Abstract
Objective
To identify the patients at greatest odds for SIRS and examine the association between Systemic Inflammatory Response Syndrome (SIRS) and outcomes in patients presenting with intracerebral hemorrhage (ICH).
Methods
We retrospectively reviewed consecutive patients presenting to a tertiary care center from 2008-2013 with ICH. SIRS was defined according to standard criteria as 2 or more of the following: (1) body temperature < 36°C or >38°C, (2) heart rate > 90 beats per minute, (3) respiratory rate > 20, or (4) white blood cell count < 4000/mm3 or > 12,000/mm3 or > 10% polymorphonuclear leukocytes for >24 hours in the absence of infection. The outcomes of interest, discharge modified-Rankin Scale (mRS 4-6), death, and poor discharge disposition (discharge anywhere but home or inpatient rehab), were assessed using logistic regression.
Results
A total of 249 ICH patients met inclusion criteria and 53 (21.3%) developed SIRS during their hospital stay. A score was developed (ranging from 0-3) to identify patients at greatest risk for developing SIRS. Adjusting for stroke severity, SIRS was associated with mRS 4-6 (OR 5.25, 95%CI 2.09-13.2) and poor discharge disposition (OR 3.74, 95%CI 1.58-4.83), but was not significantly associated with death (OR 1.75, 95%CI 0.58-5.32). We found that 33% of the effect of ICH score on poor functional outcome at discharge was explained by the development of SIRS in the hospital (Sobel 2.11, p=0.03).
Conclusion
We observed that approximately 20% of patients with ICH develop SIRS, and that patients with SIRS were at increased risk of having poor functional outcome at discharge.
Keywords: Intracerebral Hemorrhage, Stroke, Inflammation, Epidemiology, Systemic Inflammatory Response Syndrome
Introduction
Complications after intracerebral hemorrhage (ICH) contribute to the high morbidity and mortality of this condition.(1) While recent studies have identified post-ICH infectious complications as an important contributor to death and disability following ICH,(2) no studies have investigated the role of the systemic inflammatory response syndrome (SIRS) in the absence of a clinically defined infection on ICH outcome. SIRS is a systemic reaction to a stimulus (e.g., trauma, surgery) characterized by the absence of infection and the presence of 2 or more of the following: hypothermia or hyperthermia, leukocytosis or leukopenia, tachycardia, or tachypnea.(3)
SIRS without infection has been previously described in acute cerebral injury.(4) Stroke can generate an initial inflammatory response, further complicated by inflammation resulting from reperfusion injury.(5-9) Moreover, SIRS has been recognized as a risk factor for poor outcomes in patients with non-neurological critical illness, ischemic stroke, and subarachnoid hemorrhage.(4, 10, 11) In addition to the relationship with functional outcomes illustrated in other stroke types, SIRS has been shown to be related to stroke severity in subarachnoid hemorrhage patients,(4) as well as to infarct volume in acute ischemic stroke patients.(12)
It remains unclear which patients are at greatest risk for developing SIRS after an ICH, and the relationship between SIRS during hospitalization and ICH outcomes is unknown. We created a SIRS prediction score to identify those ICH patients at greatest risk for developing SIRS, explored the relationship between stroke severity and development of SIRS, and described the influence SIRS during hospitalization has on short-term ICH functional outcome.
Methods
Study Setting and Inclusion/Exclusion Criteria
This study was conducted with approval of the Institutional Review Board. We conducted a retrospective review of consecutive patients presenting to our center from 2008-2013 with non-traumatic ICH. As the focus of this paper was spontaneous ICH in adults, patients aged 19 and older were included. Patients who were enrolled in a non-observational clinical study, had a subarachnoid hemorrhage, subdural hemorrhage, epidural hemorrhage, traumatic ICH, underlying vascular malformation, or hemorrhagic conversion after an ischemic stroke were excluded. Enrollment in a clinical trial can affect outcomes, therefore patients who were enrolled in a non-observational clinical study were excluded. In the primary analyses patients who were placed on hospice, palliative care, or were clinically diagnosed with an infection at any time during their stroke hospitalization were excluded. Early care limitations have been associated with short and long term mortality.(13, 14) As this population has such an increased risk of morbidity and mortality we did not include them in the sample as to not bias the population. Infection was defined as a diagnosis of a clinical infection during the hospital course as described in the electronic medical record. We initially excluded patients with a diagnosis of an infection as we sought to assess the influence of the isolated inflammatory response on stroke outcomes. In a secondary analysis, we included patients with infection to assess the independent effects of infection, SIRS, and SIRS plus infection on short-term functional outcome in ICH patients.
