Abstract
Objective
Calabadion 2 is a new drug-encapsulating agent. In this study, we aim to assess its utility as an agent to reverse general anesthesia with etomidate and ketamine and facilitate recovery.
Methods
To evaluate the effect of calabadion 2 on anesthesia recovery, we studied the response of rats to calabadion 2 following continuous and bolus intravenous etomidate or ketamine and bolus intramuscular ketamine administration. We measured electroencephalographic predictors of depth of anesthesia (burst suppression ratio and total electroencephalographic power), functional mobility impairment, blood pressure, and toxicity.
Results
Calabadion 2 dose-dependently reverses the effects of ketamine and etomidate on electroencephalographic predictors of depth of anesthesia, as well as drug-induced hypotension, and shortens the time to recovery of righting reflex and functional mobility. Calabadion 2 displayed low cytotoxicity in 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium-based cell viability and adenylate kinase release cell necrosis assays, did not inhibit the human ether-à-go-go-related channel, and was not mutagenic (Ames test). Based on maximum tolerable dose and acceleration of righting reflex recovery, we calculated the therapeutic index of calabadion 2 in recovery as 16:1 (95% confidence interval [CI], 10–26:1) for the reversal of ketamine and 3:1 (95% CI, 2–5:1) for the reversal of etomidate.
Conclusion
Calabadion 2 reverses etomidate and ketamine anesthesia in rats by chemical encapsulation at non-toxic concentrations.
Introduction
Currently used intravenous anesthetics such as ketamine and etomidate are clinically employed in a variety of settings. Ketamine is used to induce anesthesia1, to achieve sedation and analgesia during mechanical ventilation, and to treat patients with chronic pain, or psychiatric problems including the estimated 10–30% of patients with major treatment resistant depression.2 Etomidate, a rapid acting and cardiovascular safe anesthetic, is frequently used in emergency cases3, for procedural sedation, and for anesthesia induction.4 Up to this point, these intravenous anesthetics have no mechanism of pharmacologic reversal.
Attempts to achieve faster emergence from general anesthesia have been directed towards counteracting specific physiological sedating effects by stimulating opposing systems as for example activating the arousal systems.5 In addition, other researchers develop short-acting ketamine and etomidate that achieve faster recovery.6–8
An exciting opportunity to overcome the limitations of reanimation by accomplishing an actual reduction of anesthetic agents has emerged with the characterization of the acyclic cucurbit[n]urils (CB[n]) molecular containers, which bind tightly and selectively to a variety of cations.9,10 A particularly promising new subgroup of the acyclic cucurbit[n]urils is the Calabadions.11,12 The development of narrow-spectrum high affinity macromolecular binders as antidotes has been focused mainly on neuromuscular blockers and anticoagulants13, and previous studies14 have demonstrated the effectiveness of molecular containers in scavenging excess neuromuscular blockers to speed post-surgical recovery from paralysis.
In this paper, we explore the potential use of calabadion 2 as a lead drug to inactivate ketamine and etomidate. We tested the proof of concept that acyclic cucurbit[n]urils may function as true anesthesia reversal agents by reducing levels of etomidate and ketamine in rats through encapsulation followed by renal excretion. Calabadions might have the potential to reduce operating room time and costs, the risk of postoperative complications, and to counteract accidental overdose in both clinical and recreational settings.
Materials and Methods
Chemistry
Calabadion 2 was synthesized according to the published procedure.15 The binding constants (KD) for the calabadion 2•ketamine and calabadion 2•etomidate complexes were determined by changes in UV/Vis competition assays16, with the calabadion 2•Rhodamine 6G complex (Ka = 2.3 ± 0.2 x 106 M−1), fitted to a competitive binding model as described previously.11,12,17
To establish the 1:1 stoichiometry between calabadion 2 and ketamine we used Job’s method of continuous variation.18 We maintained the total molar concentration of the ketamine and calabadion 2 constant (1 mM), but varied their mole fractions. The 1H NMR (400 MHz, 20 mM sodium phosphate buffered D2O at pD = 7.4) resonance for calabadion 2 at 7.73 ppm was monitored. The change in chemical shift is proportional to the amount of complex formed.
Animals
All studies on rats (60 adult male Sprague–Dawley rats, strain code 400; mean±SD, 294±61g) and mice (35 adult female Swiss Webster mice, strain code 551; mean±SD, 22.5±1.3g) were conducted in accordance with the Subcommittee on Research Animal Care at Massachusetts General Hospital, Boston, MA (Protocol 2011N00181), and the Subcommittee on Research Animal Care at the University of Maryland, College Park, MD (Protocol R-14–02), respectively.
Instrumentation of Sprague-Dawley rats
For placement of i.v.s, animals were anesthetized with 1.5% isoflurane. Temperature was controlled rectally and maintained at 37±1 °C with a thermostat controlled heating pad. A total of 60 rats were used in this study, of which 32 were instrumented with two i.v. lines, an arterial line and a tracheal tube. Of the remaining 28 animals, 21 rats were only instrumented with a tail vein i.v. catheter (24 gauge 19mm), while the other 7 did not undergo any instrumentation.
Effects of Calabadion 2 on electrographic metrics of unconsciousness during constant anesthetic infusion
The effects of Calabadion 2 on etomidate and ketamine evoked unconsciousness were investigated by quantified changes in electrical brain activity, measured with an epidural EEG-electrode in 26 chronically instrumented rats.
Methods described by Vijn and Sneyd and by Cotten et al. were used in a group of 13 rats to continuously estimate the burst suppression ratio (BSR), the proportion of time the EEG signal spent in suppression during each 10s-epoch for the evaluation of reversal from etomidate-evoked unconsciousness.19,20 All studies were performed in a background of inhaled isoflurane 1%.
After an initial bolus administered over 40s to achieve a BSR of approximately 70%, the infusion rate was decreased to a value between 0.1 and 0.3mg/kg/min (average dose of 183.9±28.4μg/kg/min, mean±SD) to derive at a steady state BSR higher than 40% for at least 20 minutes before test drug administration. Animals were premedicated with 5mg/kg dexamethasone to avoid symptoms of etomidate-induced adrenal suppression. After steady state recordings either a stepwise increasing calabadion 2 infusion of 40, 60, 80 and 100mg/kg/min over 5min each (n=10) or a 20min saline infusion of equivalent total fluid volume (n=3) was administered in order to reverse the effects of the constantly maintained etomidate infusion on the BSR. Additionally, the blood pressure was monitored throughout the experiment for evaluation of the reversal of effects on the cardiovascular system.
