INTRODUCTION
Fluid resuscitation with crystalloid solutions is among the most common interventions for hospitalized patients. Currently, providers choose between two classes of available crystalloid solutions: 0.9% sodium chloride (saline) and “balanced” crystalloids (such as lactated Ringer’s, Hartmann’s solution, or Plasma-lyte®). Although often used interchangeably, saline and balanced crystalloids differ in composition in ways that may impact patients. Given the similar availability and cost of each fluid, along with mounting evidence linking saline to metabolic derangements, acute kidney injury, and mortality, we will argue in this Viewpoint that saline should not be the first choice fluid for crystalloid resuscitation.
BACKGROUND
Crystalloids are solutions of ions which determine fluid tonicity but are freely permeable through capillary membranes. Early crystalloid solutions comprised of sodium, chloride, and bicarbonate in water were first prepared for treatment of cholera during the 1832 pandemic(1). Addition of calcium and potassium by Sydney Ringer in the 1870s and lactate by Alexis Hartmann in the 1930s laid the foundation for the balanced crystalloids available today. In contrast, the composition of saline derives from Hartog Hamburger’s demonstration in the 1880s that a salt concentration of 0.9% minimized in vitro erythrocyte lysis(1) and how exactly 0.9% sodium chloride entered into clinical use remains unclear(1).
With 154 mmol/L each of sodium and chloride, saline is isotonic to extracellular fluid but contains a chloride concentration 50% higher than plasma and a strong ion difference of zero (Table 1). As a result, rapid administration of large volumes of saline reliably produces a hyperchloremic metabolic acidosis(2). In contrast, the chemical composition of balanced crystalloids is designed to approximate that of extracellular fluid (Table 1). By replacing a portion of the chloride content with bicarbonate, or rapidly metabolized/excreted organic anions such as L-lactate, acetate, or gluconate, balanced crystalloids provide a more physiologic chloride concentration and strong ion difference. These differences in crystalloid composition have long been known to affect patients’ serum chloride levels and acid-base balance, but mounting data suggest that crystalloid choice may also directly impact organ function and even survival.
Table 1.
Intravenous crystalloid compositions.
| Sodium | Potassium | Calcium | Magnesium | Chloride | Acetate | Lactate | Gluconate | Bicarbonate | Osmolarity | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Plasma | 135–145 | 4.5–5.0 | 2.2–2.6 | 0.8–1.0 | 94–111 | 1–2 | 23–27 | 275–295 | ||
| 0.9% saline | 154 | 154 | 308 | |||||||
| Lactated Ringer’s | 130 | 4.0 | 1.5 | 109 | 28 | 273 | ||||
| Hartmann's solution | 131 | 5.4 | 1.8 | 112 | 28 | 277 | ||||
| Plasma-Lyte® | 140 | 5.0 | 1.5 | 98 | 27 | 23 | 294 | |||
All values are given in mmol/liter except osmolarity which is in mOsm/liter. Electrolyte concentrations of intravenous fluid preparations may differ by manufacturer – information is given for lactated Ringer’s injection (Baxter; Deerfield, IL, United States), Hartmann’s Solution (B. Braun Melsungen AG; Melsungen, Germany), Plasma-Lyte 148® (Baxter; Old Toongabbie, Australia).
SALINE CAUSES HYPERCHLOREMIA, ACIDOSIS, AND DECREASED RENAL PERFUSION
Among healthy volunteers(3, 4), patients undergoing elective surgery(2), and the critically ill(5, 6), rapid intravenous infusion of saline reliably induces hyperchloremia and metabolic acidosis, each of which is independently associated with increased mortality in at-risk patients(7, 8). The acidosis arising from saline administration may confound interpretation of underlying acid-base disorders, increase interventions by providers to correct pH(9), and potentially prolong length of stay(10). Saline also decreases renal blood flow and glomerular filtration rate (GFR)(3, 11, 12). In animal models, intrarenal infusion of chloride-rich solutions decreases renal blood flow and GFR compared with low-chloride solutions of equal tonicity(11, 12). A recent study of healthy human volunteers showed a significant reduction in renal artery flow velocity and renal cortical tissue perfusion after infusion of 2 liters of saline over an hour, but not after Plasma-Lyte® infusion(3). A similar trial demonstrated increased renal cortical tissue perfusion with starch solutions prepared in balanced crystalloids rather than saline(13). Saline may diminish renal perfusion by multiple mechanisms. Increased chloride delivery to the distal tubule may stimulate tubuloglomerular feedback, inducing afferent arteriolar vasoconstriction and reducing GFR. Additionally, saline infusions appear to cause more intracapsular renal edema than balanced crystalloids, which may further compromise renal tissue perfusion(3).