Data Collection and Variable Definition
All data collection was done through retrospective chart review by the study physicians. Demographic and clinical data collected included past medical history, imaging, and laboratory data. In-hospital treatments were documented and outcomes at discharge were assessed. Stroke severity was defined as the ICH score that was calculated on admission for each patient.(15) This score is a standard score of ICH severity with points assigned for Glasgow Coma Scale (GCS), age over 80, ICH volume greater than 30 ml, the presence of intraventricular hemorrhage, and infratentorial origin of hemorrhage.(15) The National Institutes of Health Stroke Scale (NIHSS) and GCS were obtained on admission as part of standard of care.
SIRS during hospitalization was defined according to standard criteria as having 2 or more of the following: (1) body temperature less than 36°C or greater than 38°C, (2) heart rate greater than 90 beats per minute, (3) respiratory rate greater than 20 breaths per minute, or (4) white blood cell count less than 4000/mm3 or greater than 12,000/mm3 or more than 10% polymorphonuclear leukocytes for at least 24 hours.(3, 11) We used a strict timeframe criterion to identify those patients most likely to be experiencing an inflammatory response and to prevent misclassification of patients. SIRS was considered present if the necessary criteria persisted for at least 24 hours at any time during the hospitalization. We classified SIRS on admission as being SIRS criteria met transiently with the initial ED labs and vitals. This was a more transient definition without the strict timeframe criterion.
The primary outcome was poor functional outcome at discharge, defined as a modified-Rankin Scale (mRS) score of 4-6.(16) Secondary outcomes were unfavorable discharge disposition and death. Unfavorable discharge disposition was defined as discharge anywhere but to home or an inpatient rehabilitation hospital. Death was defined as death during the hospitalization with patients who were withdrawn from full support excluded.
Statistical Analyses
The χ2 test of independence was used to assess group differences for baseline demographic categorical variables and Wilcoxon Rank Sum for continuous variables between patients with SIRS and those without SIRS. A prediction model was designed to estimate which patients would develop SIRS. All demographic, clinical, and laboratory variables available at the time of admission were examined, using logistic regression models in which the outcome was development of SIRS. Variables with p-values ≤ 0.2 were retained in the final model. Receiver operating characteristic (ROC) curves were used to evaluate continuous variables. Sensitivities were calculated to investigate the best way to categorize continuous variables. Model performance was assessed using the C-statistic, corrected for optimism.(17) To correct for optimism, reduce bias, and internally validate the model, we calculated the estimated decrease in the C-statistic that would be expected in an independent dataset using the 0.632 bootstrap method.(18) The points assigned to the variables in the score were determined using the beta coefficients from the final multivariable logistic regression model. Spearman correlation and adjusted ROC curves were used to evaluate the final score. A cut point of the SIRS prediction model was established based on sensitivity and specificity of the dichotomized score in predicting which patients developed SIRS during hospitalization.
Crude and adjusted logistic regression models were used to estimate the odds ratios and 95% confidence intervals (OR, 95% CI) for the relationship between SIRS and the outcomes of interest. A sub-analysis was conducted assessing the relationship between infection, SIRS, and SIRS plus infection on the outcomes of interest. In the sub analysis four categories were created (1) patients without an infection or SIRS, (2) patients with just an infection, (3) patients with just SIRS, and (4) patients who experienced both SIRS and an infection, although it may not have been at the same time during their hospitalization. A univariable analysis was conducted assessing the relationship between these categories and poor functional outcome using the first group (no infection/no SIRS) as a reference group. As this was an exploratory analysis, no adjustments were made for multiple comparisons.(19) An alpha of 0.05 was set as the level of significance.
