotic bipolar-I volunteers is currently under way to determine whether findings from B-SNIP are specific to psychosis.
The initial questions posed by the B-SNIP-1 study were straightforward – to examine these multiple biological measures across several psychotic illnesses, plus the putatively related axis II cluster A disorders (schizotypal, schizoid and paranoid) in non-psychotic relatives, to determine whether these indices shared commonalities across the psychosis dimension/spectrum that might clearly separate the disorders from one another more clearly than did illness symptoms, or to discover whether or not they cross traditional diagnostic boundaries. Subsidiary questions were whether similar abnormalities to those seen in subjects with psychotic disorders occurred in their unaffected relatives across disorders, (as would be expected of endophenotypes) and whether the various axis I diagnostic groups were distinguished by differences in type of biological abnormalities, differences in degree of severity of these measures, or neither of these.
Major findings
Clinical symptoms evident at the time of testing did not prominently distinguish DSM-IV-TR based diseases from one another, whether using the Positive and Negative Syndrome Scale (PANSS) for any of it subscales, the Young Mania Rating Scale, or the Montgomery-Asberg Depression Rating Scale. The Birchwood Social Functioning Scale revealed greatest impairment in schizophrenia subjects and least in psychotic bipolars, but again, all axis I groups appeared rather similar when compared to normalcy21. A novel illness rating scale (the Schizo-Bipolar Scale)7 designed specifically to separate prototypic schizophrenia from bipolar illness along a continuum, and based on both current and historical information on typical illness symptoms, inter-episode recovery, etc. revealed no points of separation between the three DSM disorders, which instead blurred into each other. Clearly then, and contrary to clinical expectation, symptom measures of various types performed very poorly in separating the DSM syndromes. Interestingly, examining proband family lineages revealed some kindreds with “pure” (i.e., consistent) psychosis diagnoses, but also numerous families with mixtures of schizophrenia/bipolar diagnoses21, as had previously been reported by Lichtenstein14.
Equally unexpectedly all the major biological measures, while discriminating axis I subjects robustly from healthy controls, performed at best modestly for separating diagnostic groups, as summarized in Tamminga, et al. 22, whether the dimension chosen was cognition23, oculomotor24, structural MRI25, resting state functional MRI26, or P 300 evoked potential27. In many cases abnormalities were relatively greater or lesser in one DSM-based group compared to another, but the general trend across all endophenotypes was one of only modest differences in degree of severity, marked similarity and overlap among groups, and often similar (although lesser) findings in unaffected relatives, with those meeting cluster-A criteria resembling axis I subjects more than controls. To summarize, the observed differences in biological deviation were in degree, rather than in kind (analogous to symptom distribution) and biomarkers performed modestly overall in distinguishing DSM clinical psychosis diagnoses from one another.
How are these biology-based results to be best understood? The most parsimonious explanation is that the investigators had fallen into the conceptual trap of using biology to validate existing, syndrome-based diagnostic categories, where measures show only blurred differences in degree across psychoses. Biological data in other words, fail to validate conventional psychiatric diagnoses as a “gold standard”, but instead revealed a single severity continuum, most often with schizophrenia at one end and psychotic bipolar illness at the other.
Reconceptualization: start with the biology
An obvious alternative strategy was for the B-SNIP investigators to start with an agnostic, bottom-up reclassification, using the same endophenotype data, but setting aside conventional, phenomenologically-based, categorical diagnostic entities, to see if we could derive distinctive entities based purely on biology. This is a strategy that has been advocated for 30 or more years28 in classifying the psychoses. An obvious recent parallel within clinical medicine is that of breast cancer, that was redefined as different disease entities with distinct treatment responses based on biological evidence29. Such an analysis was carried out on patients in the B-SNIP endophenotype data set30 using multivariate taxometric analyses, and revealed three neurobiologically distinct biologically-defined psychosis categories, termed “Biotypes,” that crossed clinical diagnostic boundaries (i.e. every Biotype contained subjects with all three conventional diagnoses). The derived Biotypes were distinctly separate from each other in a manner that was statistically vastly superior to that obtained (using the same measures) with DSM diagnoses. In addition, the Biotypes did not fit a simple severity continuum. The Biotypes appeared to be heritable, in that their unaffected first-degree relatives (who were not used in the Biotype construction) strongly resembled them on the biomarkers, and additional endophenotypes that were also not used in defining the original Biotype categories captured this statistical typology as secondary validators30.
Of particular interest is that some of the deviations from normal within different Biotype domains were in opposite directions; within one such domain (sensorimotor reactivity), one of the Biotype groups scored significantly below healthy control values, while another scored significantly above them. One can imagine that collapsing neurobiological measurements from such individuals across conventional DSM diagnoses would lead to the confusing results that have bedeviled the research field in psychosis, notably in the areas of drug and gene discovery. The obvious next step is to define the genetics and molecular biological underpinnings of each distinct Biotype, and to construct treatment trials that specifically address unique mechanisms. One of the three Biotypes appeared to be relatively similar to healthy controls across the majority of neurobiological measures; patients in this category may be “phenocopies” especially sensitive to environmental psychosis risk factors.
