Abstract
Purpose/Objectives
To examine the late effects of intensity modulated radiation therapy (IMRT) in pediatric patients with rhabdomyosarcoma of the head and neck.
Materials/Methods
All one-year survivors of pediatric head and neck rhabdomyosarcoma treated with IMRT at a single institution from 1999-2014 were assessed for long term complications. Late toxicities were graded according to CTCAE version 4.03.
Results
Among 30 patients, median age at IMRT was 7.4 (1.5-20.8) years, median follow-up was 7.7 (1.2-14.4) years, and median IMRT dose was 50.4 (36-50.4) Gy. Tumor subsites included parameningeal (80%), orbit (13%), and other (7%). Common late toxicities were facial disfigurement (n=23, 77%), growth hormone deficiency (n=11, 37%), cataract (n=10, 34%), and dental problems (n=10, 33%). Twenty-two patients (73%) had ≥ 2 late toxicities and fourteen patients (47%) had ≥ 3 late toxicities. Seventeen patients (57%) experienced grade 2 toxicity and ten patients (33%) had grade 3 toxicity. Grade 3 toxicities included visual disturbance, cataract, facial disfigurement, chronic sinusitis/otitis, and hearing loss. Severe facial deformity was noted in nine patients (30%), and three patients underwent cosmetic surgery. Patients with severe facial deformity were treated at younger ages (median 6.0 years versus 8.1 years for patients with no/non-severe facial deformity) and more likely to have infratemporal fossa tumors. There were no secondary solid malignancies.
Conclusions
Late radiation toxicities are common in survivors of pediatric head and neck rhabdomyosarcoma treated with IMRT. While the majority of late effects are mild-moderate, they can significantly impact quality of life, particularly facial disfigurement.
Keywords: intensity modulated radiation therapy, pediatric rhabdomyosarcoma, late radiation effects
Introduction
Rhabdomyosarcoma is the most common soft-tissue sarcoma in pediatric patients. Approximately 350 children and adolescents younger than age 20 are diagnosed with rhabdomyosarcoma each year in the United States. [1] Thirty-five percent of rhabdomyosarcomas are located in the head and neck, with subsites including parameningeal (15%), orbit (10%), and other head and neck sites (10%). Treatment includes multimodality therapy with chemotherapy for all patients, surgery if possible, and radiation, depending on stage, Intergroup Rhabdomyosarcoma Study Group (IRSG) surgical-pathological grouping, and primary tumor site as defined by the IRSG. Survival rates for pediatric patients with rhabdomyosarcoma are approximately 70%. [2-3] Excellent local control can be achieved for patients with head and neck rhabdomyosarcoma treated with intensity modulated radiation therapy (IMRT). [4]
In an effort to reduce radiation doses to normal tissue, and thereby minimize the acute and long-term toxicity of radiotherapy, radiation techniques for patients with head and neck rhabdomyosarcoma have significantly evolved over the past 20 years from three-dimensional (3D) conformal planning to IMRT and also proton radiotherapy. [4-7] While Paulino et al. has published long-term radiation effects in children with head and neck rhabdomyosarcoma treated with 3D-conformal radiation techniques and several authors have reported late toxicities for children with rhabdomyosarcoma treated with proton radiotherapy, there are limited data detailing the late toxicities for head and neck rhabdomyosarcoma treated with IMRT, with most reports instead focusing on survival and local control rates. [4, 6, 8-12] The objective of the current report is to fill this gap by delineating the late effects of IMRT in a single-institutional cohort of pediatric patients with rhabdomyosarcoma of the head and neck.
Methods
Patients
We identified 30 eligible patients treated with IMRT for rhabdomyosarcoma of the head and neck between 1999 and 2014. Waiver approval was obtained from the Memorial Sloan Kettering (MSK) Institutional Review Board/Privacy Board. Eligible patients were ≤21 years of age, treated with IMRT at MSK, and had detailed follow-up data within our institution for at least one year after initiation of IMRT. Twenty-two patients (73%), in addition to the minimum follow-up of one year, also had at least one evaluation in our institutional pediatric or adult “long-term follow-up clinics”, which provide rigorous screening, monitoring, and treatment for potential late complications of cancer therapy in childhood cancer survivors. All patients who enter the pediatric long term follow-up clinic are given a referral for neurocognitive testing. Patients with locally recurrent disease to the head and neck were excluded from this cohort, which could confound reporting of late effects within the radiation field.
Radiation techniques and treatment
Patients received IMRT as previously described with plans designed using an inverse planning algorithm and delivered by a 6-mv linear accelerator with dynamic multileaf collimators. [4] All patients received once-daily fractions of 1.8 Gy with median dose of 50.4 Gy (range, 36 Gy to 50.4 Gy). Twenty-two patients (73%) received 50.4 Gy and one patient received an abbreviated course of 48.6 Gy due to the onset of severe mucositis prior to receipt of the final prescribed fraction. Three patients with orbital embryonal rhabdomyosarcoma received 45 Gy. One patient with alveolar rhabdomyosarcoma of the left neck received 5 Gy of intraoperative radiation therapy followed by 41.4 Gy of IMRT. One patient with nasopharyngeal botryoid rhabdomyosarcoma had a gross total resection and received 36 Gy; another patient with paranasal sinus alveolar rhabdomyosarcoma had a complete response to neoadjuvant chemotherapy and thus also received 36 Gy.