Mediation Analysis
A mediation analysis using the Sobel test was performed to assess the complex relationships between ICH score on admission, development of SIRS, and poor functional outcome at discharge. The Sobel test, which is capable of handling dichotomous mediators and outcomes, was used to test whether the indirect effect of ICH score on poor functional outcome at discharge through the mediator (SIRS during hospitalization) was significantly different than zero.(20, 21)
Results
Of 384 consecutive patients who presented with a ICH, a total of 249 ICH patients met inclusion criteria for the primary analysis. There were 81 people excluded from the primary analysis for a diagnosis of infection during their hospital admission, and 54 people excluded from both analyses for being placed on hospice or palliative care after their ICH. The median age was 61 (range 19-94) years. Of the 249 patients in this study, 53 (21.3%) developed SIRS during hospitalization. Patients with SIRS during hospitalization had higher baseline stroke scale scores (NIHSS 17 vs. 6; p<0.0001), lower baseline GCS scores (10 vs. 15; p<0.0001), higher baseline ICH scores (2 vs. 1; p=0.0042), and larger ICH volumes (18 vs. 9 cm3; p=0.0012). There were no statistically significant differences in demographics or past medical history between patients who did and did not develop SIRS during their hospitalization (Table 1).
Table 1.
Demographic and Baseline Characteristics Stratified by Diagnosis of Systemic Inflammatory Response Syndrome after Admission for Intracerebral Hemorrhage
| No SIRS 196 (78.7%) | SIRS 53 (21.3%) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, median (range) | 63 (23-99) | 60 (19-93) | 0.1423 |
| Female Sex, N (%) | 119 (60.7%) | 23 (44.2%) | 0.6680 |
| Black Race, N (%) | 89 (45.4%) | 27 (50.9%) | 0.3823 |
| Past Medical History | |||
| History of Stroke, N (%) | 42 (21.5%) | 8 (15.1%) | 0.2998 |
| History of Atrial Fibrillation, N (%) | 15 (7.6%) | 2 (3.8%) | 0.3205 |
| History of Hyperlipidemia, N (%) | 45 (22.9%) | 9 (16.9%) | 0.3488 |
| History of Diabetes, N (%) | 48 (24.5%) | 13 (24.5%) | 0.9954 |
| History of Hypertension, N (%) | 149 (76.0%) | 41 (77.4%) | 0.8389 |
| History of Coronary Artery Disease, N (%) | 32 (16.3%) | 3 (5.7%) | 0.0475 |
| Previous History of Smoking, N (%) | 63 (36.2%) | 23 (50%) | 0.0882 |
| Clinical Information | |||
| NIHSS at Baseline, median (range) | 6 (0-41) | 17 (0-40) | <0.0001 |
| GCS at Baseline, median (range) | 15 (3-15) | 10 (3-15) | <0.0001 |
| ICH Score, median (range) | 1 (0-5) | 2 (0-4) | 0.0042 |
| ICH Volume (ml), median (range) | 9 (1-214) | 18 (3-177) | 0.0012 |
| ICH Classification | 0.3476 | ||
| Hypertension, N (%) | 130 (67.4%) | 32 (61.5%) | |
| Amyloid, N (%) | 16 (8.3%) | 6 (11.5%) | |
| Underlying Structural Abnormality, N (%) | 8 (4.2%) | 6 (11.5%) | |
| Secondary to Anticoagulation, N (%) | 8 (4.2%) | 1 (1.9%) | |
| Other, N (%) | 10 (5.2%) | 3 (5.8%) | |
| Cryptogenic, N (%) | 21 (10.9%) | 4 (7.7%) |
