Abstract
BACKGROUND & AIMS
It is a challenge to predict how patients with small bowel Crohn’s disease (CD) will respond to intensified medical therapy. We aimed to identify factors that predicted surgery within 2 years of hospitalization for CD, to guide medical vs surgical management decisions.
METHODS
We performed a retrospective review of adults hospitalized for small bowel CD from 2004 through 2012 at a single academic referral center. Subjects underwent abdominal computed tomography or magnetic resonance imaging within 3 weeks of hospitalization. Imaging characteristics of small bowel dilation, bowel wall thickness, and disease activity were assessed by a single, blinded radiologist. Multivariate analysis by Cox proportional hazard regression techniques were used to generate a prediction model of intestinal resection within 2 years.
RESULTS
A total of 221 subjects met selection criteria, with 32.6% undergoing surgery within 2 years of index admission. Bivariate analysis showed high-dose steroid use (>40 mg), ongoing treatment with anti-tumor necrosis factor agents at admission, platelet count, platelet:albumin ratio, small bowel dilation (≥35 mm), and bowel wall thickness to predict surgery (P≤.01). Multivariate modeling demonstrated small bowel dilation greater than 35 mm (hazard ratio [HR], 2.92; 95% confidence interval [CI], 1.73–4.94) and a platelet:albumin ratio ≥125 (HR, 2.13; 95% CI, 1.15–3.95) to predict surgery. Treatment with anti-tumor necrosis factor agents at admission conferred a non-significant increased trend for risk of surgery (HR, 1.61; 95% CI, 0.994–2.65).
CONCLUSIONS
Small bowel dilation greater than 35 mm and high platelet:albumin ratios are independent and synergistic risk factors for future surgery in patients with structuring small bowel CD. Platelet:albumin ratios may capture the relationship between acute inflammation and cumulative damage and serve as markers of intestines that cannot be salvaged with medical therapy.
Keywords: TNF, IBD, bowel obstruction, predictive model, outcome
INTRODUCTION
Hospitalization for severe manifestations of Crohn’s disease (CD) is common. Yet, even experienced specialists can have difficulty predicting whether an individual will respond to anti-inflammatory medical therapy, or instead is destined for surgical bowel resection in the immediate future. Early in the disease course, approximately 80% of patients demonstrate a predominantly inflammatory disease. Over time, cycles of inflammation result in progressive irreversible fibrostenotic bowel damage, with nearly 60% of patients requiring surgical management within 10 years of diagnosis1. Indirect evidence suggests that, when used sufficiently early, anti-TNF therapy can reduce the risk of future surgery, hospitalization, and disability2.
Not infrequently, anti-inflammatory therapies prove insufficiently effective, often in the patient with obstructive symptoms, and ultimately require surgical bowel resection for symptom relief. Further, empiric medical therapy, compared to bowel resection, is not without hazard. Risks of using immunosuppressive agents in those destined for surgery include an increase in spontaneous bowel perforation, prolonged suffering and the unique risks of each medication3,4. Patients appearing sufficiently improved at discharge, often on high-dose steroids and liquid diets, may undergo repeated hospitalization despite escalation of steroid-sparing therapy. In hindsight, such patient may have been best served by preparing for surgery. Avoiding (or perhaps delaying) surgery and rescuing salvageable intestine when possible is a priority of CD management. Yet, the empiric use of costly biologic therapies for CD, ranging between $19,000-34,000 per year using standard dosing, raises questions of cost-effectiveness and utility in those requiring hospitalization for stricturing CD5. The factors accurately predicting whether intensification of medical therapy will adequately relieve symptoms and avoid future hospitalization and surgery in real-world settings remain unclear.
Multi-disciplinary IBD teams of gastroenterologists, surgeons, and radiologists, experienced in the management of inflammatory bowel disease, use both clinical and diagnostic data points to guide decisions between medical intensification vs. bowel resection in hospitalized patients with CD. Unfortunately, symptom indices poorly predict response to medical therapy6. While inflammatory biomarkers are important prognostic elements, the presence of inflammation does not exclude the existence of enough intestinal fibrosis such that anti-inflammatory therapy alone will be successful7. Despite these limitations, treatment decisions must still be made. The intensity of a patient’s prior medical regimen, surrogates of intestinal inflammation, laboratory data suggesting malnutrition or disease chronicity, and imaging findings describing structural features are assessed in unison to make management decisions. In this study, we aimed to identify clinical, laboratory, and imaging predictors of medical therapeutic failure (requiring surgery) within 2 years of index admission in patients hospitalized for CD primarily affecting the small intestine.
