Abstract
Introduction
To inform historical trends and racial disparities in Prostate cancer (CaP) across time and geography.
Materials and Methods
Data from 58 autopsy studies of latent CaP from 1898-2013 were identified and analyzed accounting for histopathological methods, which may have varied over time.
Results
CaP is most prevalent in African descent men, less prevalent in European descent men, and least prevalent in Asian descent men. 50% of Asian men have latent prostate tumors by 90. This 50% prevalence is reached by age 80 in Caucasians, and by age 60 in African descent men. While men are rarely diagnosed with CaP before age 40, 4% of Asian men, 9% of Caucasian men, and 37% of African descent men have latent CaP before age 40. However, CaP is under-ascertained in Africa. An increase in CaP prevalence was observed by observing historical trends in CaP prevalence. This increase is only significant in men of European descent, who experienced a 0.3% increase in CaP prevalence per calendar year since the 1930's (p=0.043). Evaluation of incidence-prevalence-duration data suggest that men are living longer with CaP in recent years, perhaps due to early detection and improved treatment.
This information has relevance for the design of clinical trials of prostate cancer detection, chemoprevention and treatment, and has been incorporated into recent guidelines for the early detection of prostate cancer and biopsy recommendations.
Conclusions
CaP is common at all ages and varies by race, and prevalence is increasing over time. Autopsy-based prevalence data provide a means of examining historical trends and comparison of diverse demographic groups and help guide clinical practice and trial design for the diagnosis and treatment of prostate cancer.
Keywords: Autopsy, Prostate Cancer Prevalence, Temporal Trends, Disparities
Introduction
Prostate cancer (CaP) was first reported in 1817 by Langstaff and rigorously defined by Thompson in 1857[1]. Albarran and Hallé[2] first suggested that CaP was likely to be a common disease in 1898 after identifying 12 (16%) cases among 86 autopsied prostates. Since that time, it has become clear that CaP is the most common non-cutaneous male cancer in most populations[3]. African Americans suffer from among the highest incidence rates of CaP in the world, with an average annual incidence rate of 165 per 100,000 in the period 2004-2008 and 229 per 100,000 in the period 2006-2010[4, 5]. The International Agency for Research on Cancer estimates that CaP is also the leading cancer in terms of incidence and mortality in men from Africa and the Caribbean[6]. Incidence and mortality rates are generally lower in men of European descent and even lower in men of Asian descent[4, 5].
Estimates of CaP incidence are highly variable around the world, and are influenced by health care access including screening and detection practices[7]. Detection of indolent prostate tumors has been common in the US, where prostate-specific antigen (PSA) screening has been widely used[8]. On the other hand, is likely that CaP rates in Sub-Saharan Africa (SSA) are substantially higher than those reported in existing registries[9]. In SSA, the infrastructure for population-based (or even hospital-based) capture of cancer cases is limited, and clinic-based studies are subject to referral and other forms of bias that may not accurately represent CaP rates in a population. Autopsy studies provide an alternative and valid metric of CaP in populations.
The prevalence of CaP can be accurately determined if a representative cross section of a population is evaluated in post-mortem examination. If comparable techniques are used to analyze autopsied prostate specimens, comparisons of CaP rates can be made across time, geography and other demographic characteristics. Here, a summary of CaP prevalence is provided that informs differences across populations and changes over time that is difficult to obtain using other approaches.
Accurate knowledge of the true prevalence of prostate cancer among different populations and age groups provide important information for the design and conduct of clinical trials and therapeutic decision making. Autopsy studies may indicate not only the presence or absence of prostate cancer in given population, but can characterize these cancers to a degree that is often not possible in clinical studies. For example, data from autopsied prostates can characterize grade, stage, multifocality and geographical locations of tumors within the prostate gland, and thus direct detection efforts.
Materials and Methods
Search Strategy
A systematic literature search of the PubMed and Web of Science databases was conducted searching on relevant terms “CaP”, “latent”, “autopsy”, and “prevalent”. Relevant articles included those that examined autopsy (cadaver) prostates and did not include data ascertained via surveys using cystoprostatectomy or other clinical approaches including treatment or screening series. In addition, reference and citation lists of all relevant publications found by this search (including review articles) were manually reviewed to identify additional articles. Summaries, comments and reviews were reviewed for reference but not used for the analysis in this study if they did not present primary data. All potentially eligible articles were reviewed in detail to confirm that the abovementioned inclusion/exclusion criteria were met. After exclusions were applied, 58 papers were identified that met all inclusion criteria (Table 1).
Table 1. Prevalence of Latent CaP As Estimated by Autopsy Studies.
