Abstract
Objective
To assess the value of positive family history (FH) as a risk factor for prostate cancer (PCa) incidence and grade among men undergoing organized PSA-screening in a population-based study.
Patients and Methods
The study cohort comprised all attendees of the Swiss arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) with systematic PSA-tests every 4 years. Men reporting first-degree relative(s) diagnosed with PCa were considered to have a positive FH. Biopsy was exclusively PSA-triggered with a threshold of 3 ng/ml. Primary endpoint was PCa diagnosis. Kaplan-Meier and Cox regression analyses were used.
Results
Of 4,932 attendees with a median age of 60.9 (IQR 57.6–65.1) years, 334 (6.8%) reported a positive FH. Median follow-up duration was 11.6 years (IQR 10.3–13.3). Cumulative PCa incidence was 60/334 (18%, positive FH) and 550/4,598 (12%, negative FH) (OR 1.6, 95% CI 1.2–2.2, p=0.001), respectively. In both groups, most PCa diagnosed had a low grade. There were no significant differences in PSA at diagnosis, biopsy Gleason score or Gleason score on pathologic specimen among men who underwent radical prostatectomy between both groups, respectively. On multivariable analysis, age (HR 1.04, 95% CI 1.02–1.06), baseline PSA (HR 1.13 95% CI 1.12–1.14), and FH (HR 1.6, CI 1.24–2.14) were independent predictors for overall PCa incidence (p<0.0001 each). Only baseline PSA (HR 1.14, 95% CI 1.12–1.16, p<0.0001) was an independent predictor of Gleason score ≥7 PCa on prostate biopsy. The proportion of interval PCa diagnosed in between the screening rounds was non-significantly different.
Conclusion
Irrespective of the FH status, the current PSA-based screening setting detects the majority of aggressive PCa and missed only a minority of interval cancers with a 4-year screening algorithm. Our results suggest that men with a positive FH are at increased risk for low grade but not aggressive PCa.
Keywords: prostate cancer screening; positive family history; prostate cancer aggressiveness; prostate-specific antigen, screening intensity
Introduction
With an annual incidence of 233,000 in the United States [1] and 382,000 in Europe [2] prostate cancer (PCa) presents a major health issue. Population-based screening with prostate-specific antigen (PSA) has been shown to reduce cancer-specific mortality [3]. However, the main drawback of mass screening is the high rate of overdiagnosis of approximately 50% [4]. Overdiagnosis implies that the cancer detected by screening would have never become harmful during a man`s lifetime. One reason for this is the high prevalence of undiagnosed PCa shown in autopsy studies which indicates a partly low-aggressive biology [5]. Consequently, in population-based screening studies, the majority of detected PCa have low risk features [3] most of which harbour a negligible risk to metastasize [6]. Ideally, these low-risk PCa should remain undetected which points at the need of risk-stratified screening [7].
Current urologic guidelines consider family history (FH) a strong risk factor for PCa [8, 9]. Evidence for this comes from a large twin study, showing that a positive FH is an important risk factor for future PCa development, particularly in men who have first-degree relatives affected from PCa [10]. Several other studies have confirmed FH as a risk factor of PCa [11, 12]. However, most data on this topic were collected from population registers before or at the beginning of the PSA-screening era evaluating clinically diagnosed PCa while nowadays, opportunistic PSA-screening is increasing in Western countries [13, 14]. This in turn, has led to a shift towards more localized disease and therefore dramatically changed the face of PCa in terms of a sharp increment in incidence and stage migration over the past decades [15].
There is controversial data on whether FH has an effect on cancer aggressiveness [16]. For instance, FH was an independent predictor for biochemical relapse only in the early PSA era whereas men diagnosed later on presented with more favourable cancer characteristics [17]. Moreover, the relative risk for men with a FH for PCa decreased throughout the pre-PSA era suggesting a stage migration [18].
Even more importantly, a PCa diagnosis raises the awareness of the disease in family members as well as GPs of the index patients and thereby may expose male relatives to increased PSA-testing and subsequent prostate biopsy [19]. In this regard, it is noteworthy that FH is incorporated in current available risk calculators [20].
We hypothesize that due to the increasing screening attitude with detection of particularly low-risk disease at an earlier stage, FH might have a reduced effect on aggressive PCa incidence.
The particular strength of the current study is a uniform screening protocol for every attendee where decision for prostate biopsy was based exclusively upon PSA-values without any accounting for FH status, thus eliminating any possible biases towards this particular risk factor.
