Abstract
Type II photodynamic therapy (PDT) is used for cancer treatment based on the combined action of a photosensitizer, a special wavelength of light, oxygen (3O2) and generation of singlet oxygen (1O2). Intra-patient and inter-patient variability of oxygen concentration ([3O2]) before and after the treatment as well as photosensitizer concentration and hemodynamic parameters such as blood flow during PDT has been reported. Simulation of these variations is valuable, as it would be a means for the rapid assessment of treatment effect. A mathematical model has been previously developed to incorporate the diffusion equation for light transport in tissue and the macroscopic kinetic equations for simulation of [3O2], photosensitizers in ground and triplet states and concentration of the reacted singlet oxygen ([1O2]rx) during PDT. In this study, the finite-element based calculation of the macroscopic kinetic equations is done for 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide (HPPH)-mediated PDT by incorporating the information of the photosensitizer photochemical parameters as well as the tissue optical properties, photosensitizer concentration, initial oxygen concentration ([3O2]0), blood flow changes and ϕ that have been measured in mice bearing radiation-induced fibrosarcoma (RIF) tumors. Then, [1O2]rx calculated by using the measured [3O2] during the PDT is compared with [1O2]rx calculated based on the simulated [3O2]; both calculations showed a reasonably good agreement. Moreover, the impacts of the blood flow changes and [3O2]0 on [1O2]rx have been investigated, which showed no pronounced effect of the blood flow changes on the long-term 1O2 generation. When [3O2]0 becomes limiting, small changes in [3O2] have large effects on [1O2]rx.
Keywords: tissue oxygenation, blood flow changes, PDT, in vivo mice models, singlet oxygen generation, macroscopic simulation
1. INTRODUCTION
Type II photodynamic therapy (PDT) requires three key components of drug, light, and oxygen (3O2), which interact on time scales relevant to a single treatment to generate singlet oxygen (1O2).1-4 Although an understanding of the biology of PDT has expanded tremendously over the past few years, it is still a challenge to match the extent of PDT effects to the extent of the disease being treated in the clinic. To quantitatively account for the biological damage done by PDT, it is suggested to account for 1O2 production during PDT.5, 6 However, the weak and short lifetime of the luminescence signals due to rapid reactions of 1O2 in biological environments is a major obstacle to use luminescence detection methods in clinical applications.7, 8 In this study, a macroscopic model,5, 6, 9, 10 which incorporates a complete set of kinetic PDT equations and information obtained from the explicit dosimetry of the light fluence rate (ϕ), initial photosensitizer and oxygen ([3O2]0) concentration and tissue optical properties is used to calculate the temporal changes of reacted singlet oxygen concentration ([1O2]rx) during PDT. As the generation of 1O2 depends in part on the availability of 3O2 in the target tissue, knowing the level of tissue oxygenation before, during, and after PDT is important in understanding the basic physiological mechanism and the light dosimetry.11, 12 Oxygen delivery is dependent on the metabolic requirements and functional status of each organ. During PDT, both photochemical consumption of 3O2 and microvascular shutdown can lead to depletion of molecular 3O2. Therefore, there is an immediate need for additional 3O2 in tissue, which might be accomplished by increasing blood flow.13 However, if tissue oxygenation is reduced to levels insufficient for further tissue damage, this influences the treatment outcome. In order to consider the blood flow changes during PDT, a time dependent oxygen supply rate, g, has been introduced in our model 14 based on the data published for in vivo blood flow changes in mice models.13 To assess the efficacy of our simulations, the temporal changes of [3O2] during 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide (HPPH)-mediated PDT of radiation-induced fibrosarcoma (RIF) mice tumor is measured and compared with those calculated for the same treatment condition. Then, the amounts of [1O2]rx calculated for the measured and simulated [3O2] are compared to evaluate our model and HPPH photochemical parameters that have been obtained in our previous studies.5, 15
2. MATERIALS AND METHODS
2.1 Mice tumor models for in vivo studies
RIF tumors were propagated on the shoulders of female C3H mice (~9 weeks old; NCI-Frederick, MD) by the subcutaneous injection of a suspension of 1×106 cells/ml. When the tumors reached ~ 6 mm in diameter and depth (~9 days after the injection of in vitro-maintained cells), the mice received 0.25 mg/kg HPPH via tail vein injection. Animal husbandry was provided by the University of Pennsylvania Laboratory Animal Resources in Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-accredited facilities according to protocol (803929) approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