Late toxicities
Late toxicities were defined as occurring more than 90 days after radiation treatment. All toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE), version 4.03, except for facial disfigurement and dental problems. Facial deformity was not graded according to CTCAE criteria, which grades any scarring or disfigurement of the head or neck region as grade 3. Rather, in an effort to better describe the range of facial disfigurement in this cohort, facial disfigurement was categorized as either “mild,” “moderate,” or “severe” by treating physicians. “Severe” was defined as visible from a distance or resulting in cosmetic surgery. Dental problems were also not graded according to CTCAE but rather grouped descriptively, as CTCAE only includes dental caries and not other dental abnormalities. The interval time from IMRT start to the development of the first grade 2, grade 3, and grade 4 toxicities was recorded for each patient, and a cumulative incidence curve for the development of late toxicities was made by the Kaplan-Meier method.
Results
Among 30 patients, the median age at initiation of IMRT was 7.4 years (range, 1.5 to 20.8 years), and 70% patients were ≤10 years of age at the initiation of IMRT. The median follow-up was 7.7 years from initiation of IMRT (range, 1.2 to 14.4 years). At least five years of follow-up was available for 26 patients (87%), and at least ten years of follow-up was available for thirteen patients (43%). The most common tumor subsite was parameningeal (80%), followed by orbit (13%) and non-parameningeal head and neck (7%). Embryonal and botryoid histologies were the most common (73%) compared to alveolar or undifferentiated. The median dose of IMRT was 50.4 Gy (range, 36 Gy to 50.4 Gy). All patients received chemotherapy (100%). Six patients had a surgical resection (20%), while the majority of patients underwent biopsy only. Patient characteristics are summarized in TABLE I.
TABLE I.
Patient Characteristics
| Characteristic | No. (%) |
|---|---|
| Age at initiation of IMRT | |
| 1-5 years | 9 (30.0) |
| 6-10 years | 12 (40.0) |
| 11-15 years | 6 (20.0) |
| 16-20 years | 3 (10.0) |
| Gender | |
| Male | 11 (36.7) |
| Female | 19 (63.3) |
| Race | |
| Caucasian | 26 (86.7) |
| African American | 3 (10.0) |
| Asian | 1 (3.3) |
| Tumor subsite | |
| Parameningeal | 24 (80.0) |
| Infratemporal fossa | 10 |
| Nasopharynx | 7 |
| Paranasal sinus | 3 |
| Ethmoid sinus | 2 |
| Parapharyngeal region | 2 |
| Orbit | 4 (13.3) |
| Neck | 1 (3.3) |
| Buccal | 1 (3.3) |
| Histology | |
| Embryonal/botryoid | 22 (73.3) |
| Alveolar/undifferentiated | 8 (26.7) |
| Surgery | |
| Biopsy only | 24 (80.0) |
| Resection (subtotal or total) | 6 (20.0) |
| Chemotherapy | |
| Any | 30 (100) |
| Alkylators | 29 (96.7) |
| Anthracyclines | 21 (70.0) |
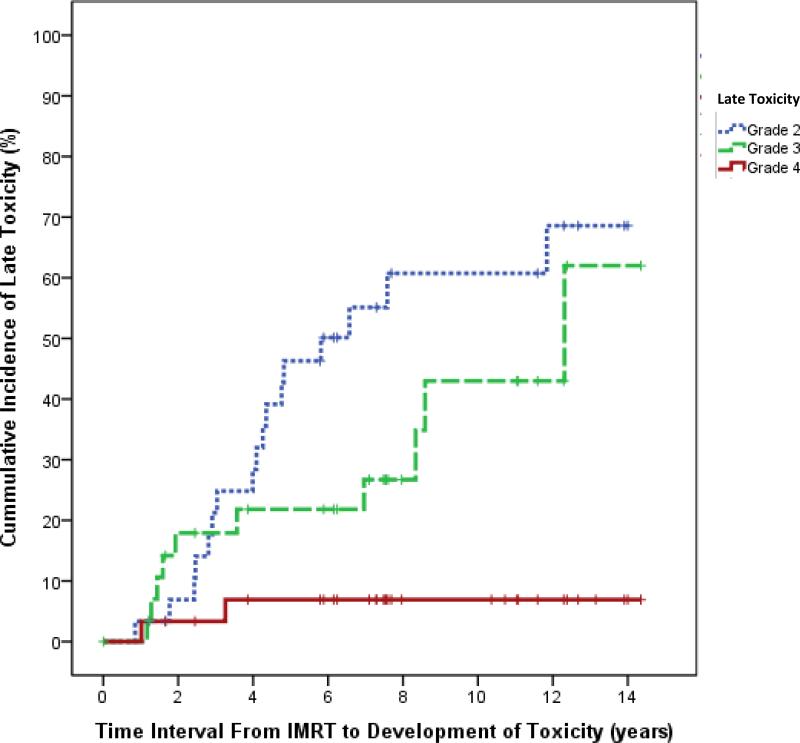