SIRS: Systemic Inflammatory Response Syndrome; NIHSS: National Institutes of Health Stroke Scale; GCS: Glasgow Coma Scale; ICH: Intracerebral Hemorrhage
Other ICH Classification includes substance abuse, vasculitis, CVST, coagulopathy due to liver failure, ethylene glycol poisoning, and brain mass
Predictors of SIRS
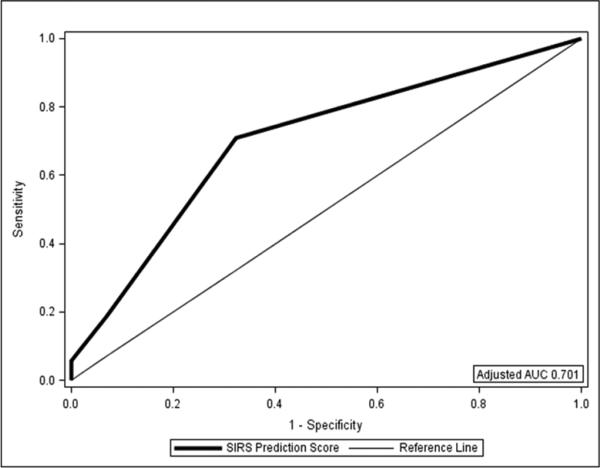
In addition to the markers of stroke severity (ICH Score) described above on univariable analysis, patients with SIRS during hospitalization had a higher frequency of intubation (50.9% vs. 18.4%; p<0.0001), but it is not known whether intubation or SIRS occurred first. Patients with SIRS during hospitalization were more likely to have abnormal vital signs (i.e., meeting SIRS vital sign criteria) obtained on admission in the emergency department (ED; 45.3% vs. 9.7%; p<0.0001). The overlap between patients with transient SIRS in the ED and persistent SIRS during hospitalization was less with only 33.3% of SIRS patients having both transient SIRS in the ED and persistent SIRS during hospitalization, 26.4% of SIRS patients who only met transient SIRS criteria in the ED, and 40.3% of SIRS patients only having persistent SIRS during their hospital stay. The variables that remained in the final multivariable prediction model were SIRS criteria in the ED and ICH score (unadjusted AUC 0.719; Figure 1). A SIRS prediction score was thus developed to predict persistent SIRS during hospitalization ranging from 0-3 with 1 point for transient SIRS criteria met in the ED, 1 point for an ICH score of 2 or 3, and 2 points for an ICH score of 4 or more. The score predicted development of SIRS during hospitalization (OR 2.86 per point on the SIRS prediction score, 95%CI 1.94-4.21, p<0.0001; adjusted AUC 0.701). A SIRS Score of 2 or more placed a patient at a 3-fold increased odds of developing SIRS during hospitalization (OR 3.05, 95%CI 1.28-6.71, p=0.0056) when compared to a score of 1.
Figure 1.
Receiver Operating Curve for the SIRS Prediction Score as a Predictor of SIRS During Hospitalization
SIRS and Outcomes
In the univariable analyses SIRS was associated with poor functional outcome (mRS 4-6 at discharge, OR 6.08, 95%CI 2.86-12.91, p<0.0001), poor discharge disposition (OR 4.41, 95%CI 1.92-4.77, p<0.0001), and death (OR 2.25, 95%CI 1.02-4.95, p=0.0432). Adjusting for ICH score at baseline, SIRS remained associated with poor functional outcome (mRS 4-6 at discharge, OR 5.25, 95%CI 2.09-13.2, p=0.0004) and poor discharge disposition (OR 3.74, 95%CI 1.58-4.83, p=0.0026), but was no longer statistically significantly associated with death (OR 1.75, 95%CI 0.58-5.32, p=0.3214).
There were 32 (11.7%) patients who met 2 SIRS criteria, 9 (3.3%) who met 3 SIRS criteria, and 3 (1.1%) who met all 4 SIRS criteria. Having only 2 SIRS criteria increased the odds for poor functional outcome at discharge (OR 4.18, 95%CI 1.94-9.03, p=0.0003) and this relationship remained after adjusting for ICH score on admission (OR 3.51, 95%CI 1.37-9.05, p=0.0092). All of the patients who met 3 SIRS criteria or 4 SIRS criteria had poor functional outcome at discharge.