Materials and Methods
This study was conducted within the European Randomized Study of Screening for Prostate Cancer (ERSPC) – Switzerland. The study protocol and the population have been described previously [21]. From September 1998 to August 2003, 10,311 Swiss men aged 55–70 years were randomized 1:1 to the screening or control group, respectively. Randomization was done after informed consent. From a total of 5,129 eligible men randomized to the screening arm, 4,932 (96.2%) men underwent baseline PSA-screening and were included for further analysis. In accordance with the main protocol of the ERSPC [22] a 6 core transrectal ultrasound guided lateralized prostate biopsy (or 8 core if prostate volume was >40cc) was performed if the PSA-value was ≥3.0 ng/ml. Biopsy was exclusively PSA-driven as per study protocol and not performed upon positive FH. PSA-screening was continued every four years until the age of 75. In a side study, men with baseline PSA of 1–3 ng/ml and free-to-total ratio ≤20% were also offered prostate biopsy at baseline (1998–2003) [23]. We also analyzed the rate of cancers emerging clinically between the screening visits. These PCa were diagnosed outside the screening protocol either by opportunistic screening, by transurethral resection of the prostate (TUR-P) or clinically, when organized PSA-screening missed the diagnosis. This type of cancer was termed "interval PCa". PCa risk was stratified according to the D’Amico classification [24]. Aggressive PCa was defined as Gleason score ≥ 7 PCa.
All prostate biopsies were externally reviewed by an experienced uro-pathologist at the University Hospital Basel, Switzerland. Through periodic linkage of all men with the cancer registries, complete information on cancer incidence was obtained until December 2012. Several committees of the ERSPC accounted for the surveillance and quality of the data such as Epidemiology Committee, Pathology Committee, PSA Committee, Quality Control Committee, Causes of Death Committee with an independent Data Monitoring Committee. The Scientific Committee had access to the data and kept overview at any time [22]. The study protocol was approved by the local ethical committee.
Statistics
Comparisons between patient characteristics for men with positive and negative FH were made using the Chi square test for proportions and Mann-Whitney U-test for continuous variables. Univariable and multivariable Cox regression analysis was used to examine the relationship between FH and time to PCa diagnosis during follow-up, with age, FH, International Prostate Symptom Score (IPSS) and baseline PSA-value as covariates. Kaplan-Meier curves were used to estimate the cancer-free survival function.
The Statistical Package for Social Sciences (SPSS) version 20 (IBM Corporation, NY, USA) was used. All tests were two-sided with a significance level set at 0.05.
Self-reported data
A standardized, non-validated questionnaire including FH on first and second-degree relatives affected from PCa including also the (IPSS) amongst other parameters was mailed prior to PSA-testing to all attendees. In addition at each screening visit, all attendees underwent a structured personal interview by a trained study nurse who verified the reported data. Men who reported one or more first-degree relative(s) (father or brother) diagnosed with PCa were considered as having a positive FH.
Results
Clinical characteristics of 4,932 attendees at baseline
All men gave information about their relatives (response rate 100%). Overall, 334 (6.8%) reported a positive FH. Most men had their father affected, n=242 (72.5%) while n=70 (21%) had brother(s) affected from the disease (table 1). Interestingly, the baseline IPSS score was higher in men with a positive FH (median 6 [IQR 3–10] vs. 5 [3–9], p<0.0001) (table 2). PSA at baseline was comparable between both groups. PSA-velocity at follow-up visit after 4 years was higher in men with positive FH (0.32 ng/ml/year vs 0.19 ng/ml/year, p=0.05). This difference disappeared during follow-up visit 8 years from baseline (0.07 ng/ml/year vs. 0.06 ng/ml/year; p=0.8). When PSA converted to values ≥3ng/ml, biopsy compliance was comparable between both groups (77.8% [negative FH] vs. 77.3% [positive FH], respectively).
Table 1.
Distribution of family history among n=4,932 study attendees
| Family History | n | % | |
|---|---|---|---|
| Negative FH group (overall) | 4,598 | 93.2% | |
| First degree-realtive affected | 334 | 6.8% | |
| Constellation of affected first degree-relative(s) | |||
| One brother affected | 65 | 19.5% | |
| Several brothers affected | 5 | 1.5% | |
| Only father affected | 242 | 72.5% | |
| Father and brother(s) affected | 8 | 2.4% | |
| Father and grandfather affected | 14 | 4.2% | |
Table 2.