Late toxicities and CTCAE grades are shown in TABLE II. The number of evaluable patients indicates the patients were screened for a specific late effect. For example, if a patient did not have a thyroid stimulating hormone (TSH) and T4 documented, that patient was excluded from the number of evaluable patients for hypothyroidism. Common late toxicities were facial disfigurement (n=23, 77%), growth hormone deficiency (n=11, 37%), cataract (n=10, 34%), and dental problems (n=10, 33%). There were 22 patients (73%) with ≥ 2 late toxicities and fourteen patients (47%) with ≥ 3 late toxicities. Seventeen patients (57%) had ≥ 1 grade 2 toxicity and ten patients (33%) had ≥ 1 grade 3 toxicity. Grade 3 late toxicities included visual disturbance, cataract, chronic sinusitis/otitis, and hearing loss. A cumulative incidence curve for the first development of grade 2, grade 3, and grade 4 toxicities is shown in Figure 1. At ten years post-radiation, the incidence of grade 2, grade 3, and grade 4 toxicities approached approximately 60%, 43%, and 6% respectively.
TABLE II.
Late Toxicities After Head and Neck Intensity Modulated Radiation Therapy for Pediatric Rhabdomyosarcoma
| Late Toxicity | No. of Evaluable Patients | Any Grade No. (%) | Grade 1 No. | Grade 2 No. | Grade 3 No. | Grade 4 No. |
|---|---|---|---|---|---|---|
| Facial disfigurement | 30 | 23 (76.7) | - | - | - | - |
| Growth hormone deficiency | 30 | 11 (36.7) | 1 | 9 | 0 | 0 |
| Cataract | 29 | 10 (34.5) | 7 | 2 | 0 | 0 |
| Dental problems | 30 | 10 (33.3) | - | - | - | - |
| Psychological/behavioral problems | 30 | 9 (33.0) | 6 | 3 | 0 | 0 |
| Central hypothyroidism | 29 | 7 (24.1) | 0 | 6 | 0 | 0 |
| Chronic sinusitis | 30 | 7 (23.3) | 3 | 3 | 1 | 0 |
| Chronic otitis | 30 | 6 (20.0) | 1 | 2 | 3 | 0 |
| Hearing loss | 30 | 6 (20.0) | 0 | 3 | 2 | 0 |
| Visual disturbance | 30 | 6 (20.0) | 3 | 1 | 2 | 0 |
| Trismus | 30 | 4 (13.3) | 4 | 0 | 0 | 0 |
| Epistaxis | 30 | 4 (13.3) | 4 | 0 | 0 | 0 |
| Cognitive deficits | 30 | 4 (13.3) | 3 | 0 | 0 | 0 |
| ACTH deficiency | 30 | 4 (13.3) | 0 | 3 | 0 | 0 |
| LH/FSH deficiency | 30 | 4 (13.3) | 1 | 2 | 0 | 0 |
| Primary hypothyroidism | 29 | 3 (10.3) | 0 | 3 | 0 | 0 |
| Nasal congestion | 30 | 3 (10.0) | 3 | 0 | 0 | 0 |
| Ptosis | 30 | 2 (6.7) | 0 | 0 | 2 | 0 |
| Voice changes | 30 | 2 (6.7) | 2 | 0 | 0 | 0 |
| Secondary malignancy | 30 | 2 (6.7) | 0 | 0 | 0 | 2 |
| Thyroid nodule | 28 | 1 (3.6) | 1 | 0 | 0 | 0 |
| Glaucoma | 30 | 1 (3.3) | 1 | 0 | 0 | 0 |
| Amblyopia | 30 | 1 (3.3) | 1 | 0 | 0 | 0 |
| Schwannoma | 30 | 1 (3.3) | 1 | 0 | 0 | 0 |
Figure 1.
Cumulative incidence curve for the development of late toxicities following intensity modulated radiation therapy (IMRT) for head and neck rhabdomyosarcoma in pediatric patients.
Facial disfigurement
Some degree of facial disfigurement was the most common late toxicity in our study, and was noted in 23 patients (77%). Facial disfigurement was categorized as “mild” in nine patients (30%), “moderate” in six patients (20%), and “severe” in nine patients (30%). Patients with severe facial deformity are further described in TABLE III. Three of the patients with “severe” facial disfigurement underwent subsequent cosmetic surgery – one received definitive radiation to the orbit and two received definitive radiation to the infratemporal fossa. Patients with severe facial deformity had a trend toward younger age at treatment; the median age at initiation of IMRT was 6.0 years for patients with severe facial deformity versus 8.1 years for patients with no or non-severe facial deformity. The most common tumor site was the infratemporal fossa for patients with severe facial deformity.
