Abstract
AALL08P1 was designed to determine if biweekly intensified pegaspargase (I-PEG) was feasible and safe in pediatric patients with newly diagnosed high risk B-precursor lymphoblastic leukemia when given with Children’s Oncology Group hemi-augmented BFM therapy. High Risk-Average (HR-Avg) patients received standard pegaspargase (S-PEG) dosing (6 doses) while High Risk-High (HR-High) patients received I-PEG biweekly from the start of Consolidation until day 1 of Maintenance. Feasibility and safety were defined in advance as ≥ 65% of patients tolerating at least 8 doses of I-PEG and 90% requiring ≤49 weeks from day 1 of Consolidation to the initiation of Maintenance. Targeted toxicities included allergic reactions, anaphylaxis, pancreatitis, thrombosis, bleeding, central nervous system events and sepsis. AALL08P1 enrolled 104 patients; 54 were classified as HR-Avg and 30 as HR-High after completion of induction therapy. Only 53% (16/30) of the HR-High patients received ≥ 8 total doses of I-PEG and 50% (15/30) took ≤ 49 weeks from start of Consolidation to the initiation of Maintenance. I-PEG did not significantly increase grade 2–5 targeted toxicities. I-PEG was not feasible or safe as defined in AALL08P1. Complete assessment of this regimen was limited due to removal of patients from I-PEG regimen and early closure of the study.
Keywords: intensified asparaginase, acute lymphoblastic leukemia, high risk, toxicities
Introduction
Relapse is the most common cause of treatment failure for children and adolescents with high risk B-precursor acute lymphoblastic leukemia (HR-B-ALL).1–8 Intensification of asparaginase therapy has been shown to improve outcomes across multiple studies, both when used in a continuous or discontinuous fashion.1–4, 9–14 The Dana-Farber Cancer Institute (DFCI) study 77–01 demonstrated that continuous weekly asparaginase decreased relapse rates and subsequent DFCI studies including further intensification of asparaginase have led to additional improvements in outcomes.3,4,10 The Children’s Cancer Group (CCG) 1882 demonstrated that intensification through an augmented Berlin-Frankfurt-Munster (ABFM) backbone including the addition of discontinuous administration of asparaginase improved outcomes in patients with slow early response to therapy and subsequent CCG and Children’s Oncology Group high risk (HR) B-ALL studies have included this intensification.1,2,6 We hypothesized that further intensification of asparaginase therapy through continuous every other week administration on the COG hemi-ABFM (hABFM) regimen would improve outcome for children, adolescents and young adults with HR-B-ALL by diminishing relapse rates.
AALL08P1 was a COG limited institution safety and feasibility pilot study designed for the treatment of newly diagnosed patients with HR-B-ALL ages 1–30 years. The COG hABFM backbone with one interim maintenance (IM) and one delayed intensification (DI) phase is significantly more myelosuppressive than the DFCI backbone, potentially increasing the risk of infectious complications when adding additional asparaginase doses in this pilot study. The primary aim of AALL08P1 was to determine whether adding biweekly intravenous (IV) intensive pegasparagase (I-PEG) beginning in Consolidation and ending at day 1 of Maintenance to the hABFM regimen was safe and feasible in children with HR-B-ALL. Targeted toxicities including allergic reactions, anaphylaxis, thromboembolic events, bleeding, CNS events, sepsis and pancreatitis are described.
Materials and Methods
Patients
Subjects 1 to 30.99 years old at one of the COG centers involved in this limited institution pilot study were eligible to enroll in AALL08P1 if they had newly diagnosed, previously untreated B-precursor ALL with NCI HR features (initial white blood cell count (WBC) ≥50,000/microliter and/or age at least 10 years). The diagnosis of ALL was based on morphology, cytochemistry and immunophenotyping. Minimal residual disease (MRD) testing and cytogenetic analyses were performed at COG reference laboratories and COG approved local institutional cytogenetic laboratories.5 The protocol was approved by the National Cancer Institute (NCI) and the Institutional Review Boards at COG member institutions. Informed consent was obtained from the patients and /or their parent(s)/guardian(s) according to federal guidelines.
Treatment
The treatment backbone for AALL08P1 was the hABFM-based prednisone/Capizzi methotrexate plus asparaginase (PC) regimen of COG AALL0232.6 The PC arm included a 4-drug prednisone-based Induction with a single dose of IV pegaspargase on Day 4, followed by an 8-week Consolidation phase, a single 8-week IM phase with Capizzi escalating IV methotrexate (MTX) and an eight week DI phase (Table 1).
TABLE 1.