SIRS and ICH Score
The relationship between ICH score and outcomes was mediated by the development of SIRS during hospitalization. For each point increase in the ICH Score on admission, there was a 45% increase in odds of developing SIRS during hospitalization (OR 1.45, 95% 1.07-1.98, p=0.0169). For each point increase in the ICH Score, adjusting for SIRS, there was a 2.68 fold increase in the odds of poor functional outcome (mRS 4-6 at discharge, OR 2.68, 95%CI 1.82-3.96, p<0.001). Using a mediation analysis we found that 33% of the effect of ICH score on poor functional outcome at discharge was explained by the development of SIRS in the hospital (Sobel 2.11, p=0.03).
Sub-analysis of SIRS and Outcomes accounting for Infections
In a sub-analysis that included patients who experienced an infection (n=330 total) there were 37 (11.2%) who had an infection, 53 (16.1%) who met SIRS criteria during their hospital admission and was not diagnosed with an infection, and 44 (13.3%) who had both an infection and met SIRS criteria. In this larger sample including patients with an infection, SIRS remained a significant independent predictor of poor functional outcome at discharge on univariable analysis (OR 5.56, 95%CI 3.10-9.99, p<0.0001). Furthermore, a clinically diagnosed infection was a significant independent predictor of poor functional outcome at discharge on univariable analysis (OR 2.84, 95%CI 1.56-5.17, p=0.0007). Adjusting for ICH score at baseline, infection remained a predictor of poor functional outcome at discharge (mRS 4-6 at discharge, OR 2.20, 95%CI 1.07-4.53, p=0.0329), as did SIRS (OR 5.16, 95%CI 2.52-10.58, p<0.0001). In the final model adjusting for both infection and ICH score on admission, SIRS remained a strong predictor of poor functional outcome at discharge (OR 4.78, 95%CI 2.25-10.15, p<0.0001), whereas infection had no statistically significant effect on discharge poor functional outcome in the presence of SIRS (OR 1.29, 95%CI 0.58-2.85, p=0.5307).
Four categories were created (1) patients without an infection or SIRS, (2) patients with just an infection, (3) patients with just SIRS, and (4) patients who experienced both SIRS and an infection, although it may not have been at the same time during their hospitalization. A univariable analysis was conducted assessing the relationship between these categories and poor functional outcome using the first group (no infection/no SIRS) as a reference group (Table 2). After adjusting for ICH score at baseline, patients with an infection were no longer at statistically significantly higher odds of poor functional outcome; however, patients with only SIRS (OR 5.16, 95%CI 1.91-13.9, p=0.0012) and patients with both SIRS and an infection were at higher odds of poor functional outcome (OR 5.93, 95%CI 2.14-16.4, p=0.0006).
Table 2.
Sub analysis of the Relationship Between Infection, SIRS, SIRS plus Infection on Poor Functional Outcome at Discharge
| Crude | Adjusted* | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| No Infection or SIRS | ref | ref | ||||
| Infection Only | 2.38 | 1.01-5.61 | 0.0468 | 1.51 | 0.55-4.16 | 0.4306 |
| SIRS Only | 5.56 | 2.39-12.9 | <0.0001 | 5.16 | 1.91-13.9 | 0.0012 |
| SIRS and an Infection | 6.55 | 2.86-15.0 | <0.0001 | 5.93 | 2.14-16.4 | 0.0006 |
The final model includes SIRS, Infection and ICH score at baseline
Discussion
Over 20% of patients in this ICH cohort developed SIRS, and the development of SIRS conferred an increased risk of poor discharge outcome. Furthermore, we developed a simple score to predict the development of SIRS during hospitalization. The prevalence of SIRS in ICH patients found in this study was similar to the prevalence of SIRS found in acute ischemic stroke patients treated with tissue plasminogen activator (18%), but was lower than the prevalence found in subarachnoid hemorrhage patients (54-86%).(4, 11, 22, 23)