TABLE III.
Severe Facial Disfigurement After Head and Neck Intensity Modulated Radiation Therapy for Pediatric Rhabdomyosarcoma
| Tumor site | Age at IMRT, years | Description | |
|---|---|---|---|
| 1 | Left infratemporal fossa | 9.8 | Hypoplasia of left frontolateral facial bones |
| 2 | Right infratemporal fossa | 6.0 | Underwent facial reconstructive surgery; right orbital hypoplasia; also with related depression |
| 3 | Left orbit | 8.4 | Underwent facial reconstructive surgery left orbital hypoplasia |
| 4 | Nasopharynx, right>left | 3.0 | Mid-facial hypoplasia |
| 5 | Left ethmoid sinus | 8.7 | Left orbital and mid facial hypoplasia |
| 6 | Left orbit/infratemporal fossa | 1.7 | Left hypoplasia and mild facial asymmetry |
| 7 | Nasopharynx and ethmoid sinus, left >right | 4.4 | Facial hypoplasia and small jaw |
| 8 | Left infratemporal fossa | 3.4 | Facial hypoplasia and asymmetry |
| 9 | Left infratemporal fossa/orbit | 7.3 | Left facial and orbital hypoplasia; underwent facial reconstructive surgery |
Endocrinopathies
Thirteen patients (43%) experienced endocrine dysfunction after the completion of therapy. The most common endocrine dysfunction was growth hormone deficiency (n=11, 37%), followed by central hypothyroidism (n=7, 24%), primary hypothyroidism (n=3, 10%), adrenocorticotropic hormone (ACTH) deficiency (n=4, 13%), and luteinizing and follicle stimulating hormone (LH/FSH) deficiency (n=4, 13%). Nine of these thirteen patients had endocrine dysfunction in ≥ 2 categories.
Eye problems
Fourteen patients (47%) developed a late toxicity of the eye, including cataracts, visual disturbance, glaucoma, amblyopia, or ptosis, as detailed by patient in TABLE IV. Ten patients developed cataracts (34%), the majority of which were grade 1 (80%). Two patients underwent removal of their cataracts (grade 3) – one patient had a history of left orbital rhabdomyosarcoma and the other had left ethmoid sinus rhabdomyosarcoma. Six patients developed visual disturbances (20%). The majority of visual disturbances were decreased visual acuity, except for one patient who developed diplopia requiring prism glasses. The two patients who developed grade 3 visual disturbance were all treated for rhabdomyosarcoma of the orbit. One patient with a left ethmoid sinus tumor developed grade 1 glaucoma (3%), and one patient with an infratemporal fossa tumor developed grade 1 amblyopia (3%). Two patients (7%) with orbital rhabdomyosarcoma developed ptosis requiring surgical correction.
TABLE IV.
Orbital Toxicities After Head and Neck Intensity Modulated Radiation Therapy for Pediatric Rhabdomyosarcoma
| Tumor site | Tumor laterality | Toxicity | Toxicity grade | Toxicity laterality | Comments | |
|---|---|---|---|---|---|---|
| 1 | Orbit | Left | Cataract Ptosis |
1 3 |
Left Left |
Status post surgical correction |
| 2 | Orbit | Left | Cataract | 1 | Bilateral | |
| 3 | Nasopharynx | Right>left | Cataract | 1 | Right | |
| 4 | Infratemporal fossa | Left | Am blyopia | 1 | Right | Refractory amblyopia |
| 5 | Infratemporal fossa | Right | Visual disturbance | 1 | Unknown | Wears glasses |
| 6 | Orbit | Left | Cataract | 1 | Left | Blind in left eye pre-radiation |
| 7 | Nasopharynx/right orbit | Right>left | Visual disturbance Cataract |
3 1 |
Right Left |
Blind in right eye |
| 8 | Ethmoid sinus | Left | Visual disturbance Cataract Glaucoma |
1 3 1 |
Left Left Unknown |
Decreased visual acuity Status post cataract removal Diagnosed at outside institution |
| 9 | Orbit | Left | Visual disturbance Cataract Ptosis |
1 3 3 |
Left Left Left |
Decreased visual acuity Status post cataract removal Status post surgical correction |
| 10 | Ethmoid sinus/orbit | Left | Cataract | 1 | Unknown | |
| 11 | Infratemporal fossa/orbit | Left | Visual disturbance | 3 | Left | Left eye decreased visual acuity |
| 12 | Nas opharynx | Left>right | Cataract | 1 | Bilateral | Left greater than right |
| 12 | Skull base | Left | Visual disturbance | 2 | - | Diplopia requiring glasses |
| 13 | Infratemporal fossa/orbit | Left | Cataract | 1 | Left | Blind in left eye pre-radiation |
Ear disorders
Ten patients (33%) developed a late toxicity of the ear, including hearing loss or chronic otitis. Hearing loss was reported in six patients (20%), primarily in those with tumors located in the skull base as shown in TABLE V. Three patients experienced unilateral hearing loss ipsilateral to the tumor. Chronic otitis was reported in six patients (20%), and three were characterized as grade 3 requiring myringotomy tubes.