2.2 Measurements of the photosensitizer concentration prior and after the photodynamic therapy
In order to determine the HPPH concentration in the tumor, a catheter was inserted into the center of the tumor. Then, a single side-cut fiber connected to a dichroic filter was placed into the catheter, which served as both an excitation source and a detector to record the interstitial HPPH fluorescence emission spectrum along the catheter axis. The photosensitizer was excited using a 405 nm diode laser. The raw spectrum was fitted to the basis spectrum of HPPH and autofluorescence in the absence of HPPH using singular value decomposition (SVD). The attenuation of the photosensitizer fluorescence signal due to the light absorption and scattering by tissue was corrected by applying a correction factor, which is a function of tissue optical absorption (µa) and scattering (µ′s) coefficients. Then, the spectra were compared with those measured in phantoms of known HPPH concentration to extract the in vivo drug concentration. More detailed description of the measurements and calculations has been published previously.16, 17
2.3 Measurements of the tissue oxygenation prior, during and after the photodynamic therapy
Following the HPPH drug-light interval of 24 h, PDT was performed and the tissue oxygenation was measured prior, during, and post treatment. For direct measurements of the tissue oxygen saturation of hemoglobin pO2, the point measurements were made each 3 min using OxyLite 1 Channel tissue pO2 monitor (OXFORD OPTRONIX). Based on our simulations, the tissue oxygenation decreases dramatically at the first 5 min of the PDT; the [3O2] drop varies with the light delivery regimen and [3O2]0. In order to measure this [3O2] drop in mice tissue, the point measurements were performed each 1 min at the first 5 min of the treatment. As the OxyLite probe is a phosphorescence-based probe, the PDT excitation light was paused for each measurement. The mice were treated on a heated water pad under anesthesia in all studies. Then, [3O2] was calculated by multiplying the measured pO2 with 3O2 solubility in tissue, which is 1.295 μM/mmHg.5, 7
2.4 Photodynamic therapy
PDT was performed by surface illumination of the tumors using a diode laser (B&W Tek, Newark, DE 19713 USA) emitting an 8-Watt maximum power and 665 nm beam. The light was delivered via a 140 μm-diameter optical fiber and the laser beam was collimated through a coupling lens on the end of the fiber to a 1cm diameter beam spot over the tumor surface. An in-air fluence rate of 80 mW/cm2 and total fluence of 160 J/cm2 was used to induce 3O2 consumption during PDT in five tumor-bearing mice.
2.5 Photodynamic equations
A set of equations is produced by simplifying and combining the energy transfer processes in PDT to describe the changes of [3O2] and creation of 1O2 during the treatment.6, 7 These equations are dependent on various parameters such as the ground state photosensitizer (S0), light source (s), μa, μ′s and photosensitizer photochemical parameters:
| (1) |
| (2) |
| (3) |
| (4) |
where [1O2]rx is defined as 1O2 effectively leading cell death. β represents the ratio of triplet state (T) phosphorescence to reaction between T and 3O2. δ is the low concentration correction parameter and σ is the ratio of photobleaching to reaction between 1O2 and cellular targets. ξ is the initial oxygen consumption rate, and g represents the oxygen supply rate to tissue. Different photosensitizers have different photochemical parameters.
2.6 Simulation of blood flow changes during PDT
Oxygen delivery is dependent on the metabolic requirements and functional status of each organ. Due to the PDT oxygen consumption, there is an immediate need for additional 3O2 in tissue, which is accomplished by increasing blood flow. The change of the blood flow during PDT was simulated in our macroscopic model by making g to be time dependent. The g function was obtained from the best fit to the data presented in Ref. 13 for Photofrin-mediated PDT in RIF tumors:
| (5) |
where, t′ = (t - 750)/632.1 is normalized by mean 750 and standard deviation 632.1 (with 95% confidence bounds).14
2.7 Use of COMSOL Multiphysics
The forward calculation of the macroscopic kinetic equations was done in COMSOL Multiphysics 5.0 for the modeling of [3O2] and [1O2] generated during PDT. The finite-element based calculation was implemented within COMSOL by including the measured [3O2]0, μa, μ′s, ϕ and photosensitizer concentration and varying the input parameters such as ξ, σ, g and β. COMSOL was run on an iMAC OSX version 10.9.5 (Processor 3.1 GHz Intel Core 17 and Memory 16 GB 1600 MHz DDR3). The calculation time was in seconds for the coupled differential equations.