Treatment Summary for HR-High ** and HR-Avg Patients
| Induction (35 days) |
| Vincristine 1.5 mg/m2 (max 2 mg) days 1, 8, 15, 22 |
| Prednisone 30 mg/m2/dose bid days 1–28 |
| Daunorubicin 25 mg/m2/dose days 1, 8, 15, 22 |
| PEG asparaginase 2,500 IU/m2/dose IV day 4 |
| Intrathecal Methotrexate (age adjusted) Day 8, 29; days 15 and 22 for CNS3 |
| Consolidation (56 days or 8 weeks)* |
| Cyclophosphamide 1000 mg/m2/dose days 1 and 29 |
| Cytarabine 75 mg/m2/dose days 1–4, 8–11, 29–32, 36–39 |
| Mercaptopurine 60 mg/m2/dose days 1–14 and 29–42 |
| Vincristine 1.5 mg/m2/dose days 15, 22, 43, 50 |
| PEG asparaginase 2500 IU/m2/dose IV days 15 and 43 (HR-high: *** Intensified PEG days 1 and every 14 days thereafter) |
| Intrathecal Methotrexate (age adjusted) days 1, 8, 15 and 22 |
| Interim Maintenance (56 days or 8 weeks) |
| Vincristine 1.5 mg/m2/dose days 1, 11, 21, 31 and 41. |
| Methotrexate starting dose 100 mg/m2/dose escalate by 50 mg/m2/dose days 1, 11, 21, 31 and 41. |
| PEG asparaginase 2500 IU/m2/dose IV days 2 and 22 (HR-high: Intensified PEG every 14 days) |
| Intrathecal Methotrexate (age adjusted) days 1 and 31 |
| Delayed Intensification (56 days or 8 weeks) |
| Vincristine 1.5 mg/m2/dose days 1, 8, 15, 43, 50 |
| Dexamethasone 5 mg/m2/dose bid days 1–7 and 15–21 |
| Doxorubicin 25 mg/m2/dose days 1, 8 and 15 |
| PEG asparaginase 2500 IU/m2/dose IV days 4 and 43 (Intensified PEG every 14 days) |
| Cyclophosphamide 1000 mg/m2/dose day 29 |
| Thioguanine 60 mg/m2/dose days 29–42 |
| Intrathecal Methotrexate (age adjusted) days 1, 29 and 36 |
| Maintenance (each cycle 84 days or 12 weeks) |
| Vincristine 1.5 mg/m2/dose days 1, 29, 57 |
| Prednisone 20 mg/m2/dose bid × 5 days every 4 weeks |
| Mercaptopurine 75 mg/m2/dose days 1–84 |
| Intrathecal methotrexate (age adjusted) Days 1 and 29 Cycle 1–4; then Day 1 only in subsequent cycles) |
| Methotrexate 20 mg/m2/dose PO weekly (omit days of intrathecal Methotrexate) |
Patients with CNS3 disease would receive cranial XRT (1800 cGy) during Consolidation. Patients with testicular disease would receive testicular XRT during Consolidation (2400 cGy)
Patients HR-High (except for CNS3) would receive cranial prophylactic XRT (1200 cGy) during the first four weeks of Maintenance
A maximum of 14 doses of I-PEG was allowed if patients did not encounter delays in therapy
Following completion of Induction, patients were stratified into two groups based on clinical features, molecular characteristics and early treatment response. Patients were defined as HR-High if they had any of the following features: corticosteroid treatment prior to study enrollment, central nervous system (CNS) or testicular leukemia, end induction bone marrow (BM) MRD ≥ 0.01% by flow cytometry or M2 (5–25% blasts) by morphology, an MLL gene rearrangement, severe hypodiploidy with < 44 chromosomes and/or DNA index < 0.81 or other evidence of a hypodiploid clone. The remaining patients were deemed HR-Average (HR-Avg). Patients found to be Philadelphia chromosome positive were not eligible to receive post-induction therapy on AALL08P1.
The HR-Avg group received standard hABFM therapy (AALL0232 PC arm) with pegaspargase given 2500 IU/m2/dose IV for a total of 6 doses post-Induction, two each in Consolidation, IM and DI (S-PEG). The HR-High patients received hABFM with pegaspargase 2500 IU/m2/dose given IV every other week beginning with Consolidation and continuing through completion of DI (I-PEG) (Table 1 and Figure 1). Patients continued to receive pegaspargase during delays in chemotherapy, and were limited to a maximum of 14 doses of I-PEG.
FIGURE 1. Treatment Schema Overview (includes prophylactic cranial radiation for HR-High and therapeutic cranial radiation for CNS 3).
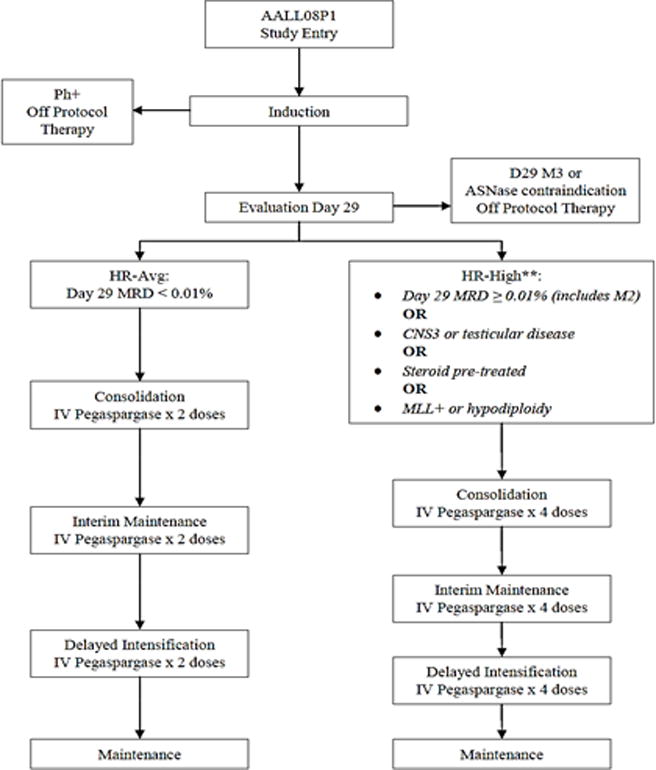
**HR-High patients with pegaspargase allergy during post-induction therapy were taken off protocol therapy and treated with best available therapy
The study temporarily closed 4/1/2010 to investigate due two post-Induction deaths on the HR-High arm. After investigation, these deaths were not deemed directly attributable to I-PEG and the study reopened 9/13/2010. Toxicity was originally reported using the NCI Common Toxicity Criteria Adverse Event (CTCAE) version 3.0, but, at the direction of the NCI, was changed to CTCAE version 4.0 effective in October 2011 with mapping of previously reported adverse events (AEs) from version 3.0 to version 4.0. HR-Avg patients who developed an anaphylaxis/allergic reaction to pegaspargase were switched to receive Erwinia asparaginase and remained on study. HR-High patients who developed an anaphylaxis/allergic reaction to pegaspargase were removed from AALL08P1, as the intent of the study was to determine feasibility of intensified pegaspargase and one determinant of feasibility was the ability to receive intensified pegaspargase therapy without developing an allergic reaction. In the setting of pancreatitis grade 3–4, PEG-aspargase was discontinued if this occurred in either arm. For mild pancreatitis, asparaginase was recommended to be held until symptoms resolved and amylase returned to normal. For other asparaginase related toxicity such as thrombosis grade 3–4, asparaginase had to be withhold until symptoms resolved or proper antithrombotic therapy initiated. With CNS related bleeding, thrombosis or infarction, asparaginase could be restarted after resolution of symptoms or treatment of adverse event. Asparaginase silent inactivation and pharmacokinetics were not monitored as part of the study.