TABLE V.
Hearing Loss After Head and Neck Intensity Modulated Radiation Therapy for Pediatric Rhabdomyosarcoma
| Tumor Site | Tumor Laterality | Hearing Loss Grade | Hearing Loss Laterality | Comments | |
|---|---|---|---|---|---|
| 1 | Neck | Left | 2 | Bilateral | Received vincristine; no cisplatin or carboplatin |
| 2 | Skull base | Left | 2 | Bilateral | Right mixed hearing loss, left conductive hearing loss; received carboplatin and vincristine |
| 3 | Skull base | Right | 3 | Unilateral | Right conductive hearing loss; received carboplatin and vincristine |
| 4 | Infratemporal fossa | Left | 3 | Unilateral | Left mixed hearing loss; received carboplatin and vincristine |
| 5 | Skull base | Left | 3 | Unilateral | Requires left hearing aid; received cisplatin and vincristine |
| 6 | Infratemporal fossa | Left | 1 | Bilateral | Bilateral conductive hearing loss |
Nose
Late toxicities included chronic sinusitis (n= 7, 23%), epistaxis (n=4, 13%), and chronic nasal congestion (n=3, 10%). Grade 3 chronic sinusitis occurred in one patient with rhabdomyosarcoma of the right skull base requiring sinoplasty. All cases of epistaxis and chronic nasal congestion were grade 1.
Oral Cavity/Throat
Ten patients (33%) experienced dental problems. Dental problems included atrophic maxilla and failing dentition in the maxilla, requiring dental implants, in two patients; dental caries in two patients; shortened roots in two patients; pyogenic granuloma in one patient; delayed dental eruption in one patient; and poor oral hygiene with plaque accumulation and gingivitis in one patient. Trismus occurred in four patients (13%), all of whom experienced grade 1 trismus that did not interfere with eating. Two patients developed grade 1 voice changes (7%).
Neurocognition
A total of 28 patients (93%) in our cohort received >10 Gy to the temporal lobe. Of the 30 patients in our cohort, 28 patients had documented neurocognitive testing available post-radiation. Four patients (13%) had grade 1 neurocognitive dysfunction documented post-radiation, which was defined as mild cognitive disability not inferring with work, school, or life performance. These patients were < 8 years of age at time of treatment (range, 3.0 to 7.3 years). Two patients with rhabdomyosarcoma of the infratemporal fossa exhibited decreased processing speed with no special education requirements. One patient with nasopharyngeal rhabdomyosarcoma exhibited slowed processing speed and weakness in memory retrieval with recommendation of Section 504 Plan and is currently an honors student in the high school. Of note, this patient's medical history was complicated by a fall causing an epidural hematoma requiring craniotomy. One patient with rhabdomyosarcoma of the infratemporal fossa also had a Section 504 Plan in place and is currently enrolled in college. Of the 30 patients in our cohort, 14 patients were >18 years old at the time of last follow-up, and all are currently enrolled in college or have graduated college. Two of these patients completed masters degrees and are considering doctorate programs, and one patient is currently in law school.
Psychological/behavioral problems
Nine patients (30%) were noted to have psychological or behavioral problems, which were likely multifactorial in nature. The median age of patients with psychological or behavioral issues at initiation of IMRT was 11.3 years (range, 3.0 – 16.0 years). Six cases were grade 1, and three cases were grade 2 requiring medication. Two of the patients had a history of developmental delay pre-dating treatment, and their psychological disorders included attention deficit hyperactivity disorder and behavioral outburst in one patient (grade 1) and depression (grade 2) in one patient. The other grade 2 psychological disorders included bipolar disorder (grade 2) and depression (grade 2). Grade 1 other psychological disorders included anxiety (3 patients), labile mood (1 patient), and depression (1 patient).
Secondary malignancy
There were no secondary solid malignancies but two patients (7%) developed secondary acute myeloid leukemia and myelodysplastic syndrome. Both patients received alkylating agents and anthracyclines. There were no other grade 4 toxicities. One patient, whose primary tumor site was the nasopharynx, developed a radiation-induced WHO grade 1 schwannoma of the right C2-C3 neural foramen nine years following IMRT. One patient developed a benign thyroid nodule.