METHODS
Selection Criteria
This study was approved by the University of Michigan Institutional Review Board. In this retrospective study, adult patients with CD who were hospitalized between January 1, 2004 and December 31, 2012 at the University of Michigan Health System were identified. CD diagnosis was verified using one inpatient and outpatient ICD-9 code of 555.X with confirmation by review of medical records8. Selection criteria included the requirement that subjects had an encounter with a University of Michigan gastroenterologist at their index hospitalization and then at least one encounter (inpatient or outpatient) a minimum of 2 years following the index admission to ensure adequate interval follow up. Included patients were required to have imaging by either standard or enterography protocolled CT or MRI of the abdomen a maximum of 3 weeks prior to hospitalization, identified by CPT procedure codes (74150-74190 and 76376, 76377). To minimize phenotypic heterogeneity, selection required patients have ileocecal or small intestinal disease distribution; patients with exclusively colonic disease were excluded. Patients with an intra-abdominal abscess or perianal fistulizing disease, often requiring surgical management, were excluded. Patients with a history of prior intestinal resection or endoscopic balloon dilation (prior to or following hospitalization) were also excluded from the study to select a more homogeneous phenotype in the study sample.
Clinical Data Collection
University of Michigan Health System electronic medical records were reviewed using a combination of automated data extraction (University of Michigan Data Warehouse Service), natural language search engines (EMERSE), and manual chart review9. Patient demographics, medication use, smoking history, and laboratory data at the time of admission were collected. Baseline admission medication data collected included use of thiopurines (azathioprine, mercaptopurine), methotrexate, anti-tumor necrosis factor alpha agents (infliximab, adalimumab, certolizumab pegol) and prednisone use (categorized as 0-19mg daily, 20-39mg, and 40mg or more). Medication use prior to the index admission was recorded. No anti-TNF use was defined as the absence of anti-TNF use within 1 year prior to index hospitalization and no use during the entire study period. New anti-TNF use was defined as anti-TNF initiation within three months following the index admission and without exposure in the year prior to hospitalization. Admission laboratory values were collected, including white blood cell count (WBC), hemoglobin (HGB), platelets (PLT), and albumin (ALB), and C-reactive protein (CRP). Imaging findings were reviewed by a single radiologist (JR) blinded to medical records and the primary outcome of surgery within two years of index hospitalization. Small bowel dilation was measured at the point of maximal intestinal distention. Bowel wall thickness was measured at the point of maximal thickness within radiographically most diseased segment. The presence or absence of inflammatory disease activity by imaging was determined by the presence of one of the following within the most diseased segment: mucosal hyperenhancement, mesenteric hypervascularity, mesenteric fat stranding, or mesenteric lymphadenopathy. A history of perianal fistulas was determined by review of gastroenterologist clinical documentation, as sufficiently sensitive imaging (e.g. 3-Tesla MR-pelvis or EUS) was not consistently available on all subjects.
Primary Outcome Assessment
The primary outcome in this study was the occurrence of surgical resection of intestine within two years of the index hospitalization. Surgical interventions included bowel resection, diverting ileostomy, and surgical stricturoplasty. Fistulotomies, ostomy revisions, and non-CD related surgeries (e.g. hernia repair, cholecystectomy) were not included. The time (days) from admission to surgery was measured in all patients who underwent surgery. Manual chart review, augmented by natural language search techniques (EMERSE), was used to capture the small portion of surgical events occurring outside the institution.
Statistical Analysis
All analyses considered a p-value of ≤0.05 as statistically significant, and were conducted using SAS 9.4 (Cary, NC). Demographic, clinical, laboratory, and imaging features at the index hospitalization were compared between patients who avoided or underwent surgery within two years of hospitalization. Bivariate analysis used Cox proportional hazards regression models, comparing each individual predictor to the time to surgical intestinal resection. Kaplan-Meier curves stratifying the time to surgery by the presence of small bowel dilation and platelet-to-albumin ratios were produced. Multivariate Cox proportional hazards regression model building utilized a backward variable selection process with forced inclusion of age, gender, and other covariates deemed clinically relevant a priori. Continuous variables were also explored as categorical variables (with and without ordinal features) to provide the best model fit. Analysis of maximum likelihood estimates provided hazard ratios and confidence limits for each parameter within the model. The discriminatory power of the regression models were evaluated by receiver operating characteristic curve c-statistics.