In 13 rats used for the evaluation of reversal of ketamine anesthesia, we quantified the total EEG power during a continuous ketamine infusion titrated to abolishment of response to tail clamping. After all surgical procedures were completed the dose of isoflurane was stepwise reduced and discontinued while a 0.67mg/kg/min ketamine infusion was started. After 10min of a sole ketamine infusion, we applied intermittent standardized tail-clamping (25N) every 2min to identify depth of anesthesia. Depending on response, the infusion rate was increased or decreased by 0.33mg/kg/min, until no response to 6 consecutive tail clamps during a constant dose of ketamine were observed.21
After steady state recordings, we administered an escalating calabadion 2 infusion with 20, 40, 60 and 80mg/kg/min over a period of 5min each with 40sec breaks in-between (n=10) or a saline infusion of equivalent volume and timing (n=3). EEG and arterial blood pressure were continuously measured throughout the experiment.
EEG recordings were analog filtered between 0.3 and 300Hz, and digitized with a bandpass filter between 0.5 and 55 Hz. The spectrum of visually identified artifact free episodes was then calculated using a Fast-Fourier-Transformation with a 1024 bit Hann (cosine-bell) window.
Changes in total EEG power and MAP were quantified in response to test drug injection in comparison to steady state ketamine.
To ensure that the observed effects were not caused by an interaction of calabadion 2 with isoflurane, we administered increasing amounts of calabadion 2 (20, 40, 60 and 80mg/kg/min for 5min each) in 3 rats anesthetized with a constant isoflurane anesthesia titrated to the abolishment of tail-clamping and quantified EEG power, mean arterial blood pressure and heart rate.
Additionally we administered an escalating phenylephrine infusion (4 to 10μg/kg/min) in 3 rats anesthetized with a continuous ketamine infusion, to ensure that our changes in EEG can be interpreted as a result of shallower anesthesia, rather than nonspecific hemodynamic reactions.
Effects of Calabadion 2 on time to regain righting reflex following single bolus anesthesia
We examined the effects of calabadion 2 on time to recovery from loss of righting reflex (LORR) following a single i.v. bolus of etomidate or ketamine in 14 adult male Sprague-Dawley rats. After instrumentation, animals were randomized to receive either an i.v. etomidate bolus (4mg/kg) over 10sec or a one minute infusion of ketamine (30mg/kg). Once placed in the supine position, animals were randomized to receive either an i.v. infusion of calabadion 2 (80mg/kg/min dissolved in distilled H2O) or saline, beginning 3min following the anesthetic injection. Recovery from LORR was taken as the moment when the rat regained a standing or sternally recumbent position.22
Additionally, we tested in crossover experiments the effect of calabadion 2 on propofol anesthesia in 5 adult Sprague-Dawley rats randomized to receive an i.v. infusion of calabadion 2 (80mg/kg/min dissolved in distilled H2O) and saline beginning 3min following the i.v. injection of 20mg/kg propofol. This was performed in two study days with 48h recovery time between the experiments.
Effects of Calabadion 2 on functional mobility after ketamine and etomidate anesthesia on the balance beam
Recovery of functional mobility impairment was quantified as previously described23 using the balance beam test, a common method to assess motor coordination and balance of animals24, used as a predictor for pharmacologic impact on recovery.25 The time rats remained on a wooden rod (diameter 2.5 cm) was measured to evaluate balance and body strength. After recovery from LORR animals were placed on the beam every 4min starting at the i.v. anesthetic agent injection, or every 10min starting at the i.m. ketamine injection. Test performance was scored by a team member blinded to the study treatment as unable to maintain grip or balance on the beam (0 points), able to remain on the beam for up to 10 sec (1 point), 11 to 20 sec (2 points), or 21 to 30 sec or reaches support (3 points).24
Toxicology
We analyzed the effect of calabadion 2 on human white blood cells (THP-1), liver cells (HepG2) and kidney cells (HEK293).
The cell viability was measured using a MTS based assay (CellTiter 96® AQueous Kit assay from Promega G3580) and cell necrosis was determined via the quantification of the release of cytosolic adenylate kinase enzyme (Toxilight® BioAssay from Lonza LT07–117). These cells were exposed to 0.16mM, 0.4mM, 1mM, and 2.5mM calabadion 2, hydroxypropyl-β-cyclodextrin (HP-β-CD) or erythromycin, as a point of comparison for a FDA approved drug. In each cell type the cell viability was normalized to the average values obtained from untreated cells. The cell lysis on the other hand was normalized to the values obtained from the incubation of the cells with distilled water, which induces cells lysis via osmotic shock.
In order to test the effect of calabadion 2 on Ether-à-go-go-Related Gene (hERG) currents we used a Chinese Hamster Ovary cell line transfected with the hERG ion channel. The potassium flow was analyzed with patch clamp technology. The activity of the hERG channel of untreated cells was used to normalize the effect of increasing doses of calabadion 2 or the hERG inhibitor quinidine, both up to a dose of 25μM.
In order to determine the mutagenic properties of calabadion 2, we used the bacteria reverse mutation assay (Ames Test MOLTOX® 31–100.2, histidine auxotroph strains of S.typhimurium - not able to grow on histidine deficient agar without a mutation). The mutagenicity of a compound was addressed by the ratio of the number of colonies growing after treatment with the test compound relative to untreated bacteria. Compounds that give ratios greater than 2.0, of 1.6–1.9 or of less than 1.6 are considered mutagenic, potentially mutagenic or not mutagenic, respectively. In addition, the potential of calabadion 2 to be metabolized by the liver into a more toxic metabolite was assessed by incubation with rat liver extract (+S9) before treatment of bacteria. We used four different bacterial test strains to assess the mutagenicity of the compound (TA1535, TA 1537, TA 98 and TA 100) and administered 0.012, 0.037, 0.11, 0.33 or 1mg calabadion 2 per plate plus 1.5μg Sodium Azide, 6μg Daunomycin, 1μg CR 191 Acridine or 10μg 2-Aminoanthracene per plate as control.