3. RESULTS
RIF tumors were propagated on the shoulders of female C3H mice by the subcutaneous injection of a cell suspension. Upon reaching the predetermined diameter of ~ 6 mm, the photosensitizer concentration and the amount of [3O2]0 in the tumor were measured. The average HPPH concentration and [3O2]0 in five mice were measured to be 1.1 ± 0.5 μM and 20.5 ± 2.4 μM, respectively. After the measurements, HPPH-PDT was immediately performed with an in-air fluence rate of 80 mW/cm2 and a total in-air fluence of 160 J/cm2. The changes of [3O2] were also measured during the treatment by stopping the excitation light. In order to evaluate our macroscopic model, the amounts of [3O2] and [1O2] were also calculated using the same treatment condition and photosensitizer concentration. The tissue optical properties, g0, [3O2]0, HPPH concentration and photochemical parameters used in the simulation have been shown in Table 1.
Table 1.
Tissue optical properties, initial oxygen and HPPH concentrations as well as photochemical parameters used in the simulations
|
μa
(cm−1) |
μ′s
(cm−1) |
ε
(cm−1μM−1) |
ξ
(cm2mW−1s−1) |
σ
(μM −1) |
g0
(μM s−1) |
β
(μM) |
δ
(μM) |
[3O2]0
(μM) |
S0
(μM) |
In-air ϕ
(mWcm−2) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPPH | 0.8 ± 0.1 | 9.3 ± 0.9 | 0.108 | (110 ± 20)×10−3 | (4.5 ± 3.0)×10−5 | 0.8 ± 0.2 | 11.9 | 33 | 20.5 ± 2.4 | 1.1 ± 0.5 | 80 |
* PDT treatment time is 2000 s
The spatial distribution of ϕ in the tumor calculated by using the diffusion equation (Eq. (1)) has been shown in Fig. 1(a). Fig. 1(b) shows the temporal changes of the normalized g (g/g0) obtained from Eq. (5). The changes of photosensitizer concentration versus total fluence calculated from Eq. (3) can be found in Fig. 1(c).
Figure 1.
(a) Spatial distribution of the light fluence rate (ϕ) in the RIF tumor. (b) Temporal changes of the normalized oxygen supply rate (g/g0) obtained from Ref. [13]. (c) Temporal changes of the HPPH photosensitizer concentration versus total fluence.
The measured [3O2] has been compared with those simulated with the macroscopic model with and without considering the blood flow changes. The symbols shown in Fig. 2(a) are the average [3O2] measured in five mice. The purple and blue lines present the temporal changes of [3O2] simulated with the model incorporating the blood flow changes during the treatment and [3O2] simulated without considering the changes of the blood flow, respectively. Both measured and calculated [3O2] show the dramatic changes in tissue oxygenation during PDT. Although the simulations show a reasonably good agreement with the measurements, a peak measured in the region of 700-1200 s could not be calculated with our model. [1O2]rx generated during the treatment was also calculated based on the measured [3O2] (black line) as well as the simulated ones (blue and purple lines) as shown in Fig. 2(b); the gray area shows the upper and lower bounds of the [1O2]rx calculated using the measured [3O2]. Although the difference between the [1O2]rx is more pronounced after 800 s PDT, [1O2]rx calculated based on the simulated [3O2] is still within the gray region of the measurement uncertainty.
Figure 2.
(a) A comparison of the temporal changes of oxygen concentration ([3O2]) measured (the symbols) during HPPH-PDT with those simulated for the same treatment condition. (b) Temporal changes of the singlet oxygen concentration ([1O2]rx) during HPPH-mediated PDT calculated with the macroscopic model. The purple and blue lines present the calculation with and without considering the blood flow changes, respectively. The black line is [1O2]rx calculated based on the measured [3O2]; the gray area is the upper and lower bounds of the [1O2]rx. In-air fluence rate is 80 mW/cm2 and total in-air fluence is 160 J/cm2.