Given the high prevalence of SIRS in this sample and the association we observed between SIRS and poor functional outcome, the ability to identify patients at greatest risk for developing SIRS after ICH may prove useful in prognosticating outcomes in ICH patients. The score we developed can identify patients who are at a 3 fold higher odds of developing SIRS after an ICH. To our knowledge, this is the first study to investigate the predictors of SIRS in ICH patients using a strict definition, as well as a diagnostic evaluation that rules out sepsis and infection during the hospital stay. Using information available at the time of admission, we developed a simple, easy to use score for ICH patients that can provide clinicians with the probability that a non-infected ICH patient will develop SIRS during hospitalization. While there were some differences between groups with regard to demographics (e.g. gender), these differences were not statistically significantly different. The components of the SIRS score, SIRS criteria on admission, and ICH score on admission suggest that SIRS during ICH hospitalization is a function of stroke severity. This is supported by previous research in acute ischemic stroke and subarachnoid hemorrhage that identified strong positive associations between stroke severity and development of SIRS.(5, 24) While studies in ischemic stroke and subarachnoid hemorrhage reported that prior history of stroke hypertension, and chronic disease burden as predictors of SIRS, we did not observe this.(24-26) The influence of stroke severity on the inflammatory process suggests that SIRS in ICH patients may be related to an inflammatory reaction to the stroke itself, as opposed to pre-existing chronic inflammatory conditions. Further research will determine whether the score can be used to direct intervention to prevent the development of SIRS and improve ICH outcomes in the presence of SIRS.
Consistent with studies in other stroke samples, patients who developed SIRS were at greater odds of being discharged with poor functional outcome, even after adjusting for covariates that have been identified to influence ICH outcomes. Our findings are consistent with associations previously reported in ischemic stroke and subarachnoid hemorrhage. (4, 11) Considering that individual components of the SIRS classification (e.g. body temperature, leukocytosis) have been linked to poor outcomes, the relationship between the combined components and outcomes was anticipated.(27, 28),22, 23 The relationship between elevated body temperature and poor outcomes after ICH has also been well described, with one multicenter study highlighting a 40% increased odds of poor outcomes at 90 days in patients with elevated body temperature.(27, 29) In a study of multiple types of stroke, tachycardia was identified as a predictor of mortality at 3 months,(30) though no study to our knowledge has linked tachypnea with outcomes after stroke. Leukocytosis after ICH has been linked to secondary injury and poor outcomes.(31, 32) Further research is needed to assess whether it is just the specific components of the SIRS classification driving the relationship with poor functional outcome, or if there is a dose response effect with number of SIRS criteria and the relationship with poor functional outcome. Unfortunately, the relatively low numbers of ICH patients with 3 or more SIRS criteria in our sample did not permit us to explore this issue.
As expected, the prevalence of SIRS was higher in patients presenting with greater severity of ICH. In our cohort, we observed poor outcome to be associated with ICH score, a marker of stroke severity. The results of the mediation analysis suggest that SIRS may be one mechanism by which the association of rising ICH score and worsening functional outcome can be explained. Further, this is consistent with our finding that the development of SIRS in ICH patients is strongly associated with stroke severity. This finding is in keeping with previous research between individual components of the SIRS classification and stroke severity.(33) Elevated body temperature alone has been associated with stroke severity; fever may be a manifestation of stroke severity, hematoma growth due to the breakdown of the blood-brain barrier, or cell death due to inflammation.(34, 35) A relationship between leukocytosis and stroke severity has been reported, with larger baseline hemorrhage volumes being strongly associated with leukocytosis.(36, 37) This association could be due to the presence of leukocytes around the injury within hours of the ICH, with a direct relationship between number of leukocytes present, and volume of hemorrhage.(38) To our knowledge, no prior study has identified a relationship between ICH severity and tachycardia or tachypnea.