During conduct of AALL08P1, results from the parallel COG AALL0232 HR B-ALL trial became available demonstrating improved EFS for all HR-ALL patients treated with high dose MTX (HDMTX) compared with Capizzi MTX during IM.6 As well, children <10 years old treated with HD-MTX during IM and with two weeks of dexamethasone during Induction, rather than 4 weeks of prednisone, had improved EFS.7 Consequently, AALL08P1 closed to new patient enrollment on 3/21/2011 and therapy was modified to incorporate HDMTX in IM for all patients with HR B-ALL who had not yet completed the first cycle of Maintenance therapy or received cranial radiation (see Figure, Supplemental Digital Content 1).
Statistical Methods
Data frozen as of 9/30/2013 are included in this report. Accrual of 150 patients (45 HR-High and 105 HR-Avg) was originally planned; the results from 104 eligible patients are reported here. The study design defined I-PEG as feasible if ≥ 65% of the patients assigned to receive I-PEG tolerated at least 8 of the 12 (or 8 of the maximum 14 doses I-PEG) planned total doses of every other week pegaspargase therapy. Another a priori endpoint was completion of the intensive phases of therapy without unacceptable delays. The study design defined I-PEG therapy as safe if ≥90% of patients required ≤49 weeks to complete the specified therapy from day 1 of Consolidation to day 1 of Maintenance. Descriptive statistics were calculated for baseline characteristics including frequencies and medians. Two-sided chi-square tests were used to compare key frequencies and proportions between the risk groups. Two-sided Fisher’s exact tests were performed when expected sample sizes were inadequate. Statistical significance was defined as p<0.05. All analyses were performed using SAS® software v. 9.2.
Results
Patient population
AALL08P1 enrolled a total of 104 eligible patients between 2/23/09 and 3/21/11 at 18 COG member institutions. Characteristics of the patients at ALL diagnosis are summarized in Table 2. Three patients found to have Philadelphia chromosome positive ALL did not receive post-Induction therapy on AALL08P1. Factors such as gender, race, ethnicity, age and total WBC were not significantly different between HR-Avg and HR-High arms (Table 2).
TABLE 2.
Patient Characteristics for HR-Avg and HR-High ALL#
| Total* (n=104) |
HR-Avg (n=54) |
HR-High (n=30) |
* P values | |
|---|---|---|---|---|
| Gender | 0.41 | |||
| Male | 62 (60%) | 31 (57%) | 20 (67%) | |
| Female | 42 (40%) | 23 (43%) | 10 (33%) | |
| Race | 0.53 | |||
| White | 68 (65%) | 36 (67%) | 22 (73%) | |
| African American | 7 (7%) | 3 (6%) | 2 (7%) | |
| Asian Indian | 2 (2%) | 1 (2%) | 1 (3%) | |
| Vietnamese | 1 (1%) | 0 (0%) | 1 (3%) | |
| Other Asian | 3 (3%) | 2 (4%) | 1 (3%) | |
| Pacific Islander | 2 (2%) | 0 (0%) | 0 (0%) | |
| Other | 15 (14%) | 9 (17%) | 2 (7%) | |
| Unknown | 6 (6%) | 3 (6%) | 1 (3%) | |
| Ethnicity | 0.08 | |||
| Non Hispanic | 67 (64%) | 39 (72%) | 16 (53%) | |
| Hispanic | 37 (36%) | 15 (28%) | 14 (47%) | |
| Age | 0.61 | |||
| 10+ years | 69 (66%) | 33 (61%) | 20 (67%) | |
| < 10 years | 35 (34%) | 21 (39%) | 10 (33%) | |
| WBC (per μl) | 0.95 | |||
| <50,000 | 60 (58%) | 31 (57%) | 17 (57%) | |
| ≥50,000 | 44 (42%) | 23 (43%) | 13 (43%) | |
| CNS Status | ||||
| 1 | 87 (84%) | 47 (87%) | 22 (73%) | |
| 2 | 13 (13%) | 7 (13) | 6 (20%) | |
| 3 | 4 (4%) | 0 (0%) | 2 (7%) | |
| Testicular | ||||
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | |
| No | 62 (60%) | 31 (57%) | 19 (63%) | |
| N/A | 42 (40%) | 23 (43%) | 9 (37%) | |
| Steroid Pre-treated | ||||
| Yes | 5 (5%) | 0 (0%) | 5 (17%) | |
| No | 99 (95%) | 54 (100%) | 25 (83%) | |
| Hypodiploidy | ||||
| Yes | 3 (3%) | 0 (0%) | 2 (7%) | |
| No | 101 (97%) | 54 (100%) | 28 (93%) | |
| MLL-Rearrangement | ||||
| Yes | 4 (4%) | 0 (0%) | 4 (13%) | |
| No | 100 (96%) | 54 (100%) | 26 (87%) | |
| Age | ||||
| 10+ years | 69 (66%) | 33 (61%) | 20 (67%) | |
| < 10 years | 35 (34%) | 21 (39%) | 10 (33%) |
No between risk-group comparisons for patient characteristic distributions were statistically significant. Risk group assignment variables including CNS Status, Testicular, Steroid, Hypodiploidy, and MLL-R were not tested.
104 patients enrolled, 20 patients off protocol therapy post-induction, thus 84 proceeded to post-induction therapy.
P values comparison among HR-Avg and HR-High groups.
