Abstract
Objectives
To investigate the change in cartilage T2-values and structural degeneration in knee joints over 72 months in women of African American (AA) vs. Caucasian American (CA) ethnicity.
Methods
Knee 3T MRIs from baseline, 24, 48 and 72 month visits of 100 AA and 100 CA women from the OAI were assessed for cartilage T2-values and whole-organ magnetic resonance imaging (WORMS) score. Subjects were pair-matched by age, BMI, Kellgren-Lawrence (KL) score, clinical site and subcohort within the OAI. We compared the rate of change in whole knee cartilage T2-values and WORMS cartilage, bone marrow edema pattern (BMEP) and meniscus scores between the two ethnic groups using mixed random effects models.
Results
At 24 and 48 months 60 subjects and at 72 months 45 subjects per group were available for analysis resulting in 38 complete pairs with data of all time points. Compared to CA, cartilage T2-values in AA increased at a significantly faster rate at baseline (AA: 0.45ms/y, CA: 0.35ms/y, p=0.029) and averaged over 6 years (AA: 0.36ms/y, CA: 0.27ms/y, p=0.039) with changes in both groups reaching a plateau by 48 months. Cartilage, meniscus and BMEP scores tended to increase in both groups during follow-up, but rates of change did not differ by ethnicity.
Conclusion
Cartilage T2-values increased faster over 72 months in AA than CA, however changes in WORMS cartilage, meniscus and BMEP scores did not differ. T2-values may be able to distinguish ethnicity-related differences of cartilage degeneration at an early stage before differences in structural joint degeneration appear.
Introduction
Osteoarthritis (OA) is a chronic degenerative form of arthritis; it is a complex entity evolving as a collection of multiple etiologies. These etiologies can be categorized by local factors such as joint injury and joint instability, constitutional factors such as muscle weakness, misalignment and obesity as well as systemic factors such as sex, age, hormonal status, bone mineral density, inflammatory, metabolic and genetic factors, all of which contribute to the onset and development of the disease1. Genetic factors may be responsible for up to 65% of the variance of OA in women2 and there is evidence that the prevalence and degree of OA varies between ethnic groups with a higher prevalence of knee OA among African American women3 and a higher prevalence of hip OA in African American men4. While these ethnic differences may in part be explained by behavioral, socioeconomic and constitutional factors, genetic differences have to be considered as a contributing factor.
A recent study on ethnic differences in cartilage composition showed that MRI T2 relaxation times of the knee cartilage in African American women were significantly lower compared to Caucasian women5. In the context of a higher prevalence of knee OA in African American as reported in several studies3,4 the finding of lower T2 values in this group was surprising since degradation of the cartilage matrix6 and progression of structural changes with knee OA7 have been shown to be associated with increased T2 values raising the question of whether the natural history of cartilage degeneration is different in African Americans.
To further explore differences in joint degeneration and cartilage biochemical composition over 6 years we performed a longitudinal study using 3T MRI for the measurement of cartilage T2 and the WORMS scoring system for the assessment of structural joint degeneration in African American and Caucasian American women.
Materials and Methods
Subjects