In the sub-analysis investigating the relationship between SIRS, infection, and outcomes our study highlights the strong association between SIRS and outcomes in the absence of infection. Patients with SIRS and patients with SIRS and infections have a much higher odds of poor functional outcome than patients with only an infection even after adjusting for baseline stroke severity. This finding is consistent with prior research in subarachnoid patients indicating that SIRS is nonspecific and not necessarily an indicator of infection.(23) The close relationship between stroke severity and SIRS provides further indirect evidence that SIRS is not an indicator of infection, but an inflammatory response related to the stroke itself. Further research is needed to explore temporal relationships among SIRS, infection development, and functional outcomes.
Limitations
Our study is limited by the retrospective nature and small sample size involving only one academic center. Validation in additional cohorts is needed to determine the generalizability of our SIRS prediction score to ICH patients in general and other patient populations. Additionally, we did not have information on inflammatory biomarkers. We defined infections by clinical diagnosis, although there could have been patients with infections that were not diagnosed during their hospital stay. While distinguishing infection from SIRS is never perfect, this research provides estimates from a real world perspective and is an important contribution despite the limitations. We do not have information on duration of SIRS, or whether SIRS occurred before, during, or after infection in the sub-analysis including those with infections. Our study is further limited by the lack of data on seizure activity during hospitalization and the short-term nature of our outcomes data. This study is limited by the potential bias of having differing measures of vital signs, particularly among the sicker patients, that could under report the prevalence of SIRS in the healthier stroke patients. We attempted to decrease this bias as much as possible by including the 24-hour requirement for SIRS criteria to be classified as having SIRS. Furthermore, infections were not assessed in a systematic manner and therefore some infections could be missed. This study is limited to only outcomes at discharge as there is no follow up information on this patient population. Despite these limitations, this is the first study to develop a SIRS prediction score for ICH stroke patients and to describe SIRS as a predictor of outcomes in ICH patients—independent of infection status.
Conclusion
The high prevalence of SIRS after ICH, in addition to the increased risk of poor outcomes, necessitates further research to determine whether early intervention in treating SIRS will reduce the poor functional outcome observed with patients who develop SIRS during ICH hospitalization. This observation raises the question of whether early intervention to prevent or treat incipient SIRS may reduce morbidity and mortality in this high-risk population.
Supplementary Material
Acknowledgements
Dr. Boehme is supported by NINDS NIH T32 NS007153-31. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
Footnotes
Disclosures
There are no conflicts of interest to disclose.
References
- 1.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. The Lancet Neurology. 2012;11(1):101–18. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 2.Lord AS, Langefeld CD, Sekar P, Moomaw CJ, Badjatia N, Vashkevich A, et al. Infection After Intracerebral Hemorrhage: Risk Factors and Association With Outcomes in the Ethnic/Racial Variations of Intracerebral Hemorrhage Study. Stroke; a journal of cerebral circulation. 2014 doi: 10.1161/STROKEAHA.114.006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemrrhage. Stroke; a journal of cerebral circulation. 2001;32(9):1989–93. doi: 10.1161/hs0901.095646. [DOI] [PubMed] [Google Scholar]
- 5.Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke; a journal of cerebral circulation. 2004;35(9):2128–33. doi: 10.1161/01.STR.0000137607.61697.77. [DOI] [PubMed] [Google Scholar]
- 6.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. Journal of neuropathology and experimental neurology. 2003;62(2):127–36. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 7.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke; a journal of cerebral circulation. 2003;34(10):2518–32. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 8.D'Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Molecular medicine. 2001;7(6):367–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen ED, Waje-Andreassen U, Vedeler CA, Aamodt G, Mollnes TE. Systemic complement activation following human acute ischaemic stroke. Clinical and experimental immunology. 2004;137(1):117–22. doi: 10.1111/j.1365-2249.2004.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Diepen S, Vavalle JP, Newby LK, Clare R, Pieper KS, Ezekowitz JA, et al. The systemic inflammatory response syndrome in patients with ST-segment elevation myocardial infarction. Critical care medicine. 2013;41(9):2080–7. doi: 10.1097/CCM.0b013e31828a67b2. [DOI] [PubMed] [Google Scholar]