We evaluated the impact of the platelet-to-albumin ratio as a prognostic indicator of future surgery. Covariates from the final multivariate model were used to estimate the probability of avoiding surgery in six common clinical scenarios of patients hospitalized with small bowel CD based on the presence of small bowel dilation, anti-TNF exposure (none, ongoing use, post-hospitalization start), and the platelet-to-albumin ratio. The platelet-to-albumin ratio was used as a categorical variable at three levels (less than 75, 75-125, and greater than 125), defined by variable tertiles.
RESULTS
Patient Characteristics
A total of 509 admissions of patients with a primary diagnosis of CD were identified during the retrospective study interval (2004-2012). Of the identified patients, 128 patients were excluded due to prior surgical resection, 59 were excluded due to the absence of small bowel disease, 48 were excluded for inadequate follow up, 34 patients were excluded for endoscopic balloon dilation, and 19 were excluded due absence of imaging within the required window. Of the remaining 221 subjects meeting selection criteria, 72 (32.6%) underwent surgery within two years of admission with a median follow up time (time between index hospitalization and most recent encounter with a gastroenterologist) of 1,203 days (±523), or approximately 3.3 years. Demographic variables including age, gender and history of perianal fistulizing disease were not significant in bivariate analysis (Table 1). Examining medications, while the use of prednisone 40 mg or more at index admission was associated with eventual bowel resection (HR 2.30, 95%CI 1.23, 4.32), existing immunomodulator and anti-TNF use was not associated with surgery. The initiation of thiopurines (HR 1.53, 95%CI 0.93, 2.52) or anti-TNF agents (HR 1.49, 95%CI 0.734, 3.017) following hospitalization exhibited non-significant trends of failure to avoid surgery in bivariate analysis.
Table 1.
Patient characteristics
| Risk Factor | Surgical Status | HR | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Avoid (n =149) | Undergo (n =72) | ||||||
| Gender | 62 (41.61%) | 30 (41.67%) | 1.02 | 0.639 | 1.632 | 0.929 | |
| Age | 42.05 (14.65) | 38.71 (17.52) | 0.99 | 0.973 | 1.005 | 0.173 | |
| Perianal Fistula | 17 (11.49%) | 16 (22.22%) | 1.88 | 0.839 | 3.286 | 0.124 | |
| Medication Use Prior to Hospitalization | |||||||
| Prednisone | |||||||
| Omg | 117 (78.5%) | 52 (72.2%) | reference | ||||
| 0-19mg | 12 (8.1%) | 3 (4.2%) | 0.66 | 0.205 | 2.110 | 0.482 | |
| 20-39mg | 10 (6.7%) | 5 (6.9%) | 1.23 | 0.489 | 3.079 | 0.662 | |
|
| |||||||
| >40mg | 10 (6.7%) | 12 (16.7%) | 2.30 | 1.225 | 4.322 | 0.010 | |
|
| |||||||
| Thiopurines | 70 (47.0%) | 36 (50.0%) | 1.12 | 0.707 | 1.782 | 0.623 | |
| Methotrexate | 11 (7.4%) | 4 (5.6%) | 0.77 | 0.279 | 2.098 | 0.604 | |
| Anti-TNF | 34 (22.8%) | 18(25.0%) | 1.10 | 0.645 | 1.876 | 0.727 | |
| Medicatians Started Followina Hospitalization (within 3 months) | |||||||
| Thiopurines | 33 (22.2%) | 23 (31.9%) | 1.53 | 0.934 | 2.515 | 0.091 | |
| Methotrexate | 9(6.1%) | 4 (5.6%) | 0.83 | 0.303 | 2.278 | 0.719 | |
| Anti-TNF | 24(16.1%) | 16(22.2%) | 1.49 | 0.734 | 3.017 | 0.269 | |
| Imaging Characteristics | |||||||
|
| |||||||
| Dilation ≥35mm | 66 (±44.6) | 52 (±72.2) | 2.77 | 1.652 | 4.639 | <.001 | |
| Diameter (mm) | 33.9 (±12.78) | 40.4 (±12.03) | 1.03 | 1.007 | 1.044 | 0.006 | |
| Thickness (mm) | 5.9 (±2.76) | 7.2 (±2.47) | 1.16 | 1.054 | 1.268 | 0.002 | |
|
| |||||||
| Disease Activity | 98 (66.2%) | 55 (76.4%) | 1.49 | 0.864 | 2.565 | 0.152 | |
| Laboratory | |||||||
| WBC | 10.56 (±4.96) | 11.00 (±5.86) | 1.02 | 0.974 | 1.064 | 0.420 | |
| HGB | 13.02 (±1.84) | 12.66 (±2.27) | 0.93 | 0.822 | 1.046 | 0.217 | |
|
| |||||||
| PLT | 309.2 (±116.1) | 375.2 (±177.8) | 1.01 | 1.000 | 1.016 | <.001 | |