4. DISCUSSIONS
The relationship between tissue oxygenation, blood flow variation, and PDT effect is complex, and remarkably few studies have directly correlated 3O2 and blood flow changes during PDT with the final biological effect.13, 14 The photochemical 3O2 consumption and its importance for PDT were first demonstrated experimentally in multi-cell tumor spheroids,12 which does not account for the blood flow changes during the treatment. To our knowledge, there is no real time monitoring to ensure adequate oxygenation at strategic points in tumor tissues during PDT. In this study, we measured the oxygen consumption during PDT of RIF tumors in five C3H mice administered with HPPH. Based on the results, tumor oxygen status before PDT, which has been measured to be 20.5 ± 2.4 μM, markedly deteriorated at the first 400 s of the treatment; the drop was 94 ± 6.9 percent of the pre-treatment oxygenation. After this immediate drop, an increase in the tissue oxygenation occurred, which reached 17.77 ± 3.42 μM by the end of the treatment. In all PDT treatments, the irradiation was performed with an in-air fluence of 80 mW/cm2 and a total energy of 160 J/cm2. In order to evaluate our macroscopic model, the temporal changes of [3O2] was also calculated using the same treatment condition, initial photosensitizer, and [3O2]0. In our model, the blood flow changes during PDT was simulated by making g to be time dependent based on the best fit to the in vivo data presented in Ref. 13 for Photofrin-mediated PDT; to our knowledge there is no published data for the blood flow changes during the HPPH-mediated PDT. Although both macroscopic models with and without blood flow changes show a reasonably good agreement with the measurements, a peak measured in the region of 700-1200 s could not be calculated with our simulations. As different photosensitizers might impact the blood flow differently, we think [3O2] peak at 700-1200 s might be due to a blood flow increase during the HPPH-PDT that present for the case for Photofrin-PDT. However, detailed studies are required to investigate the blood flow changes during HPPH-PDT and its impact on the [3O2] consumption. The amount of [1O2]rx simulated using measured [3O2] showed a pronounced difference after 800 s PDT as compared with those simulated using the calculated [3O2]. However, [1O2]rx calculated based on the simulated [3O2] is still within the gray region of the measurement uncertainty.
It is reported that different photosensitizers affect the [3O2] consumption differently.18 The mean [3O2] drops to 33.9 ± 12.0 percent of the pretreatment level in complement-proficient C57BL/6 (B6) hosts bearing lewis lung carcinoma and MCA205 fibrosarcoma following Photofrin-PDT.18 The drop in the mean [3O2] following benzoporphyrin derivative (BPD)-PDT in B6 is reported to be 53.2 ± 19.1 percent of the pretreatment level at the same tumor type.18 Tumor oxygenation before the treatment (3.25-26 μM) was not significantly affected with B6 mice administered with Photofrin or BPD in the absence of light exposure or by tumor light treatment alone.18 This information has been used in our simulations to investigate the temporal changes of [3O2] during Photofrin-PDT and their effect on [1O2]rx. We also studied the impact of the blood flow changes during PDT on our simulations. Table 2 shows the tissue optical properties, g0, photochemical parameters, [3O2]0, drug concentration and in-air fluence rate and fluence used in our simulation for Photofrin-PDT.6
Table 2.
Tissue optical properties, initial oxygen and Photofrin concentrations as well as photochemical parameters and the treatment protocol used in the simulations.6, 15
| Sensitizer |
μa
(cm−1) |
μ′s
(cm−1) |
ε
(cm−1μM−1) |
ξ
(cm2 mW−1 s−1) |
σ
(μM s−1) |
g0
(μM s−1) |
β
(μM) |
δ
(μM) |
[3O2]0a
(μM) |
In-air ϕ
(mW/cm2) |
Fluence (J/cm2) |
S0
b
(μM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photofrin | 1.03 | 13.46 | 0.0035 | 3.7 × 10−3 | 7.6 × 10−5 | 0.7 | 11.9 | 33 | 3.25-26 | 80-90 | 150 | 7 |
The partial pressure of oxygen pO2 in tumors, measured with polarographic needle electrodes using an Eppendorf histograph Model KIMOC 6650 (Eppendorf-Nethereler-Hinz GmbH, Hamburg, Germany), have been taken from Ref. 18. The initial tumor oxygenation ([3O2]0) have been calculated by multiplying the measured pO2 with 3O2 solubility in tissue, which is 1.295 μM/mmHg.