We analyzed the toxicity of calabadion 2 in 35 Swiss Webster mice by performing a dose escalation study. Groups (n=7) of 4–6 week old female mice were injected intraperitoneally daily with 29mg/kg, 87mg/kg, 145mg/kg and 203mg/kg of calabadion 2 or not injected (untreated) for 14 consecutive days. The weight of each mouse was determined over a period of 28 days.
We further analyzed the toxicity of calabadion 2 in rats (n=10) by performing a maximal tolerated dose escalation study. Adult male Sprague-Dawley rats (n=6) were injected with escalating doses of calabadion 2 by i.v. injection for 5 consecutive days until the lethal dose was reached (100, 500, 1000, 1500 and 2000 mg/kg). In the 4 remaining rats we administered a nonlethal cumulative dose of 1.6 g/kg on 3 consecutive days (100, 500 and 1000 mg/kg).
Based on the ratio of median lethal dose (LD50) and median dose of calabadion 2 required to achieve an accelerated recovery from LORR with a 50% probability (ED50) we calculated the therapeutic index of calabadion 2 in reversing etomidate and ketamine anesthesia.
The heart, lungs, liver, kidneys and spleen of all 10 animals were harvested and fixated in 10% neutral buffered formalin. Samples were stained with hematoxylin and eosin (H&E), embedded in paraffin slides, and the organ tissue toxicity of calabadion 2 was evaluated by an independent pathologist.
Statistical analysis
All data are reported as means ± standard deviation unless otherwise specified. Statistical analysis was performed using SPSS 22.0 (SPSS Inc. Chicago, IL) and GraphPad Prism 6.0 (GraphPad Software, Inc. LaJolla, CA). Descriptive analytics and visual inspection of the distribution including histogram, density plots, Q-Q plots were applied. Normality was tested for using the Shapiro-Wilk test and data was considered normally distributed when P>=0.05.
To assess the effects of calabadion 2 on EEG/BSR and blood pressure during a continuous infusion of etomidate or ketamine we used a mixed linear model with an identity link function for normally distributed probability. Our mixed model included main effects of reversal agent (calabadion 2 vs. placebo) and dose, and the interaction term reversal of agent and dose as fixed effects while allowing intercepts to vary (random intercepts model). Goodness-of-fit was established using the likelihood ratio test to compare the fit of the final model to the intercept only model. The same model was used to evaluate the effects on blood pressure.
We used a paired t-test to assess differences in recovery time from LORR after etomidate, ketamine, and propofol anesthesia when administering calabadion 2 compared to saline in cross-over experiments in the same animals at different study days.
To evaluate the effect on post-anesthetic etomidate and ketamine induced balance- and coordination-impairment, we used a mixed linear model with an identity link function for normally distributed probability. We tested for a fixed main effect of the reversal agent on the time needed to reach recovery milestones (score of 1, 2 and 3 after Combs et al.)24, while allowing a subject specific intercept to vary as random effects.
All model assumptions were examined through model diagnostic plots including residual plots and Q-Q plots. We examine whether the variance estimate was indistinguishable from zero (P>0.05). If so, fixed effects model is applied instead of the mixed model. Model comparison, if applied, was presented and conducted using ANOVA and comparing BIC values.
Inhibition of the hERG channel was analysed using GraphPad Prism 6 to calculate statistical significance and IC50 values via nonlinear regression analysis, using a least squares (ordinary) fit. Goodness-of-fit was assessed using the R2 value for the nonlinear regression and the standard deviation of residuals (sy.x). Medium convergence criteria were used. That is, regression concluded when five consecutive iterations altered the sum-of-squares by <0.0001%. Model normality assumptions were examined and visualized through model diagnostic plots including residual plots and Q-Q plots. Additionally, normality of residuals was tested for using the Shapiro-Wilk test and residuals were considered normally distributed when P>=0.05. The nonlinear regression used for quinidine passed the Shapiro-Wilk normality test in GraphPad Prism.
A student’s t-test was used to detect differences between treatment conditions and untreated or distilled water-treated cells for the cell death and cell viability assay, respectively. The maximum tolerated dose study data was plotted as the average change in weight for each group plus or minus one standard deviation. A student’s unpaired t test was performed to compare each dosage group to the untreated mice. A P-Value <0.05 was considered significant.
Results
Chemistry
The dissociation constants (KD) of the calabadion 2•ketamine and calabadion 2•etomidate complexes were determined to be 5.1±0.3 μM and 27.2±5.0 μM, respectively (Fig. 1; see Fig. S1 and S2, Supplemental Digital Content 1, showing the binding assays for both complexes).
Figure 1.
Chemical structures of Calabadion 2, ketamine, and etomidate.
The Job plots for the calabadion 2•ketamine and calabadion 2•etomidate complexes, showed maxima at mole fractions of 0.5, which establishes the 1:1 nature of the calabadion 2•drug complexes (see Fig. S3 and S4, Supplemental Digital Content 1, establishing the stoichiometry of calabadion 2 and ketamine).
Calabadion 2 reverses electrographic metrics of unconsciousness during constant anesthetic infusion
Deepening anesthesia with etomidate is marked by lengthening of suppression periods in the EEG quantifiable as the burst-suppression ratio (BSR). An average dose of 183.9±28.4μg/kg/min was used to maintain the BSR at a stable rate of 63% [95% confidence interval (CI), 62–65%], deep enough such that a partial reversal could be achieved without awakening the animal. Calabadion 2, but not saline control, induced a dose-dependent decrease in BSR to 38%% [95% CI, 24–51%] (reversal agent*dose, P=0.001, Fig. 2A; n=10; likelihood ratio test (LRT) P<0.001; see table S1, Supplemental Digital Content 1, displaying the effect sizes of fixed effects), while the mean arterial blood pressure (MAP) returned from 83% [95% CI, 80–86%] to 101% [95% CI, 96–105%] of pre-etomidate baseline (reversal agent*dose P=0.033, Fig. 2A; n=10; LRT P<0.001). These changes in brain function and blood pressure objectively demonstrate the ability of calabadion 2 to reverse the effects of etomidate.
Figure 2. Calabadion 2 decreases levels of unconsciousness during continuous administered anesthesia with etomidate and ketamine.