| Age-Specific Prevalence (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| First Author | Year | N | Ethnicity/Location | Ages | Datesa | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | 90+ | Overall | Section Methodg |
|
| |||||||||||||||
| Albarran[2] | 1898 | 86 | France | NR | NR | 16.3 | NR | ||||||||
|
| |||||||||||||||
| Neller[31] | 1926 | 40 | Caucasian | 30-70 | NR | 17.5 | NR | ||||||||
|
| |||||||||||||||
| Caulk[32] | 1932 | NR | Caucasian | NR | NR | 20.0 | S | ||||||||
|
| |||||||||||||||
| Mintz[33] | 1934 | 100 | Caucasian | NR | NR | 13.0 | NR | ||||||||
|
| |||||||||||||||
| Muir[1] | 1934 | 54 | English | >50 | NR | 13.0 | NR | ||||||||
|
| |||||||||||||||
| Rich[34] | 1935 | 292 | Americanh | >50 | NR | 5.4 | 8.1 | 20.3 | 0f | 14.0 | R:3-4mm | ||||
|
| |||||||||||||||
| Moore[35] | 1935 | 375 | Austrian | 20-90 | 1931-32 | 0 | 0 | 17 | 14 | 23 | 21 | 29 | 20.5 | R:4 mm | |
|
| |||||||||||||||
| Graves[36] | 1935 | NR | Caucasian | NR | NR | 17.5 | S | ||||||||
|
| |||||||||||||||
| D'Abreu[37] | 1936 | NR | Caucasian | NR | NR | 16.0 | S | ||||||||
|
| |||||||||||||||
| Barringer[38] | 1937 | NR | Caucasian American | NR | NR | 17.4 | S | ||||||||
|
| |||||||||||||||
| Myers[39] | 1937 | NR | Caucasian American | NR | NR | 29.4 | S | ||||||||
|
| |||||||||||||||
| Walthard[40] | 1937 | 100 | Swiss | >40 | NR | 30.0 | S:3-4 mm | ||||||||
|
| |||||||||||||||
| Gaynor[41] | 1938 | 1040 | Austrian | 20-90 | 1935-37 | 0 | 4.0 | 4.9 | 10.4 | 17.8 | 28.3 | 38.7 | 40.0 | 18.4 | R:3-4 mm |
|
| |||||||||||||||
| Yotsuyanagi[42] | 1938 | 100 | Japanese | NR | NR | 3.0 | NR | ||||||||
|
| |||||||||||||||
| Kahler[43] | 1939 | 195 | Caucasian American | >50 | NR | 17.3 | R:3-4 mm | ||||||||
|
| |||||||||||||||
| Baron[44] | 1941 | 364 | Caucasian American | >50 | 1935-39 | 6.4 | 8.1 | 16 | 21 | 9.9 | R | ||||
| 50 | 42.1 | 38.1 | 66.7 | 100 | 46.0 | S | |||||||||
|
| |||||||||||||||
| Lowsley[45] | 1941 | 120 | Caucasian | >40 | NR | 12.5 | NR | ||||||||
|
| |||||||||||||||
| Abe[37] | 1943 | 550 | Japanese | NR | NR | 1.8 | R | ||||||||
|
| |||||||||||||||
| Quinland[46] | 1943 | 188 | African American | >50 | NR | 18.0 | NR | ||||||||
|
| |||||||||||||||
| Vernet[47] | 1944 | 210 | Caucasian | NR | NR | 25.0 | S | ||||||||
|
| |||||||||||||||
| Buchert[48] | 1947 | 135 | Caucasian | NR | NR | 13.0 | R | ||||||||
|
| |||||||||||||||
| Mathé[49] | 1947 | 130 | Caucasian | NR | NR | 8.4 | R | ||||||||
|
| |||||||||||||||
| Meyenburg[50] | 1948 | 100 | Caucasian | >40 | NR | 23.0 | NR | ||||||||
|
| |||||||||||||||
| Andrews[51] | 1949 | 142 | English | 15-79 | 1949 | 0 | 4 | 5.3 | 17.9 | 31.8 | 48 | 83 | 12.0 | S:3-4 mm | |
|
| |||||||||||||||
| Horstman[52] | 1952 | 118 | Caucasian | 40-86 | NR | 10.2 | NR | ||||||||
|
| |||||||||||||||
| Labess[53] | 1952 | 98 | Caucasian | 44-95 | NR | 9.2 | R | ||||||||
|
| |||||||||||||||
| Edwards[54] | 1953 | 81 | Canadian | >40 | 1942-45 | 14.8 | R:4 mm | ||||||||
| 173 | 3.9 | 5.2 | 9.1 | 8.1 | 2.9 | 16.7 | S:4 mm | ||||||||
|
| |||||||||||||||
| Hirst[11] | 1953 | 39 | Caucasian American | >80 | 1950-51 | 42.7 | 80.0 | 35.6 | S:4 mm | ||||||
|
| |||||||||||||||
| Franks[55] | 1954 | 178 | English | >50 | 1954 | 0 | 0 | 0 | 28.9 | 30.2 | 40.0 | 66.7 | 100 | 37.6 | S:3-4 mm |
|
| |||||||||||||||
| Oota[56] | 1958 | 203 | Japanese | NR | NR | 13.3 | NR | ||||||||
|
| |||||||||||||||
| Viitanen[57] | 1958 | NR | Finnish | >50 | NR | 14 | 21 | 30 | 0 | 22.0 | S | ||||
|
| |||||||||||||||
| Butler[46] | 1959 | 220 | Americanh | >50 | NR | 32.2 | R:6-8 mm | ||||||||
|
| |||||||||||||||
| Sugihara[58] | 1959 | 157 | Japanese | >40 | NR | 2.5 | 4.9 | 19.4 | 30.0 | 0.0 | 10.9 | NR | |||
|
| |||||||||||||||
| Imai[59] | 1960 | 129 | Japanese | >40 | NR | 0 | 8.8 | 9.7 | 16.7 | 25.0 | 7.7 | NR | |||
|
| |||||||||||||||
| Oota[59] b | 1961 | 259 | Japanese-Japan | >45 | 1959 | 5.0 | 6.6 | 13.6 | 35.8 | 45.5 | 50.0 | 18.1 | S:3-4 mm | ||
|
| |||||||||||||||
| Karube[60] | 1961 | 229 | Japanese-Japan | >40 | 1954-58 | 2.2 | 5.1 | 18.2 | 22.9 | 0 | 10.9 | S:3-4 mm | |||
|
| |||||||||||||||
| Strahan[61] | 1963 | 85 | Caucasian | 60-80 | NR | 17.6 | S:5 mm | ||||||||
| Schmalhorst[62] | 1964 | 98 | Americanh | 80-90 | NR | 17.5 | R | ||||||||
| 57.0 | S | ||||||||||||||
|
| |||||||||||||||
| Halpert[63] | 1966 | 407 | Americanh | 70-79 | NR | 17.4 | R | ||||||||
| 100 | 41.0 | S | |||||||||||||
|
| |||||||||||||||
| Scott[10] | 1969 | 5000 | Americanh | 30-80+ | 1949-63 | 1 | 1 | 3 | 5 | 9 | 10 f | R | |||
| 158 | 70+ | 36 | 45 f | 39 | S:4 mm | ||||||||||
|
| |||||||||||||||
| Halpert[64] | 1963 | 4696 | Caucasian American | 33-91 | NR | 8.8 | S:3-4 mm | ||||||||
|
| |||||||||||||||
| Liavåg[65] | 1968 | 324 | Norwegian | >40 | NR | 8.0 | 20.4 | 23.7 | 28.9 | 48.8 | 66.7 | 26.9 | R:4 mm | ||