Subjects for this study were from the Osteoarthritis Initiative (OAI), a multi center cohort study consisting of 4796 participants divided into a progression cohort (subjects with symptomatic knee OA), an incidence cohort (subjects without symptomatic knee OA but risk factors for OA) and a normal cohort without knee OA or risk factors8. We used the same sample as our recent study of 200 African-American and Caucasian-American women selected from the OAI incidence and progression subcohorts5 (Figure 1).
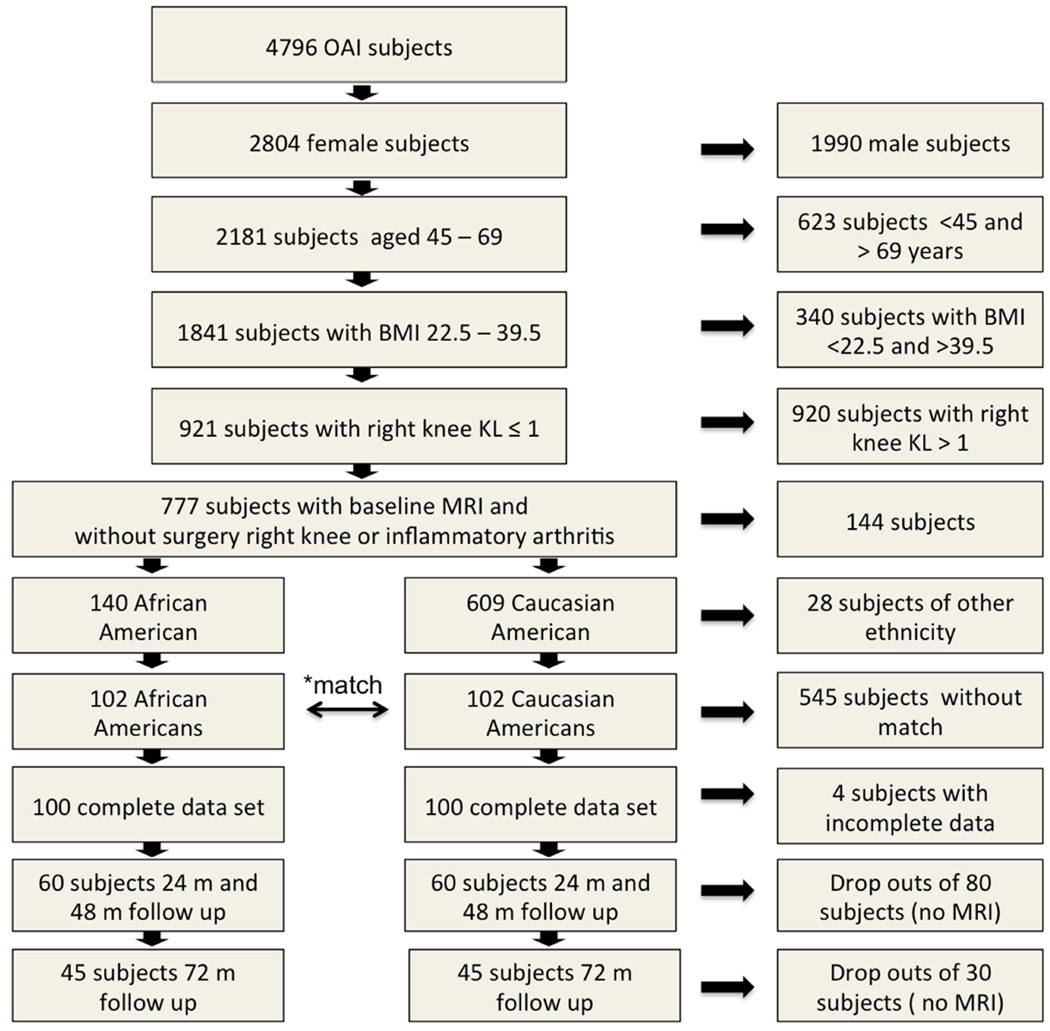
Figure 1.
Selection of subjects. *pairwise matching by age, BMI, KL grade, subcohort and clinical site. Overall 200 subjects in 100 matched pairs were included in the analysis. Due to dropouts the data set contained 38 matched pairs with a complete MRIs at all time points. At month 72, the data of 45 individuals was available in each group including 38 complete pairs and seven individuals each, who lost their matching partner.
The inclusion criteria for that study were: Females who were either of African-American or Caucasian-American ethnicity, age 45 to 69 and a body mass index (BMI) of 22.5–39.5 kg/m2. Subjects also had to have low Kellgren-Lawrence (KL) scores of 0 (definitively radiographically absent OA), and 1 (doubtful radiographic OA) to ensure that only participants with low cartilage lesion load were included and were suitable for T2 relaxation time measurements. Exclusion criteria were history of inflammatory arthritis and knee surgery at the right knee. The resulting selection of 140 African-American and 609 Caucasian American women was matched pairwise by KL grade (0 or 1), baseline age (5 year strata from 45 to 69 years) and BMI (5 point strata from 22 to 40 kg/m2), subcohort and clinical site. For each stratum Caucasian subjects were randomly selected. A total of 100 Caucasian American women could be identified to fulfill the matching criteria, thus 38 African American women had to be excluded. Two subjects had to be excluded because of incomplete data sets resulting in a total of 100 pairs at baseline.
For the present study, 60 pairs (120 subjects) had follow-up MRI data in both members of the pair at both 24 and 48 months. At month 72, the data of 45 individuals was available in each group including 38 complete pairs and seven individuals each, who lost their matching partner. These individuals were kept in the study groups to maintain a sufficient sample size. A retrospective analysis of the demographic data showed that both groups remained balanced. (Figure 1, Table 1).
Table 1.
Subject characteristics
| Ethnicity | |||
|---|---|---|---|
| African American | Caucasian American | p-value | |
| Baseline | |||
| N | 100 | 100 | |
| Age | 55.89 (6.02) | 55.32 (6.46) | 0.138 |
| BMI* | 29.20 (4.02) | 29.16 (3.81) | 0.816 |
| KL* | 0.29 | 0.29 | 1.000 |
| PASE* | 151.2 (80,4) | 167.1 (78.7) | 0.160 |
| Occupational act. level | 2.17 | 2.16 | 0.888 |
| 24 months follow up | |||
| N | 60 | 60 | |
| Age* | 57.7 (5,8) | 57.2 (6.3) | 0.690 |
| BMI* | 29.6 (0.56) | 29.0 (0.53) | 0.393 |
| KL* | 0.54 (0.7) | 0.48 (0.74) | 0.658 |
| PASE* | 154.4 (73.0) | 164.7 (87.8) | 0.491 |
| PASE Occupational act. level | 2.26 | 2.17 | 0.685 |
| 48 months follow up | |||
| N | 60 | 60 | |
| Age* | 59.4 (6.0) | 59.0 (6.2) | 0.733 |
| BMI* | 29.5 (0.53) | 29.3 (0.51) | 0.832 |
| KL* | 0.55 (0.77) | 0.63 (0.96) | 0.619 |
| PASE* | 159.0 (79.2) | 164.2 (80.1) | 0.727 |
| PASE Occupational act. Level** | 2.24 | 2.26 | 0.905 |
| 72 months follow up | |||
| N | 45 | 45 | |
| Age* | 61.1 (5.5) | 60.3 (6.2) | 0.388 |
| BMI* | 30.0 (3.7) | 29.3 (4.1) | 0.360 |
| KL* | 0.5 (0.68) | 0.66 (0.77) | 0.348 |
| PASE* | 156.2 (76.4) | 170.1 (81.4) | 0.407 |
| PASE Occupational act. level | 2.38 | 2.34 | 0.796 |
numbers are mean (SD).