Effect of an escalating calabadion 2 intravenous (i.v.) infusion on (A) burst suppression ratio (BSR) and mean arterial blood pressure (MAP) during continuous etomidate i.v. infusion (titrated to an average dose of 184 μg/kg/min; n=13) and on (B) electroencephalographic power (EEG-power) and mean arterial blood pressure (MAP) during continuous ketamine infusion (titrated to an average dose of 122 μg/kg/min; n=13). Calabadion 2 decreased BSR during etomidate infusion and EEG power during ketamine infusion in a dose dependent fashion and increased the MAP accordingly (*** p < 0.001). BSR and EEG power are displayed as % values from baseline (average value during continuous etomidate/ketamine infusion before test drug infusion). MAP is displayed as % of mean MAP before start of etomidate/ketamine administration. Error bars represent the 95% confidence intervals.
Unlike etomidate, ascending levels of ketamine gradually increase EEG power. During continuous ketamine infusion titrated to abolish responses to a noxious stimulus (tail clamping), calabadion 2 induced a dose-dependent decrease in total EEG power to 63% [95% CI, 54–72%] of steady-state-ketamine EEG-power, indicating that calabadion 2 reversed the typical effects of ketamine in the EEG (reversal agent*dose P<0.001, Fig. 2B; n=10; LRT P<0.001, see table S1, Supplemental Digital Content 1, displaying the effect sizes of fixed effects). During both calabadion 2 (n=10) and saline (n=3), all frequency bands behaved very similarly, without significant differences between individual bandwidths (Fig. 3). In parallel, calabadion 2 injection resulted in a dose dependent increase in MAP to almost 130% [95% CI, 117–142%] compared to pre-ketamine baseline (96 mmHg) at the highest dose (n=10), also indicating reversal of anesthesia (reversal agent*dose P<0.001, Fig. 2B; n=10; LRT P<0.001).
Figure 3. Calabadion 2 effects on electroencephalographic frequency bands during continuous administered anesthesia with ketamine.
During both calabadion 2 (n=10) and saline (n=3), all frequency bands (delta, 1–4 Hz; theta, 4–8 Hz; alpha, 8–12 Hz; beta, 12–25 Hz; and fast beta, 25–30 Hz) behaved very similarly, without significant differences between individual bandwidths. Error bars represent the 95% confidence intervals. (Abs=absolute)
No significant changes in BSR (n=3, P=0.22), EEG-power (n=3, P=0.08) or MAP (during etomidate, n=3, P=0.939; during ketamine, n=3, P=0.697) were observed during saline infusion (Fig. 2A+B).
In contrast, continuous phenylephrine infusion during steady state shallow ketamine anesthesia resulted in significant MAP increases without effects on EEG power (n=3, P=0.024). We did not observe any effects of calabadion 2 on EEG-power, BSR and MAP during and after the highest dose of the stepwise increasing calabadion 2 infusion when administered during constant isoflurane anesthesia.
Effects of Calabadion 2 on time to emergence from anesthesia
Emergence from etomidate and ketamine anesthesia was assessed by measuring time to recovery from LORR, frequently used as a predictor for the level of anesthesia.26,27 Relative to saline, calabadion 2 significantly decreased the time to recovery from LORR by almost 50% in etomidate-anesthetized rats (15.2±1.4min vs. 26.9±2.3min, n=7, P<0.001, Fig. 4) and by about 30% in ketamine anesthetized rats (6.0±0.7min vs. 8.4±1.6min, n=7, P=0.023, Fig. 4). The median dose of calabadion 2 required to achieve the described accelerated recovery from LORR with a 50% probability (ED50) was 984mg/kg [95%CI 976–991mg/kg] and 167mg/kg [95%CI 161–173mg/kg] for the reversal of a 4mg/kg i.v. etomidate bolus and a 30mg/kg i.v. bolus of ketamine, respectively.
Figure 4. Calabadion 2 accelerated recovery from single bolus anesthesia with etomidate and ketamine, and did not affect recovery from propofol anesthesia.
Effect of calabadion 2 (80 mg/kg/min, intravenous (i.v.)) on time to recovery from loss of righting reflex (LORR) following administration of a single i.v. bolus of etomidate (4 mg/kg, n= 7), ketamine (30 mg/kg, n=7) or propofol (30 mg/kg, n=5). In the cases of etomidate and ketamine recovery time was significantly shorter following Calabadion 2 administration vs. placebo (***p < 0.001, **p = 0.023). Compared to saline, Calabadion 2 did not affect the time to recovery from LORR after a propofol bolus (P=0.672). Data are ± standard deviation.
Calabadion 2 did not affect the time to recovery from LORR after a single bolus of propofol compared to saline (13.0±1.3min vs. 12.6±1.6min, n=5, P=0.672, Fig. 4).
Effects of Calabadion 2 on post-anesthesia functional mobility impairment
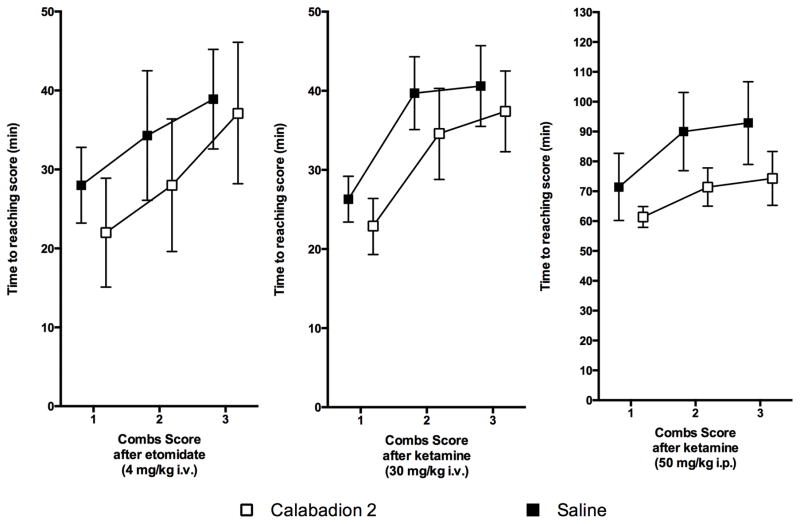
We observed a significantly faster recovery of balance after anesthesia, when injecting Calabadion compared to saline. Calabadion 2 significantly reduced the time slope of recovery by 4.9 min [95% CI, 1.1–8.6 min] (P=0.013; LRT P=0.002,) after 4mg/kg etomidate i.v., by 3.9 min [95% CI, 1.5–6.3 min] (P=0.002; LRT P<0.001) after 30mg/kg ketamine i.v. and by 15.7 min [95% CI, 9.4–22.0 min] (P<0.001; LRT P<0.001,) after 50mg/kg ketamine i.m., as compared to saline (Fig. 5 and see table S2, Supplemental Digital Content 1, displaying the effect sizes of fixed effects). The faster recovery of balance may suggest a faster recovery of muscle strength and/or motor coordination after Calabadion 2 injection for both anesthetics.