|
| |||||||||||||||
| Lundberg[66] | 1970 | 3034 | Swedish | >40 | 1962-66 | 0.8 | 7.4 | 14.8 | 21.9 | 35.5 | 20.9 | R:5 mm | |||
|
| |||||||||||||||
| Akazaki[67] | 1973 | 158 | Japanese-Hawaii | >50 | 1969-72 | 10.7 | 20.8 | 22.9 | 57.1f | 29.1 | S:3 mm | ||||
| 239 | Japanese-Japan | 12.3 | 18.0 | 18.3 | 35.9f | 28.4 | |||||||||
|
| |||||||||||||||
| Breslow[18] | 1977 | 145 | German | >45 | NR | 28.4 | S:5 mm | ||||||||
| 306 | Swedish | 31.6 | |||||||||||||
| 173 | Hong Kong | 15.8 | |||||||||||||
| 242 | Singapore | 13.2 | |||||||||||||
| 168 | Jamaican | 29.8 | |||||||||||||
| 150 | Ugandan | 19.5 | |||||||||||||
| 143 | Israeli | 22.0 | |||||||||||||
|
| |||||||||||||||
| Guileyardo[19] | 1980 | 207 | African American | >50 | NR | 0.0 | 50c | 20d | 34.4 | 44.2e | 31.4 | S:3 mm | |||
| 293 | Caucasian American | >50 | NR | 0.0 | 0.0c | 23d | 31.6 | 40.7e | 29.0 | S:3 mm | |||||
|
| |||||||||||||||
| Yatani[68] b | 1982 | 253 | Caucasian American | >50 | 1969-78 | 34.6 | S:3 mm | ||||||||
| 178 | African American | 1969-78 | 36.9 | ||||||||||||
| 182 | Colombian | 1967-70 | 31.5 | ||||||||||||
| 417 | Japanese- Hawaii | 1969-78 | 25.6 | ||||||||||||
| 576 | Japanese-Japan | 1965-79 | 20.5 | ||||||||||||
|
| |||||||||||||||
| Yatani[69] b | 1988 | 576 | Japanese-Japan | >50 | 1965-79 | 22.5 | S:3 mm | ||||||||
| 576 | Japanese-Japan | 1982-86 | 34.6 | ||||||||||||
|
| |||||||||||||||
| Gatling[70] | 1990 | 1641 | American | NR | 1974-87 | 10.5 | R | ||||||||
|
| |||||||||||||||
| Stemmermann[71] | 1992 | 293 | Japanese-Hawaii | NR | 1970-99 | 19.0 | 22.0 | 33.0 | 63.0f | 27.0 | NR | ||||
|
| |||||||||||||||
| Takahashi[72] | 1992 | 29 | Japanese | >90 | NR | 58.6 | S | ||||||||
| Sakr[20] b | 1994 | 249 | Caucasian American | 20-69 | NR | 0.0 | 33.0 | 36.0 | 62.0 | 60.0 | 36.0 | S:2-3 mm | |||
| 249 | African American | 3.0 | 26.0 | 29.0 | 44.0 | 67.0 | 23.0 | ||||||||
|
| |||||||||||||||
| Shiraishi[73] | 1994 | 18 | Caucasian American | <50 | 1965-79 | 0.0 | 0.0 | 0.0 | S:3 mm | ||||||
| 18 | African American | 0.0 | 20.0 | 16.7 | |||||||||||
| 16 | Japanese-Hawaii | 0.0 | 9.0 | 6.3 | |||||||||||
| 47 | Japanese-Japan | 0.0 | 4.0 | 4.3 | |||||||||||
| 128 | Japanese-Japan | 6.0 | 17.0 | 14.1 | |||||||||||
|
| |||||||||||||||
| Sanchez-Chapado[14] | 2003 | 162 | Spanish | 20-80 | NR | 3.6 | 8.8 | 14.3 | 23.8 | 31.7 | 33.3 | 18.5 | S:3-4 mm | ||
|
| |||||||||||||||
| Stamatiou[15] | 2006 | 212 | Greek | 30-98 | 2002-04 | 0.0 | 0.0 | 2.6 | 5.2 | 13.8 | 30.9 | 40.0f | 18.8 | S:4 mm | |
|
| |||||||||||||||
| Konety[74] | 2005 | 3307 | Americanh | >40 | 1955-60 | 0 | 1.0 | 4.5 | 7.4 | 8.9 | 22.9 | 4.8 | R:4 mm | ||
| 2938 | 1991-01 | 0 | 0.8 | 2.0 | 2.5 | 3.2 | 0.0 | 1.2 | |||||||
|
| |||||||||||||||
| Soos[16] | 2005 | 139 | Hungarian | 18-95 | NR | 0.0 | 15.0 | 26.6 | 32.1 | 50.0 | 64.7 | 86.6f | 38.8 | S:3-5 mm | |
|
| |||||||||||||||
| Haas[75] | 2007 | 164 | Mixed | 54-73 | NR | 29.0 | S:4 mm | ||||||||
|
| |||||||||||||||
| Zare-Mirzaie[17] | 2012 | 149 | Iranian | 50-91 | 2008-09 | 9.4 | S:3-5 mm | ||||||||
|
| |||||||||||||||
| Zlotta[76] | 2013 | 220 | Russian | 22-80 | 2008-11 | 37.3 | S:4 mm | ||||||||
| 78 | Japanese-Japan | 24-89 | 34.6 | ||||||||||||
Period prevalence interval.
Only the larger or more recent of studies from a group are included here; earlier studies with possible sample overlap are not presented.
Ages 30-44
Ages 44-60
Ages 60+
Ages 80+
S=Step Sectioned; R=Single, Routine or Random Sections. Value in mm indicates the section interval, if available.
Race/Ethnicity not specified
NR: Not reported
It has been well-documented that differences in methods for the detection of tumors in autopsy prostates can lead to very different estimates of CaP prevalence[10-12]. Therefore, autopsy evaluation technique information was determined that included step-sectioning methods[12] (“S”, Table 1), random/single sections (“R”) of the prostate.
Analysis of Prevalence, Incidence, and Duration
Incidence is defined here as the number of new cases of a disease in a population, and the incidence (density) rate is the number of new cases of disease in given time period divided by the total number in population at risk at that time period (i.e., person-time)[13]. Prevalence is defined here as the number of individuals in a population who have the condition in a particular time period, i.e., the number of cases existing at the start of the period and the number of new cases that arise during that period[13]. The prevalence rate is the proportion of a population affected by the condition in a specified time period. We use here the term “prevalence” to be the period prevalence rate, defined as the total number of individuals accrued over a time period. This period differed from study to study.