Although these results are novel and promising, they are still preliminary, and require replication. An NIMH-funded study, B-SNIP-2, is therefore currently underway to test Biotype replicability in a new sample. Conventional schizophrenia subtypes (such as paranoid, hebephrenic etc.) are highly unstable over time, which is one reason they were omitted from DSM-5; it certainly will be necessary to demonstrate the longitudinal stability of Biotypes. Another obvious question is whether non-psychotic bipolar subjects, who were not examined in B-SNIP, are biologically distinct from psychotic bipolar individuals. A separate NIMH funded study is currently underway to address that question.
Take-Home Messages – and the Future
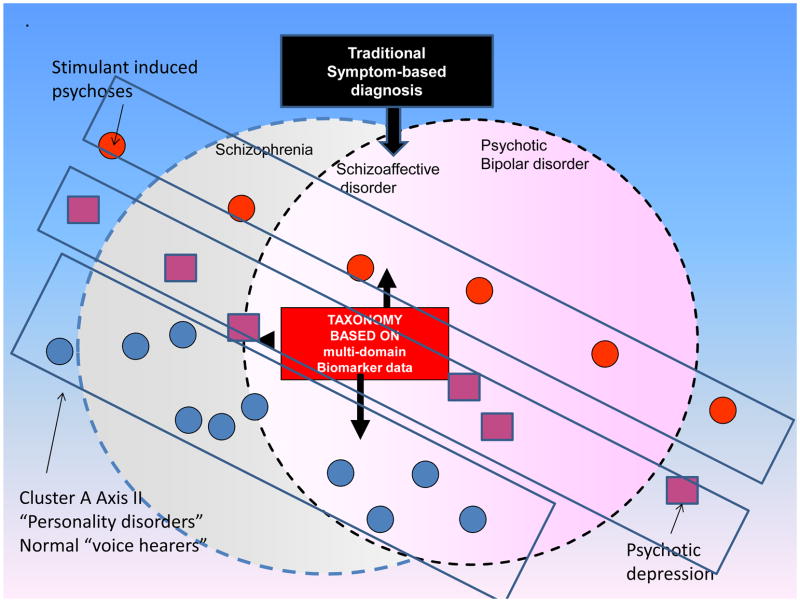
First, there is diminishing support for the familiar, clinical phenomenology-based, classical Kraepelinean diagnostic model still used as the gold standard in DSM-5. Using multiple biological classifiers reveals an absence of true points of diagnostic rarity across the most prevalent psychotic disorders, a finding that would not be expected if these were truly distinct entities. Another problem for classic diagnostic categories, not explored in the above article, is the existence of psychosis continua versus what we are accustomed to thinking of as categorical diagnoses. For example cluster-A “personality disorders”, are likely positioned on a continuum of severity with psychotic illnesses, as individuals who meet diagnostic criteria for them manifest similar if lesser biological abnormalities to patients with the disorders31. This is analogous perhaps to the elevated but not pathological blood sugars found in non-diabetic relatives of type-2 diabetic patients. There is also the phenomenon of non-psychotic “voice-hearers”, who also may belong on an extended psychosis spectrum32. These and allied phenomena have led some observers to argue that dimensional or spectrum concepts may describe psychotic disorders more realistically than our current categorical classifications. It will be also important to see how psychotic major depressive disorder patients fit into any new schema. See Figure 1 below:
Figure 1.
A possible example of a biomarker-based classification, agnostic to conventional diagnostic categories, illustrating how dimensional or spectrum concepts might plausibly delineate psychotic disorders differently than current, symptom-based categorical classifications. Such a novel taxonomy would be based on multi-domain endophenotype measures as described in the text. In addition to the three major diagnoses examined in BSNIP-1 (schizophrenia, schizoaffective disorder and psychotic bipolar disorder), theoretically allied conditions are incorporated into this schema as illustrated, including psychostimulant-induced psychoses (upper left) and psychotic depression (lower right). Additional conditions portrayed include individuals who can be conceived of as existing on the mild end of a theoretical continuum with psychotic disorders, for example, persons lacking a psychiatric diagnosis but who experience phenomena allied to auditory hallucinations (so-called “voice-hearers”) as well as the cluster-A “personality disorders” listed in DSM-IV (schizotypal, paranoid and schizoid).
Courtesy of M. Keshavan, MD, Boston, MA.