BMI = body mass index, KL = Kellgren Lawrence score, PASE = physical activity scale for the elderly.
Graded with 1 = sitting, 2 = sitting/standing/walking, 3 = walking/handling < 50 lbs., 4 = walking/handling >50 lbs.
Radiographs
Standing postero-anterior fixed flexion knee radiographs were acquired as described in detail in the OAI Radiographic Procedure Manual freely accessible at http://www.oai.ucsf.edu. All knee radiographs were analyzed centrally and graded with regard to the degree of joint degeneration using the Kellgren-Lawrence score (KL) score9,10.
MR imaging protocol
MR images of the right knee were obtained using four identical 3.0 Tesla scanners (Trio, Siemens) and quadrature transmit-receive coils (USA Instruments, Aurora, Oh, USA) at one of four sites (Ohio State University, Columbus, OH; University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; and Memorial Hospital of Rhode Island, Pawtucket, RI). Details of the acquisition protocol have been published11 and included the following sequences: 1) coronal proton density-weighted fast spin-echo (FSE); 2) sagittal 3-D dual echo in the steady state (DESS) with selective water excitation; 3) sagittal intermediate-weighted FSE with fat suppression; and 4) sagittal T2-weighted multi-echo spin-echo (SE) for quantitative T2 relaxation time measurements.
Quantitative T2 relaxation time measurements
The sagittal 2-D multi-echo SE of the right knee was used for segmentation and quantification of T2-relaxation time using an in-house developed spline-based, semi-automated software segmentation algorithm in MATLAB (Mathworks Inc, El Segundo, CA)12,13. Segmentation of the cartilage was performed on the first echo sequence to maximize signal-to-noise ratio. All segmentations were performed by four individuals (MK, AY, UH, MS) in the following compartments: patella, medial/lateral femoral condyle, medial/lateral tibia. The trochlea was not segmented because of interfering flow artifacts from the popliteal artery. Inter- reader (1.57%) and intra-reader reproducibility errors of the (1.46%) of this technique were minimal as reported in a prior study14.
Whole-Organ Magnetic Resonance Imaging Score (WORMS) grading
MR images were evaluated for the grade of cartilage, meniscal and bone marrow edema pattern (BMEP) lesions using WORMS15, modified as previously described16. Two radiologists (MK with 11 and AY with 9 years of experience) who were blinded to the ethnicity of subjects analyzed separately an equal number of subjects (AA and CA subjects all time points). Problematic cases were reviewed with TML (24 years of experience) and a final consensus diagnosis was obtained. Inter- and intra-observer agreement data of the UCSF modified WORMS score were published previously.16 Meniscus lesions were graded 0–4 in each of 6 regions (medial/lateral and anterior/body/posterior). Cartilage grades (0–6) and BMEP grades (0–3) were also scored in 6 regions (patella, trochlea, medial/lateral femur, and medial/lateral tibia). BMEP was defined as areas of poorly marginated increases in T2 signal intensity in the fat suppressed imaging sequences. For each type of lesion (meniscus, cartilage, BMEP), a sum score per knee was defined as the sum of scores in all regions. We also calculated a total score (WORMS sum) by summing scores for menisci, cartilage, ligaments, BMEP, cysts, effusion and baker cysts for each knee over all regions
Statistical analysis
Statistical analysis was performed using JMP version 11 (SAS Institute, Cary, NC, USA) and STATA version 12 software (StataCorp LP, College Station, TX, USA). In addition to descriptive statistics, differences of demographic variables between ethnic groups were determined using the Kruskal-Wallis test. Based on the previously published significant differences in baseline cartilage T2 values5 we wanted to investigate the development of T2 values and WORMS grades in the two different ethnic groups. Mean T2 values and WORMS scores of AA and CA subjects were compared at each time point using paired t-test. Significance of differences of Mean T2 values and WORMS scores from baseline to 72 month follow-up was tested using ANOVA, level of confidence was set to p < 0.05. Mixed models were used to assess the differences in the rates of change of (1) cartilage T2 and (2) WORMS scores (using all four 4 time points) between the AA and CA subjects. We assumed a nonlinear increment of T2 values and found a significant quadratic relationship with time while WORMS changes followed a significant linear pattern. We controlled for random effects with multiple measurements per subjects in adding the identification code for the individual subject as a covariate. Pairs were identified with a numerical code and included as a covariate. Since the T2 increments followed a non-linear quadratic relationship, we reported the (i) difference in the baseline rate of change in T2 between both groups and (ii) difference in the average annual rate of change averaged over 6 years between both groups.
Results
Subject characteristics
Subject characteristics at baseline are summarized in Table 1. Although drop out changed group composition slightly at the different follow up time points, there were no significant differences in BMI, age, KL-grade or PASE physical activity scores including the subscore for occupational physical activity between AA and CA at any of the time points.