Clinical characteristics of 4,932 men stratified according to family history
| Variable | pos FH | neg FH | p-value |
|---|---|---|---|
| Subjects, No. (%) | 334 (6.8) | 4,598 (93.2) | - |
| Age at baseline, years | |||
| median [IQR] |
60.8 [57.1 – 64.4] |
61.0 [57.6 – 65.2] |
0.07 |
| PSA at baseline, ng/ml, median [IQR] |
1.13 [0.57 – 2.23] |
1.01 [0.57 – 1.87] |
0.3 |
| PSA-Conversion rate to ≥3ng/ml 4 years from baseline, No (%) | 54 (20.2) | 776 (19.4) | 0.7 |
| PSA-Conversion rate to ≥3ng/ml 4 to 8 years from baseline, No (%) | 57 (27.4) | 680 (22.4) | 0.1 |
| PSA-velocity 4 years from baseline (ng/ml/year) median [IQR] |
0.32 [−0.02 – 0.84] |
0.19 [−0.05 – 0.67] |
0.05 |
| PSA-velocity 8 years from baseline (ng/ml/year) median [IQR] |
0.07 [−0.01 – 0.2] |
0.06 [0.01 – 0.2] |
0.8 |
| IPSS* at baseline median [IQR] |
6 [3–10] |
5 [3–9] |
<0.0001 |
| Cumulative detection rate of prostate cancer, No. (%) | 60 (18.0) | 550 (12.0) | 0.001 |
| PSA at diagnosis, ng/ml, No. (%) | |||
| median [IQR] |
4.70 [3.50 – 6.88] |
4.55 [3.48 – 7.28) |
0.1 |
| Age at diagnosis, years | |||
| median [IQR] |
66.0 [62.3 – 69.0] |
67.0 [63.0 – 70.0] |
0.1 |
| 50 – 59, No. (%) | 9 (15.0) | 47 (8.5) | |
| 60 – 69, No. (%) | 39 (65.0) | 341 (62.0) | |
| ≥ 70, No. (%) | 12 (20.0) | 162 (29.5) | |
IPSS = International Prostate Symptom Score
In 122 men IPSS questionnaire could not be evaluated (neurogenic bladder dysfunction, diuretics, etc.)
PCa incidence and cancer characteristics among men with positive vs. negative FH
During a median follow-up duration of 11.6 years (IQR 10.3–13.3), more men with positive FH (18.0%) had been diagnosed with PCa as compared to men with negative FH (12.0%) (unadjusted OR 1.61, 95% CI 1.20–2.16; p=0.001). In case of PCa diagnosis, age at diagnosis and Gleason score were not significantly different between both groups (table 2 and 3).
Table 3.
Unadjusted risk for PCa, aggressive PCa, all-cause mortality and PCa death among 4932 men stratified by family history status
| Positive FH (n = 334) |
Negative FH (n = 4,598) |
OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| PCa, No (%) | 60 (18.0) | 550 (12.0) | 1.61 | 1.20–2.16 | 0.001 |
| Interval PCa | 14 (4.2) | 142 (3.1) | 1.46 | 0.83–2.56 | 0.2 |
| Aggressive* interval PCa | 6 (42.9) | 61 (43.0) | 1.00 | 0.33–3.02 | 0.99 |
| > T2 PCa | 0 (0) | 5 (0.1%) | 0.93 | 0.92–0.94 | 0.5 |
| Aggressive PCa*, No (%) | 17 (5.1) | 183 (4.0) | 1.30 | 0.77–2.15 | 0.3 |
| Deaths from PCa, No (%) | 3 (0.9) | 14 (0.3) | 2.97 | 0.85–10.38 | 0.07 |
| Overall deaths, No (%) | 40 (12.0) | 526 (11.4) | 1.05 | 0.75–1.48 | 0.8 |
Aggressive was defined as Gleason score ≥ 7.
Of 334 men with positive FH, 17 (5.1%) were found to have aggressive disease defined as a Gleason score of >=7 as compared to 183 of 4,598 (4.9%) men with negative FH (OR 1.30; 95% CI 0.77–2.15; p=0.3) (table 3). Seven (1.3%) men with negative FH were found to have metastasis at the time of diagnosis (none in the positive FH group). Three of 334 (0.9%) and 14 (0.3%) of 4,598 died from PCa during follow-up (OR 2.97; 95% CI 0.85–10.38, p=0.07). In case of radical prostatectomy (n=334), Gleason score, T-stage and frequency of positive lymph nodes were not significantly different between both groups (table 4).
Table 4.