|
| |||||||
| Albumin | 3.99 (±0.67) | 3.85 (0.72) | 0.82 | 0.597 | 1.121 | 0.211 | |
|
| |||||||
| PLT-Alb Ratio | 81.0 (±39.7) | 103.4 (±61.2) | 1.01 | 1.003 | 1.012 | <.001 | |
|
| |||||||
| PLT-Albumin Ratio (categorical) | |||||||
| 0-75 | 81 (54.4%) | 31 (43.1%) | reference | ||||
| 75-125 | 50 (33.6%) | 21 (29.2%) | 1.07 | 0.619 | 1.834 | 0.818 | |
|
| |||||||
| >125 | 18 (12.1%) | 20 (27.8%) | 2.05 | 1.321 | 3.651 | 0.010 | |
|
| |||||||
Rows highlighted in gray indicate statistical significance at or below the .05 level.
Of those undergoing surgery, 29 (40.3%) underwent surgery during the index hospitalization, while 43 (59.7%) underwent surgery after the index hospitalization. The median time between index hospitalization and surgery was 142 days (IQR, 60-356), or approximately 5 months, for those undergoing surgery following the index admission. Bivariate analysis did not demonstrate meaningful differences between risk factors of immediate vs. eventual (within 2 years) surgery (Supplementary Table 1). There was no difference in the decision to create an ileostomy between those undergoing surgery during index admission 34.5%) compared to post-hospitalization surgery (30.2%, p=0.7044). Comparing complications by timing of surgery (post-operative anastomotic leak, abscess, fistula, wound infection or dehiscence), there was no difference between index (20.7%) and post-hospitalization (18.6%) surgical groups (p=0.8265).
Bivariate Analysis of Imaging and Laboratory Results Relationship to Future Surgery
Imaging from CT and MR studies (both standard and enterography protocols) revealed that both bowel wall dilation and bowel wall thickness were both predictive of eventual surgery on bivariate analysis (Figure 1). Increasing bowel wall thickness had a modest linear impact on the predicted probability of surgery (HR 1.16, 95%CI 1.06-1.27 per mm thickness). Dichotomizing bowel dilation as greater or less than 35mm provided the greatest discrimination of surgical outcomes on unadjusted analysis (HR 2.77, 95%CI 1.65-4.64). Radiologist assessment of the presence or absence of inflammatory activity was not a significant predictor of future surgery. Of all radiographic studies performed, 128 of 221 (57.9%) were enterography protocols (86.7% were CT- vs. 13.3% MR-enterography); the remainder were standard positive oral contrast CT-scans of the abdomen. Bivariate analysis of subjects with enterography-protocolled studies revealed no meaningful differences between the effect size or statistical significance of reported disease activity, bowel dilation >35mm, or bowel wall thickness compared to analyses including both enterography and standard studies (Supplementary Table 2). Laboratory values at the time of admission including WBC, HGB, and albumin were not predictive of future surgery. Elevated platelet count was a predictor of future surgery. Examining the platelet-to-albumin ratio (PltAlb-ratio), a high platelet count relative to albumin level at index hospitalization increased the risk of surgery over time (Figure 2). Categorizing the PltAlb-ratio based on tertiles, those with a PltAlb-ratio of greater than 125 exhibited a hazard ratio of 2.05 (95%CI 1.32, 3.65) for future surgery on bivariate analysis. CRP data was collected, however only 136 of 221 subjects had complete data; considering the small study size we elected not to impute missing data and did not analyze the predictive role of CRP.
Figure 1. Avoidance of surgery within two years stratified by small bowel dilation.
Unadjusted Kaplan-Meier analysis demonstrating that patients hospitalized for Crohn’s disease exhibiting small bowel dilation equal or greater than 35mm were more likely to undergo bowel resection within two years (56.8% vs. 82.4%, p<.0001).