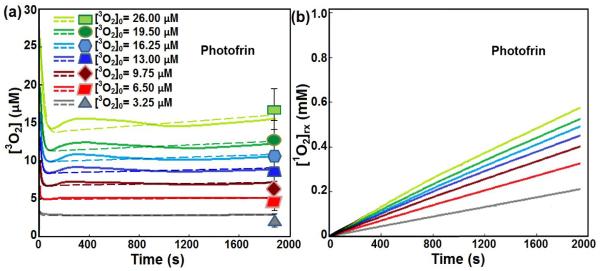
Figs. 3 (a) compares the simulated [3O2] with those measured post Photofrin-mediated PDT for ϕ = 80 mW/cm2 and total energy of 150 J/cm2. The solid and dashed lines show the calculated [3O2] with and without considering the blood flow changes, respectively; the symbols are the measured values post treatment. Incorporating the information of the blood flow changes in our model did not have a large impact on the calculation of the [3O2]. The theoretical model describes with excellent agreement the changes in 3O2 consumption that have been measured. Fig. 3(b) shows [1O2]rx calculated for each [3O2]0. Based on our simulations, [1O2]rx was not affected with the blood flow changes during the treatment. In the well-oxygenated tissue, [3O2]0 has low influence on the amounts of [1O2]rx. When [3O2]0 becomes limiting (3.25-9.75 μM), small changes in [3O2]0 have large effects on the generation of 1O2.
Figure 3.
Temporal changes of (a) oxygen concentration ([3O2]) and (b) reactive singlet oxygen concentration ([1O2]rx) during Photofrin-mediated PDT. Initial oxygen concentration ([3O2]0) has been changed from 3.25 to 26 μM. The in-air fluence rate and total fluence are considered to be 80 mW/cm2 and 150 J/cm2, respectively. The solid and dashed lines are the calculations with and without considering the blood flow changes, respectively; the symbols are the measured [3O2] post PDT for different [3O2]0, reported by Ref.18
5. CONCLUSION
In PDT, the spatial distribution of light in tissue is determined by the light source characteristics and the tissue optical properties.4, 19 The tissue optical properties, in turn, are affected by the concentration of photosensitizer, as well as the concentration and oxygenation state of the blood in the tissue. Therefore, tissue optical properties, the variability in time and/or space of ground-state [3O2], sensitizer availability and light delivery to the treatment area during typical therapy should be incorporated in PDT dosimetry.20 To quantitatively account for the biological damage done by PDT, some researchers are considering the measurement of a quantity that depends on all, or at least most, of the above factors.5, 6 The measurement of [1O2]rx is one of the suggestions that could allow for the determination of the biological response that a specific treatment protocol would evoke. As it is extremely difficult to monitor [1O2]rx during treatment, a macroscopic model was developed for the calculation of [1O2]rx that incorporates the effects of tissue optical properties, initial drug concentration and photosensitizer photochemical parameters. To employ [1O2]rx as a dose metrics for PDT or to simply incorporate it into a dosimetry model, it is essential to understand how the different [3O2] and blood flow changes that could be involved in PDT process affect the production and deposition of [1O2]rx. In this study we evaluated our macroscopic model and our HPPH photochemical parameters that we have obtained in our previous studies 5 by comparing the calculated [3O2] with those measured during in vivo HPPH-PDT; the macroscopic model showed a reasonably good agreement with the measurements. A comparison of the [1O2]rx simulated using calculated and measured [3O2] showed a reasonably good agreement; [1O2]rx obtained based on the simulated [3O2] was within the region of the measurement uncertainty. The Photofrin photochemical parameters calculated with the macroscopic model were also assessed by comparing the simulated [3O2] post-PDT with those measured for the same treatment condition in Ref. 18. To our knowledge, this is the first study that uses the real time monitoring of [3O2] for the adequate calculation of the [1O2]rx in tumor tissues during PDT.
ACKNOWLEDGEMENTS
This work is supported by grants from the National Institute of Health (NIH) R01 CA154562 and P01 CA87971. The authors would like to thank Theresa M. Busch and Keith Cengel for their help in the animal studies and providing the HPPH photosensitizer.