- 11.Boehme AK, Kapoor N, Albright KC, Lyerly MJ, Rawal PV, Bavarsad Shahripour R, et al. Systemic inflammatory response syndrome in tissue-type plasminogen activator-treated patients is associated with worse short-term functional outcome. Stroke; a journal of cerebral circulation. 2013;44(8):2321–3. doi: 10.1161/STROKEAHA.113.001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347(8999):422–5. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 13.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68(20):1651–7. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill JC, 3rd, Newman J, Zhao S, Johnston SC. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2004;35(5):1130–4. doi: 10.1161/01.STR.0000125858.71051.ca. [DOI] [PubMed] [Google Scholar]
- 15.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2001;32(4):891–7. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 16.Savitz SI, Lew R, Bluhmki E, Hacke W, Fisher M. Shift analysis versus dichotomization of the modified Rankin scale outcome scores in the NINDS and ECASS-II trials. Stroke; a journal of cerebral circulation. 2007;38(12):3205–12. doi: 10.1161/STROKEAHA.107.489351. [DOI] [PubMed] [Google Scholar]
- 17.Y M, I C, K K, WJ B. Estimating Harrell's Optimism on Predictive Indices Using Bootstrap Samples. SAS Global Forum; 2013. [Google Scholar]
- 18.Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. Journal of clinical epidemiology. 2001;54(8):774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 19.Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of clinical epidemiology. 2001;54(4):343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 20.DP M, JH D. Estimating Mediated Effects in Prevention Studies. Evaluation Review. 1993;17:144–58. [Google Scholar]
- 21.Mackinnon DP, Warsi G, Dwyer JH. A Simulation Study of Mediated Effect Measures. Multivariate behavioral research. 1995;30(1):41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam AK, Ilodigwe D, Mocco J, Mayer S, Kassell N, Ruefenacht D, et al. Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. Neurocritical care. 2010;13(2):182–9. doi: 10.1007/s12028-010-9402-x. [DOI] [PubMed] [Google Scholar]
- 23.Guterman EL, Kamel H, Azran C, Shah MP, Claude Hemphill J, 3rd, Smith WS, et al. Time from onset of SIRS to antibiotic administration and outcomes after subarachnoid hemorrhage. Neurocritical care. 2014;21(1):85–90. doi: 10.1007/s12028-013-9846-x. [DOI] [PubMed] [Google Scholar]
- 24.Boehme AK, Kapoor N, Albright KC, Lyerly MJ, Rawal PV, Bavarsad Shahripour R, et al. Predictors of systemic inflammatory response syndrome in ischemic stroke undergoing systemic thrombolysis with intravenous tissue plasminogen activator. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23(4):e271–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocritical care. 2008;8(3):404–12. doi: 10.1007/s12028-008-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Chronic medical conditions and risk of sepsis. PLoS One. 2012;7(10):e48307. doi: 10.1371/journal.pone.0048307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke; a journal of cerebral circulation. 2008;39(11):3029–35. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54(2):354–61. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 29.Rincon F, Lyden P, Mayer SA. Relationship between temperature, hematoma growth, and functional outcome after intracerebral hemorrhage. Neurocritical care. 2013;18(1):45–53. doi: 10.1007/s12028-012-9779-9. [DOI] [PubMed] [Google Scholar]
- 30.Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. Journal of the neurological sciences. 2005;234(1-2):99–103. doi: 10.1016/j.jns.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Dore S. Inflammation after intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 32.Adeoye O, Walsh K, Woo JG, Haverbusch M, Moomaw CJ, Broderick JP, et al. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23(2):e107–11. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claassen J, Albers D, Schmidt JM, De Marchis GM, Pugin D, Falo CM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Annals of neurology. 2014;75(5):771–81. doi: 10.1002/ana.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Annals of neurology. 2003;53(6):731–42. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 35.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, et al. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Annals of neurology. 2002;51(4):517–24. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 36.Agnihotri S, Czap A, Staff I, Fortunato G, McCullough LD. Peripheral leukocyte counts and outcomes after intracerebral hemorrhage. Journal of neuroinflammation. 2011;8:160. doi: 10.1186/1742-2094-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leira R, Davalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461–7. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26(6):811–20. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.