Discussion
In this review of 30 patients treated with IMRT for rhabdomyosarcoma of the head and neck, we found that these patients commonly experience late toxicities from IMRT. As the radiation techniques for rhabdomyosarcoma of the head and neck have evolved from 3D-conformal planning to IMRT, it is increasingly important to examine how the late toxicities of treatment have changed. [4-5] In seventeen children treated with 3D-conformal radiation techniques for head and neck rhabdomyosarcoma, Paulino et al. observed a wide range of severe late toxicities occurring greater than 10 years after radiation, including midbrain hemorrhage, chondronecrosis, pontine hemorrhage, or esophageal stenosis. [8] While these toxicities were not observed in our cohort, it is possible that such issues will become apparent with longer follow-up since 10-year follow-up was only available for 12 patients in our study. Additionally, the patient who developed chondronecrosis and esophageal necrosis in their report received a higher dose per fraction of 250 cGy. Our study otherwise reported similar late effects, such as neuroendocrine dysfunction, hearing loss, visual problems, facial asymmetry, hypothyroidism, and impaired dentition.
There are limited data detailing the late toxicities for head and neck rhabdomyosarcoma treated with IMRT, with most reports focusing on survival and local control rates and with limited follow-up. [4, 11-12] Combs et al. reported late toxicities in nine patients with head and neck rhabdomyosarcoma treated with IMRT or fractionated stereotactic radiotherapy with a median follow-up of 17 months. [11] Late toxicities included reduced lacrimation, chronic eye swelling, visual disturbance, trismus, facial asymmetry, and growth hormone deficiency. Curtis et al. reported late toxicities in six children treated with IMRT for head and neck rhabdomyosarcoma with a median follow-up of 4.6 years. [12] Late toxicities included cataracts, hypothyroidism, growth hormone deficiency, facial asymmetry, and secondary malignancy. These studies are limited by relatively short median follow-up, thus preventing comparison to our findings.
In addition to comparing late toxicities associated with IMRT to those observed with 3D conformal techniques, it would also be useful to compare these results to proton therapy data. Dosimetric comparison of IMRT to proton therapy treatment plans in a phase II trial of patients with pediatric rhabdomyosarcoma demonstrated the mean integral dose was 1.8 times higher for IMRT to the head and neck (p<0.01), and significant tissue sparing was seen in many critical structures such as chiasm, pituitary, hypothalamus, brainstem, cerebellum, maxilla, mandible, contralateral optic nerve, and temporal lobe with the use of protons. [7] Similarly, Kozak et al. in a dosimetric comparison of IMRT and proton therapy for parameningeal rhabdomyosarcomas reported a significant decrease in mean dose in all normal structures except for ipsilateral cochlea and mastoid with proton radiotherapy. [13] Childs et al. reported the late toxicities of proton radiation in 10 pediatric patients with parameningeal rhabdomyosarcoma including decreased height velocity, endocrinopathies, facial hypoplasia, chronic nasal congestion, and dental problems, with a median follow-up time of 5 years. [9] The late toxicities of 43 patients with any tumor site enrolled in this phase II trial have been reported by Ladra et al. and include: grade 3 late toxicities, including cataract, chronic otitis, and retinopathy with decreased visual acuity, in 7% of patients; grade 2 late toxicities, including dry eye, facial asymmetry, epistaxis, dry skin, chronic otitis, endocrine abnormalities, hearing loss, and cognitive disturbance, in 20% of patients. [6] For 39 pediatric patients with parameningeal rhabdomyosarcoma treated with pencil beam scanning proton radiotherapy, Weber et al. reported grade 1 late toxicities in 21% of patients, grade 2 late toxicities in 26% of patients, and grade 3 late toxicities in 8% of patients with no secondary malignancies at median follow-up of 3.4 months. Grade 3 toxicities included cataract and hearing loss. [10]
The most common late toxicity observed in our study was facial disfigurement in 77% of patients; three patients subsequently underwent facial reconstructive surgery. Paulino et al. similarly reported a high percentage of facial asymmetry in 73% of patients treated with 3D conformal radiation, including three patients who underwent facial reconstruction, and Childs et al. reported facial hypoplasia in 70% of patients for whom late toxicities were reported treated with proton radiation at a median follow-up of 5 years. [8-9] Combs et al. reported facial asymmetry in only one patient (5%) with a median follow-up of 17 months after IMRT, [11] suggesting that longer follow-up may be needed to observe this late effect. We noted that patients with “severe” facial disfigurement in our study had a trend toward younger age at treatment with median age of 6.0 years for those with severe facial disfigurement versus 8.1 years for those with no or non-severe facial deformity. Often facial deformities do not become apparent until after the adolescent growth spurt. The median age of our patients at last follow-up was 17.0 years (range, 5.4 to 32.0 years), and 24 patients (80%) were older than 12 years of age at last follow-up.