Pathologic characteristics of 334 men (38 of positive FH group and 296 of negative FH group) detected with PCa after radical prostatectomy
| Variable | positive FH (overall PCa n=60) |
negative FH (Overall PCa n=550) |
overall | p-value | |
|---|---|---|---|---|---|
| Subjects, No. (%) | 38 (100) | 296 (100) | 334 | - | |
| Gleason score | |||||
| 3+3 | 18 (47.4) | 146 (49.3) | 164 | 0.8 | |
| 3+4 | 11 (28.9) | 84 (28.4) | 94 | ||
| 4+3 | 5 (13.2) | 33 (11.1) | 38 | ||
| ≥ 4+4 | 2 (5.3) | 28 (9.5) | 30 | ||
| T-stage | |||||
| pT2 | 31 (81.6) | 244 (82.4) | 275 | ||
| ≥ pT3 | 7 (18.4) | 52 (17.6) | 59 | 0.9 | |
| N-stage | |||||
| pN + | 2 (5.3) | 11 (3.7) | 13 | ||
| pN0 | 32 (84.2) | 248 (83.8) | 0.9 | ||
| pNx | 4 (10.5) | 37 (12.5) | |||
Of 5 men RP Gleason score was not available (negative FH)
Of 2 men RP Gleason score was not available (positive FH)
Incidence of interval PCa
Interval PCa were diagnosed outside the screening protocol either by opportunistic screening, by TUR-P or clinically, when organized PSA-screening missed the diagnosis. During the entire follow-up period, 156 cancers (3.2%) surfaced in between the screening rounds or after termination of screening due to age >75 years and were therefore termed interval cancer. Of those, 