Figure 2. Avoidance of surgery within two years stratified by platelet-to-albumin ratio.
Unadjusted Kaplan-Meier analysis where Crohn’s patients are stratified by the platelet-to-albumin ratio demonstrated those with a ratio greater than 125 had a lower probability of avoiding surgery within two years of index hospitalization compared to less than 125 (47.4% vs.74.1%, p=0.007).
Multivariate Model for Predicting Future Surgery
Proportional hazard Cox regression providing a multivariate analysis adjusting for covariates, identified intestinal dilation greater than 35mm and a PltAlb-ratio ≥125 as predictors of surgery within 2 years of index hospitalization (Table 2). Additionally, a non-significant trend suggested that pre-existing anti-TNF use at the time of hospitalization may be associated with future surgery (HR 1.61, 95%CI 0.99, 2.65, p=0.059). Models of surgery during the index hospitalization revealed intestinal dilation (HR 4.21, 95%CI 1.59, 11.16, p=0.0038) and high-dose prednisone use at index presentation (HR 3.12, 95%CI 1.26, 7.76, p=0.0143), but not PltAlb-ratio (HR 2.19, 95%CI 0.84, 5.76, p=0.1109), as predictors of surgery (Supplementary Table 3a). However, of those avoiding surgery during the index hospitalization, intestinal dilation (HR 2.33, 95%CI 1.23, 4.34, p=0.0099), PltAlb-ratio (HR 2.10, 95%CI 1.11, 4.71, p=0.0486), and also pre-existing anti-TNF use at the time of hospitalization (HR 2.20, 95%CI 1.08, 4.47, p=0.0294) were significant risk factors of subsequent surgery within 2 years (Supplementary Table 3b). Alternative models were examined, forcing the addition of thiopurine and methotrexate use prior to and following index admission, imaging characteristics, and other laboratory covariates which did not improve model fit. The platelet count alone was a significant risk factor for surgery on adjusted analysis (HR 1.22, 95%CI 1.01, 1.44, p=0.0241). The choice to use the PltAlb-ratio was because it exhibited a greater effect (HR 2.13, 95%CI 1.15, 3.95, p=0.0168) and improved overall model fit.
Table 2.
Multivariate model predicting surgery within two years in patients hospitalized for Crohn’s disease.
| Risk Factor | HR | 95% CI | p-value | |
|---|---|---|---|---|
| Bowel Dilation >35mm | 2.92 | 1.731 | 4.939 | <.001 |
|
| ||||
| Anti-TNF use | ||||
| Prior to hospitalization | 1.61 | 0.994 | 2.651 | 0.059 |
| Within three months of hospitalization | 1.22 | 0.671 | 2.22 | 0.520 |
| Prednisone >40mg at presentation | 1.85 | 0.977 | 3.502 | 0.120 |
|
| ||||
| Platelet-Albumin Ratio >125 | 2.13 | 1.146 | 3.949 | 0.017 |
|
| ||||
| Age | 0.99 | 0.977 | 1.009 | 0.362 |
| Gender | 1.02 | 0.625 | 1.657 | 0.943 |
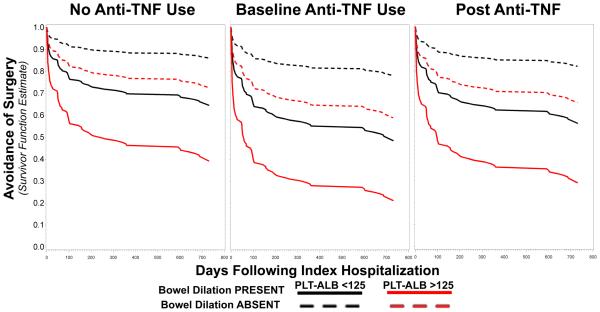
Estimated Probability of Avoiding Surgery by Platelet-to-Albumin Ratio in Common Clinical Scenarios
Across all modeled clinical scenarios, the presence of small bowl dilation ≥35mm was associated with lower estimated probabilities of avoiding surgery (Figure 3). Among those with small bowel dilation and no anti-TNF use, a PltAlb-ratio of ≥125 resulted in a lower estimated probability of avoiding surgery than PltAlb-ratios of <125 (39.8% vs. 67.4%, p<.0001, Table 3). Of those with intestinal dilation and high PltAlb-ratio (high risk for future surgery), naïve patients who then initiate anti-TNF therapy were estimated to have no reduction in the probability of surgery (39.8% vs. 28.7%, p=0.288). In addition, compared to anti-TNF naïve patients, those presenting with intestinal dilation and high PltAlb-ratios who are already using anti-TNF therapy were estimated to have a significantly lower probability of avoiding surgery (39.8% vs. 19.7%, p=0.009). Across all scenarios modeled, there were no significant differences between PltAlb-ratios of <75 and 75-125.