Assuming a population is stable and the incidence and prevalence rates are unchanging, the relationship of incidence and prevalence rates are well known as It=Pp/D(1–Pp),where It is the person-time incidence rate, Pp the period prevalence rate, and D the average duration of the disease from diagnosis until recovery or death[13]. This relationship was used to evaluate prevalence data with a subset of studies for which incidence data were available.
Statistical analyses were performed to evaluate the relationship of prevalence rates by age, year of report, and race using descriptive methods, non-parametric Kruskal-Wallis analysis of variance, and linear regression. Analytic weights were used to consider the sample size involved in the reported averages. We used weights that were proportional to the variance of an observation (e.g., mean prevalence in a group) such that the variance of the jth observation is assumed to be σ2/wj, where σ2 is the total variance and wj are the weights defined by the number of observations used to determine the sample means and variances. Spearman rank correlation coefficients were also estimated. All computations were undertaken using STATA 12.0 (College Station, TX).
Results
Table 1 summarizes the literature of latent CaP identified by autopsy studies. Data in African descent men (particularly at older ages) are limited, with more data in Asian men, and the most data in European descent men. Earlier studies tended to use random or single section prostate evaluations, while more recent data rely on serial section evaluations.
The reasons for differences in cancer rates across time or by geography can be explained by a number of factors, including differences in methods for the detection of prostate tumors in an autopsy sample[10-12]. A comparison of step section vs. random/single section data (Table 1) indicate the mean prevalence of CaP is higher (25.2%) among step sectioned tissues than random/single section tissues (16.1%; χ22=0.02, p=0.002). This difference remained even after adjustment for study year. Therefore, most analyses considered the method of sectioning used to report prevalences. However, year of publication was significantly correlated with section technique (Spearman's rho=0.3755, p=0.0014), so the potential for mulitcollinearity among these variables limited out ability to include both in the same linear model.
Unlike incidence, it is not expected that the use of PSA screening should change CaP prevalence. A comparison of prevalences before and after the widespread use of PSA screening suggested no substantial differences before or after 1990 (χ21=0.021, p=0.885). Therefore, all subsequent analyses considered only step-section data, and did not consider whether the study occurred before or after the PSA era.
Effect of Age, Race, Geography, and Time on Prevalence
Using only prevalences estimated from serial section data (Table 1), the overall weighted prevalence of CaP was 19.9% in men of Asian descent, 26.7% in men of European descent, and 26.2% in men of African descent. These prevalences were not significantly different from one another overall (χ22=4.05, p=0.132), but prevalences were higher in African and European descent men combined (26.6%) than in Asian descent men (19.9%, χ21=4.05, p=0.044)
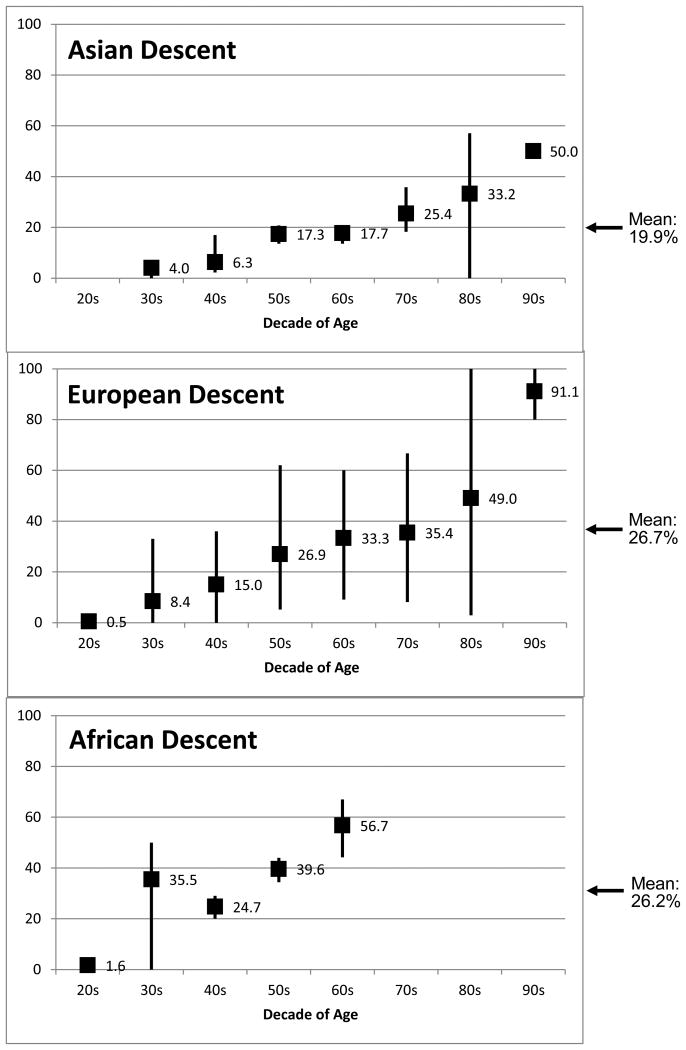
The age-specific prevalence distribution reflects these overall race-specific prevalences (Figure 1). The age-specific distribution of mean CaP prevalence was generally highest at all ages in African, lower in European, and lowest in Asian descent populations. Asian descent men reached a mean peak value of 50% prevalence in men over age 90, while the prevalence at the oldest age group reached a mean of 91.1% in European descent men. Prevalences in older African descent men (i.e., over age 70) have not been reported in the literature. CaP prevalence in African descent men in their 60's (56.7%) was similar to European descent men in their 80's (49%) and Asian descent men in their 90's (50%; Figure 1). While essentially no men are clinically diagnosed with CaP before age 40, 4% of Asian descent men, 8% of European descent men, and 35% of African descent men were estimated to have latent CaP in their 30's.
Figure 1. CaP Prevalence Estimates by Decade of Age and Race: Weighted mean percentage of prostates found to have CaP; Vertical bars denote observed ranges.