T2 relaxation time and ethnicity
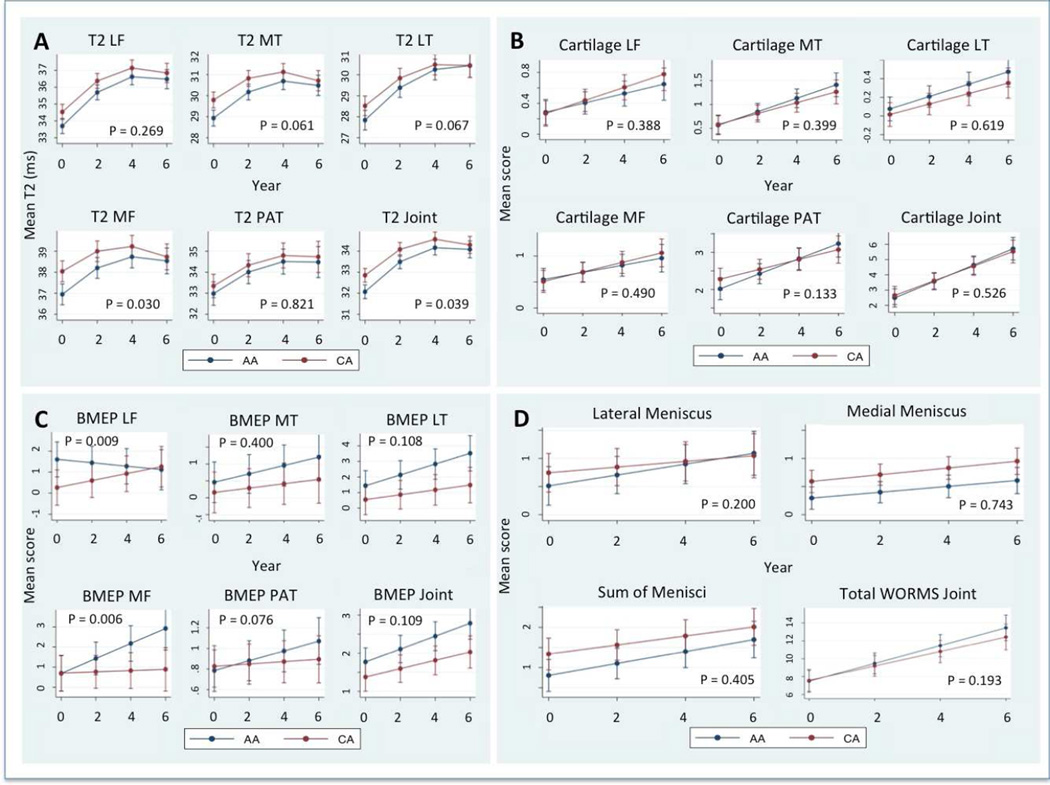
AA cartilage T2 values remained lower at all time points. However, the significance of differences disappeared after 24 months (p = 0.617), which was mainly due to the increase of T2 values in AA cartilage (Table 2, 4, Fig. 2A). There was a significant increase of T2 values averaged over the joint in both groups over the 6 years (Table 3, Figure 2 A) (p <0.0001). The slope followed a non-linear pattern and gradually decreased with time building a plateau after 4th year (Figure 2 A). Compared to the Caucasian Americans the baseline rate of increase in the African Americans was significantly higher in the total cartilage per joint (AA 0.45 ms/y [CI 0.38–0.5], CA 0.35 ms/y [CI 0.29–0.41], p = 0.029). Also the average annual rate of change of the total cartilage T2 was significantly higher in AA (AA 0.36, [CI 0.30–0.43], CA 0.27, [CI 0.20–0.33], p = 0.039, Table 4) so that while total cartilage T2 values were lower in AA at baseline the values converged over time. In addition, baseline and overall annual rates of change were greater in AA in the medial femoral condyle (baseline rate p = 0.025, average annual rate p = 0.030) and there were trends for a greater baseline and average annual rates of change in the medial and lateral tibia in the African Americans subjects (Table 4, Figure 2 A).
Table 2.
Differencese in T2 values and WORMS scores at time points baseline to 6 years
| Ethnicity | |||||
|---|---|---|---|---|---|
| African Americans | Caucasian Americans | ||||
| mean | 95% CI | mean | 95% CI | p | |
| Baseline | |||||
| Average T2 (ms) | 32.07 | 31.7–32.4 | 32.85 | 32.5–33.2 | <0.0001 |
| WORMS sum | 7.79 | 6.47–9.12 | 7.57 | 6.58–8.56 | 0.755 |
| Cartilage sum | 3.61 | 2.97–4.23 | 3.62 | 2.96–4.28 | 0.969 |
| BMEP sum | 1.81 | 1.37–2.24 | 1.35 | 0.99–1.71 | 0.069 |
| Meniscus sum | 0.9 | 0.54–1.26 | 1.36 | 0.89–1.83 | 0.065 |
| Medial Meniscus sum | 0.34 | 0.16–0.52 | 0.61 | 0.38–0.84 | 0.031 |
| Lateral Meniscus sum | 0.56 | 0.25–0.87 | 0.75 | 0.33–1.16 | 0.379 |
| 24 months | |||||
| WORMS sum | 33.49 | 33.2–33.8 | 33.97 | 33.8–34.4 | 0.617 |
| Average T2 (ms) | 8.66 | 6.91–10.39 | 8.91 | 7.44–10.38 | 0.687 |
| Cartilage sum | 4.29 | 3.47–5.11 | 4.54 | 3.69–5.38 | 0.556 |
| BMEP sum | 2.17 | 1.60–2.74 | 1.71 | 1.25–2.16 | 0.231 |
| Meniscus sum | 0.66 | 0.18–1.13 | 1.42 | 0.82–2.02 | 0.003 |
| Medial Meniscus sum | 0.26 | 0.02–0.49 | 0.55 | 0.26–0.84 | 0.017 |
| Lateral Meniscus sum | 0.4 | −0.01–0.8 | 0.87 | 0.34–1.40 | 0.034 |
| 48 months | |||||
| WORMS sum | 34.16 | 33.8–34.5 | 34.43 | 34.2–34.9 | 0.259 |
| Average T2 (ms) | 12.15 | 10.48–13.82 | 10.93 | 8.98–12.89 | 0.268 |
| Cartilage sum | 5.88 | 5.09–6.67 | 5.75 | 4.90–6.60 | 0.749 |