Available evidence shows multiple shared neurobiological signatures across the psychoses in a manner that offers very little support for conventional symptom-based diagnostic categories such as those bequeathed to us from the Kraepelinean dichotomy and instantiated today in the latest edition of the DSM. Although Kraepelin hoped that biology would ultimately validate the syndromes that he described as distinct entities, the available facts do not support his belief. An alternative means to attack the problem of classifying psychosis is an approach that uses reliable and valid neurobiological tests to derive a bottom-up, statistically-driven novel categories, agnostic to phenomenological symptom-based diagnostic criteria. This type of alternative taxonomic approach is necessarily based on multiple endophenotypes rather than any simple, single measure. This is not to minimize the utility of biological disease markers that are not also heritable indicators of illness predisposition. While, the latter more readily lend themselves to the discovery of disease risk genes, non-inherited biomarkers may be uniquely useful for disease classifications for those illness features that lack a direct genetic antecedent.
This strategy offers promising preliminary data that suggest a means to derive a biologic redefinition of psychotic syndromes, in a manner consistent with the Research Domain Criteria35 (RDoC), proposed by NIH33. This type of process is actually one that has characterized much of the history of medicine. For example, “dropsy” is now recognized not to be a distinct disease, but rather a syndrome with multiple causes, including cardiac and renal diseases and protein deficiency, each of which responds to a specific type of treatment. Perhaps a more apt example is medicine’s ability to use biological tests such as chest x-rays and sputum cultures to parse a heterogeneous group of patients all presenting with the symptom of severe cough, into individuals with the diseases of viral pneumonia, pulmonary tuberculosis and lung cancer, requiring different treatments. A salutary fact is that a substantial minority of psychotic patients are treatment-resistant, despite similar clinical presentations. Recent arguments have been made to repurpose existing drugs for the treatment of schizophrenia, based on emerging genetic and molecular biological mechanisms34. Logically, discovery of the molecular biological underpinnings of Biotypes would move in a similar direction, or even to novel drug-designs based on a finer-grained trans-diagnostic classification scheme. The rest of medicine made the transition from syndromes to diseases many years ago as the underlying pathology and biology of particular syndromes was discovered, but the great complexity and inaccessibility of the brain renders this enterprise a necessarily complex and daunting one for psychiatry. Previously-mentioned efforts from the National Institute of Mental Health to reclassify mental disorders as a whole based on biological observations, in their Research Domain Criteria35, represents a parallel effort in this direction, although psychosis does not necessarily fit comfortably in this schema. Initial efforts to fit specific psychotic symptoms such as alogia/ blunted affect and hallucinations within RDoC are being made36, 37 and the general strategy that biological data should be gathered across current diagnostic categories is shared by both RDoC and B-SNIP38. The reconceptualization of psychosis categories based on biology holds the promise of new directions in disease classification and hopefully will lead to novel treatment strategies that one hopes will emerge over the next few years.
Key Points.
Psychotic disorders overlap considerably in terms of clinical symptoms, familial patterns, risk genes and treatment response.
Numerous neurobiological measurements also fail to distinguish the most prevalent classic psychotic disorders (schizophrenia, schizo-affective and psychotic bipolar) patients from each other.
Statistical methods applied to such biological measurements in large numbers of these patients, result in novel classifications that cut across traditional diagnostic boundaries, to reveal “Biotypes”, i.e. biologically-defined entities.
Such new types of classification approaches within psychotic illnesses hopefully represent an opportunity to move away from phenomenologically-defined syndromes in psychiatry and towards neurobiologically-defined diseases.
Footnotes
Potential Conflicts of interest: Dr. John Sweeney has received support from Takeda, BMS, Lilly, Roche and Janssen. Dr. Matcheri Keshavan has received support from Sunovion. Dr. Carol Tamminga has received funding from Astellas, Eli Lilly, Intracellular Therapies, Lundback and Pure Tech Ventures. Other authors declare no financial interest in relation to the work described in this manuscript other than NIH grant funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kraepelin E. Psychiatry: A textbook for Students and Physicians. 7. Leipzig: Barth: McMillan; 1912. [Google Scholar]
- 2.EB Dementia praecox or the group of schizophrenias. Vertex. 1911;21:394–400. [PubMed] [Google Scholar]
- 3.Pearlson GD. Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annual review of clinical psychology. 2015;11:251–281. doi: 10.1146/annurev-clinpsy-032814-112915. [DOI] [PubMed] [Google Scholar]
- 4.Fischer BA, Carpenter WT., Jr Will the Kraepelinian dichotomy survive DSM-V? Neuropsychopharmacology. 2009 Aug;34(9):2081–2087. doi: 10.1038/npp.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zendig E. Beitrage zur differentialdiagnose des manisch-depressiven irreseins und der dementia praecox. Allg Z Psychiatr. 1909;66:932–833. [Google Scholar]