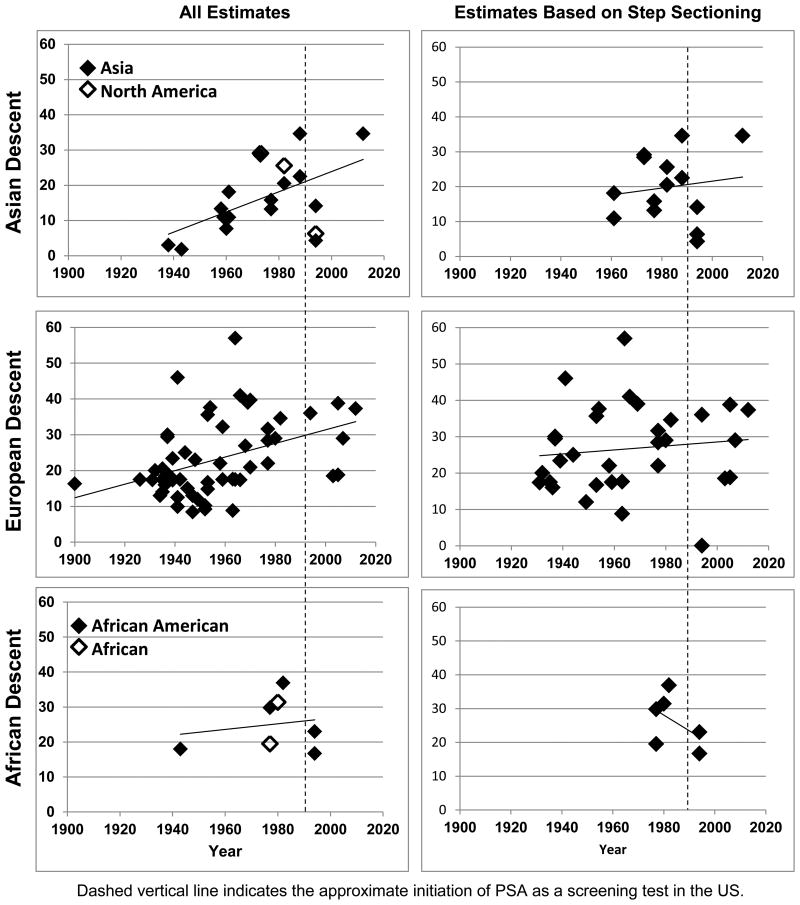
As suggested by the near complete overlap of prevalence estimates by geography in Figure 2, there was no difference in prevalence between Asians living in Asia or North America (including Hawaii; χ21=0.451, p=0.502), or African Americans vs. Africans (χ21=0.150, p=0.699). However, the sample sizes available for these comparisons remain small. While not depicted in Figure 2, there was also no difference in prevalence between European descent men living in Europe vs. North America (χ21=0.001, p=0.970).
Figure 2. CaP Prevalence Estimates by Year and Race.
Figure 2 suggests that there have been increases in CaP prevalence over time. However, these effects are enhanced when including older, non-step-section data. Using step section data, only European descent men showed significant weighted regression effects with time (β=0.32, p=0.043), with non-significant regression coefficients for men of Asian descent (β=0.31, p=0.137) or African descent (β=-0.30, p=0.420). These data suggest that for every year of observed data, there has been an increase in CaP prevalence of 0.32% in European descent men.
Incidence – Prevalence - Duration
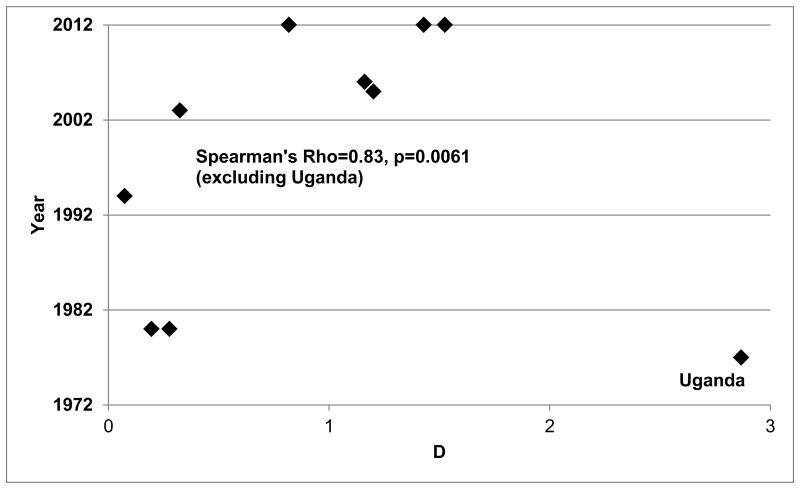
Incidence and prevalence data representative of major ethic/geographical groups representing Europeans[14-16], Iranians[17], Ugandans[18], Japanese, Caucasian Americans[19], African Americans[19, 20]. Only prevalence studies that used a consistent step-sectioning method for prostate evaluation were included. Incidence rates were obtained for US men by using SEER[4] data and for non-US men using GLOBOCAN data[3]. Ugandan incidence rates were obtained from the report of Wabinga et al.[21] Incidence rates estimated closest to the period in which the prevalences were estimated. Figure 3 shows that over time, the duration of disease, measured by the metric D, has increased, with a correlation between year and D of 0.826 (p-value=0.0061). However, this strong relationship was only apparent when one data point (Uganda[21]) was excluded. As shown in Figure 3, the estimate of D (2.87) for Uganda is substantially greater than in any other country or time period. This suggests either that CaP duration was substantially longer among Ugandans in the 1970s than in any other group, or the estimate of prevalence is inflated, or the reported estimate of CaP incidence is lower than it should be. It is unlikely that CaP duration in that period was significantly longer in Ugandan men than in any other population, including more recent data. It is possible that the prevalence estimate was inflated if the ascertainment of autopsy subjects was preferential toward men at high risk of CaP. However, there is no evidence from the original publication that this was the case[18]. While the data reported by Wabinga et al.[21] appear to be accurate given the information that was available (i.e., CaPs that came to clinical attention), the most likely explanation is that the incidence of CaP was underestimated in Uganda. This inference is supported by other data that support the hypothesis that CaP is under-ascertained in Sub-Saharan Africa[7]. These data also support the hypothesis that the duration of CaP is increasing with time (i.e., men are living longer with CaP than in the past). This may in part be explained by the observation that screening and improved treatment have had beneficial effects on CaP survival.