SALINE MAY INCREASE ACUTE KIDNEY INJURY AND MORTALITY
Whether saline’s effects on hyperchloremia, acidosis, and renal perfusion impact clinically-significant measures of organ function and patient outcomes has been the subject of increasing study. In a propensity-matched analysis of administrative data from over 30,000 adults undergoing major open abdominal surgery, the rate of acute kidney injury requiring dialysis was 1.0% with balanced crystalloids compared to 4.8% with saline (p<0.001)(14). A ‘before-and-after’ study of around 1,500 critically ill adults found use of chloride-restricted fluids was associated with less stage 2 or 3 acute kidney injury (8% vs 14%, p<0.001) and less renal replacement therapy (6% vs 10%, p=0.005)(15), even after accounting for other changes in practice over time(16). Finally, in a recent meta-analysis involving over 6,000 patients from 15 randomized trials, 5 observational reports, and the above ‘before-and-after’ study, high-chloride fluids were associated with an increased risk of acute kidney injury (RR 1.64, 95% CI 1.27 – 2.13, p<0.001)(5).
The available evidence linking high-chloride solutions to increased mortality is similar. In a retrospective analysis of over 50,000 ICU patients with septic shock, receipt of balanced crystalloids was associated with lower in-hospital mortality (19.6% vs 22.8%, p=0.001), with a “dose response” such that each incremental increase in the proportion of IVF that was balanced rather than saline was associated with an additional decrease in mortality(17). The administration of higher IV chloride load remains associated with increased mortality in a dose-response type relationship even after carefully accounting for the volume of fluid administrated(18). A recent network meta-analysis involving over 18,000 patients from 14 studies confirmed the finding of lower mortality with balanced crystalloids compared with saline(19).
Although the above evidence consistently suggests an association between saline and organ injury and mortality among acutely ill adults, it is essentially all observational. The only large, high-quality randomized trial to date comparing saline with balanced crystalloids among critically ill adults is the 0.9% Saline vs Plasma-Lyte 148® (PL-148) for ICU fluid Therapy (SPLIT) trial(20). Designed as a pilot study to provide preliminary data on recruitment rates and effect sizes for the planning of a definitive phase III trial(21), SPLIT assigned 2,278 adults from four New Zealand ICUs to receive either 0.9% saline or Plasma-lyte® whenever an IV crystalloid was needed during their ICU stay. Overall, patients were at low risk for acute kidney injury and mortality (primarily admitted after elective surgery, average APACHE II score of 14) and experienced limited exposure to the assigned intervention (around 2 liters of total crystalloid during the ICU stay). Rates of acute kidney injury (9%) and renal replacement therapy (3%) were similar between groups. The observed point estimate of a 12% relative reduction in in-hospital mortality in favor of balanced crystalloids, however, led the authors to recommend a “pivotal randomized clinical trial” adequately powered, and with enough exposure to the study fluids, to detect differences in patient-centered outcomes between balanced crystalloids and saline(20).