14 (4.2%) PCa emerged among men with positive FH and 142 (3.1%) among men with negative FH, respectively (OR 1.46, 95% CI 0.83–2.56; p=0.2) (table 3). Biopsy Gleason scores of interval PCa were 3+3 (n=8 [57.1%]), 3+4 (n=5 [35.7%]), 4+3 (n=1 [7.1%]), ≥4+4 (n=0[0%]) (positive FH) and 3+3 (n=79 [55.6%]), 3+4 (n=31 [21.8%]), 4+3 (n=6 [4.2%]) and ≥4+4 (n=24 [16.9%] (negative FH), respectively (table 3). For 2 men, Gleason score could not be classified as diagnosis was achieved by clinical symptoms and PSA >100ng/ml.
Risk factor analysis for the indidence of PCa
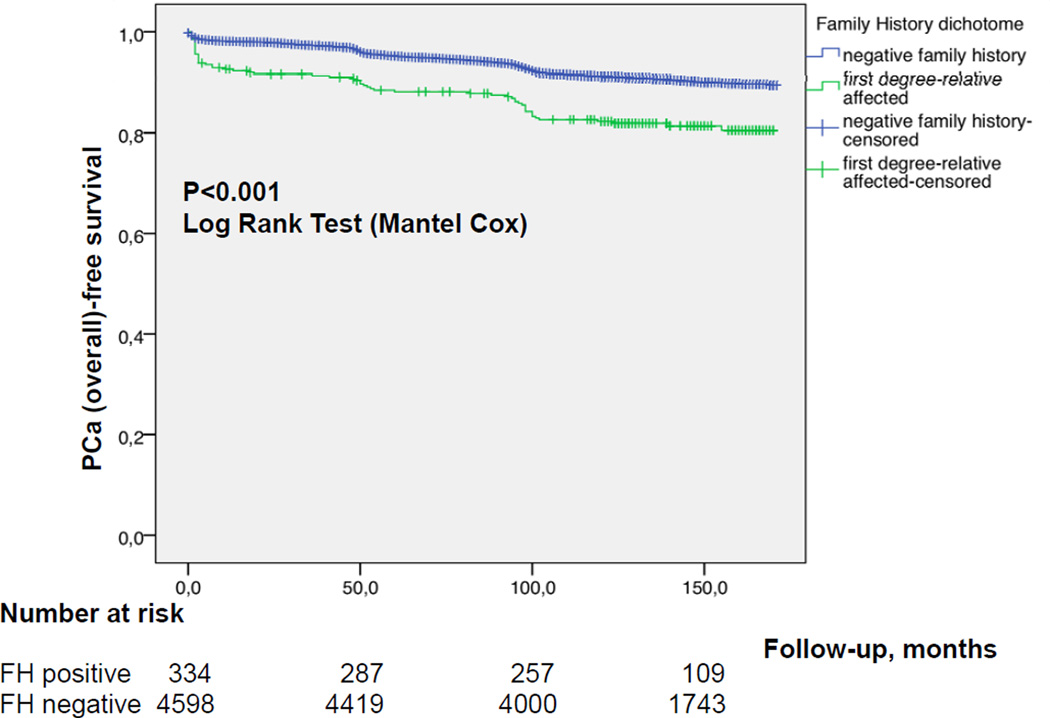
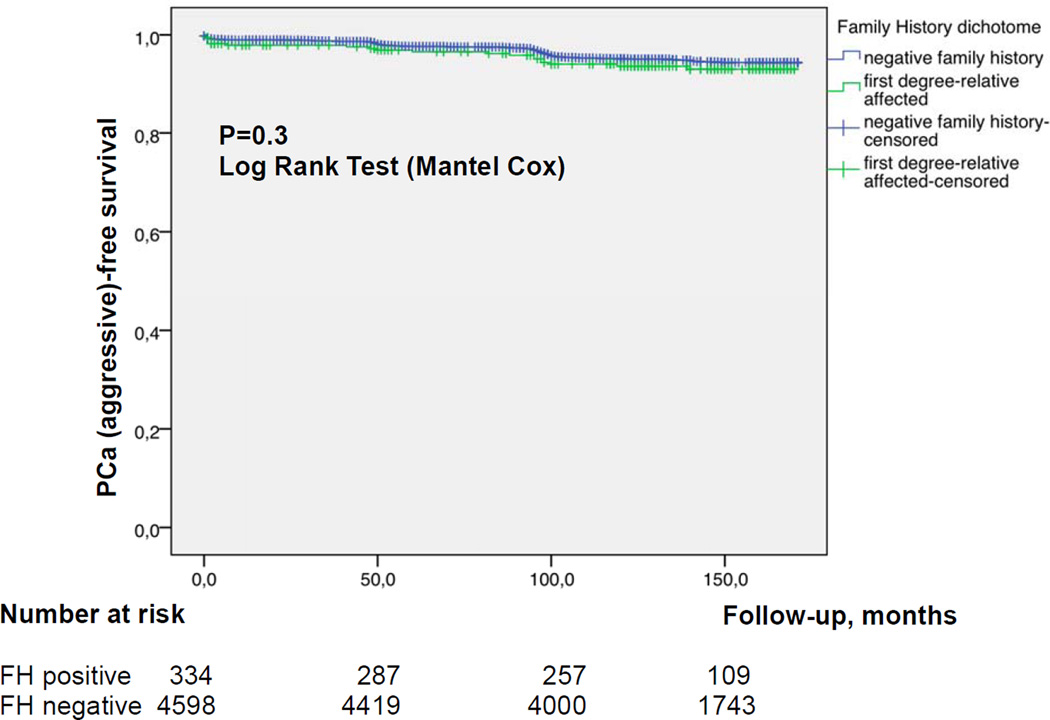
Figures 1a and b show the cumulative incidence of overall (a) and aggressive (b) PCa, respectively, stratified by FH status. In multivariable analysis age, PSA and positive FH at baseline were all strong independent predictors for overall PCa detection during follow-up (Table 5a; p<0.0001 each). However, for time to aggressive PCa, only PSA at baseline (p<0.0001), but not FH (p=0.1) remained an independent predictor (Table 5b).
Figure 1.
a Kaplan Meier estimate for PCa-free survival comparing positive vs negative FH b Kaplan Meier estimate for aggressive PCa-free survival comparing positive vs negative FH
Table 5.