Figure 3. Relationship of Incidence, Prevalence and Duration.
Discussion
Most of the data used to characterize prostate cancers in clinical populations come from either biopsy or radical prostatectomy specimens. This information, however, is only available for those tumors which became clinically evident and detected; the characteristics of early prostate cancers which have not been diagnosed are much more difficult. Only cystoprostatectomy and autopsy studies can accurately describe the characteristics of these clinically undetected tumors. Recent studies[22] evaluated size, grade, multifocality, location and even optimal biopsy strategies to locate such lesions. This knowledge has been incorporated into strategies of risk stratification[23], prostate biopsy strategies[24] and guidelines for the early detection of prostate cancer[25]
This report demonstrates that autopsy studies of latent CaP can provide information about differences by race as well as changes in CaP occurrence over time using data from historical data. The data presented here confirm that CaP prevalence is highest in men of African descent, lower in men of European descent, and lowest in men of Asian descent. These data also demonstrate that these relative differences are present at most ages, and that CaP increases with age in all races. Why may these trends have been observed? Changes in methodology and clinical practice could change the probability of detecting a prostate tumor. This is likely given the effects on prevalence we identified that compared serial section vs. random or single section methods. However, the effect of technique cannot completely explain the differences observed here. PSA screening would be likely to change the clinical detection of cancers, but it is not expected to influence the prevalence of cancer in a population. The present data suggest no differences in CaP prevalence before or after the onset of widespread PSA screening. Demographics or changes in lifestyle or exposure may also explain increases in CaP over time or with age. Increasing lifespan is one reasonable explanation for increasing CaP prevalence. Since the data presented here stretch back as far as 1900, these differences could well reflect lifespan changes. According to the US Census, European descent men in the US had a life expectancy at birth of 48.2 years in 1900, 66.3 years in 1950 and 74.8 years in 2000. Given that age is the single most salient risk factor for CaP, this is a likely explanation for the temporal trends in prevalence.
Changing lifestyle is also a potential explanation for differences in the occurrence of CaP over time. However, few if any risk factors have been identified that may explain changes in CaP occurrence (risk)[26]. Thus, if changes in exposure or lifestyle explain these changes over time, it is not possible at this time to know which factors may have played a role. Finally, the short period of time over which these data were collected suggests that genetics is not a major explanation for the increase in CaP prevalence, although genetic susceptibility to changing environments could explain these observed patterns.
While these data present a unique perspective about CaP, there are limitations that temper the inferences that can be made using the information presented here. While an advantage of autopsy studies is that a relatively consistent approach can be used across time and populations to estimate cancer prevalence, there is still likely to be a great deal of variability in methods and data quality that cannot be adequately evaluated. This is particularly true for older studies (e.g., before 1960) where methods may not have been presented in detail. Thus, increases in CaP prevalence over time could reflect actual changes to the prevalence of CaP, or improvements in pathology methods that allowed improved detection of tumors over time. The data presented here clearly suggest that systematic step sectioning methods may have improved the capture of prostate tumors compared with random or single sections that have been used in some reports (Table 1). In addition, the epidemiological methods for many studies do not clearly define the sampling design or any inclusion/exclusion criteria used. In addition, data from populations in the developing world (e.g., Africa, South America, South and Southeast Asia) are particularly limited.
Information derived from autopsy studies also has relevance to the design of recent clinical trials which can lead to public health policy, detection and treatment guidelines, and everyday clinical practice. The recently conducted Prostate Cancer Prevention Trial[27] utilized data from autopsy studies to predict baseline prostate cancer prevalence in the target population. Their finding of nearly 25% prostate cancers in the control arm of the study (in men aged 55-70 with PSA <4 ng/ml, who received placebo only), presumably represent typical men biopsied only due to study requirement, is consistent with data from the autopsy study of Sakr et al.[28, 29].
Recent efforts to develop nomograms and Risk Calculators to help guide physician recommendation and patient decision to undergo prostate biopsy and to select appropriate management also rely on autopsy studies to inform of the prevalence and characteristics of prostate cancers in particular populations[23].
American Urological Association (AUA) recommendations on the technique of prostate biopsies to detect cancer were based, at least partially on information on the location of cancers and optimum targeting of specific regions of the prostate as derived from autopsy data[22, 30]. The autopsy prevalence of prostate cancer in young US and European men was also a key consideration in the recently published AUA Guidelines for the Early Detection of Prostate Cancer. The guidelines advised against prostate cancer screening in men under the age of 40 and referred to the low prevalence of cancers found in US and European men under the age of 40 in autopsy studies[28]. These examples demonstrate the importance of the identification and characterization of prostate cancers in autopsy studies for the design of rational studies and clinical recommendations. CaP is common in men at all ages, even in young men, and varies by race. Temporal trends in prostate cancer prevalence in all populations suggest that increased use of screening is the only explanation for increased prostate cancer occurrence. Autopsy studies of latent CaP can provide valuable information to inform epidemiological, etiological, and clinical research at a variety of levels. Limitations in the data, including more limited data in men of African descent, are required to fill gaps in this body of knowledge.
Acknowledgments
Funding: This work was supported grants from the National Institutes of Health R01-CA085074, P50-CA105641, and P60-MD006900, the Commonwealth of Pennsylvania, and the AACR-Landon Foundation.