- 6.Kasanin J. The acute schizoaffective psychoses. 1933. Am J Psychiatry. 1994 Jun;151(6 Suppl):144–154. doi: 10.1176/ajp.151.6.144. [DOI] [PubMed] [Google Scholar]
- 7.Keshavan MS, Morris DW, Sweeney JA, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011 Dec;133(1–3):250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coryell W, Lavori P, Endicott J, Keller M, VanEerdewegh M. Outcome in schizoaffective, psychotic, and nonpsychotic depression. Course during a six- to 24-month follow-up. Arch Gen Psychiatry. 1984 Aug;41(8):787–791. doi: 10.1001/archpsyc.1984.01790190061008. [DOI] [PubMed] [Google Scholar]
- 9.Keck PE, Jr, McElroy SL, Havens JR, et al. Psychosis in bipolar disorder: phenomenology and impact on morbidity and course of illness. Compr Psychiatry. 2003 Jul-Aug;44(4):263–269. doi: 10.1016/S0010-440X(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin FK, Jamison KR. Manic-depressive illness. New York: New York: Oxford University Press; 1990. [Google Scholar]
- 11.Guze SB, Woodruff RA, Jr, Clayton PJ. The significance of psychotic affective disorders. Arch Gen Psychiatry. 1975 Sep;32(9):1147–1150. doi: 10.1001/archpsyc.1975.01760270079009. [DOI] [PubMed] [Google Scholar]
- 12.Glahn DC, Williams JT, McKay DR, et al. Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biol Psychiatry. 2014 Jul 21; doi: 10.1016/j.biopsych.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majadas S, Olivares J, Galan J, Diez T. Prevalence of depression and its relationship with other clinical characteristics in a sample of patients with stable schizophrenia. Compr Psychiatry. 2012 Feb;53(2):145–151. doi: 10.1016/j.comppsych.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein P, Yip BH, Bjork C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009 Jan 17;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consortium C-DgotPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013 Apr 20;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow TJ, Chance SA, Priddle TH, Radua J, James AC. Laterality interacts with sex across the schizophrenia/bipolarity continuum: an interpretation of meta-analyses of structural MRI. Psychiatry Res. 2013 Dec 30;210(3):1232–1244. doi: 10.1016/j.psychres.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Kraepelin E. Der erschenungsformen der irreseins. (The manifestations of insanity) Hist Psychiatry. 1920;3:509–529. [Google Scholar]
- 18.Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970 Jan;126(7):983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- 19.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: Am. Psychiatr. Publ; 2013. [Google Scholar]
- 20.Glahn DC, Knowles EE, McKay DR, et al. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014 Mar;165B(2):122–130. doi: 10.1002/ajmg.b.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the Bipolar- Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013 Nov;170(11):1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 22.Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014 Mar;40(Suppl 2):S131–137. doi: 10.1093/schbul/sbt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill SK, Reilly JL, Keefe RS, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013 Nov 1;170(11):1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly JL, Frankovich K, Hill S, et al. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014 Sep;40(5):1011–1021. doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013 Nov 1;170(11):1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khadka S, Meda SA, Stevens MC, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013 Sep 15;74(6):458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ethridge LE, Hamm JP, Pearlson GD, et al. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2014 May 4; doi: 10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendell RE. Diagnosis and classification of functional psychoses. Br Med Bull. 1987 Jul;43(3):499–513. doi: 10.1093/oxfordjournals.bmb.a072198. [DOI] [PubMed] [Google Scholar]
- 29.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012 Jun 21;486(7403):346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA. Identification of distinct psychosis biotypes using brain-based biomarkers. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2015.14091200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarley RW, Niznikiewicz MA, Salisbury DF, et al. Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. European archives of psychiatry and clinical neuroscience. 1999;249(Suppl 4):69–82. doi: 10.1007/pl00014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen P, Modinos G, Hubl D, et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012 Jun;38(4):695–703. doi: 10.1093/schbul/sbs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular psychiatry. 2012 Dec;17(12):1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- 34.Lencz T, Malhotra AK. Targeting the schizophrenia genome: a fast track strategy from GWAS to clinic. Molecular psychiatry. 2015 Jul;20(7):820–826. doi: 10.1038/mp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009 Dec 1;66(11):988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Ford JM, Morris SE, Hoffman RE, et al. Studying hallucinations within the NIMH RDoC framework. Schizophr Bull. 2014 Jul;40(Suppl 4):S295–304. doi: 10.1093/schbul/sbu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen AS, Najolia GM, Kim Y, Dinzeo TJ. On the boundaries of blunt affect/alogia across severe mental illness: implications for Research Domain Criteria. Schizophr Res. 2012 Sep;140(1–3):41–5. doi: 10.1016/j.schres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter WT. RDoC and DSM-5: what’s the fuss? Schizophr Bull. 2013 Sep;39(5):945–6. doi: 10.1093/schbul/sbt101. [DOI] [PMC free article] [PubMed] [Google Scholar]