Abstract
Trimethoprim-sulfamethoxazole (TMP/SMX) is used as prophylaxis against Pneumocystis jiroveci during chemotherapy. Many groups recommend withholding TMP/SMX during high-dose methotrexate (HDMTX) for concerns that it will delay methotrexate clearance. We compared methotrexate exposure following HDMTX (NCT00549848) in 424 patients including 783 courses that were and 602 courses that were not given concurrently with TMP/SMX. Among 176 patients (555 courses) on the low-risk arm (HDMTX = 2.5 g/m2/24 hours), there was no difference in clearance (110.7 (1.8%) vs. 108.2 (0.9%) mL/min/m2, p=0.3) nor in 42 hour methotrexate concentration (0.37 (5.1%) vs. 0.40 (5.0%) μM, p=0.23). Among 248 patients (830 courses) on the standard/high-risk arm (HDMTX ~5 g/m2/24 hours), there was slightly higher clearance (95.5 (1.4%) vs. 91.2 (0.8%) mL/min/m2, p=0.005) in those receiving TMP/SMX, with no difference in the 42 hour methotrexate concentration (0.59 (4.1%) vs. 0.66 (4.2%) μM, p=0.06). There was no difference in neutrophil counts based on TMP/SMX during HDMTX (p = 0.83). TMP/SMX also did not have a significant impact on myelosuppression of low-dose methotrexate (40 mg/m2) given during continuation therapy among 230 patients enrolled on a prior study (NCT00137111). Thus, we found no evidence for an interaction between methotrexate and TMP/SMX given prophylactically.
Introduction
High-dose methotrexate (HDMTX) is used to treat acute lymphoblastic leukemia (ALL)(1–4) as well as other malignancies. In St. Jude’s front-line protocol for ALL, Total Therapy XVI (Total XVI), HDMTX is given for 4 doses during the consolidation phase of therapy,(5, 6) similar to other groups.(7)
Because ALL therapy is immunosuppressive, thrice-weekly TMP/SMX is utilized as prophylaxis for Pneumocystis jiroveci pneumonia (PCP) throughout therapy.(8) Many groups withhold TMP/SMX prophylaxis during HDMTX(9) administration due to concerns that TMP/SMX may inhibit methotrexate clearance, displace methotrexate from plasma protein binding sites, (10–12)or cause toxicity via inhibition of dihydrofolate reductase.(13, 14)
Previously, we showed that TMP/SMX did not affect methotrexate at a dose of ~1500 mg/m2 over 24 hours.(15) Based on this lack of interaction, our practice at St. Jude has been to administer TMP/SMX during HDMTX, though other groups withhold TMP/SMX. Hence, we extended our studies to test whether there was any interaction with higher doses of HDMTX, and to test for any impact of TMP/SMX on blood neutrophil counts (ANC) after HDMTX or low-dose methotrexate (LDMTX).
Methods
We prospectively estimated HDMTX clearance and 42 hour plasma methotrexate concentrations in all patients enrolled on the Total XVI protocol from October 2007 through December 2014. Informed consent was obtained according to institutional guidelines. Consolidation therapy consisted of daily oral mercaptopurine along with HDMTX administered every-other-week for 4 courses (16) Patients on the low-risk arm of the protocol received HDMTX at 2500 mg/m2 whereas patients on the standard/high-risk arms received 5000 mg/m2 for the first course, followed by doses individualized to achieve a steady-state plasma concentration of 65 μM.5 Ten percent of the HDMTX was given over one hour and 90% over the subsequent 23 hours, with hydration, alkalinization, and leucovorin initiated at hour 42.5 Methotrexate plasma concentrations were measured at hours 6, 23, and 42 hours using the TDx assay (Abbot Laboratories, Abbott Park, IL) and pharmacokinetic parameters were estimated.(5) All patients had complete blood count with differential measured at least weekly after each HDMTX.
TMP/SMX at 75 mg (TMP)/m2/dose every 12 hours was administered on Monday, Tuesday, and Wednesday throughout all of consolidation and maintenance therapy. We considered HDMTX courses that started on Monday or Tuesday as receiving TMP/SMX, and courses that started on Thursday or Friday as not receiving TMP/SMX; courses started on Wednesday, Saturday, or Sunday were not considered. The effects of TMP/SMX on HDMTX pharmacokinetics, leukocyte count, and ANC were determined using linear mixed-effects modeling (Matlab R2015a).