Figure 3. Adjusted Cox models for probabilities of avoiding surgery stratified by small bowel dilation, platelet-to-albumin ratio, and anti-TNF exposure.
Panels provide adjusted estimated probabilities of avoiding surgery over a two-year period from the index hospitalization of patients presenting with stricturing Crohn’s disease. Small bowel dilation is defined as ≥35mm. No anti-TNF use is defined as the absence of recorded medication exposure for a minimum of 1 year and no subsequent exposure during the study period. Baseline anti-TNF use is defined as the use of anti-TNF during and prior to index hospitalization; post anti-TNF use is defined as initiation of anti-TNF therapy within 3 months of index hospitalization. Across all scenario models, having a platelet-to-albumin ratio ≥125 significantly reduced the probability of avoiding surgery (p<.0001). There were no significant differences between estimates of avoiding surgery between the platelet-to-albumin ratios of less than 75 and 75-125 (p=0.397). Of those with small bowel dilation, platelet-to-albumin ratios of ≥125, and baseline anti-TNF use at the time of index hospitalization had the lowest probability of avoiding surgery at 2 years (19.7%). Holding other conditions constant, there was no difference in estimated avoidance of surgery between no anti-TNF and baseline anti-TNF use groups (p=0.2108).
Table 3.
Estimated percent probability of avoiding surgery two years following hospitalization for Crohn’s disease.
| Small Bowel Dilation Present | Absent | |||
|---|---|---|---|
| Platelet-Albumin Ratio | No Anti-TNF | Baseline Anti-TNF | Post Anti-TNF |
|
| |||
| Less than 75 | 67.4 | 87.8 | 50.1 | 79.4 | 59.1 | 84.9 |
| Between 75-125 | 61.6 | 85.3 | 43.5 | 75.8 | 52.2 | 80.1 |
| Greater than 125 | 39.8 | 72.2 | 19.7 | 57.9 | 28.7 | 65.7 |
DISCUSSION
Our single-center retrospective study examining real-world data and practice behaviors suggests that the presence of small bowel dilation ≥35mm and the platelet-to-albumin ratio are important predictors of future surgery in patients hospitalized for small bowel CD. Whether a CD patient will achieve durable relief from obstructive symptoms and avoid bowel resection is often a function of the ability to sufficiently improve luminal diameter10. Chronic intestinal wall thickening seen in stricturing disease is a mixture of both inflammatory and fibrotic elements, which are believed to be responsive and non-responsive, respectively, to anti-inflammatory therapy. Both bowel wall diameter and thickness were greater in those undergoing surgery within two years of hospitalization, which agrees with existing studies11. Despite expecting progressively increasing bowel diameter to incrementally increase the risk of surgery, neither linear, non-linear, or categorical associations were observed. Variations in the timing between imaging and the onset of obstructive symptoms, as well as potential decompression by fasting, vomiting, or NG-tube suction, could confound a potential relationship between the degree of bowel dilation and the likelihood of surgery. Considering these real-world clinical caveats, small bowel dilation of ≥35 mm may provide a practical threshold of mechanical obstruction reflecting a reduced probability of medical therapy being sufficient to avoid surgery.