Figure 5. Calabadion 2 accelerated recovery from post-anesthetic functional mobility impairment.
Effect of calabadion 2 on time to recovery of balance described by the Combs score following administration of a single bolus of etomidate (4 mg/kg intravenous (i.v.); n=7) or ketamine (30 mg/kg i.v..; n=7; 50 mg/kg intramuscular (i.m.); n=7) vs. placebo. Test performance was scored as unable to maintain grip or balance on the beam (0 points), able to remain on the beam for up to 10 sec (1 point), 11 to 20 sec (2 points), or 21 to 30 sec or reaches support (3 points). Recovery time was significantly shorter following calabadion 2 vs. placebo. Following an intraperitoneal (i.p.) injection of calabadion 2 we observed the effect in accelerating recovery time to be significantly higher compared to i.v. administration (p <0.001). Error bars represent the 95% confidence intervals.
Calabadion 2 is not toxic or mutagenic
Even under stringent conditions (calabadion 2 up to 1 mM), we did not observe a significant reduction in cell viability of THP-1 and HepG2- cells, and only a slight dip on the HEK293 cells, and no cell lysis (Fig. 6A+B). These results were very comparable to the toxicity observed after incubation of the same cell lines with the antibiotic erythromycin and the cyclodextrin, HP-β-CD (Fig. 7).
Figure 6. Calabadion 2 does not induce acute cytotoxity, inhibit the human ether-à-go-go-related (hERG) channel or cause pathology in mice.
The cell viability (A) or cell lysis (B) of human white blood cells (THP-1), liver cells (HepG2) and kidney cells (HEK293) after treatment with increasing doses (0.16 mM to 2.5 mM) of Calabadion 2 is shown and compared to untreated (UT) and deionised water (DI). (C) The hERG assay was conducted using transfected Chinese Hamster Ovary (CHO)-hERG cells in an automated patch clamp study, with quinidine as positive control (IC50=1.66μM; best-fit, nonlinear line of regression, Data are ± standard deviation). (D) Swiss Webster mice were injected intraperitoneally (i.p.) for 14 days either with increasing doses of calabadion 2 (mg/kg body weight) or left untreated (UT). The weight of each mouse was followed daily until 14 days post-treatment. The average and standard deviation of the change in weight of the mice per treatment group (n=7) is graphed. For A–C the values are an average of at least three replicates with corresponding standard deviation values (*p=0.01–0.05; **p=0.001–0.01; ***p<0.001).
Figure 7. Toxicity of Erythromycin and HPβCD in in vitro cell assays.
Monocytes (THP-1), liver (HepG2) and kidney (HEK293) cell lines were incubated with indicated doses (0.16mM to 2.5mM) of erythromycin (A+C) and cyclodextrin (HPβCD) (B+D). The untreated (UT) and cell death-inducing conditions (DI) are indicated as appropriate controls. The cell viability (A+B) and cell death (C+D) were analyzed and results were normalized to untreated groups or death induction controls, respectively. For A–D the values are an average of at least three replicates with corresponding standard deviation values (*p=0.01–0.05; **p=0.001–0.01; ***p<0.001).
Treatment with calabadion 2 up to a concentration of 25μM did not result in significant differences in the observed current at the hERG channel (IC50>25μM), indicating no inhibition of the hERG channel. In contrast, the positive control, quinidine, showed a distinct decrease from an average of 90±4% to 1±6% in the post-treatment current across the ion channel with increasing concentrations of the compound (IC50=1.66μM, Fig. 6C).
The ratio of the amount of colonies growing after treatment with calabadion 2 in the Ames test relative to untreated bacteria did not exceed 1.1 even at the highest dose (1 mg/ml), which indicates that calabadion 2 has no mutagenic potential (Table 1).
Table 1.
Bacteria reverse mutation assay (Ames test) for Calabadion 2, in the presence or absence of rat liver S9 fraction (−/+S9) in order to test for metabolic activation. Displayed are numbers of colonies growing after treatment with the test compound (Data are ± SD) and the ratio of the number of colonies growing after treatment with the test compound relative to untreated bacteria.
| TA 1535 | TA 1537 | TA 98 | TA 100 | |||||
|---|---|---|---|---|---|---|---|---|
| Calabadion 2 concentration (mg) | −S9 | +S9 | −S9 | + S9 | −S9 | + S9 | −S9 | + S9 |
| 0 | 11 ± 2 | 8 ± 2 | 10 ± 5 | 7 ± 1 | 19 ± 2 | 27 ± 3 | 100 ± 27 | 109 ± 4 |
| 0.012 | 6 ± 1 | 10 ± 2 | 7 ± 2 | 2 ± 1 | 24 ± 4 | 24 ± 4 | 92 ± 11 | 114 ± 2 |
| 0.037 | 10 ± 5 | 6 ± 2 | 8 ± 2 | 4 ± 1 | 18 ± 8 | 27 ± 3 | 100 ± 6 | 112 ± 8 |
| 0.11 | 8 ± 3 | 7 ± 1 | 7 ± 2 | 6 ± 1 | 30 ± 10 | 23 ± 5 | 94 ± 4 | 91 ± 6 |
| 0.33 | 7 ± 4 | 10 ± 5 | 8 ± 1 | 6 ± 1 | 21 ± 5 | 24 ± 7 | 105 ± 9 | 101 ± 7 |
| 1 | 8 ± 3 | 7 ± 3 | 3 ± 2 | 5 ± 2 | 20 ± 5 | 29 ± 6 | 106 ± 6 | 97 ± 4 |
| Ratio | 0.72 | 0.87 | 0.3 | 0.71 | 1.05 | 1.07 | 1.06 | 0.88 |
| Positive Control | SA 446 |
2-AA 91 |
191-A 250 |
2-AA 141 |
D-myc 505 |
2-AA 1101 |
SA 282 |
2-AA 1307 |
191-A = ICR 191 Acridine; 2-AA = 2-Aminoanthracene; D-myc = Daunomycin; SA = Sodium Azide; TA 98-1535=Strain number of Salmonella thyphimurium
Additionally, a maximum tolerated dose study in mice revealed a good tolerance of calabadion 2 without obvious side effects. The average weight change for mice in all groups did not fall below 95% after 28 days (Fig. 6D).