We also assessed the impact of TMP/SMX on the myelosuppressive effects of LDMTX given during continuation among patients treated on the St. Jude Total XV protocol.(3) Patients received LDMTX 40 mg/m2/week IV or IM for 2 or 3 weeks out of every 4 for 120 weeks for girls and 146 weeks for boys.(3) Blood leukocyte counts and ANC were obtained at least weekly. During continuation, each LDMTX was characterized as to day of week delivered; for each phase, patients were categorized as on TMP/SMX if LDMTX was always given on Monday-Wednesday, off TMP/SMX if LDMTX was always given on Thursday-Sunday, and excluded if LDMTX and TMP/SMX were sometimes given together and sometimes not. This resulted in 121 to 230 patients per phase that were evaluable for blood counts.
Results and Discussion
A total of 1,385 courses of HDMTX (n=424 patients) were evaluated; 176 patients (555 courses) on the low-risk arm and 248 patients (830 courses) on the standard/high-risk arm. Among patients on the low-risk arm, there was no difference in the methotrexate clearance between the group that did vs did not receive concurrent TMP/SMX (110.7 (1.8%) vs 108.2 (0.9%) mL/min/m2, mean (standard error CV%); p=0.3; Figure 1A). There was also no difference in the methotrexate 42 hour plasma concentrations between the group that did vs did not (0.37 (5.1%) vs 0.40 (5.0%) μM, mean (standard error CV%); p=0.23; Figure 1B) receive TMP/SMX. Among patients on the standard/high-risk arms, the methotrexate clearance was slightly higher in the group that did vs did not receive TMP/SMX (95.5 (1.4%) vs 91.2 (0.8%) mL/min/m2, mean (standard error CV%); p=0.005; Figure 1A). But, there was no significant difference in the 42 hour plasma concentrations between the group that did vs did not (0.59 (4.1%) vs 0.66 (4.2%) μM, mean (standard error CV%); p=0.06; Figure 1B) receive TMP/SMX. Moreover, by repeated measures analyses, there were no differences in the lowest ANC (p = 0.83) (Figure 2), the mean ANC (p = 0.95), the lowest leukocyte count (p=0.60), nor in the mean leukocyte count (p =0.54) based on TMP/SMX use.
Figure 1. MTX clearance and 42 hr exposure subdivided by risk group and TMP/SMX status.
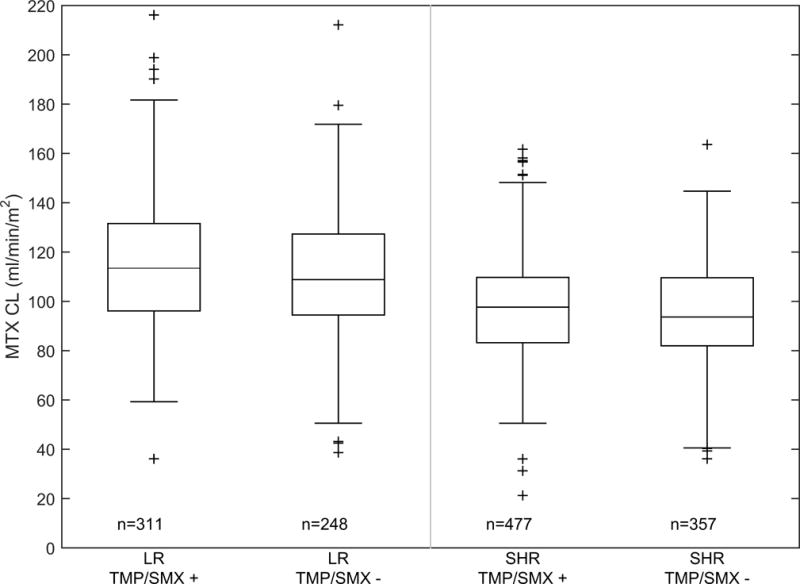
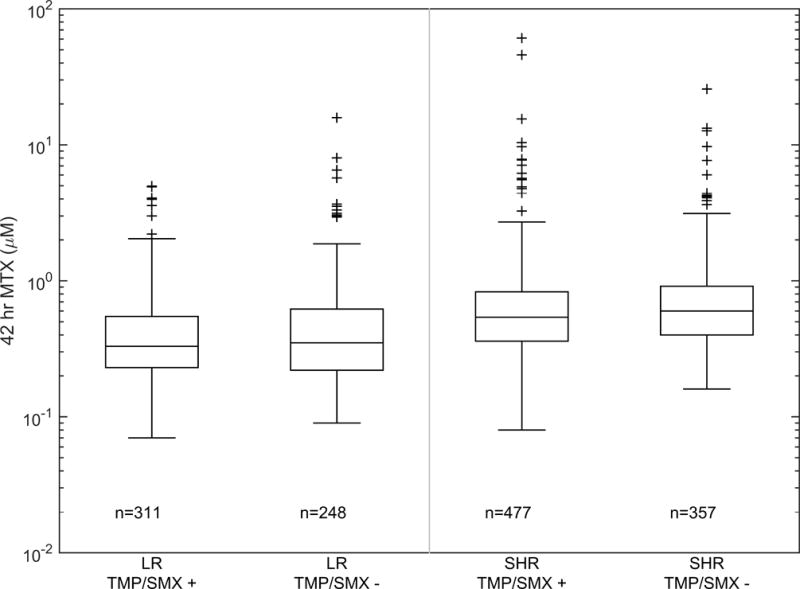
(A) Boxplot of individual methotrexate clearance estimates subdivided by risk group and TMP/SMX status. The bar, box, and whiskers represent the median, 25th–75th percentile, and non-outlier range respectively. n is the number of courses in each group. (B) Boxplot of individual methotrexate 42 hour plasma concentration subdivided by risk group and TMP/SMX status. The bar, box, and whiskers represent the median, 25th-75th percentile, and non-outlier range respectively. n is the number of courses in each group.
Figure 2. Lowest AUC subdivided by treatment phase and TMP/SMX status.
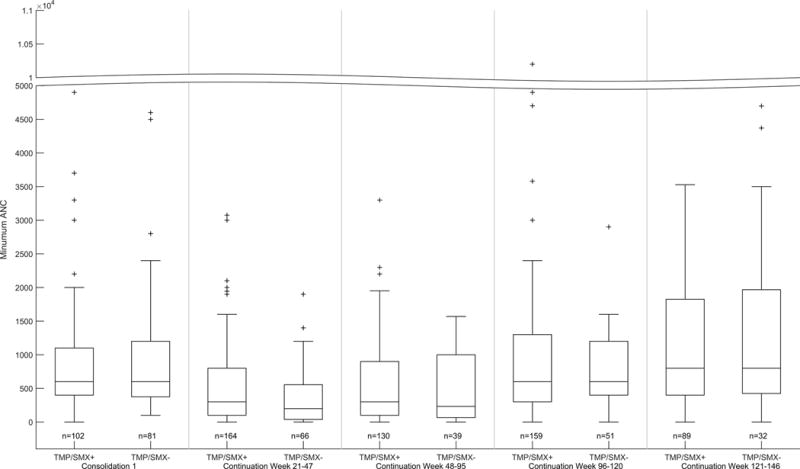
Boxplots of lowest ANC per phase during HDMTX on Total XVI (left panel, shown for consolidation #1 only) and for LDMTX on Total XV (right panels, shown for weeks 21–47, 48–95, 96–120, and 121–140). The bar, box, and whiskers represent the median, 25th-75th percentile, and non-outlier range respectively. n is the number of patients in each group.
We also compared blood counts in patients who received LDMTX with or without TMP/SMX. Whether comparing the highest, median, lowest, or mean leukocyte count or ANC, during any of the 4 phases of continuation therapy, there were no differences in blood counts when LDMTX was given with or without TMP/SMX (data shown for lowest ANC, Figure 2).