REFERENCES
- 1.Penjweini R, Loew H-G, Breit P, Kratky KW. Optimizing the antitumor selectivity of PVP-Hypericin re A549 cancer cells and HLF normal cells through pulsed blue light. Photodiagnosis Photodyn Ther. 2013;10(4):591–599. doi: 10.1016/j.pdpdt.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 3.Penjweini R, Loew H-G, Eisenbauer M, Kratky KW. Modifying excitation light dose of novel photosensitizer PVP-Hypericin for photodynamic diagnosis and therapy. J. photochemistry and photobiology B, Biology. 2013;120:120–129. doi: 10.1016/j.jphotobiol.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Finlay JC, Darafsheh A. Light sources, drugs, and dosimetry. In: Wong B, Ilgner J, editors. Biomedical Optics in Otorhinolaryngology: Head and Neck Surgery. Springer; New York: 2016. [Google Scholar]
- 5.Penjweini R, Liu B, Kim MM, Zhu TC. Explicit dosimetry for 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a-mediated photodynamic therapy: macroscopic singlet oxygen modeling. Journal of Biomedical Optics. 2015;20(12):128003. doi: 10.1117/1.JBO.20.12.128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang KK-H, Finlay JC, Busch TM, Hahn SM, Zhu TC. Explicit dosimetry for photodynamic therapy: macroscopic singlet oxygen modeling. Journal of Biophotonics. 2010;3(5-6):304–318. doi: 10.1002/jbio.200900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu TC, Liu B, Penjweini R. Study of tissue oxygen supply rate in a macroscopic photodynamic therapy singlet oxygen model. Journal of Biomedical Optics. 2015;20(3):38001. doi: 10.1117/1.JBO.20.3.038001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochemistry and Photobiology. 2002;75(4):382–391. doi: 10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Penjweini R, Kim MM, Zhu TC. In-vivo outcome study of HPPH mediated PDT using singlet oxygen explicit dosimetry (SOED) Proc. SPIE. 2015;9308 doi: 10.1117/12.2076441. 93080N-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MM, Penjweini R, Zhu TC. In vivo outcome study of BPD-mediated PDT using a macroscopic singlet oxygen model. Proc. SPIE. 2015;9308 doi: 10.1117/12.2077803. 93080A-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodhams JH, Macrobert AJ, Bown SG. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochemical & Photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. 2007;6(12):1246–1256. doi: 10.1039/b709644e. [DOI] [PubMed] [Google Scholar]
- 12.Georgakoudi I, Foster TH. Singlet oxygen-versus nonsinglet oxygen-mediated mechanisms of sensitizer photobleaching and their effects on photodynamic dosimetry. Photochem. Photobiol. 1998;67(6):612–625. [PubMed] [Google Scholar]
- 13.Yu G, Durduran T, Zhou C, et al. Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy. Clin Cancer Res. 2005;11(9):3543–3552. doi: 10.1158/1078-0432.CCR-04-2582. [DOI] [PubMed] [Google Scholar]
- 14.Penjweini R, Zhu TC. PDT study using a model incorporating initial oxygen concentration and blood flow increase. Proceedings of the COMSOL: 2015:1–5. [Google Scholar]
- 15.Liu B, Kim MM, Gallagher-Colombo SM, Busch TM, Zhu TC. Comparison of PDT parameters for RIF and H460 tumor models during HPPH-mediated PDT. Proc SPIE. 2014;8931 doi: 10.1117/12.2040241. 89311C-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimofte A, Finlay JC, Zhu TC. A method for determination of the absorption and scattering properties interstitially in turbid media. Physics in Medicine and Biology. 2005;50(10):2291–2311. doi: 10.1088/0031-9155/50/10/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay JC, Zhu TC, Dimofte A, et al. Interstitial fluorescence spectroscopy in the human prostate during motexafin lutetium-mediated photodynamic therapy. Photochem. Photobiol. 2006;82(5):1270–1278. doi: 10.1562/2005-10-04-RA-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecic I, Minchinton AI, Korbelik M. The impact of complement activation on tumor oxygenation during photodynamic therapy. Photochem. Photobiol. 2007;83(5):1049–1055. doi: 10.1111/j.1751-1097.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- 19.Darafsheh A, Kraus EA, Kim MM, Zhu TC, Finlay JC. Performance characterization of a low-cost spatial frequency domain imaging system for the determination of optical properties in tissue-simulating phantoms and in vivo. Photodiagnosis and Photodynamic Therapy. 2015;12(3):331–332. [Google Scholar]
- 20.Penjweini R, Loew H-G, Hamblin MR, Kratky KW. Long-term monitoring of live cell proliferation in presence of PVP-Hypericin: a new strategy using ms pulses of LED and the fluorescent dye CFSE. Journal of Microscopy. 2012;245(1):100–108. doi: 10.1111/j.1365-2818.2011.03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]