Outcome of Induction therapy
A single death occurred during Induction therapy on day 23 following a cerebrovascular accident and another death occurred eight days following completion of Induction therapy, prior to beginning Consolidation, due to respiratory failure (presumed fungal infection). Ninety-eight of the remaining 103 patients (95.1%) enrolled had day 29 bone marrow evaluation/response completed (Figure 2). Ninety-six of the 98 patients (98%) entered complete remission at end Induction with one patient having an M2 marrow and another with an M3 (≥25% blasts) marrow. Twenty patients came off protocol therapy prior to post-Induction treatment of which 5 were due to adverse events (Figure 2). Thus, 84/104 (80.8%) subjects continued with post-Induction therapy with 54 HR-Avg assigned to S-PEG and 30 HR-High assigned to I-PEG.
FIGURE 2. Consort Flowchart.

Feasibility
The Consolidation, IM, and DI phases were each scheduled to take 8 weeks to complete; thus, HR-High patients on I-PEG should have received at least 4 doses of pegaspargase during each phase. However, this was found not to be feasible as defined in the protocol. Sixty percent (18/30) of HR-High patients starting Consolidation therapy received ≥ 4 doses of pegaspargase however 12/30 (40%) patients came off protocol therapy during this phase. During IM, 77.7% (14/18) and during DI, 61.1% (11/18) of patients completed ≥ 4 doses of pegaspargase (Table 3). Only 16/30 (53.3%) of those who started Consolidation on I-PEG and 14/15 (93.3%) of those completing DI received 8 or more total doses of pegaspargase. HR-High patients removed from study prior to completion of planned therapy (n=16) included: physician’s choice (n=2); parent refusal (n=1); second malignancy (n=2); death (n=1); non-compliance (n=1); relapsed (n=1) and adverse events (n=8) of which 6 were anaphylaxis-allergic reactions (5 in Consolidation and 1 in DI), 1 pancreatitis and 1 due to seizure/thromboembolic event. Fourteen HR-High patients (46.6%) completed planned protocol therapy. In summary, 53% of I-PEG patients were removed early from study with about half of this group (27%) secondary due to an adverse event. Nine of 54 (16.7%) patients in the S-PEG arm were removed prior to completion of planned therapy for the following reasons: physician’s choice (3); parent refusal (1); relapse (3); patient moved to non-participating facility (1) and therapy error (1). Comparison of percentage of patients coming off study was significantly higher in the I-PEG arm compared to the S-PEG arm (53% vs 17%, p<0.001).
TABLE 3.
Summary of Doses of Asparaginase Received per Phase for S-PEG and I-PEG Treatment Groups
| S-PEG | I-PEG | |||||
|---|---|---|---|---|---|---|
| Reporting Period | Starting RP | Off Tx during RP | Received 2 doses of PEG in RP | Starting RP | Off Tx during RP | Received ≥ 4 doses of PEG in RP |
| Consolidation | 54 | 0 | 41 | 30 | 12 | 18 |
| IM | 54 | 0 | 36 | 18 | 0 | 14 |
| DI | 54 | 1 | 34 | 18 | 3 | 11 |
RP: reporting period
The backbone hABFM regimen included two planned doses of pegaspargase during each of the 3 phases, which was received by 41/54 (75.9%) HR-Avg patients during Consolidation, 36/54 (66.6%) during IM, and 34/54 (63%) during DI (one HR-Avg patient went off protocol therapy during DI) (Table 3).
Safety
The primary determinant of safety was taking ≤49 weeks from start of Consolidation to initiation of Maintenance. This was achieved by 15/30 HR-High (50%) patients. Fourteen of these patients completed planned protocol therapy. No patients took longer than 49 weeks to reach Maintenance. The time from beginning of Consolidation to coming off therapy for the patients on I-PEG ranged from 1 week to 30.9 weeks. As described previously, of the sixteen HR-High patients removed from protocol therapy eight were due to adverse events. The time period for successful completion of the specified therapy (Consolidation to initiation of Maintenance) varied between 25.6 and 39.6 weeks (see Figure, Supplemental Digital Content 2). The average time for completion of therapy from beginning Consolidation to first Maintenance for 53 HR-Avg was 30.0 weeks (sd=1.98) and for the 15 HR-High patients 31.2 weeks (sd=3.9).
Toxicity
Post-Induction targeted toxicities are summarized in Table 4. Anaphylaxis and CNS toxicities were the most frequently reported targeted toxicities and were comparable among the S-PEG and I-PEG arms.
TABLE 4.
Summary Targeted Toxicities Grade 2–5 During Each Reporting Phase*
| Reporting Period | S-PEG | I-PEG |
|---|---|---|
| Consolidation | N=54 | N=30 |
| Anaphylaxis | 9 (17%) | 5 (17%)** |
| Allergy | 4 (7%) | 2 (6%)** |
| Pancreatitis | 1 (2%) | 1 (3%) |
| Thrombosis | 2 (4%) | 1 (3%) |
| Bleeding | 0 (0%) | 1 (3%) |
| CNS | 1 (2%) | 2 (7%) |
| Sepsis | 0 (0%) | 0 (0%) |
| Interim Maintenance | N=54 | N=18 |
| Anaphylaxis | 0 (0%) | 0 (0%) |
| Allergy | 2 (4%) | 0 (0%) |
| Pancreatitis | 0 (0%) | 0 (0%) |
| Thrombosis | 0 (0%) | 0 (0%) |
| Bleeding | 0 (0%) | 0 (0%) |
| CNS | 1 (2%) | 1 (6%) |
| Sepsis | 0 (0%) | 0 (0%) |
| Delayed Intensification | N=54 | N=18 |
| Anaphylaxis | 1 (2%) | 0 (0%) |
| Allergy | 1 (2%) | 1 (3%)** |
| Pancreatitis | 2 (4%) | 1 (6%) |
| Thrombosis | 0 (0%) | 0 (0%) |
| Bleeding | 0 (0%) | 0 (0%) |
| CNS | 1 (2%) | 1 (6%) |
| Sepsis | 0 (0%) | 1 (6%) |
No comparisons were statistically significant P>0.05
Four of five patients with anaphylaxis in Consolidation were removed from study. One was not removed as it was felt to be unrelated to treatment. One grade 3 allergic adverse event during Consolidation was also removed from study. One grade 2 allergy adverse event during DI, patient was removed from study.