| a. Uni- and multivariable Cox proportional hazard analysis of predictors for overall PCa incidence | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Baseline Characteristics | ||||||
| Age, years | 1.05 | 1.03 – 1.07 | < 0.0001 | 1.04 | 1.02 – 1.06 | < 0.0001 |
| PSA, ng/ml | 1.13 | 1.12 – 1.14 | < 0.0001 | 1.13 | 1.12 – 1.14 | < 0.0001 |
| Family History | 1.58 | 1.21 – 2.07 | 0.001 | 1.63 | 1.24 – 2.14 | < 0.0001 |
| IPSS | 1.02 | 1.01–1.04 | 0.002 | 1.01 | 0.99 – 1.02 | 0.3 |
| b. Uni- and multivariable Cox proportional hazard analysis of predictors for aggressive* PCa incidence | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Baseline Characteristics | ||||||
| Age, years | 1.04 | 1.01 – 1.07 | 0.003 | 1.03 | 1.00 – 1.07 | 0.06 |
| PSA, ng/ml | 1.14 | 1.13 – 1.16 | < 0.0001 | 1.14 | 1.12 – 1.16 | < 0.0001 |
| Family History | 1.30 | 0.82 – 2.06 | 0.26 | 1.52 | 0.92 – 2.51 | 0.1 |
| IPSS | 1.00 | 0.97–1.03 | 0.9 | 0.98 | 0.95 –1.01 | 0.2 |
Aggressive PCa was defined as Gleason score ≥ 7
Discussion
In the current study, men with positive FH had a higher unadjusted risk for overall PCa diagnosis as compared to men with negative FH (OR 1.61, 95% CI 1.20–2.16; p=0.001). The PCa detection rate among men with FH is comparable to previous reports although considerably below the calculated risk of former epidemiological studies of more than twice the risk [25]. The overall prevalence of FH of 6.8% in the Swiss cohort is in line with other series and appears to be representative [26–28]. When we adjusted for other parameters, a positive FH remained an independent predictor for overall, but not aggressive PCa. Importantly, the frequency of "interval PCa” indicating a more aggressive cancer biology was only slightly but not significantly higher in men with positive FH (4.2% vs 3.1%, OR 1.46; 95% CI 0.83–2.56, p=0.2 respectively). Thus, systematic PSA-screening with a 4-year algorithm seems to detect potentially aggressive PCa at an earlier stage. Without this organized screening schedule, we might expect more aggressive cases among men with positive FH as compared to negative FH as it was demonstrated in studies before the screening era [18]. However, the current screening attitude in the western world clearly drifts towards opportunistic screening with early retest intervals far below 4 years [13]. This intensive screening strategy seems to weaken the real predictive effect of a first-degree relative affected from PCa.
Meta-analysis support the fact of having a first-degree relative diagnosed with PCa to be a significant risk factor for future PCa development to the index patient [29, 30]. This effect was particularly true for (clinically diagnosed) disease before the PSA-screening era. Reported risk ratios varied from 2.5 to 3.4 times as compared to men with negative FH with a greater number of family members affected or younger age at diagnosis increasing this risk even further [12]. Basically, a positive FH seems therefore to be a key factor to identify those individuals who have inherited predisposition to PCa and the goal of FH inventory is to provide enough information for a risk assessment with respect to further investigations. However, the predominant part of the underlying data of those studies was gathered before or at the beginning of the PSA era. Having said that, some noteworthy contemporary studies have failed to reproduce the correlation between a positive FH and PCa aggressiveness [31]; for instance, the Prostate Cancer Prevention Trial (PCPT) found PSA-levels, digital-rectal examination and previous biopsy but not positive FH as significant predictors for high grade disease despite a very high rate of positive FH of 17% [20]. However, a limitation of PCPT was the high rate of positive FH and the randomization of men with exclusively “low-risk” baseline PSA-values less or equally to 3ng/ml which does not reflect the characteristics of the general population. In another study by Roehl et al., clinicopathologic features and 7-year progression-free survival were similar between sporadic and familial PCa cases after surgery [32]. Likewise, Siddiqui et al. found equivalent long-term oncological outcomes in patients with familial, hereditary and sporadic PCa after radical prostatectomy [33].
With the growing frequency of opportunistic PSA-based screening worldwide [13, 14, 34], the PCa incidence increased rapidly and most of PCa are nowadays detected at an earlier stage and lower grade [15]. Mass screening is associated with an overdiagnosis rate of roughly 50% [4]. Every overdiagnosed PCa in turn induces a switch from negative to “falsely-positive” FH in relatives, which seems to dilute the true effect of FH on the biological aggressiveness of PCa. The results of the current study underline this effect. Whereas in early days, the odds for PCa was 2.5 to 3-fold higher in men having a 1st degree relative affected from PCa [12], it dropped to 1.6 in our trial or 1.3 in the Finnish arm of the ERSPC [28]. This slightly lower RR of 1.3 as compared to the current study can be explained by less intensive screening protocol in Finland as men in range 3–4 ng/ml underwent prostate biopsy only if free-to-total PSA ratio was less than 16%. There are still true hereditary PCa having shorter lead-time and an increased risk of developing an aggressive disease [35, 36]. However, in population-based studies, this proportion represents only a minority of all PCa diagnosed. Additionally, the only slightly higher percentage of aggressive PCa in the current study of 5.1% (positive FH) versus 4.0% (negative FH) suggests that at least a part of these cancers among men with a positive FH were detected in their pre-clinical development phase which is actually one of the very purposes of the screening intervention.
While FH should still be considered as a risk factor in daily practice, the assessment should become much more sophisticated and detailed, including the exact origins of diagnosis, that is whether prostate cancer in relatives has been detected by PSA-screening or clinically; a detailed analysis of tumor characteristics; the exact age at diagnosis of the first degree relative affected etc.