These observations do not support the hypothesis that TMP/SMX interferes with methotrexate clearance or its myelosuppressive effects. The HDMTX pharmacokinetic data are consistent with our prior results with HDMTX at 1.5 g/m2/24 hours.(15) The NOPHO ALL-92 reported more myelosuppression and a lower cumulative dose of mercaptopurine in patients receiving TMP/SMX (TMP 5 mg/kg/day) than in those who did not receive TMP/SMX, with some NOPHO patients received daily rather than 2 to 3 days per week of TMP/SMX. Heterogeneity among TMP/SMX prophylaxis regimens exists, although the majority of centers use a regimen similar to ours (TMP at 5 mg/kg/day or 150 mg/m2/day, divided into two daily doses, given 3 days per week (summarized in [ref Levinson et al]). Many confounding factors can contribute to myelosuppression, including the dose of myelosuppressive chemotherapy such as methotrexate and thiopurines, and host factors such as use of thiopurine methyltransferase activity.[Levinson et al] Although our study was not intentionally randomized, patients were generally assigned a fixed “clinic day” to receive scheduled therapy on the same day of the week, and thus whether they received TMP/SMX concurrent with HDMTX (or LDMTX) was essentially a randomly assigned event. In our study, unlike in some others, neither the daily dose nor weekly regimen of TMP/SMX varied among patients. Thus the lack of association with myelosuppression that we observed was unlikely to be confounded by variation in factors other than TMP/SMX, such as underlying predisposition to myelosuppression, or in any changes to maintenance therapy such myelosuppression might have triggered. Based on our study, we have no data to support that TMP/SMX contributed to myelosuppression during consolidation or during continuation therapy.
Renal methotrexate clearance is through active transport by the organic anion transport system and through glomerular filtration.(11, 17, 18) (19) Previous studies have demonstrated that some organic acids, including sulfonamides, can inhibit the renal clearance of methotrexate.(20) Also, sulfamethoxazole can compete with methotrexate for plasma protein binding,(11) although such interactions are unlikely to significantly affect free drug exposure due to the modest protein binding and low unbound intrinsic clearance exhibited by methotrexate. In one evaluation, TMP/SMX resulted in a 60% increase in methotrexate exposure;(11) however, other evaluations have found less significant increases.(10, 21)
Although an increase in methotrexate exposure with TMP/SMX at 2–3 mg/kg/day and methotrexate at 20–75 mg/m2/dose was observed in nine children,(11) this was not observed in another study with methotrexate 3.7–20 mg/m2 and TMP/SMX at 4.4–6.2 mg (TMP)/kg/day.(21) We acknowledge that myelosuppression has been attributed to TMP/SMX by others. There are case reports and a study in rheumatoid arthritis of increased hematologic toxicities with LDMTX (5 to 15 mg per week) with TMP/SMX.(22) However, these reports used therapeutic (rather than the lower prophylactic) doses of TMP/SMX, and may not be relevant to oncology patients receiving many myelosuppressive agents. In a randomized crossover study of ALL using LDMTX at 20 mg/m2/week, TMP/SMX resulted in more myelosuppression, but doses of TMP/SMX were higher (150 mg/m2/day TMP)(13) than those given at St. Jude. In our current study, using LDMTX at 40 mg/m2 and prophylaxis doses of TMP/SMX, we were unable to demonstrate any pharmacodynamic interaction, with no changes in leucocyte count or ANC attributable to TMP/SMX.
We did not evaluate mucositis or liver function tests in those on vs off TMP/SMX. Mucositis is of course a function of several variables, including methotrexate and leucovorin exposure; we have previously reported our very low frequency of grade 3 or 4 mucositis (3%) with our method of methotrexate administration, including targeting systemic exposure based on individual clearance and pharmacokinetically-directed leucovorin rescue.(5) Serum liver function tests were not uniformly evaluable in all patients in an unbiased fashion, and thus the data are not available for an unbiased comparison in those on vs off TMP/SMX.
In conclusion, with the HDMTX and LDMTX regimens and prophylactic doses of TMP/SMX described herein, there is no evidence for a pharmacokinetic interaction between TMP/SMX and methotrexate, and no evidence of an impact on myelosuppression. Therefore, continuation of PCP prophylaxis with TMP/SMX on a schedule of twice daily for three consecutive days, without regard to methotrexate, remains a reasonable strategy.
Acknowledgments
Supported by NIH CA 21975 and ALSAC.