Post-Induction Grade 2–5 non-targeted/non-hematologic toxicities for each phase are described below. During Consolidation there were 30 evaluable patients on the I-PEG arm and 54 on the S-PEG arm with toxicities as follows: typhlitis: S-PEG (4%) and I-PEG (0%); hyperbilirubinemia: S-PEG (4%) and I-PEG (17%) (Fisher’s exact test 2- sided p=0.092). Hypoalbuminemia was observed on I-PEG only (3%).
During IM there were 18 evaluable patients on I-PEG and 54 on S-PEG. Grade 2–5 toxicities as described above included: ALT elevation-S-PEG (13%) and I-PEG (6%); AST elevations-S-PEG (7%) and I-PEG (0%): hyperbilirubinemia S-PEG (4%), I-PEG (11%).
Seventy-two patients were evaluable for DI with 18 on I-PEG and 54 on S-PEG. Grade 2–5 toxicities included: ALT elevation S-PEG (9%), I-PEG (33%) (Fisher’s exact test 2-sided p=0.023); AST elevation in 1 patient on S-PEG (2%) and 2(11%) on the I-PEG (Fisher’s exact test 2-sided p=0.19); hyperbilirubinemia: S-PEG (4%) and I-PEG (17%) (Fisher’s exact test 2-sided p=0.10). Pancreatitis occurred in two patients on S-PEG (4%) and 1 patient (6%) on I-PEG.
There were 68 evaluable patients who entered at Maintenance Cycle 1 including only half (15/30) of patients on I-PEG and 53/54 (98%) on S-PEG. Of those, the following non-hematologic toxicities were observed: ALT and AST elevations were rare though ALT elevations were slightly more common on S-PEG (9%) than I-PEG (7%); bilirubin elevations were rare on both arms: S-PEG (2%) and I-PEG (7%). Grade 2–4 osteonecrosis/avascular necrosis (AVN) was reported in 8 patients throughout maintenance with all but one patient affected on S-PEG. Additionally, 2 patients on the I-PEG arm had osteonecrosis/AVN reported at 2 years follow-up (with none in follow-up on the S-PEG arm). A total of 9 patients received HDMTX as per study guidelines after the results of AALL 0232 became known: 6 HR-Avg and 3 HR-High.
Two post-induction deaths were reported on I-PEG: one death occurred during Consolidation (day 57) due to gram negative sepsis and multiorgan system failure and the other death occurred 44 days after last protocol therapy due to Candida tropicalis cerebral microabscesses and Candida sepsis (off protocol therapy day 15 Consolidation). Neither death was attributed to the I-PEG therapy. There was one additional death 32 days after last protocol therapy (off protocol therapy day 30 Induction) due to respiratory failure. In summary, 5 deaths occurred during treatment or after completion of treatment: Induction (n=1), post-induction prior to Consolidation (n=1), Consolidation I-PEG (n=1), follow-up period (n=2) with one on I-PEG and one that was removed during Induction.
Three secondary malignancies were reported. One HR-High patient was diagnosed with secondary acute myeloid leukemia (AML) during Consolidation after receiving only 2 doses of pegaspargase. A second HR-High patient was diagnosed with therapy related myelodysplastic syndrome (MDS) during Maintenance cycle 9. A third patient was diagnosed with acute biphenotypic leukemia 2 years after completion of therapy though the patient was removed prior to post-induction therapy due to an adverse event and was not risk stratified.
Twenty-five osteonecrosis/AVN events were reported in 10 patients: 13% (7/54) HR-Avg and 10% (3/30) HR-High. The average age at diagnosis was: 16.0 years in HR-Avg and 14.2 years in the HR-High patients. Two of the seven HR-Avg and one of 3 HR-High patients developing osteonecrosis/AVN were female. Most of the events were reported during the first few cycles of Maintenance therapy.
Although hematological toxicity was not the primary toxicity focus of the study, grade 2–5 toxicities were similar between arms: I-PEG grade 3 (47%) and grade 4 (70%); S-PEG grade 3 (61%) and grade 4 (74%). Myelosuppression grade 2–5 was comparable across the different phases in both I-PEG and S-PEG (see Table 6, Supplemental Digital Content 1). The overall grade 2–5 infection rate (febrile neutropenia and sepsis) was low with no differences between HR-Avg and HR-High arms. Infectious toxicity during DI in both treatment arms was not different: I-PEG 50% (9/18) vs S-PEG 41% (22/54) (p=0.49).
The 3-year overall survival (OS) (n=104) was 83.5 ± 4.2% and the 3-year event free survival for the entire cohort of patients was 75.1 ± 5.0%. The 3-year OS using the beginning of consolidation for the HR-Avg (n=50) was 100% and for the HR-High (n=34) 80 ± 8.2%. The 3-year EFS was 92.0 ± 4.5% for the HR-Avg and 69.0 ± 9.6% for the HR-High.
Adverse events reported to AdEERS include the following: six events Grade 5: I-PEG: encephalitis (n=1), infection NOS (n=1). S-PEG arm: no Grade 5 events. Induction: CNS cerebrovascular ischemia (n=1), pneumonitis (n=1), disseminated aspergillosis (n=1), death (n=1). Grade 4: twenty-six events total. I-PEG: hypotension (n=2), typhlitis (n=1), intracranial hemorrhage (n=2), adult respiratory distress syndrome (n=1), hypoxia (n=1), neutrophil count decreased (n=1), platelet count decreased (n=1), thromboembolic event (n=1), seizure (n=1). S-PEG: seizure (n=1), CNS ischemia (n=1). Induction: hypotension (n=1), bilirubin increased (n=1), posterior reversible encephalopathy (n=2), adult respiratory distress syndrome (n=1), hypoxia (n=1), neutrophil count decreased (n=1), disseminated intravascular coagulation (DIC) (n=1), thromboembolic (n=1), infection blood (n=1), infection paranasal (n=1), infection trachea (n=1), sepsis (n=1). Grade 3: eight events: I-PEG: colitis (n=1), fibrinogen (n=1). S-PEG: none. Induction: infection blood (n=1), seizure (n=2), hypertension (n=1), infection blood (n=1), lower gastrointestinal bleed (n=1); Grade 2: Induction: hypertension (n=1).