Soft tissue sarcoma survivors are known to be at risk of developing subsequent cancers, as reported by the Childhood Cancer Survivor Study. [14] As IMRT increases the volume of normal tissue exposed to low-dose radiation compared to 3D-conformal techniques, it is particularly import to monitor for the development of secondary solid malignancies within the radiation field. None of the patients in our cohort developed a secondary solid malignancy but two patients (7%) developed secondary acute myeloid leukemia and myelodysplastic syndrome (grade 4), presumably secondary to chemotherapy. While even wide-field radiation in pediatric patients with Hodgkin's disease has not been associated with development of leukemia, chemotherapy is known to be associated with subsequent leukemia. [15] One patient developed a secondary radiation-induced neoplasm, a WHO grade 1 schwannoma, within the radiation field nine years following IMRT. Paulino et al. reported the development of only one secondary solid malignancy in their study of late effects of 3D-conformal radiation, which was a parotid mucoepidermoid carcinoma at the edge of the radiation field twenty years following radiation therapy. [8] In a report by Casey et al. on 242 pediatric patients treated with IMRT at our institution, six patients developed a secondary malignancy, four with secondary solid malignancies within the radiation field and two with myelodysplastic syndrome. [16] The median time from IMRT to a secondary solid malignancy was 7.2 years in that report, which is comparable to the median follow-up time in the current study. Curtis et al. reported the development of an osteosarcoma four years post-radiation therapy within the radiation field for a patient with a p53 mutation following IMRT. [12] Combs et al. reported no secondary malignancies in a study of 19 patients with head and neck rhabdomyosarcoma, but with median follow-up of only 17 months. [11] Ladra et al. reported no secondary malignancies in the phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma with median follow-up of 3.9 years. [6] For all of these studies, including the present report, longer follow-up is needed to better assess the risk of secondary solid cancers after radiotherapy.
Our study has a number of limitations which must be considered. One important limitation is the lack of a validated grading system for facial disfigurement, as the CTCAE simply grades any scarring or disfigurement of the head or neck region as a grade 3. Our evaluation of facial disfigurement was subjective and based on the consensus of treating physicians. Additionally, we were unable to document the precise age at development of facial disfigurement which may be useful when comparing our results to other patient cohorts, as patients treated at younger ages may skew results for a higher incidence of facial disfigurement. Another limitation of our study was the inability to evaluate the patients’ feelings about their facial appearance and the impact this may have on their quality of life. Facial asymmetry has considerable impact on quality of life, as three of our patients underwent subsequent facial reconstructive surgery and one of these patients developed depression in part related to facial disfigurement. The largest limitation of our study is the retrospective nature. A major strength of this study is the detailed screening for late toxicities provided by our institutional pediatric or adult “long term follow-up clinics”, which included 73% of patients in this study. These patients are comprehensively examined and tested for known late effects of their cancer therapy in our “long term follow-up clinics.” Routine screening and monitoring is critical for these patients to recognize and treat late toxicities of their cancer treatment. Additionally, lengthy follow-up is paramount, as studies have shown complications arising even >10 years following radiation therapy. [8] Though we required at least one year of follow-up post-IMRT, only three patients in our cohort had less than three years of follow up. With longer duration of follow-up, particularly for children treated in their first decade of life, more late sequelae are likely to emerge. Another limitation of our retrospective review was that the majority of patients did not have neurocognitive testing at baseline prior to radiation treatment, but rather neurocognitive testing was performed post-radiation. In the future, this would be helpful to assess in a prospective study.
It is imperative to closely observe the long-term effects of treatment for pediatric patients, who are at risk for adverse quality of life and even increased risk of death following cancer survival. [17-18] It should also be considered that even with evolving and more conformal radiation techniques, the delivery of radiation to normal structures directly abutting or involved by tumor cannot be spared in order to adequately treat the tumor according to current treatment standards. Unfortunately, we do not know which patients can safely have radiation eliminated or meaningfully dose-reduced. For parameningeal tumors where local recurrence is the dominant risk of treatment failure, some radiation late sequelae may be the unfortunate price of successful treatment of the tumor.
Conclusions
Late radiation toxicities are commonly seen in pediatric rhabdomyosarcoma survivors treated with IMRT to the head and neck. The majority of late effects are mild-moderate in severity. Facial disfigurement was the most common late effect of IMRT, especially in younger children with infratemporal fossa tumors. Future research efforts are needed to minimize the magnitude of radiation-related late effects. These data on late effects with IMRT for head and neck rhabdomyosarcoma will be useful for future comparison to other tissue sparing techniques such as proton therapy.
Acknowledgements
This work was funded in part by NIH/NCATS grant KL2 TR000458 of the Clinical and Translational Science Center at Weill Cornell and the NIH/NCI Cancer Support Grant P30 CA008748.
Abbreviations
- ACTH
adrenocorticotropic hormone
- CTCAE
Common Terminology Criteria for Adverse Events
- FSH
follicle stimulating hormone
- Gy
gray
- IMRT
intensity modulated radiation therapy
- LH
luteinizing hormone
- MSK
Memorial Sloan Kettering
- TSH
thyroid stimulating hormone
- WHO
World Health Organization
Footnotes
Conflicts of interest
Leonard Wexler reports personal fees from Astra-Zeneca, outside the submitted work.