Footnotes
Conflict-of-interest disclosure: The authors declare no conflict of interest.
References
- 1.Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240–50. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. The New England journal of medicine. 2009;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118(4):874–83. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauley JL, Panetta JC, Crews KR, Pei D, Cheng C, McCormick J, et al. Between-course targeting of methotrexate exposure using pharmacokinetically guided dosage adjustments. Cancer chemotherapy and pharmacology. 2013;72(2):369–78. doi: 10.1007/s00280-013-2206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St. Jude Children’s Research Hospital. Total Therapy Study XVI for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia. http://clinicaltrialsgov/show/NCT00549848.
- 7.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–14. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 8.Hughes WT. Pneumocystis carinii pneumonitis. The New England journal of medicine. 1987;317(16):1021–3. doi: 10.1056/NEJM198710153171608. [DOI] [PubMed] [Google Scholar]
- 9.Vrooman LM, Stevenson KE, Supko JG, O’Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(9):1202–10. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans WE, Christensen ML. Drug interactions with methotrexate. The Journal of rheumatology Supplement. 1985;12(Suppl 12):15–20. [PubMed] [Google Scholar]
- 11.Ferrazzini G, Klein J, Sulh H, Chung D, Griesbrecht E, Koren G. Interaction between trimethoprim-sulfamethoxazole and methotrexate in children with leukemia. The Journal of pediatrics. 1990;117(5):823–6. doi: 10.1016/s0022-3476(05)83351-7. [DOI] [PubMed] [Google Scholar]
- 12.Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. The New England journal of medicine. 1983;309(18):1094–104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- 13.Woods WG, Daigle AE, Hutchinson RJ, Robison LL. Myelosuppression associated with co-trimoxazole as a prophylactic antibiotic in the maintenance phase of childhood acute lymphocytic leukemia. The Journal of pediatrics. 1984;105(4):639–44. doi: 10.1016/s0022-3476(84)80439-4. [DOI] [PubMed] [Google Scholar]
- 14.Levinsen M, Shabaneh D, Bohnstedt C, Harila-Saari A, Jonsson OG, Kanerva J, et al. Pneumocystis jiroveci pneumonia prophylaxis during maintenance therapy influences methotrexate/6-mercaptopurine dosing but not event-free survival for childhood acute lymphoblastic leukemia. European journal of haematology. 2012;88(1):78–86. doi: 10.1111/j.1600-0609.2011.01695.x. [DOI] [PubMed] [Google Scholar]
- 15.Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12(8):1667–72. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 16.Jeha S, Pui CH. Risk-adapted treatment of pediatric acute lymphoblastic leukemia. Hematology/oncology clinics of North America. 2009;23(5):973–90, v. doi: 10.1016/j.hoc.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Mikkelsen TS, Thorn CF, Yang JJ, Ulrich CM, French D, Zaza G, et al. PharmGKB summary: methotrexate pathway. Pharmacogenetics and genomics. 2011;21(10):679–86. doi: 10.1097/FPC.0b013e328343dd93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanWert AL, Sweet DH. Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharmaceutical research. 2008;25(2):453–62. doi: 10.1007/s11095-007-9407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. Journal of the American Society of Nephrology : JASN. 2002;13(3):595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 20.Liegler DG, Henderson ES, Hahn MA, Oliverio VT. The effect of organic acids on renal clearance of methotrexate in man. Clinical pharmacology and therapeutics. 1969;10(6):849–57. doi: 10.1002/cpt1969106849. [DOI] [PubMed] [Google Scholar]
- 21.Beach BJ, Woods WG, Howell SB. Influence of co-trimoxazole on methotrexate pharmacokinetics in children with acute lymphoblastic leukemia. The American journal of pediatric hematology/oncology. 1981;3(2):115–9. doi: 10.1097/00043426-198100320-00001. [DOI] [PubMed] [Google Scholar]
- 22.Bourre-Tessier J, Haraoui B. Methotrexate drug interactions in the treatment of rheumatoid arthritis: a systematic review. The Journal of rheumatology. 2010;37(7):1416–21. doi: 10.3899/jrheum.090153. [DOI] [PubMed] [Google Scholar]