Discussion
Several previous pediatric ALL studies have investigated intensification of asparaginase therapy.1–4, 8–13 Protocol 77-01 (DFCI study) used an intensified asparaginase approach in pre-B ALL. Patients received asparaginase during consolidation (50,000 IU/m2 IV every other day children < 6 years old and 25,000 IU/m2 IV every other day for children > 6 years old 5 doses total). All patients received prolonged intensification with vincristine, prednisone and doxorubicin and half were randomized to receive weekly high-dose asparaginase versus no asparaginase. There were fewer treatment failures in the asparaginase treatment arm (2-sided p=0.04). Asparaginase toxicity occurred in 8% of the patients and was self-limited but precluded further asparaginase use in these patients. The major toxicity encountered was cardiomyopathy in 14% of the patients related to doxorubicin use.10 DFCI 91-01 compared L-asparaginase 25,000 IU/m2 weekly for 30 consecutive doses to pegaspargase 2500 IU/m2 every other week for 15 doses with a backbone chemotherapy consisting of vincristine, steroids (prednisone or dexamethasone), doxorubicin, mercaptopurine and methotrexate.3 The 5-year EFS for all patients (standard and high risk ALL) was 83% ± 2 % which was superior to their predecessor studies (p=0.03). In multivariate analysis, patients receiving at least 26 weeks of asparaginase therapy (pegaspargase or E coli asparaginase) had improved survival compared to those receiving less therapy (<26 weeks) (p<0.01).3 The Pediatric Oncology Group (POG) study 8704 had a backbone with alternating combinations of vincristine, doxorubicin, cyclophosphamide, prednisone, cytarabine, mercaptopurine, teniposide and a randomization with native asparaginase (25000 IU/m2 for 20 weeks) during Consolidation.9 The 4-year EFS for patients with T cell- ALL treated in the intensified asparaginase arm was 68% compared to 55% in the standard arm (p=0.002). Allergic reactions and pancreatitis were more common in the intensified arm but with no significant difference in the reported incidence of neutropenia or sepsis. However, there was an overall incidence of secondary MDS/AML in 3.3% of patients with a fourfold increase for those in the intensified arm, suggesting that intensified asparaginase might augment the leukemogenic potential of epipodophyllotoxins.9,14 POG 9296 attempted to use prolonged asparaginase depletion like POG 8704 but using methotrexate and mercaptopurine to replace teniposide and cytarabine in order to reduce the risk for a secondary malignancy.15 Pegaspargase dosing was modified from the originally planned 20 weeks (Pegaspargase × 10 doses every 2 weeks) to monthly dosing for 5 doses over 20 weeks due to concerns of excessive toxicity with the biweekly schedule (particularly pancreatitis and severe malnutrition). The incidence of allergic reactions was 2.3% and pancreatitis 0.7%. Of note, no patients developed secondary MDS/AML. The 5-year EFS was 68% for patients with T-cell ALL and 81% for patients with non-Hodgkin’s lymphoma.
The POG 9203 pilot study (n=34) investigated a combined biweekly intensified pegaspargase with IV methotrexate (1 gm/m2 over 24 hours) plus IV mercaptopurine (1gm/m2 over 6 hours) and cytarabine IV (1 gm/m2 over 24 hours) alternating every 2 weeks in HR ALL patients. Excessive toxicities attributed to pegaspargase and cytarabine due to associated myelosuppression resulted in early closure of the study.8 Asparaginase-associated myelosuppression independent of the type of asparaginase product (E. coli vs pegaspargase) was described in the DFCI ALL Consortium Protocol 05-01.16 Intensified asparaginase had also demonstrated its efficacy in relapse ALL. POG 9310 was designed for patients with pre-B ALL with first bone marrow relapse or extramedullary relapse.11 Patients were reinduced with doxorubicin (day 1), prednisone (28 days), vincristine (weekly × 4) and PEG-aspargase weekly or biweekly by randomization. Asparaginase levels and antibodies were measured weekly and before asparaginase administration. Complete remissions were significantly higher in the weekly arm (97%) versus biweekly (82%) (p=0.003) and responses were also significantly higher in those patients with higher asparaginase levels (p=0.012). Grade 3–4 infectious toxicities were common (50%) with 4 deaths related to sepsis during induction. Hypersensitivity to asparaginase was rare (4%).