References
- 1.Ries L, Smith M, Gurney J, Linet M, Tamra T, Young JL, Bunin GR, editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER program; Bethesda, MD.: 1999. NIH Pub. No. 99-4649. [Google Scholar]
- 2.Arndt CA, Stoner JA, Hawkins DS, Rodeberg DA, Hayes-Jordan AA, Paidas CN, Parham DM, Teot LA, Wharam MD, Breneman JC, Donaldson SS, Anderson JR, Meyer WH. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: a children's oncology group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raney RB, Walterhouse DO, Meza JL, Andrassy RJ, Breneman JC, Crist WM, Maurer HM, Meyer WH, Parham DM, Anderson JR. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29:1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolden SL, Wexler LH, Kraus DH, Laguaglia MP, Lis E, Meyers PA. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiation Oncol Biol Phys. 2005;61:1432–1438. doi: 10.1016/j.ijrobp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Michalski JM, Sur RK, Harms WB, Purdy JA. Three-dimensional conformal radiation therapy in pediatric parameningeal rhabdomyosarcomas. Int J Radiation Oncol Biol Phys. 1995;33:985–991. doi: 10.1016/0360-3016(95)00551-X. [DOI] [PubMed] [Google Scholar]
- 6.Ladra MM, Syzmonifka JD, Mahajan A, Friedman AM, Yong Yeap B, Goebel CP, MacDonald SM, Grosshans DR, Rodriquez-Galindo C, Marcus KJ, Tarbell NJ, Yock TI. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32:3762–3770. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladra MM, Edgington SK, Mahajan A, Grosshans D, Szymonifka J, Khan F, Moleabbed M, Friedmann AM, MacDonald SM, Tarbell NJ, Tock TI. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled in a prospective phase II proton study. Radiother Oncol. 2014;113:77–83. doi: 10.1016/j.radonc.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulino AC, Simon JH, Zhen W, Wen BC. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiation Oncol Biol Phys. 2000;48:1489–1495. doi: 10.1016/s0360-3016(00)00799-9. [DOI] [PubMed] [Google Scholar]
- 9.Childs SK, Kozak KR, Friedmann AM, Yeap BY, Adams J, MacDonald SM, Liebsch NJ, Tarbell NJ, Yock TI. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82:635–642. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber DC, Ares C, Albertini F, Frei-Welte M, Niggli FK, Schneider R, Lomax AJ. Pencil beam scanning proton therapy for pediatric parameningeal rhabdomyosarcomas: clinical outcome of patients treated at the Paul Scherrer Institute. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25864. doi: 10.1002/pbc.25864. [DOI] [PubMed] [Google Scholar]
- 11.Combs SE, Behnisch W, Kulozik AE, Huber PE, Debus J, Schulz-Ertner D. Intensity modulated radiotherapy (IMRT) and fractionated stereotactic radiotherapy (FSRT) for children with head-and-neck-rhabdomyosarcoma. BMC Cancer. 2007;7:177. doi: 10.1186/1471-2407-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis AE, Okcu MF, Chintagumpala M, Teh BS, Paulino AC. Local control after intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiation Oncol Biol Phys. 2009;73:173–177. doi: 10.1016/j.ijrobp.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Kozak KR, Adams J, Krejcarek JJ, Tarbell NJ, Yock TI. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179–186. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 14.Bassal M, Mertens AC, Taylor L, Neglia JP, Greffe BS, Hammond S, Ronckers CM, Friedman DL, Stovall M, Yasui YY, Robinson LL, Meadows AT, Kadan-Lottick NS. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 15.Wolden SL, Lamborn KR, Cleary SF, Tate DJ, Donaldson SS. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998;16:536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 16.Casey DL, Friedman DN, Moskowitz CS, Hilden PD, Sklar CA, Wexler LH, Wolden SL. Second cancer risk in childhood cancer survivors treated with intensity-modulated radiation therapy (IMRT). Pediatr Blood Cancer. 2015;62:311–316. doi: 10.1002/pbc.25285. [DOI] [PubMed] [Google Scholar]
- 17.Hudson MM, Mulrooney DA, Bowers DC, Sklar CA, Green DM, Donaldson SS, Oeffinger KC, Neglia JP, Meadows AT, Robison LL. High-risk populations identified in childhood cancer survivor study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27:2405–2414. doi: 10.1200/JCO.2008.21.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertens AC, Yong J, Dietz AC, Kreiter E, Yasui Y, Bleyer A, Armstrong GT, Robison LL, Wasilewski-Masker K. Conditional survival in pediatric malignancies: analysis of data from the childhood cancer survivor study and the surveillance, epidemiology, and end results program. Cancer. 2015;121:1108–1117. doi: 10.1002/cncr.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]