| BMEP sum | 2.83 | 0.28–3.38 | 2.02 | 1.56–2.48 | 0.024 |
| Meniscus sum | 1.39 | 0.94–1.85 | 1.85 | 1.25–2.45 | 0.252 |
| Medial Meniscus sum | 0.49 | 0.27–0.71 | 0.74 | 0.44–1.03 | 0.287 |
| Lateral Meniscus sum | 0.9 | 0.52–1.29 | 1.11 | 0.58–1.65 | 0.506 |
| 72 months | |||||
| WORMS sum | 34.08 | 33.7–34.5 | 34.23 | 33.9–34.7 | 0.386 |
| Average T2 (ms) | 13.84 | 11.88–15.79 | 12.38 | 9.57–15.19 | 0.251 |
| Cartilage sum | 6.79 | 5.87–7.71 | 6.44 | 5.45–7.43 | 0.866 |
| BMEP sum | 3.09 | 2.44–3.73 | 2.11 | 1.58–2.65 | 0.065 |
| Meniscus sum | 1.54 | 1.01–2.08 | 2.02 | 1.32–2.72 | 0.639 |
| Medial Meniscus sum | 0.63 | 0.37–0.89 | 0.87 | 0.52–1.21 | 0.216 |
| Lateral Meniscus sum | 0.91 | 0.46–1.37 | 1.15 | 0.54–1.78 | 0.871 |
Significants of differences were tested with the paired t test, level of confidence p < 0,05. Part of baseline data were reported in Yu et al. 20157
Table 4.
Rate of change in T2 values at baseline and over 6 years
| Ethnicity | |||||
|---|---|---|---|---|---|
| African Americans | Caucasian Americans | ||||
| Rate of change at baseline | slope coeff.* | 95% CI | slope coeff.* | 95% CI | p |
| Average of all regions | 0.45 | 0.38–0.51 | 0.35 | 0.29–0.41 | 0.029 |
| LFC | 0.62 | 0.53–0.72 | 0.55 | 0.45–0.65 | 0.238 |
| LT | 0.53 | 0.44–0.62 | 0.42 | 0.34–0.51 | 0.058 |
| MFC | 0.37 | 0.28–0.42 | 0.23 | 0.13–0.33 | 0.025 |
| MT | 0.37 | 0.28–0.45 | 0.26 | 0.18–0.34 | 0.054 |
| P | 0.33 | 0.19–0.47 | 0.32 | 0.18–0.45 | 0.835 |
| Average change rate over 6 years | rate/year* | 95% CI | rate/year* | 95% CI | p |
| Average of all regions | 0.36 | 0.30–0.43 | 0.27 | 0.20–0.33 | 0.039 |
| LFC | 0.51 | 0.41–0.61 | 0.43 | 0.33–0.53 | 0.269 |
| LT | 0.46 | 0.37–0.54 | 0.35 | 0.26–0.43 | 0.067 |
| MFC | 0.29 | 0.20–0.39 | 0.14 | 0.05–0.24 | 0.030 |
| MT | 0.29 | 0.21–0.37 | 0.18 | 0.10–0.26 | 0.061 |
| P | 0.27 | 0.15–0.40 | 0.25 | 0.13–0.38 | 0.821 |
Numbers of slope coefficients at baseline and average rate /year are least square means adjusted for multiple measurements per ID and matched pairing between groups pairs using a mixed random effects model. Units are ms/year.
Figure 2.
Mean Cartilage T2 (A) and the WORMS scores for cartilage (B), bone marrow edema pattern (C) menisci (D) and the sum of all WORMS scores (D) in African American (AA) and Caucasian American women (CA) over 6 years. Values are least square means adjusted for multiple measurements per ID and matched pairing between groups pairs using a mixed random effects model. Error bars indicate standard errors. P values indicate significance of differences of the rate of change (at baseline in non linear increments). LF = lateral femur, MF = medial femur, LT = lateral tibia, MT = medial tibia, PAT = patella, Joint = mean of all compatments.
Table 3.