Finally, a dose escalation study on ten male Sprague-Dawley rats suggested a median lethal dose of 2.7g/kg (LD50=2.7g/kg [95%CI, 1.8–4.3]). Calabadion did not induce apparent toxic effects in efficacy experiments. The histopathological evaluation of organs showed no significant lesions (i.e. within normal limits) in the heart and spleen and mild to moderate vacuolation in the liver and kidney. In animals receiving lethal doses of calabadion 2 in escalating dose experiments, we observed mild cellular necrosis of parts of the lungs with fluid in the alveolar spaces, and occasional distension of the pulmonary alveolar capillaries with red blood cells, which may be the consequence of pulmonary embolism when supratherapeutic, toxic doses are administered.
The therapeutic index of calabadion 2 in accelerating recovery of righting reflex was 16:1 (95% CI, 10–26:1) for the reversal of 30 mg/kg i.v. ketamine and 3:1 (95% CI, 2–5:1) for the reversal of 4 mg/kg i.v. etomidate. Calabadion 2 was well tolerated at effective doses. The detailed results of the histopathology studies are listed in table 2.
Table 2. Effects of lethal doses of Calabadion 2 on rat organs.
Pathological evaluation of heart, lungs, liver, kidneys and spleen of 10 rats after dose escalation study to determine the maximal tolerated dose with intravenous injection of Calabadion 2 up to 5.1 g/kg. Organs were fixated in 4% formaldehyde, stored in 70% ethanol, and stained with hematoxylin and eosin (H&E), embedded in paraffin slides.
| Organ | Calabadion dose in which the pathology finding was present | Pathology finding |
|---|---|---|
| Heart |
|
|
| Lung |
|
|
| Liver |
|
|
| Kidney |
|
|
| Spleen |
|
|
P1 and P2 = Segment 1 and 2 of the proximal tubule
Discussion
The in vitro binding data show that calabadion 2 encapsulates etomidate and ketamine molecules. In vivo encapsulation translates to inactivation of clinical etomidate and ketamine anesthesia. Our data indicate that calabadion 2 raises the level of consciousness during continuous anesthesia of etomidate and ketamine, decreases the time to emergence and mitigates lingering effects on motor- and cognitive function by sequestrating anesthetic agents so they cannot act at the effect compartment. These reversal effects were dose-dependently achieved by non-toxic concentrations of calabadion 2. We provide the proof-of-concept that acyclic cucurbit[n]urils can function as true anesthesia reversal agents by reducing levels of etomidate and ketamine in rats through encapsulation followed by renal excretion.
In clinical practice, emergence from general anesthesia is considered a passive process governed by anesthetic drug pharmacokinetics. Recently, Brown and Solt have described “reanimation” from general anesthesia: an active emergence with methylphenidate.26,27 Methylphenidate inhibits reuptake transporters for dopamine and norepinephrine in the brain28, and both neurotransmitters are known to promote arousal.29 This was also observed after administration of a D1 dopamine receptor agonist30 as well as electrical stimulation of the ventral tegmental area (VTA)31, suggesting that dopamine release by VTA neurons causes a profound arousal response sufficient to reverse the behavioral effects of general anesthesia.
While reanimation from general anesthesia aims to overpower the anesthetics at the receptor level by stimulation of this dopamine mediated arousal pathway, calabadion 2 encapsulates the anesthetic agent without receptor interactions. This allows a reduction of anesthetic effects and potential side effects by decreasing the concentration of active molecules rather than stimulating other pathways. The encapsulation complex of calabadion 2 and molecules bound to it is excreted in the urine.
We defined emergence in rats as recovery of etomidate and ketamine specific EEG measures to levels reflecting higher consciousness, reversal of blood pressure effects of the anesthetic agents, recovery of the righting reflex and of coordination. We found calabadion 2 encapsulation of ketamine and etomidate on EEG measures of brain function to be consistent with higher levels of consciousness. Ketamine and etomidate both disrupt frontal-parietal communication, leading to unconsciousness.32 However, their neurophysiological mechanisms of action are quite different, likely accounting for their different EEG effects, and requiring different techniques for EEG quantification. Deep etomidate anesthesia is characterized by alternating periods of EEG suppression and activity, referred to as a burst suppression pattern, similarly observed with most GABA types of anesthetics.33 As opposed to anteriorization, the shift in occipital alpha activity to frontal alpha coherence also characteristic for GABA anesthetics, which develops rather abruptly as a function of anesthetic infusion34,35, the BSR progressively and continuously increases with deeper levels of anesthesia, reflecting a decrease in cerebral metabolic rate coupled with the stabilizing properties of ATP-gated potassium channels.36,37 Unlike etomidate, sedation with ketamine does not produce a pattern of burst suppression.21,33 Instead, ascending levels of ketamine gradually increase EEG power likely due to inhibition of NMDA-mediated glutamatergic inputs to GABAergic interneurons, leading to aberrant excitatory activity in the cortex, hippocampus and limbic system.38 Therefore, we quantified electrographic depth of ketamine by measuring total EEG power.21 Calabadion 2 both dose-dependently decreased periods of suppression (BSR) during deep etomidate anesthesia and total EEG power in ketamine anesthetized rats, showing a reversal of these anesthetics’ EEG effects.
Because lingering post anesthetic effects may be caused by residual anesthetic molecules, we hypothesized drug encapsulation with calabadion 2 would mitigate post emergence motor impairment. Towards this end, we evaluated the effects of calabadion 2 on functional mobility with the balance beam test, which has previously been used as a predictor for pharmacologic impact on the recovery process.25 The balance beam test is indicative of subtle deficits in motor skills due to age, central nervous system lesions, and pharmacological manipulations with a higher sensitivity for coordination impairment than other motor tests.39 One group of experiments was conducted in order to analyze the encapsulation and reversal ability of calabadion 2 (i.p.) even when not administered by the same route as the anesthetic (ketamine i.m.). This could be of high clinical importance in emergency situations, when intravenous injection is not possible (e.g. after recreational ketamine overdose).