The platelet-to-albumin ratio represents a novel descriptor of CD activity and was a significant predictor in the adjusted model of future surgery. Platelets are an acute phase reactant, induced by core inflammatory cytokines including IL-1, IL-6, INF-γ, and TNF, and can be used as a coarse indicator of inflammatory activity12,13. Albumin levels in CD are influenced by several factors including both sub-acute and long-term nutrition, intestinal absorptive capabilities, and potentially protein-losing enteropathies. These contributors to albumin level may serve to reflect the chronicity of severe CD both in terms of bowel damage and mechanically limiting obstruction14. Our interest in examining this ratio was to capture the relative intensity of acute inflammation and chronic bowel damage in a more parsimonious term. Interestingly, while platelets are well described as acute phase reactants, a growing body of evidence supports platelets having an impact on fibrosis in gastrointestinal disease. Activated platelets liberate CXC Chemokine Ligand 4, a profibrogenic mediator of hepatic fibrosis in animal models15. Activated platelets also release significant amounts of serotonin, which activates TGF-β in inducing fibrosis, and antagonists of 5HT2A receptors ameliorate fibrosis in rodent models 16,17. In addition, platelets have been shown to drive epithelial-to-mesenchymal and fibroblast-to-myofibroblast transition in models of endometriosis18. In our retrospective study, platelet-to-albumin ratios ≥125, independent of small bowel dilation or anti-TNF exposure, doubled the hazard of surgery within 2 years. While only 27% of our surgical population exhibited ratios ≥125, its impact appears meaningful, as highlighted by the nearly 30% increase in estimated future surgery compared to those with PltAlb-ratios less than 125.
In this retrospective study, the initiation of anti-TNF following hospitalization for obstruction did not reduce the risk of eventual surgery. In fact, of those avoiding surgery during index hospitalization, the initiation of new anti-TNF following hospitalization exhibited a non-significant trend of increasing the risk of future surgery (HR 2.04, 95%CI 0.91, 4.548, p=0.0834). Further, existing anti-TNF use at the time of index hospitalization also trended towards an association with future surgery, with a statistically significant doubling of future surgery in those avoiding immediate surgery even after adjusting for steroid use (HR 2.20, 95%CI 1.082, 4.473, p=0.0294). Anti-TNF therapy has been shown in prospective observational settings to reduce the risk of surgery in newly diagnosed patients 19. However, other studies examining the impact of adding an anti-TNF to existing regimens have not demonstrated a reduction in future surgery 20. Once stricturing causes bowel dilation, anti-TNF therapy may not provide sufficient benefit to change surgical outcomes21. Our study examines real-world practices in patients presenting with stricturing CD, attempting to discriminate those able to benefit from escalation of medical therapy from those highly likely to require near-term surgery. Considering the well demonstrated efficacy of anti-TNF therapies, the results suggest that disease modifying therapy is often initiated too late. Also highlighted is the need for predictive tools to stratify patients to early, high potency therapies while the opportunity to avoid the development of medically unsalvageable bowel damage still exists.
Several limitations require consideration when evaluating the results. Controlling for surgical decision-making is difficult as patient preference for surgical (or medical) management and individual surgeon assessments for timing and candidacy of an operation vary. Acknowledging this limitation, it is difficult to avoid surgery in situations where patients are obstructed and often have exhausted medical options. Medication use timing, optimization, and compliance can not be verified in this retrospective design; potentially relevant granular medication use details may not have been captured. Reasons for non-use of anti-TNF therapy, which could include patient preference, financial limitation, prior allergy or non-response in the remote past, were unable to be reliably extracted. In addition, we were unable to account for the development of immunogenicity causing secondary loss of response to anti-TNF. Radiographic data is subject to inter-observer variation. Bowel dilation and wall thickness measurements were recorded at their greatest observed values in efforts to achieve uniformity and reproducibility. We did not assess the length of intestinal disease as enterography protocols, more sensitive to mild disease, were used in approximately 50% of the cohort given the time period of our study. Finally, while platelets provide a course marker of inflammation, it is possible that ESR, CRP, or fecal calprotectin would have provided better prognostic accuracy in comparison to albumin levels.
In conclusion, in patients hospitalized for small bowel CD, bowel dilation of ≥35mm and platelet-to-albumin ratios ≥125 are independent and synergistic predictors of future surgical bowel resection. Future prospective work could determine whether advanced bowel imaging techniques to identify fibrosis, including MR-based diffusion weighted imaging (DWI), magnetic transference (MT-MRI) sequences, and both MR and ultrasound-based elastography can improve similar predictive models 22. Further exploration is needed to determine whether CRP, ESR, or fecal calprotectin can outperform platelets in predicting clinical outcomes in stricturing CD. The ratio of inflammatory biomarkers to albumin, in conjunction with imaging, endoscopic, and patient-reported measures, could contribute to decision support tools for determining whether patients are best served by intensification of anti-inflammatory therapy or timely surgical management.
Supplementary Material
Acknowledgments
Grant Support: Crohn’s and Colitis Foundation of America Career Development Award 3375 (Stidham), National Institutes of Health K23-DK101687
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No potential conflicts of interest relevant to this manuscript are present.