Development of T2 values and WORMS scores over 6 years
| Follow up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| baseline | 24 months | 48 months | 72 months | ||||||
| mean | 95% CI | mean | 95% CI | mean | 95% CI | mean | 95% CI | p | |
| African Americans | |||||||||
| Average T2 ms | 32.07 | 31.7–32.4 | 33.49 | 33.2–33.8 | 34.16 | 33.8–34.5 | 34.08 | 33.7–34.5 | <0.0001 |
| WORMS sum | 7.79 | 6.47–9.12 | 8.66 | 6.91–10.39 | 12.15 | 10.48–13.82 | 13.84 | 11.88–15.79 | <0.0001 |
| WORMS Cartilage sum | 3.61 | 2.97–4.23 | 4.29 | 3.47–5.11 | 5.88 | 5.09–6.67 | 6.79 | 5.87–7.71 | <0.0001 |
| WORMS BMEP sum | 1.81 | 1.37–2.24 | 2.17 | 1.60–2.74 | 2.83 | 0.28–3.38 | 3.09 | 2.44–3.73 | 0.003 |
| Meniscus sum | 0.9 | 0.54–1.26 | 0.66 | 0.18–1.13 | 1.39 | 0.94–1.85 | 1.54 | 1.01–2.08 | 0.033 |
| Medial meniscus | 0.34 | 0.16–0.52 | 0.26 | 0.02–0.49 | 0.49 | 0.27–0.71 | 0.63 | 0.37–0.89 | 0.148 |
| Lateral meniscus | 0.56 | 0.25–0.87 | 0.4 | −0.01–0.8 | 0.9 | 0.52–1.29 | 0.91 | 0.46–1.37 | 0.191 |
| Ligament sum | 0.22 | 0.10–0.34 | 0.12 | −0.02–0.26 | 0.16 | 0.02–0.30 | 0.04 | −0.04–0.13 | 0.396 |
| Cyst sum | 0.63 | 0.31–0.94 | 0.57 | 0.15–0.98 | 0.95 | 0.56–1.35 | 1.35 | 0.88–1.81 | 0.043 |
| Effusion | 0.12 | 0.04–0.19 | 0.05 | −0.04–0.14 | 0.11 | 0.02–0.19 | 0.13 | 0.03–0.23 | 0.629 |
| Baker cyst | 0.65 | 0.42–0.87 | 0.79 | 0.53–1.06 | 0.83 | 0.57–1.08 | 0.89 | 0.59–1.18 | 0.563 |
| Caucasian Americans | |||||||||
| Average T2 ms | 32.85 | 32.5–33.2 | 33.97 | 33.8–34.4 | 34.43 | 34.2–34.9 | 34.23 | 33.9–34.7 | <0.0001 |
| WORMS sum | 7.57 | 6.58–8.56 | 8.91 | 7.44–10.38 | 10.93 | 8.98–12.89 | 12.38 | 9.57–15.19 | 0.0003 |
| WORMS Cartilage sum | 3.62 | 2.96–4.28 | 4.54 | 3.69–5.38 | 5.75 | 4.90–6.60 | 6.44 | 5.45–7.43 | <0.0001 |
| WORMS BMEP sum | 1.35 | 0.99–1.71 | 1.71 | 1.25–2.16 | 2.02 | 1.56–2.48 | 2.11 | 1.58–2.65 | 0.052 |
| Meniscus sum | 1.36 | 0.89–1.83 | 1.42 | 0.82–2.02 | 1.85 | 1.25–2.45 | 2.02 | 1.32–2.72 | 0.328 |
| Medial meniscus | 0.61 | 0.38–0.84 | 0.55 | 0.26–0.84 | 0.74 | 0.44–1.03 | 0.87 | 0.52–1.21 | 0.494 |
| Lateral meniscus | 0.75 | 0.33–1.16 | 0.87 | 0.34–1.40 | 1.11 | 0.58–1.65 | 1.15 | 0.54–1.78 | 0.626 |
| Ligament sum | 0.28 | 0.17–0.39 | 0.08 | −0.07–0.23 | 0.15 | 0.00–0.29 | 0.09 | −0.08–0.26 | 0.117 |
| Cyst sum | 0.29 | 0.05–0.53 | 0.42 | 0.11–0.72 | 0.48 | 0.17–0.78 | 0.82 | 0.46–1.18 | 0.119 |
| Effusion | 0.06 | 0.03–0.09 | 0.00 | −0.04–0.04 | 0.00 | −0.04–0.04 | 0.00 | −0.05–0.05 | 0.042 |
| Baker cyst | 0.66 | 0.43–0.88 | 0.77 | 0.48–1.05 | 0.69 | 0.41–0.97 | 0.89 | 0.56–1.21 | 0.68 |
Significance of differences over all time points was tested with ANOVA, level of confidence p < 0.05. Part of baseline data were reported in Yu et al. 20157
Progression of WORMS lesions and ethnicity
Both the African and the Caucasian American group started with a nearly identical total WORMS score of 7.57 and 7.79 (p = 0.755, Table 2) and increased significantly in a linear pattern over the time interval of 6 years (p <0.0001 and p = 0.0003 in the two groups respectively, Table 3, Figure 2 D). However, there were no significant differences in the rate of change over 6 years between the two groups (AA 0.99/y [CI 0.79–1.19], CA 0.81/y [CI 0.61–1.01], p = 0.193) (Table 5). The WORMS sum of the medial and lateral compartment did not significantly differ between AA and CA at all time points (Table 2). WORMS sum of both compartments increased significantly in both ethic groups (Table 3).While the sum of WORMS cartilage lesions increased significantly during follow up in both groups (p<0.0001 in both groups) the slope of increase was not significantly different between the groups (AA 0.54/y [CI 0.41–0.66], CA 0.48/y [CI 0.35–0.61], p = 0.526) (Table 5, Figure 2 B).
Table 5.