Obviously, PCa diagnosis raises the awareness of the disease in family members as well as their GPs and thereby exposes male relatives to increased PSA testing and subsequent prostate biopsy. Also, a higher socioeconomic status was shown to be associated with detection of more localized but not metastatic PCa [19]. Thus, a percentage of men with a positive FH diagnosed with PCa are explained by increased screening behaviour.
There are several limitations in our study: First, we did not have complete data on tumour characteristics among affected relatives, nor had we information on the three-generation pedigree for more detailed FH evaluation. Second, we collected data on FH at the study entrance, but this might have changed slightly over time. Therefore, there might potentially be more men with a positive FH during follow-up. However, if so, this would weaken the impact of positive FH even more. Finally, we have a relatively small sample size as far as aggressive PCa is considered.
It would be of interest to compare those with a positive FH undergoing screening to those with a positive FH not undergoing screening (the control group of ERSPC). However, in order to prevent any PSA contamination data on FH was not collected in the control group of our trial so this interesting question cannot be answered.
In conclusion, although our study has confirmed FH as a risk factor for PCa diagnosis, most of these men were classified as having low-risk disease in this contemporary population-based screening cohort. A PSA-based screening setting detects the majority of aggressive PCa and missed only a minority of interval cancers with a 4-year screening algorithm. FH as a risk factor might therefore rather be used complementary to risk factors such as prostate volume, baseline PSA and age. In order to perform a more personalized screening for PCa, information on a positive FH might need to be obtained including complete information on cancer characteristics of the relative.
Acknowledgments
We thank Professor Lukas Bubendorf, Institute of Pathology, University Hospital Basel, Switzerland, for reviewing all prostate biopsies.
Financial support ERSPC Switzerland:
The Horten Foundation, Aargau Cancer League, Swiss Cancer League (Grant Nr KFS 787-2-1999 and 01112-02-2001), Health Department of Canton Aargau, Prostate Cancer Research Foundation, Baugarten Foundation, Miss Messerli Foundation, Switzerland. Sigrid Carlsson is supported by a grant of AFA Insurance.
Conflict of interest:
Maciej Kwiatkowski is member of the advisory board at Astellas and has held a lecture at Myriad Genetics.
Footnotes
Ethical standards:
The study has been approved by the appropriate ethics committee
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014 Jan-Feb;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. European journal of cancer. 2010 Mar;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 Dec 6;384:2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003 Jun 18;95:868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 5.Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-Specific Antigen-Era. Int J Cancer. 2015 Dec 15;137:2795–2802. doi: 10.1002/ijc.29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross HM, Kryvenko ON, Cowan JE, Simko JP, Wheeler TM, Epstein JI. Do adenocarcinomas of the prostate with Gleason score (GS)</=6 have the potential to metastasize to lymph nodes? The American journal of surgical pathology. 2012 Sep;36:1346–1352. doi: 10.1097/PAS.0b013e3182556dcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randazzo M, Beatrice J, Huber A, et al. Is further screening of men with baseline PSA< 1 ng ml worthwhile? The discussion continues-Results of the Swiss ERSPC (Aarau) Int J Cancer. 2015 Jan 6; doi: 10.1002/ijc.29420. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich ABP, Bellmunt J. Guidelines on Prostate Cancer. 2013 http://www.uroweb.org/guidelines/online-guidelines/?no_cache=1. [Google Scholar]
- 9.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013 Aug;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000 Jul 13;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 11.Frank C, Fallah M, Ji J, Sundquist J, Hemminki K. The population impact of familial cancer, a major cause of cancer. Int J Cancer. 2014 Apr 15;134:1899–1906. doi: 10.1002/ijc.28510. [DOI] [PubMed] [Google Scholar]