Footnotes
Financial Disclosures: None
References
- 1.Muir E. Carcinoma of the Prostate. The Lancet. 1934:668–672. [Google Scholar]
- 2.Albarran J, Halle N. Hypertrophie et Neoplasies Epitheliales de la Prostate. Annales des Maladies Org Genitoiun. 1898;XVI:797–801. [Google Scholar]
- 3.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.SEER. Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute; [Google Scholar]
- 5.Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute; 2011. SEER Cancer Statistics Review, 1975-2008. [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2008 doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Rebbeck TR, Devesa SS, Chang BL, Bunker CH, Cheng I, Cooney K, Eeles R, Fernandez P, Giri VN, Gueye SM, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer. 2013;2013:560857. doi: 10.1155/2013/560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, Feuer EJ. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 9.Osegbe DN. Prostate cancer in Nigerians: facts and nonfacts. J Urol. 1997;157(4):1340–1343. [PubMed] [Google Scholar]
- 10.Scott R, Mutchnik DL, Laskowski TZ, Schmalhorst WR. Carcinoma of the prostate in elderly men: incidence, growth characteristics and clinical significance. J Urol. 1969;101(4):602–607. doi: 10.1016/s0022-5347(17)62388-7. [DOI] [PubMed] [Google Scholar]
- 11.HIRST AE, BERGMAN RT. Carcinoma of the prostate in men 80 or more years old. Cancer. 1954;7(1):136–141. doi: 10.1002/1097-0142(195401)7:1<136::aid-cncr2820070114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Schütze U. [Latent prostatic carcinoma--an autopsy study of men over 50 years of age] Zentralbl Allg Pathol. 1984;129(4):357–364. [PubMed] [Google Scholar]
- 13.Rothman K, Greenland S, TL L. Modern Epidemiology. Third. Philadelphia: Lippincott, Williams and Wilkins; 2008. [Google Scholar]
- 14.Sanchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238–247. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- 15.Stamatiou K, Alevizos A, Perimeni D, Sofras F, Agapitos E. Frequency of impalpable prostate adenocarcinoma and precancerous conditions in Greek male population: an autopsy study. Prostate Cancer Prostatic Dis. 2006;9(1):45–49. doi: 10.1038/sj.pcan.4500847. [DOI] [PubMed] [Google Scholar]
- 16.Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Eur Urol. 2005;48(5):739–744. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Zare-Mirzaie A, Balvayeh P, Imamhadi MA, Lotfi M. The frequency of latent prostate carcinoma in autopsies of over 50 years old males, the Iranian experience. Med J Islam Repub Iran. 2012;26(2):73–77. [PMC free article] [PubMed] [Google Scholar]
- 18.Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977;20(5):680–688. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 19.Guileyardo JM, Johnson WD, Welsh RA, Akazaki K, Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. J Natl Cancer Inst. 1980;65(2):311–316. [PubMed] [Google Scholar]
- 20.Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo. 1994;8(3):439–443. [PubMed] [Google Scholar]
- 21.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br J Cancer. 2000;82(9):1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas GP, Delongchamps NB, Jones RF, Chandan V, Serio AM, Vickers AJ, Jumbelic M, Threatte G, Korets R, Lilja H, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99(19):1484–1489. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 23.Bul M, Delongchamps NB, Steyerberg EW, de la Roza G, van Leeuwen PJ, Zhu X, van Vugt HA, Haas GP, Schröder FH, Roobol MJ. Updating the prostate cancer risk indicator for contemporary biopsy schemes. Can J Urol. 2011;18(2):5625–5629. [PubMed] [Google Scholar]
- 24.Bjurlin MA, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D, Wheeler TM, Schlossberg S, Penson DF, Taneja SS. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013;189(6):2039–2046. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boffetta P, Tubiana M, Hill C, Boniol M, Aurengo A, Masse R, Valleron AJ, Monier R, de Thé G, Boyle P, et al. The causes of cancer in France. Ann Oncol. 2009;20(3):550–555. doi: 10.1093/annonc/mdn597. [DOI] [PubMed] [Google Scholar]
- 27.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 28.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 Pt 1):379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 29.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 30.Bjurlin M, Carter H, Schellhammer P, Cookson M, Gomella L, Troyer D, Wheeler T, Schlossberg S, Penson D, Taneja S. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013;189(6):2039–2046. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neller V, Neüburger K. Ueber atypische Epithelwucherungen und beginnende Karzinome in der senilen Prostata. 1926;73:57–59. [Google Scholar]
- 32.Caulk, Bonn-Itt Latent prostatic cancer. American Journal of Cancer. 1932;16:1024. [Google Scholar]
- 33.Mintz R, Smith G. AUTOPSY FINDINGS IN 100 CASES OF PROSTATIC CANCER. New Engl J Med. 1934;211(11):479–489. [Google Scholar]
- 34.Rich On the frequency of occult carcinoma of the prostate. J Urol. 1935;33:315–323. [Google Scholar]
- 35.Moore R. Latent Prostatic Cancer. J Urol. 1935;33:224. [Google Scholar]
- 36.Graves R, Militzer R. Carcinoma of the prostate with metastases. J Urol. 1935;33:235–251. [Google Scholar]
- 37.d'Abreu Urologie Rev. 1936;40:12. [Google Scholar]
- 38.Barringer B. Prostate Cancer. Am J Roentgenol. 1937;37:49. [Google Scholar]
- 39.Myers G. Prostate Cancer. Colorado Med. 1937;34:248. [Google Scholar]
- 40.Walthard Z. Prostatenkrebs. Zeitschrift Urol Chir. 1931;32:411. [Google Scholar]
- 41.Gaynor E. Prostate Cancer. Virchows Archive Path Anat. 1938;301:602–656. [Google Scholar]
- 42.Yotsuyanagi S. Prostate Cancer. Gann. 1938;32:191. [Google Scholar]
- 43.Kahler J. Prostate Cancer. J Urol (Baltimore) 1939;41:557–574. [Google Scholar]
- 44.Baron E, Angrist A. Prostate Cancer. Arch Pathol. 1941;32:787–793. [Google Scholar]