Other groups such as the International Berlin-Frankfurt-Muenster Study Group (I-BFM-SG) and the Italian group (Associazone Italiana Ematologia Pediatrica (AEIOP) had reported their experience with asparaginase intensification in pediatric ALL.12,13 The I-BFM-SG showed an improved DFS at 10 years using a prolonged high-dose asparaginase approach during continuation (20 weekly Erwinia asparaginase 25,000 IU/m2) with a reduced BFM type therapy in children with standard risk ALL (DFS 87.5% high dose asparaginase versus 78.7% no high dose asparaginase).12 The Italian group in contrast did not show a significant difference in DFS for intermediate risk ALL treated with high dose asparaginase (25,000 IU/m2 total 20 weeks) versus standard asparaginase (4 standard doses 10,000 IU/m2) (75.7% vs 72.4% respectively, p=0.64).13
Asparaginase intensification has also been tested in adult ALL patients (ages 18–50 years) using the DFCI Pediatric ALL consortium regimen which utilized a 30-week course of pharmacokinetically dose-adjusted E. coli L-asparaginase during Consolidation.17 The primary focus of their study was to determine the feasibility of administering weekly IM E.coli asparaginase during the 30 week intensification phase. Sixty-three percent of the patients (36/57) completed all 30 doses of asparaginase. Asparaginase-related toxicities included allergic reactions (5%), thrombosis (17%) and pancreatitis (11%). The 4-year DFS was 71% (95% CI 58–81%) demonstrating that a pediatric based regimen for teens and adults was feasible and associated with tolerable toxicity with improved outcomes compared to historical regimens in young adults with ALL.17
These data suggested that intensification of pegaspargase therapy on the COG hABFM backbone might improve outcome, and AALL 08P1 was designed as a safety and feasibility pilot for this approach with I-PEG designed to be given every two weeks from the start of Consolidation until the end of DI (maximum 14 doses) regardless of blood count recovery in HR-High patients. While I-PEG did not significantly increase the likelihood of the targeted toxicities (allergic reactions, anaphylaxis, pancreatitis, thromboembolic events or sepsis) when compared to S-PEG, eight of the 16 patients coming off study were due adverse events of which 6 were anaphylaxis-allergic reactions in the HR-High arm. In contrast to some of the above referenced studies, AALL08P1 showed comparable grade 2–5 hematological and infectious toxicities between I-PEG and S-PEG. Myelosuppression was similar among the I-PEG and S-PEG arms, perhaps explaining the low and similar proportion of patients that developed sepsis/infectious complications. Despite the similar toxicity profiles, 53% of the patients receiving therapy on the I-PEG arm came off study due to an adverse event or physician/patient choice prior to starting Maintenance therapy in contrast to only 17% in the S-PEG arm. Only 16/30 patients on the I-PEG arm completed at least 8 doses of pegaspargase and only 15/30 reached Maintenance therapy in ≤49 weeks from the start of Consolidation. Perhaps, pharmacokinetically dose-adjusted asparaginase as tested in previous DFCI studies, could have helped with asparaginase dosing and administration and at the same time, limiting the amount of toxicity while allowing ongoing therapy. However, pharmacokinetics and pharmacodynamics were not part of this pilot study. These findings suggest that the addition of I-PEG to hABFM regimen was not feasible or safe as defined by the parameters of this study.
Although not a targeted toxicity, a non-significant increase in grade 2–4 hyperbilirubinemia and ALT elevation was more frequent in the I-PEG in contrast to the S-PEG. Similar to other reports, hyperbilirubinemia was more frequent in older patients (>13 years old). Older patients seemed to have more asparaginase induced toxicity compared to younger patients as demonstrated by the DFCI results.3,4, 17
Abnormalities in liver function have been previously reported in patients receiving asparaginase for ALL.17–24 Older patients (> 18 years) treated with asparaginase are more susceptible to liver toxicity when compared to children less than 18 years of age (3% versus 14%) though liver function test abnormalities were typically reversible within 2–3 weeks after administration of the drug.18–21 However, hepatic steatosis was observed up to 87% of patients for greater than 8 months after cessation of asparaginase therapy.20–24
Thrombosis and pancreatitis are potentially life-threatening toxicities encountered with asparaginase therapy, most commonly described in older patients.3,4,17,25–27 Despite reported incidences of thromboembolism in children with ALL of about 3.2% (range 1.1–36.7%), thromboembolic events were rare on AALL08P1.25–27 The concomitant administration of asparaginase and corticosteroids can increase the risk of thromboembolism.26,27 On AALL08P1, patients were rarely exposed to the concomitant use of corticosteroids except for two weeks during DI. This limited exposure may explain the rare occurrence of thromboembolic events observed on AALL08P1.
Early closure of the study and the high drop-out rate for patients on the HR-High arm limit our complete assessment of the I-PEG regimen. I-PEG with hABFM backbone demonstrated similar targeted toxicity rates when compared to S-PEG. However, a remarkably higher percentage (53% vs 17%) of patients came off study in the I-PEG arm in contrast to the S-PEG, suggesting that I-PEG with hABFM is not clinically feasible. Similar to previous studies, hepatic toxicity reflected as hyperbilirubinemia and liver transaminases elevation were more common in older children receiving I-PEG asparaginase. Given the superior EFS for high risk patients receiving HD MTX compared to Capizzi methotrexate during IM on the PC arm of AALL0232, AALL08P1 was closed to allow subsequent patients to be treated on the successor study, AALL1131, in which all patients received HD MTX. No meaningful analysis can be performed at this time for the patients switching to Erwinia asparaginase or IM HDMTX. The COG is not currently planning to test this schedule of intensified pegaspargase therapy in successor high risk ALL trials.
Supplementary Material
SDC 1. Treatment modification of AALL08P1 based on results of AALL0232 demonstrating EFS advantage for patients who received high dose methotrexate compared to Capizzi methotrexate. Therapy modifications recommended were dependent on the stage of therapy patients were currently receiving on AALL08P1.
SDC 2. Patient count versus weeks to successful completion from start of Consolidation to start of Maintenance for the 15 patients on the I-PEG arm who reached Maintenance.
SDC 3. Table. Myelosuppression Grade 3–4 (decreased WBC and/or decrease neutrophil) Rates by Treatment and Reporting Period.
SDC 4–7: Survival analysis.
Acknowledgments
Supported by grants from National Cancer Institute to the Children’s Oncology Group (Chair’s Grant CA98543 and CA180886 and Statistics and Data Center Grant CA98413 and CA180886).
Dr. S Hunger has received consulting fees from Sigma Tau Pharmaceuticals and Jazz Pharmaceuticals.