Rate of change of WORMS scores over 6 years
| Ethnicity | |||||
|---|---|---|---|---|---|
| African Americans | Caucasian Americans | ||||
| WORMS scores | rate/year* | 95% CI | rate/year* | 95% CI | p |
| Total score | 0.99 | 0.79–1.19 | 0.81 | 0.61–1.01 | 0.193 |
| Cartilage | |||||
| Sum of all regions | 0.54 | 0.41–0.66 | 0.48 | 0.35–0.61 | 0.526 |
| LFC | 0.06 | 0.02–0.89 | 0.08 | 0.04–0.12 | 0.388 |
| LT | 0.14 | 0.09–0.19 | 0.11 | 0.07–0.16 | 0.399 |
| MFC | 0.07 | 0.02–0.11 | 0.09 | 0.04–0.13 | 0.49 |
| MT | 0.07 | 0.04–0.09 | 0.06 | 0.03–0.08 | 0.619 |
| P | 0.2 | 0.14–0.26 | 0.13 | 0.07–0.19 | 0.133 |
| BMEP | |||||
| Sum of all regions | 0.17 | 0.12–0.22 | 0.11 | 0.06–0.16 | 0.109 |
| LFC | −0.01 | −0.02–0.04 | 0.02 | 0.003–0.03 | 0.009 |
| LT | 0.03 | 0.02–0.05 | 0.02 | −0.001–0.03 | 0.108 |
| MFC | 0.037 | 0.02–0.05 | 0.003 | −0.01–0.02 | 0.006 |
| MT | 0.01 | 0.003–0.02 | 0.006 | −0.003–0.02 | 0.400 |
| P | 0.05 | 0.02–0.08 | 0.01 | −0.02–0.04 | 0.076 |
| Menisci | |||||
| Meniscus sum | 0.15 | 0.09–0.21 | 0.11 | 0.05–0.17 | 0.405 |
| Medial meniscus | 0.05 | 0.02–0.08 | 0.06 | 0.03–0.09 | 0.743 |
| Lateral meniscus | 0.09 | 0.05–0.15 | 0.05 | 0–0.10 | 0.200 |
Numbers of rate/year are least square means adjusted for multiple measurements per ID and matched pairing between groups pairs using a mixed random effects model
There was a non-significant trend for BMEP lesion scores to increase faster in the African American group (AA 0.17/y [CI 0.12–0.22], CA 0.11/y [CI 0.06–0.16], p = 0.11). The rate of change was significantly higher in the medial femoral condyle of the African Americans (AA 0.037/y [CI 0.02–0.05], CA 0.003/y [CI −0.01–0.02], p = 0.006). In contrast, in the lateral femoral condyle the BMEP score decreased in the African Americans while it slightly increased in the Caucasian Americans (p = 0.009) (Table 5, Figure 2 C). CA subjects showed higher mean meniscus lesions scores form baseline to 48m follow up. The difference was predominantly found and significant in the medial meniscus. There was only a slight progression of meniscus lesions that was not significant in either group. Also the rates of change were not significantly different (p = 0.41) (Table 5, Figure 2 D).
Discussion
The results of this study show that compared to CA, AA cartilage T2 relaxation times averaged over the whole joint and in the medial femoral condyle increased at a significantly faster rate per year, so that while T2 values in the latter were lower at baseline the T2 values of the two groups converged slowly within the observation period. In contrast, the rate of progression of WORMS cartilage lesion scores was not significantly different between the two groups. There were also no differences between groups in the rate of progression in WORMS meniscus or total joint BMEP lesion scores, but BMEP lesions in the MFC increased more in the AA and BMEP lesions in the LFC increased more in the CA women.
To date there is limited knowledge on ethnic differences in cartilage composition with MRI and to the best of our knowledge only one study was performed5. This cross-sectional analysis of baseline data of AA and CA women in the OAI, a subset of whom were also included in this study, showed consistently lower T2 values in AA women in all compartments. Since the results were strictly controlled for the main confounders of age, BMI and KL grade and a sub analysis of compartments without cartilage lesions confirmed the results of lower T2 lesions in AA, the differences in cartilage T2 might reflect racial differences in cartilage composition. Interestingly data from our longitudinal study show that the initially different T2 values converged over time. This effect was due to both the significantly higher rate of increment at baseline and average change over 6 years in AA women and may be indicative for a faster progression of matrix degeneration.
The development of cartilage T2 followed a nonlinear quadratic relationship entering a plateau phase after 4 years while cartilage lesions measured with WORMS progressed constantly during follow up. This ceiling effect has been theorized in a prior study17 that followed the development of cartilage T2 over 2 years and found an inverse relationship between baseline T2 and the slope of T2 progression. The authors hypothesized a saturation effect that might occur after a certain degree of cartilage matrix degeneration prior to the onset of a cartilage lesion. There are other studies supporting this finding e.g. Dunn et al18 found significantly elevated T2 values in subjects with mild knee OA while no significant further increment could be observed from mild to severe OA and a study with a pig model reported a ceiling effect with cartilage maturation followed by a decrease of T2 with further aging19.
While cartilage T2 values increased faster in AA women the progression of structural knee degeneration as expressed with the WORMS score was comparable. This seems to be in contrast to the higher prevalence of knee OA (at least KL 2) in AA women found by Anderson et al.3. However, our study cohort consisted of subjects without definite OA (KL0-1) thus the grade of knee joint degeneration may have been too low and the observation period too short to detect significant differences. A longer follow up observation with more subjects entering the stage of definite radiographic OA is needed to estimate the predictive value of cartilage T2 changes with regard to the onset of definite knee OA in these groups.