- 45.Lowsley O. Surgical and nonsurgical treatment of prostate cancer. Bulletin of the NY Acad Sci. 1941:651–673. [PMC free article] [PubMed] [Google Scholar]
- 46.BUTLER J, BRAUNSTEIN H, FREIMAN DG, GALL EA. Incidence, distribution, and enzymatic activity of carcinoma of the prostate gland. Arch Pathol. 1959;68:243–251. [PubMed] [Google Scholar]
- 47.Vernet S. Cancer des prostata. Barcelona. 1944:17. [Google Scholar]
- 48.BUCHERT WI, CULP DA, JONES GH. Carcinoma of the prostate gland; an analysis of 135 consecutive cases. Pa Med J. 1947;51(2):165–173. [PubMed] [Google Scholar]
- 49.MATHE CP, ARDILA CE. Carcinoma of the prostate; review of 130 cases treated between 1940 and 1946. Surg Gynecol Obstet. 1947;84(3):276–282. [PubMed] [Google Scholar]
- 50.Meyenburg V, et al. Prostate Cancer. Schweiz Med Wochenschrift. 1948;78:473. [PubMed] [Google Scholar]
- 51.Andrews GS. Latent Carcinoma of the Prostate. J Clin Pathol. 1949;2(3):197–208. doi: 10.1136/jcp.2.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horstmann W. Neoplastic diseases of the prostate, especially of the carcinoma. Z Urol. 45(1):50–59. [PubMed] [Google Scholar]
- 53.LABESS M. Occult carcinoma in clinically benign hypertrophy of the prostate; a pathological and clinical study. J Urol. 1952;68(6):893–896. doi: 10.1016/S0022-5347(17)68299-5. [DOI] [PubMed] [Google Scholar]
- 54.EDWARDS CN, STEINTHORSSON E, NICHOLSON D. An autopsy study of latent prostatic cancer. Cancer. 1953;6(3):531–554. doi: 10.1002/1097-0142(195305)6:3<531::aid-cncr2820060311>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 55.FRANKS LM. Latent carcinoma of the prostate. J Pathol Bacteriol. 1954;68(2):603–616. doi: 10.1002/path.1700680233. [DOI] [PubMed] [Google Scholar]
- 56.Oota K, Misu Y. Gann. 1958;49(Suppl):283–284. [Google Scholar]
- 57.VIITANEN I, VON HELLENS A. Latent carcinoma of the prostate in Finland; preliminary report. Acta Pathol Microbiol Scand. 1958;44(1):64–67. doi: 10.1111/j.1699-0463.1958.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 58.Sugihara G. Gann. 1959;50(Suppl):163–164. [Google Scholar]
- 59.Oota K. Latent carcinoma of the prostate among the Japanese. Acta Un Int Cancer. 1961;17:952–957. [Google Scholar]
- 60.KARUBE K. Study of latent carcinoma of the prostate in the Japanese based on necropsy material. Tohoku J Exp Med. 1961;74:265–285. doi: 10.1620/tjem.74.265. [DOI] [PubMed] [Google Scholar]
- 61.STRAHAN RW. Carcinoma of the prostate: incidence, origin, pathology. J Urol. 1963;89:875–880. doi: 10.1016/S0022-5347(17)64665-2. [DOI] [PubMed] [Google Scholar]
- 62.SCHMALHORST WR, HALPERT B. CARCINOMA OF THE PROSTATE GLAND IN PATIENTS MORE THAN 80 YEARS OLD. Am J Clin Pathol. 1964;42:170–173. doi: 10.1093/ajcp/42.2.170. [DOI] [PubMed] [Google Scholar]
- 63.Halpert B, Schmalhorst WR. Carcinoma of the prostate in patients 70 to 79 years old. Cancer. 1966;19(5):695–698. doi: 10.1002/1097-0142(196605)19:5<695::aid-cncr2820190515>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 64.HALPERT B, SHEEHAN EE, SCHMALHORST WR, SCOTT R. Carcinoma of the prostate. A survey of 5,000 autopsies. Cancer. 1963;16:737–742. doi: 10.1002/1097-0142(196306)16:6<737::aid-cncr2820160608>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 65.Liavåg I. The localization of prostatic carcinoma. An autopsy study. Scand J Urol Nephrol. 1968;2(2):65–71. doi: 10.3109/00365596809136971. [DOI] [PubMed] [Google Scholar]
- 66.Lundberg S, Berge T. Prostatic carcinoma. An autopsy study. Scand J Urol Nephrol. 1970;4(2):93–97. doi: 10.3109/00365597009137581. [DOI] [PubMed] [Google Scholar]
- 67.Akazaki K, Stemmerman GN. Comparative study of latent carcinoma of the prostate among Japanese in Japan and Hawaii. J Natl Cancer Inst. 1973;50(5):1137–1144. doi: 10.1093/jnci/50.5.1137. [DOI] [PubMed] [Google Scholar]
- 68.Yatani R, Chigusa I, Akazaki K, Stemmermann GN, Welsh RA, Correa P. Geographic pathology of latent prostatic carcinoma. Int J Cancer. 1982;29(6):611–616. doi: 10.1002/ijc.2910290602. [DOI] [PubMed] [Google Scholar]
- 69.Yatani R, Shiraishi T, Nakakuki K, Kusano I, Takanari H, Hayashi T, Stemmermann GN. Trends in frequency of latent prostate carcinoma in Japan from 1965-1979 to 1982-1986. J Natl Cancer Inst. 1988;80(9):683–687. doi: 10.1093/jnci/80.9.683. [DOI] [PubMed] [Google Scholar]
- 70.Gatling RR. Prostate carcinoma: an autopsy evaluation of the influence of age, tumor grade, and therapy on tumor biology. South Med J. 1990;83(7):782–784. doi: 10.1097/00007611-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 71.Stemmermann GN, Nomura AM, Chyou PH, Yatani R. A prospective comparison of prostate cancer at autopsy and as a clinical event: the Hawaii Japanese experience. Cancer Epidemiol Biomarkers Prev. 1992;1(3):189–193. [PubMed] [Google Scholar]
- 72.Takahashi S, Shirai T, Hasegawa R, Imaida K, Ito N. Latent prostatic carcinomas found at autopsy in men over 90 years old. Jpn J Clin Oncol. 1992;22(2):117–121. [PubMed] [Google Scholar]
- 73.Shiraishi T, Watanabe M, Matsuura H, Kusano I, Yatani R, Stemmermann GN. The frequency of latent prostatic carcinoma in young males: the Japanese experience. Vivo. 1994;8(3):445–447. [PubMed] [Google Scholar]
- 74.Konety BR, Bird VY, Deorah S, Dahmoush L. Comparison of the incidence of latent prostate cancer detected at autopsy before and after the prostate specific antigen era. J Urol. 2005;174(5):1785–1788. doi: 10.1097/01.ju.0000177470.84735.55. discussion 1788. [DOI] [PubMed] [Google Scholar]
- 75.Delongchamps NB, Singh A, Haas GP. Epidemiology of prostate cancer in Africa: another step in the understanding of the disease? Curr Probl Cancer. 2007;31(3):226–236. doi: 10.1016/j.currproblcancer.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Van der Kwast TH, Roobol MJ. Defining the threshold for significant versus insignificant prostate cancer. Nat Rev Urol. 2013 doi: 10.1038/nrurol.2013.112. [DOI] [PubMed] [Google Scholar]