Writing Assistance: No writing assistance was provided.
Author Contributions
Ryan W. Stidham (corresponding author): study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision
Amanda S. Guentner: acquisition of data; analysis and interpretation of data, revision of the manuscript for intellectual content.
Julie L. Ruma: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for intellectual content.
Shail M. Govani: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Akbar K. Waljee: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision.
Peter D. R. Higgins: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis.
References
- 1.Peyrin-Biroulet L, Loftus EV, Colombel JF, et al. The Natural History of Adult Crohn's Disease in Population-Based Cohorts. Am J Gastroenterol. 2009;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 2.Colombel J-F, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoepfer AM, Dehlavi M-A, Fournier N, et al. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108:1744–53. doi: 10.1038/ajg.2013.248. quiz 1754. [DOI] [PubMed] [Google Scholar]
- 5.Tang DH, Armstrong EP, Lee JK. Cost-utility analysis of biologic treatments for moderate-to-severe Crohn's disease. Pharmacotherapy. 2012;32:515–526. doi: 10.1002/j.1875-9114.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 6.Benitez J-M, Meuwis M-A, Reenaers C, et al. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn's disease monitoring. Gut. 2013;62:1806–1816. doi: 10.1136/gutjnl-2012-303957. [DOI] [PubMed] [Google Scholar]
- 7.Adler J, R Punglia D, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn's disease. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21801. [DOI] [PubMed] [Google Scholar]
- 8.Hou JK, Tan M, Stidham RW, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn's disease in the Veterans Affairs Health Care System. Dig Dis Sci. 2014;59:2406–2410. doi: 10.1007/s10620-014-3174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanauer DA, Mei Q, Law J, et al. Supporting information retrieval from electronic health records: A report of University of Michigan's nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE) J Biomed Inform. 2015;55:290–300. doi: 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jauregui-Amezaga A, Rimola J, Ordás I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn's disease in the era of biologics. Gut. 2015;64:1397–1402. doi: 10.1136/gutjnl-2014-308101. [DOI] [PubMed] [Google Scholar]
- 12.Danese S, Motte Cd C de L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938–945. doi: 10.1111/j.1572-0241.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 13.Menchén L, Marin-Jimenez I, Arias-Salgado EG, et al. Matrix metalloproteinase 9 is involved in Crohn's disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut. 2009;58:920–928. doi: 10.1136/gut.2008.150318. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DT, Mulani P, Chao J, et al. Effect of adalimumab on clinical laboratory parameters in patients with Crohn's disease: results from the CHARM trial. Inflamm Bowel Dis. 2012;18:818–825. doi: 10.1002/ibd.21836. [DOI] [PubMed] [Google Scholar]
- 15.Ripoche J. Blood platelets and inflammation: their relationship with liver and digestive diseases. Clin Res Hepatol Gastroenterol. 2011;35:353–357. doi: 10.1016/j.clinre.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Grewal JS, Mukhin YV, Garnovskaya MN, et al. Serotonin 5-HT2A receptor induces TGF-beta1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am. J. Physiol. 1999;276:F922–30. doi: 10.1152/ajprenal.1999.276.6.F922. [DOI] [PubMed] [Google Scholar]
- 17.Fabre A, Marchal-Sommé J, Marchand-Adam S, et al. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur. Respir. J. 2008;32:426–436. doi: 10.1183/09031936.00126907. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Duan J, Liu X, et al. Platelets Drive Smooth Muscle Metaplasia and Fibrogenesis in Endometriosis Through Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation. Mol. Cell. Endocrinol. 2016 doi: 10.1016/j.mce.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Peyrin-Biroulet L, Oussalah A, Williet N, et al. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn's disease. Gut. 2011;60:930–936. doi: 10.1136/gut.2010.227884. [DOI] [PubMed] [Google Scholar]
- 20.Osterman MT, Haynes K, Delzell E, et al. Effectiveness and Safety of Immunomodulators With Anti-Tumor Necrosis Factor Therapy in Crohn's Disease. Clin Gastroenterol Hepatol. 2015;13:1293–1301. doi: 10.1016/j.cgh.2015.02.017. e5– quiz e70–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Stidham RW, Higgins PD. Imaging of intestinal fibrosis: current challenges and future methods. United European Gastroenterology Journal. 2016 doi: 10.1177/2050640616636620. 2050640616636620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.