Calabadion 2 dose-dependently also reversed the etomidate-induced decrease in MAP, indicating a reversal of anesthesia depth-associated effects on the cardiovascular system. We also observed an increase in MAP when reversing ketamine. As opposed to our BSR-monitored experiments under deep etomidate anesthesia, we titrated a shallow ketamine anesthesia to achieve abolishment of response to tail clamping. As a consequence of further lowering anesthetic levels when reversing with calabadion 2 we observed an increase in MAP, further indicating awakening due to reversal.
To ensure the awakening reaction was not caused by nonspecific effects of calabadion 2 on the animal’s hemodynamics, we applied a phenylephrine infusion in 3 rats anesthetized with an equally titrated ketamine infusion. This did not affect any ketamine-induced EEG-patterns, indicating no reversal.
We did not observe any changes in BSR, EEG or MAP during and after the highest doses (80 mg/kg/min) of calabadion 2 given during steady state isoflurane anesthesia, and no differences in recovery time during propofol anesthesia. This enforces our hypothesis that the observed effects are caused by specific encapsulation and inactivation of etomidate and ketamine molecules.
The chemical structure of calabadion 2 features a glycoluril tetramer unit which enables the compound to bind to hydrophobic and cationic species, the aromatic sidewalls impart affinity due to p-p interactions toward targets that contain aromatic rings in their structures, and finally the overall cavity size of Calabdion 2 endows it with selectivity based on size. The preference for calabadion 2 toward ketamine and etomidate relative to other molecules like isoflurane or propofol reflects the absence of one or more of the structural binding determinates in the latter compounds. The affinity of calabadion 2 for compounds that are neutral in water (e.g., propofol, isoflurane) is typically less than 0.1% of its affinity for related cationic compounds.
The design of this study allows the conclusion that reversal of etomidate and ketamine with calabadion 2 is due to specific binding. Both anesthetics bind to calabadion 2 in vitro and reverse the drugs in vivo. The similar reduction in time to recovery from LORR is the consequence of high dose of calabadion 2 given to etomidate compared to ketamine anesthetized rats – based on the different duration of action at a constant rate of calabadion 2 infusion. The therapeutic range of ketamine is pretty low in rodents, so we could only apply relatively small doses without cardiovascular compromise in rats. In contrast, at the recommended dose of etomidate used, duration of action was longer, and more calabadion 2 could be titrated to accelerate recovery from LORR.
Single boluses of both etomidate and ketamine are used during procedures of short duration, such as electroconvulsive therapy, or for emergency intubations40, and ketamine is often used as the anesthetic of choice in pediatric patients for minor surgical procedures, as well as in the developing world, where it is frequently used by non-anesthetists when monitoring equipment is poor or absent.41 Use of higher dosages are associated with longer hospitalization, and typical complications described when used in pediatric patients include emesis, vomiting, rash, as well as recovery agitation and transient airway complications (e.g. post sedative airway misalignment, or apnea).42 Maybe even more importantly, ketamine is frequently abused.43 The ability to directly reverse their effects would not only result in reduced complication rates and time to discharge, and a decrease costs of care in patients receiving etomidate and ketamine in such circumstances, but also provide an antidote in cases of abuse associated intoxication.
Maintenance of sedation with ketamine and etomidate has been achieved in humans at plasma concentrations of 2–3μg/ml and 0.3–0.6μg/ml44, respectively, and plasma concentrations in humans awakening from ketamine and etomidate were described at 1μg/ml and 0.28μg/ml (vs. 5μg/ml and 0.44μg/ml in rodents), respectively45–48. Even though we estimate one-fifth and half dosages of calabadion 2 needed in humans to achieve an equivalent reversal of ketamine and etomidate, respectively, we expect a calabadion 2 excess concentration of 5.1–10.2μmol/L and of 2.5–34.6μmol/L to achieve the 50–67% anesthesia concentration reduction needed to reverse ketamine anesthesia and the 6–53% reduction needed to reverse etomidate anesthesia in humans. The relatively high concentrations of excess calabadion 2 raise the question of its interaction with other endogenous molecules.
Given that plasma concentrations in rodents at recovery from loss of righting reflex are in the μM range, while adrenal suppression is already achieved in the nM range, we do not expect calabadion 2 to reverse etomidate-induced adrenal suppression at the doses used in this study to accelerate emergence from anesthesia.
Calabadion 2 reverses ketamine and etomidate with a narrow therapeutic index of 16:1 and 3:1, respectively, mainly explained by calabadion 2′s design to reverse the neuromuscular blocking agents rocuronium, vecuronium and cisatracurium, which is achieved at about one-tenth of the doses used here.14 At doses sufficient to reverse neuromuscular blockade, calabadion 2 has minimal effects on anesthetic depth or duration. The present studies demonstrate a proof of principle of etomidate reversal, similar to the proof of principle earlier published on the effectiveness of calabadion 1 to reverse cisatracurium49, where subsequent medicinal chemistry optimization allowed us to create a similar compound with higher affinity now used for drug development. Of note, the ED50 of calabadion 2 to reverse ketamine of 166 mg/kg is only about twice as high as the dose used to reverse cisatracurium, which might make a clinical use of calabadion 2 for the reversal of ketamine possible. Considering that lower dosages will be required to reverse anesthesia in humans, and that we plan to explore potential changes in chemical structure to increase the affinity, we do not expect the narrow therapeutic range in this study to be a limitation for the reversal of anesthesia by encapsulation of active anesthetic molecules.
We are currently developing calabadions to be used for specific indications: To reverse neuromuscular blocking agents, to reverse intoxications with stimulants of abuse (ketamine, cocaine) and to reverse side unwarranted effects of ketamine and etomidate administered in clinical medicine. Each of the above indications will require generation of dose-response relationships, in order to define indications and contraindications, and in order to avoid side-effects from displacement.
In conclusion, calabadion 2 accelerates emergence from etomidate and ketamine anesthesia and reverses evoked unconsciousness as well as lingering effects of these anesthetics that impair motor coordination in rats by chemical encapsulation at non-toxic concentrations.