There was also a difference in the distribution of lesions within the joint. Meniscus lesions were found to be significantly more frequent in the Caucasian American group while BMEP lesion scores tended to be higher in African Americans. Since potential risk factors for meniscus lesions and higher BMEP such as high physical activity20 (measured with PASE) or obesity21,22 (no significant difference in BMI) were very similar in both groups these factors are not a likely explanation for these differences. Analyzing the distribution of degeneration according to medial and lateral disease we did not find a significant discrepancy between the ethnic groups. Particularly we did not find a predominance of lateral disease in African American knees as described in Braga et al23 who analyzed knee X-rays of more than 3187 participants of the Johnston County osteoarthritis project. The reason for this may be that we included only subjects with no or doubtful signs of radiographic knee OA.
Our study has several limitations: Many subject were lost for the follow-up time points resulting in relatively small groups of 45 subjects per ethnic group at 72 months. This reduces the generalizability of our findings and may have reduced the power of the study leading to significant differences of T2 values only in the average of compartments and the MFC and borderline significant results of T2 measures in the lateral and medial tibia. Moreover the high rate of dropouts could have introduced a selection bias, e.g. changing demographic data or improving T2 values due to potential dropout of the worst cases receiving TKR. However, demographic data were equally distributed among study groups at all time points and no subject of our study cohort received a TKR during the observation period. Moreover structural degeneration proceeded constantly over the 4 observation time points. We used the arithmetic average as a parameter for the T2 cartilage composition of the whole joint. This method gives equal weight to all compartments regardless the size of the cartilage segmentations, thus regions with smaller segmentation size may have been overrepresented in the average value. Since we used the same method in both ethnic groups it should not have biased the differences between groups. The strengths of this study are the one to one matching of the African and Caucasian subjects enabling a thorough comparison between the groups and the follow up regimen with 4 measurements over 6 years that allowed a precise monitoring of the cartilage composition and structural joint degeneration.
In conclusion this study showed that in AA women without definite radiographic OA T2 values increased faster compared to CA women while the progress of cartilage degeneration and joint degeneration measured with WORMS was comparable. The results suggest that T2 values are able to distinguish ethnicity related longitudinal cartilage changes in an early stage before differences of structural joint degeneration appear.
Acknowledgments
The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) with research conducted by the Osteoarthritis Initiative Study Investigators. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants U01-AR059507, P50-AR060752 and R01-AR064771. The study was also funded by a Grant from Beijing High Levels of Health Technical Talent Team of Construction Project (no. 2013-3-033). M.K. received grants from the Gottfried and Julia Bangerter-Rhyner Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions:
Study design: MK, UH, GBJ, MN, CEM, TML
Subject Selection: MK, UH, AY, MS, FL
Image Analysis: MK, AY, UH, MS
Statistical analysis: GBJ, CEM, MK
Interpretation of data: MK, GBJ, MN, CEM, TML
Drafting of Article: MK, TML
Review/revision: MK, AY, UH, MS, FL, GBJ, MN, CEM, TML
Final Approval: MK, AY, UH, MS, FL, GBJ, MN, CEM, TML
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
M. Kretzschmar, Email: martin.kretzschmar@ucsf.edu.
U.R. Heilmeier, Email: ursula.heilmeier@ucsf.edu.
A. Yu, Email: aihongy@gmail.com.
G.B. Joseph, Email: gabby.joseph@ucsf.edu.
F. Liu, Email: fliu@psg.ucsf.edu.
M. Solka, Email: solka.martin@gmail.com.
C.E. McCulloch, Email: cmcculloch@epi.ucsf.edu.
M.C. Nevitt, Email: mnevitt@psg.ucsf.edu.
T.M. Link, Email: thomas.link@ucsf.edu.
References
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940–943. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 4.Jordan JM, Helmick CG, Renner JB, Luta G, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36:809–815. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu A, Heilmeier U, Kretzschmar M, Joseph GB, et al. Racial differences in biochemical knee cartilage composition between African-American and Caucasian-American women with 3Tesla MR-based T2 relaxation time measurements - Data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 7.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–797. vii. doi: 10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70:1884–1886. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carballido-Gamio J, Bauer JS, Stahl R, Lee K-Y, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med. 2010;63:465–472. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Stehling C, Lane NE, Nevitt MC, Lynch J, et al. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungmann PM, Kraus MS, Nardo L, Liebl H, et al. T(2) relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: longitudinal data from the osteoarthritis initiative. J Magn Reson Imaging. 2013;38:1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinar H, Navon G. Multinuclear NMR and microscopic MRI studies of the articular cartilage nanostructure. NMR Biomed. 2006;19:877–893. doi: 10.1002/nbm.1068. [DOI] [PubMed] [Google Scholar]
- 20.Kretzschmar M, Lin W, Nardo L, Joseph GB, et al. Association of physical activity measured by accelerometer, knee joint abnormalities and cartilage T2-measurements obtained from 3T MRI: Data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laberge MA, Baum T, Virayavanich W, Nardo L, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects--data from the Osteoarthritis Initiative. Skeletal Radiol. 2012;41:633–641. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim YZ, Wang Y, Wluka AE, Davies-Tuck ML, et al. Association of obesity and systemic factors with bone marrow lesions at the knee: a systematic review. Semin Arthritis Rheum. 2014;43:600–612. doi: 10.1016/j.semarthrit.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Braga L, Renner JB, Schwartz TA, Woodard J, et al. Differences in radiographic features of knee osteoarthritis in African-Americans and Caucasians: the Johnston county osteoarthritis project. Osteoarthritis Cartilage. 2009;17:1554–1561. doi: 10.1016/j.joca.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]