BALANCED CRYSTALLOIDS ARE SAFE
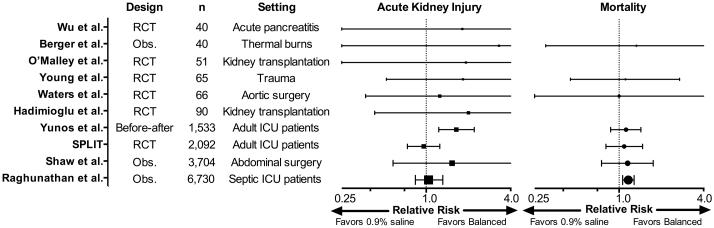
Although the available “physiologically-balanced” crystalloids contain a chemical composition closer to the extracellular fluid than that of 0.9% saline, none are truly “balanced” or “physiologic”. Theoretical concerns raised by the composition of balanced fluids include the effect on brain water of relative hypotonicity (with the low sodium content of lactated Ringer’s and Hartmann’s solution)(4), hyperkalemia, hyperlactatemia (with lactated Ringer’s or Hartmann’s solution), and cardiotoxicity (with acetate containing solutions). Fortunately, these theoretical concerns have largely not borne out in the available studies comparing saline to balanced crystalloids. Randomized trials among patients with trauma(22) and brain injury(23) have not suggested a difference between commercially prepared balanced crystalloids and saline with regard to intracranial pressure. Although balanced crystalloids contain a small amount of potassium, multiple randomized trials in patients undergoing kidney transplantation have shown significantly less hyperkalemia with balanced crystalloids compared with saline(9), perhaps due to the influence of acid-base change on potassium redistribution. The accumulation in the serum of lactate from resuscitation with lactated Ringer’s or Hartmann’s solution is rare in the absence of liver dysfunction, and is not known to be of clinical consequence. Although use of acetate as a buffer during hemodialysis is recognized to predispose to hypotension, there is no current evidence to suggest toxicity from use of acetate-containing balanced crystalloids during volume resuscitation. Perhaps the greatest reassurance of balanced crystalloids’ relative safety is that among studies involving almost fifteen thousand critically ill adults, none have demonstrated worse organ function, mortality, or other clinical outcome with balanced crystalloids compared to saline (Figure 1).
Figure 1.
Forest plot illustrating the relative risk of acute kidney injury (boxes) and mortality (circles), with 95% confidence intervals, for saline compared with balanced crystalloids (adapted from Krajewski et al(5)). Definition and timing of acute kidney injury and mortality differ between studies, for details see: Wu et al (PubMed ID: 21645639), Berger et al (11089771), O’Malley et al (15845718), Young et al (23732264), Waters et al (11574339), Hadimioglu et al (18635497), Yunos et al (23073953), 0.9% Saline vs Plasma-Lyte 148® for ICU fluid Therapy (SPLIT) by Young et al (26444692), Shaw et al (22470070), Raghunathan et al (24674927). RCT = randomized controlled trial; Obs. = observational study.
AVAILABILITY AND COST
Saline and balanced crystalloids are widely available to providers in the United States(17), Europe(24), Australia and New Zealand(25), and globally. Although the exact cost of each fluid varies by location, in general, a 1 liter bag of saline, lactated Ringer’s, or Hartmann’s solution costs just over 1 U.S. dollar and Plasma-lyte® costs around 2 U.S. dollars(6, 22). Thus, saline, lactated Ringer’s, and Hartmann’s solution are clearly cost-equivalent and the additional 80 cents for Plasma-lyte® may be viewed as an acceptable incremental expense for critically ill patients whose costs of care often range in the thousands of dollars per day.
CONCLUSION
Millions of liters of IV crystalloid are given to critically ill patients across the world every year, most of it saline. If balanced crystalloids produce even slightly better outcomes than saline, the result could be lower morbidity and mortality for thousands of patients. A large, randomized trial comparing saline to balanced crystalloids (in a manner that carefully accounts for patients’ baseline risk and “dose” of fluid exposure) is urgently needed. Until such a definitive trial is completed, the similar availability and cost of each crystalloid, established safety of balanced crystalloids, and mounting concerns about acidosis, acute kidney injury, and mortality with saline argue that saline should not be the first choice fluid for crystalloid resuscitation.
Acknowledgments
Source of Funding
M.W.S. was supported by a National Heart, Lung, and Blood Institute (NHLBI) T32 award (HL087738 09).
Footnotes
Author contributions: Drafting of the manuscript: M.W.S. and T.W.R.; Critical revision of the manuscript for important intellectual content: M.W.S. and T.W.R.