- 12.Brandt A, Bermejo JL, Sundquist J, Hemminki K. Age-specific risk of incident prostate cancer and risk of death from prostate cancer defined by the number of affected family members. European urology. 2010 Aug;58:275–280. doi: 10.1016/j.eururo.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Nordstrom T, Aly M, Clements MS, Weibull CE, Adolfsson J, Gronberg H. Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003–2011. European urology. 2013 Mar;63:419–425. doi: 10.1016/j.eururo.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Howard K, Brenner AT, Lewis C, et al. A comparison of US and Australian men's values and preferences for PSA screening. BMC health services research. 2013;13:388. doi: 10.1186/1472-6963-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass AS, Cowan JE, Fuldeore MJ, et al. Patient demographics, quality of life, and disease features of men with newly diagnosed prostate cancer: trends in the PSA era. Urology. 2013 Jul;82:60–65. doi: 10.1016/j.urology.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 16.Selkirk CG, Wang CH, Lapin B, Helfand BT. Family history of prostate cancer in men being followed by active surveillance does not increase risk of being diagnosed with high-grade disease. Urology. 2015 Apr;85:742–747. doi: 10.1016/j.urology.2014.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Kupelian PA, Reddy CA, Reuther AM, Mahadevan A, Ciezki JP, Klein EA. Aggressiveness of familial prostate cancer. J Clin Oncol. 2006 Jul 20;24:3445–3450. doi: 10.1200/JCO.2006.05.7661. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007 Oct 1;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bratt O, Garmo H, Adolfsson J, et al. Effects of prostate-specific antigen testing on familial prostate cancer risk estimates. Journal of the National Cancer Institute. 2010 Sep 8;102:1336–1343. doi: 10.1093/jnci/djq265. [DOI] [PubMed] [Google Scholar]
- 20.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. Journal of the National Cancer Institute. 2006 Apr 19;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 21.Kwiatkowski M, Huber A, Stamm B, et al. Features and preliminary results of prostate cancer screening in Canton Aargau, Switzerland. BJU international. 2003 Dec;92(Suppl 2):44–47. doi: 10.1111/j.1465-5101.2003.04395.x. [DOI] [PubMed] [Google Scholar]
- 22.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012 Mar 15;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Recker F, Kwiatkowski MK, Huber A, Stamm B, Lehmann K, Tscholl R. Prospective detection of clinically relevant prostate cancer in the prostate specific antigen range 1 to 3 ng./ml. combined with free-to-total ratio 20% or less: the Aarau experience. J Urol. 2001 Sep;166:851–855. [PubMed] [Google Scholar]
- 24.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 25.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. The New England journal of medicine. 2010 Apr 1;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med. 2006 Sep;8:571–575. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai PL, Wideroff L, Greene MH, Graubard BI. Prevalence of family history of breast, colorectal, prostate, and lung cancer in a population-based study. Public Health Genomics. 2010;13:495–503. doi: 10.1159/000294469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saarimaki L, Tammela TL, Maattanen L, et al. Family history in the Finnish Prostate Cancer Screening Trial. Int J Cancer. 2015 May 1;136:2172–2177. doi: 10.1002/ijc.29243. [DOI] [PubMed] [Google Scholar]
- 29.Bruner DW, Moore D, Parlanti A, Dorgan J, Engstrom P. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int J Cancer. 2003 Dec 10;107:797–803. doi: 10.1002/ijc.11466. [DOI] [PubMed] [Google Scholar]
- 30.Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003 Jun;91:789–794. doi: 10.1046/j.1464-410x.2003.04232.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JA, 2nd, Gerber L, Moreira DM, et al. Prostate cancer risk in men with prostate and breast cancer family history: results from the REDUCE study (R1) Journal of internal medicine. 2012 Jul;272:85–92. doi: 10.1111/j.1365-2796.2011.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roehl KA, Loeb S, Antenor JA, Corbin N, Catalona WJ. Characteristics of patients with familial versus sporadic prostate cancer. The Journal of urology. 2006 Dec;176:2438–2442. doi: 10.1016/j.juro.2006.07.159. discussion 42. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui SA, Sengupta S, Slezak JM, Bergstralh EJ, Zincke H, Blute ML. Impact of familial and hereditary prostate cancer on cancer specific survival after radical retropubic prostatectomy. The Journal of urology. 2006 Sep;176:1118–1121. doi: 10.1016/j.juro.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 34.Van der Meer S, Lowik SA, Hirdes WH, et al. Prostate specific antigen testing policy worldwide varies greatly and seems not to be in accordance with guidelines: a systematic review. BMC family practice. 2012;13:100. doi: 10.1186/1471-2296-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grin B, Loeb S, Roehl K, Cooper PR, Catalona WJ, Helfand BT. A rare 8q24 single nucleotide polymorphism (SNP) predisposes North American men to prostate cancer and possibly more aggressive disease. BJU international. 2015 Jan;115:101–105. doi: 10.1111/bju.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beebe-Dimmer JL, Isaacs WB, Zuhlke KA, et al. Prevalence of the HOXB13 G84E prostate cancer risk allele in men treated with radical prostatectomy. BJU international. 2014 May;113:830–835. doi: 10.1111/bju.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]