References
- 1.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 2.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 4.Silverman LB, Declerck L, Gelber RD, et al. Results of Dana-Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981–1995) Leukemia. 2000;14:2247–2256. doi: 10.1038/sj.leu.2401980. [DOI] [PubMed] [Google Scholar]
- 5.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen EC, Salzer WL, Devidas M, et al. Comparison of high-dose methotrexate (HD-MTX) with Capizzi methotrexate plus asparaginase (C-MTX/ASNase) in children and young adults with high-risk acute lymphoblastic leukemia (HR-ALL): A report from the Children’s Oncology Group Study AALL0232. J Clin Oncol. 2011;29(suppl) doi: 10.1200/JCO.2015.62.4544. abstract 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winick N, Salzer WL, Devidas M, et al. Dexamethasone (DEX) versus prednisone (PRED) during induction for children with high-risk acute lymphoblastic leukemia (HR-ALL): A report from the Children’s Oncology Group Study AALL 0232. J Clin Oncol. 2011;29(suppl) abstr 9504. [Google Scholar]
- 8.Salzer WL, Devidas M, Shuster JJ, et al. Intensified PEG-L-asparaginase and antimetabolite-based therapy for treatment of higher risk precursor-B acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2007;29:369–375. doi: 10.1097/MPH.0b013e3180640d54. [DOI] [PubMed] [Google Scholar]
- 9.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 10.Sallan SE, Hitchcock-Bryan S, Gelber R, et al. Influence of Intensive Asparaginase in the Treatment of Childhood Non-T-Cell Acute Lymphoblastic Leukemia. Cancer Res. 1983;43:5601–5607. [PubMed] [Google Scholar]
- 11.Abshire TC, Pollock BH, Billet AL, et al. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapse acute lymphoblastic leukemia: a pediatric oncology group study. Blood. 2000;96:1709–1715. [PubMed] [Google Scholar]
- 12.Pession A, Valsecchi MG, Masera G, et al. Long-Term Results of a Randomized Trial on Extended Use of High Dose L-Asparaginase for Standard Risk Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2005;23:7161–7167. doi: 10.1200/JCO.2005.11.411. [DOI] [PubMed] [Google Scholar]
- 13.Rizzari C, Valsecchi MG, Arico M, et al. Effect of Protracted High-Dose L-Asparaginase Given as a Second Exposure in a Berlin-Frankfurt-Münster-Based Treatment: Results of the Randomized 9102 Intermediate-Risk Childhood Acute Lymphoblastic Leukemia Study-A Report From the Associazione Italiana Ematologia Oncologia Pediatrica. J Clin Oncol. 2001;19:1297–1303. doi: 10.1200/JCO.2001.19.5.1297. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Relling MV, Behm FG, et al. L-asparaginase may potentiate the leukemogenic effect of the epipodophyllotoxins. Leukemia. 1995;9:1680–1684. [PubMed] [Google Scholar]
- 15.Winter SS, Holdsworth MT, Devidas M, et al. Antimetabolite-based therapy in childhood T-cell acute lymphoblastic leukemia: a report of POG study 9296. Pediatr Blood Cancer. 2006;46:179–186. doi: 10.1002/pbc.20429. [DOI] [PubMed] [Google Scholar]
- 16.Merryman R, Stevenson KE, Gostic WJ, et al. Asparaginase-Associated Myelosuppression and Effects on Dosing of Other Chemotherapeutic Agents in Childhood Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2012;59:925–927. doi: 10.1002/pbc.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2014;229:1–9. doi: 10.1038/leu.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oettgen HF, Stephenson PA, Schwartz MK, et al. Toxicity of E. coli L-asparaginase in man. Cancer. 1970;25:220–228. doi: 10.1002/1097-0142(197002)25:2<253::aid-cncr2820250204>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Ohnuma T, Holland JF, Freeman A, et al. Biochemical and pharmacological studies with asparaginase in man. Cancer Res. 1970;30:2297–2305. [PubMed] [Google Scholar]
- 20.Pratt CB, Simone JV, Zee P, et al. Comparison of daily versus weekly L-asparaginase for the treatment of childhood acute leukemia. J Pediatr. 1970;77:474–483. doi: 10.1016/s0022-3476(70)80023-3. [DOI] [PubMed] [Google Scholar]
- 21.Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk and Lymph. 2011;52:2237–2253. doi: 10.3109/10428194.2011.596963. [DOI] [PubMed] [Google Scholar]
- 22.Pratt CB, Johnson WW. Duration and severity of fatty metamorphosis of the liver following L-asparaginase therapy. Cancer. 1971;28:361–364. doi: 10.1002/1097-0142(197108)28:2<361::aid-cncr2820280215>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Biggs JC, Chesterman CN, Holliday J. L-asparaginase clinical experience in leukaemia, lymphoma and carcinoma. Austral N Z J Med. 1971;1:1–7. doi: 10.1111/j.1445-5994.1971.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 24.Bodmer M, Sulz M, Stadlmann S, et al. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion. 2006;74:28–32. doi: 10.1159/000095827. [DOI] [PubMed] [Google Scholar]
- 25.Athale UH, Chan AKC. Thrombosis in children with acute lymphoblastic leukemia: Part I Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res. 2003;111:125–131. doi: 10.1016/j.thromres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukemia. Br J Haematol. 2007;138:430–445. doi: 10.1111/j.1365-2141.2007.06677.x. [DOI] [PubMed] [Google Scholar]
- 27.Nowak-Göttl U, Heinecke A, von Kries R, et al. Thrombotic events revisited in children with acute lymphoblastic leukemia: impact of concomitant Escherichia coli asparaginase/prednisone administration. Thromb Res. 2001;103:165–172. doi: 10.1016/s0049-3848(01)00286-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1. Treatment modification of AALL08P1 based on results of AALL0232 demonstrating EFS advantage for patients who received high dose methotrexate compared to Capizzi methotrexate. Therapy modifications recommended were dependent on the stage of therapy patients were currently receiving on AALL08P1.
SDC 2. Patient count versus weeks to successful completion from start of Consolidation to start of Maintenance for the 15 patients on the I-PEG arm who reached Maintenance.
SDC 3. Table. Myelosuppression Grade 3–4 (decreased WBC and/or decrease neutrophil) Rates by Treatment and Reporting Period.
SDC 4–7: Survival analysis.