Copyright form disclosures:
Dr. Rice consulted for Avisa Pharma, LLC (Consultancy); GlaxoSmithKline, LLC (Chair of DSMB); and Cumberland Pharmaceuticals, LLC (Director, Medical Affairs). He received support for article research from the National Institutes of Health (NIH). Dr. Semler received support for article research from the NIH and the National Heart, Lung, and Blood Institute (NHLBI) T32 award (HL087738 09). His institution received funding from the NHLBI T32 award (HL087738 09).
Conflicts of Interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declare no potential conflicts of interest. T.W.R. reported serving on an advisory board for Avisa Pharma, LLC and Cumberland Pharmaceuticals and as a DSMB member for GlaxoSmithKline PLC.
REFERENCES
- 1.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr Edinb Scotl. 2008;27:179–188. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 4.Williams EL, Hildebrand KL, McCormick SA, et al. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Krajewski ML, Raghunathan K, Paluszkiewicz SM, et al. Meta-analysis of high-versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yunos NM, Kim IB, Bellomo R, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–2424. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 7.Neyra JA, Canepa-Escaro F, Li X, et al. Association of Hyperchloremia With Hospital Mortality in Critically Ill Septic Patients. Crit Care Med. 2015;43:1938–1944. doi: 10.1097/CCM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocktaeschel J, Morimatsu H, Uchino S, et al. Unmeasured anions in critically ill patients: can they predict mortality? Crit Care Med. 2003;31:2131–2136. doi: 10.1097/01.CCM.0000079819.27515.8E. [DOI] [PubMed] [Google Scholar]
- 9.O’Malley CMN, Frumento RJ, Hardy MA, et al. A randomized, double-blind comparison of lactated Ringer’s solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–1524. doi: 10.1213/01.ANE.0000150939.28904.81. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2011;9:710–717. doi: 10.1016/j.cgh.2011.04.026. e1. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quilley CP, Lin YS, McGiff JC. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacol. 1993;108:106–110. doi: 10.1111/j.1476-5381.1993.tb13447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 1-L infusions of 6% hydroxyethyl starch suspended in 0.9% saline (voluven) and a balanced solution (Plasma Volume Redibag) on blood volume, renal blood flow velocity, and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2014;259:881–887. doi: 10.1097/SLA.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 14.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 15.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA J Am Med Assoc. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 16.Yunos NM, Bellomo R, Glassford N, et al. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–264. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 17.Raghunathan K, Shaw A, Nathanson B, et al. Association Between the Choice of IV Crystalloid and In-Hospital Mortality Among Critically Ill Adults With Sepsis. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AD, Raghunathan K, Peyerl FW, et al. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–1905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochwerg B, Alhazzani W, Sindi A, et al. Fluid Resuscitation in Sepsis: A Systematic Review and Network Meta-analysis. Ann Intern Med. 2014 doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 20.Young P, Bailey M, Beasley R, et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA. 2015:1–10. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 21.Young P, Bailey M, Beasley R, et al. The statistical analysis plan for the 0.9%Saline vs Plasma-Lyte 148 for Intensive care fluid Therapy (SPLIT) study. [Internet]. [cited 2016 Feb 18] Available from: http://wellingtonicu.com/Data/Trials /SPLITSAP.pdf. [Google Scholar]
- 22.Young JB, Utter GH, Schermer CR, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg. 2014;259:255–262. doi: 10.1097/SLA.0b013e318295feba. [DOI] [PubMed] [Google Scholar]
- 23.Roquilly A, Loutrel O, Cinotti R, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: a randomised double-blind pilot study. Crit Care Lond Engl. 2013;17:R77. doi: 10.1186/cc12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310:1809–1817. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 25.Hammond NE, Taylor C, Saxena M, et al. Resuscitation fluid use in Australian and New Zealand Intensive Care Units between 2007 and 2013. Intensive Care Med. 2015;41:1611–1619. doi: 10.1007/s00134-015-3878-y. [DOI] [PubMed] [Google Scholar]