Supplementary Material
Acknowledgments
Funding: This study was funded by the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital, the State of Maryland Technology Development Corporation (TEDCO) and the NIH (R01 HL117871 and R01 CA168365). Support for this research was also provided by a grant to the University of Maryland from the Howard Hughes Medical Institute Undergraduate Science Education Program.
Footnotes
This study was selected for presentation in the Best Abstracts, Basic Science session of the Anesthesiology 2014 Annual Meeting on 10/13/2014 in New Orelans, LA, and has been invited for submission to Anesthesiology.
Disclosures: L.I. and M.E. hold an equity stake in Calabash Bioscience, Inc., which develops Calabadions for biomedical applications. L.I., G.H., V.B. and M.E. are inventors on patents (WO2012/051413 A1) on topics related to the use of Calabadions in biomedical applications. All other authors have no conflicts of interest.
References
- 1.Haas DA, Harper DG. Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Catena-Dell’Osso M, Fagiolini A, Rotella F, Baroni S, Marazziti D. Glutamate system as target for development of novel antidepressants. CNS Spectr. 2013;18:188–98. doi: 10.1017/S1092852912000971. [DOI] [PubMed] [Google Scholar]
- 3.Godwin SA, Caro DA, Wolf SJ, Jagoda AS, Charles R, Marett BE, Moore J American College of Emergency P. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45:177–96. doi: 10.1016/j.annemergmed.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Yang CH, Tian X, Yin HB, Gao XH, Li N. Sedation and analgesia with fentanyl and etomidate for intrathecal injection in childhood leukemia patients. Medicine (Baltimore) 2015;94:e361. doi: 10.1097/MD.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarren HS, Chalifoux MR, Han B, Moore JT, Meng QC, Baron-Hionis N, Sedigh-Sarvestani M, Contreras D, Beck SG, Kelz MB. alpha2-Adrenergic stimulation of the ventrolateral preoptic nucleus destabilizes the anesthetic state. J Neurosci. 2014;34:16385–96. doi: 10.1523/JNEUROSCI.1135-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey M, Sleigh J, Voss L, Pruijn F, Jose J, Gamage S, Denny W. Determination of the Hypnotic Potency in Rats of the Novel Ketamine Ester Analogue SN 35210. Pharmacology. 2015;96:226–32. doi: 10.1159/000439598. [DOI] [PubMed] [Google Scholar]
- 7.Harvey M, Sleigh J, Voss L, Jose J, Gamage S, Pruijn F, Liyanage S, Denny W. Development of Rapidly Metabolized and Ultra-Short-Acting Ketamine Analogs. Anesth Analg. 2015;121:925–33. doi: 10.1213/ANE.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 8.Pejo E, Ge R, Banacos N, Cotten JF, Husain SS, Raines DE. Electroencephalographic recovery, hypnotic emergence, and the effects of metabolite after continuous infusions of a rapidly metabolized etomidate analog in rats. Anesthesiology. 2012;116:1057–65. doi: 10.1097/ALN.0b013e3182515403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao L, Sekutor M, Zavalij PY, Mlinaric-Majerski K, Glaser R, Isaacs L. Cucurbit[7]uril. Guest Pair with an Attomolar Dissociation Constant. Angew Chem, Int Ed. 2014;53:988–993. doi: 10.1002/anie.201309635. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L. The Cucurbit[n]uril Family: Prime Components for Self-Sorting Systems. J Am Chem Soc. 2005;127:15959–15967. doi: 10.1021/ja055013x. [DOI] [PubMed] [Google Scholar]
- 11.Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY, Briken V, Isaacs L. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat Chem. 2012;4:503–510. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]
- 12.Shen C, Ma D, Meany B, Isaacs L, Wang Y. Acyclic Cucurbit[n]uril Molecular Containers Selectively Solubilize Single-Walled Carbon Nanotubes in Water. J Am Chem Soc. 2012;134:7254–7257. doi: 10.1021/ja301462e. [DOI] [PubMed] [Google Scholar]
- 13.Forster V, Leroux JC. Nano-antidotes for drug overdose and poisoning. Sci Transl Med. 2015;7:290ps14. doi: 10.1126/scitranslmed.3008736. [DOI] [PubMed] [Google Scholar]
- 14.Haerter F, Simons JC, Foerster U, Moreno Duarte I, Diaz-Gil D, Ganapati S, Eikermann-Haerter K, Ayata C, Zhang B, Blobner M, Isaacs L, Eikermann M. Comparative Effectiveness of Calabadion and Sugammadex to Reverse Non-depolarizing Neuromuscular-blocking Agents. Anesthesiology. 2015;123:1337–49. doi: 10.1097/ALN.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY, Briken V, Isaacs L. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat Chem. 2012;4:503–10. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]
- 16.Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic cucurbit[n]uril-type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo. Angew Chem Int Ed Engl. 2012;51:11358–62. doi: 10.1002/anie.201206031. [DOI] [PubMed] [Google Scholar]
- 17.Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic Cucurbit[n]uril-Type Molecular Containers Bind Neuromuscular Blocking Agents In Vitro and Reverse Neuromuscular Block In Vivo. Angew Chem, Int Ed. 2012;51:11358–11362. doi: 10.1002/anie.201206031. [DOI] [PubMed] [Google Scholar]
- 18.Huang CY. Determination of binding stoichiometry by the continuous variation method: the Job plot. Methods Enzymol. 1982;87:509–25. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
- 19.Vijn PC, Sneyd JR. I.v anaesthesia and EEG burst suppression in rats: bolus injections and closed-loop infusions. Br J Anaesth. 1998;81:415–21. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 20.Cotten JF, Le Ge R, Banacos N, Pejo E, Husain SS, Williams JH, Raines DE. Closed-loop continuous infusions of etomidate and etomidate analogs in rats: a comparative study of dosing and the impact on adrenocortical function. Anesthesiology. 2011;115:764–73. doi: 10.1097/ALN.0b013e31821950de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eikermann M, Grosse-Sundrup M, Zaremba S, Henry ME, Bittner EA, Hoffmann U, Chamberlin NL. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116:35–46. doi: 10.1097/ALN.0b013e31823d010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-Gil D, Mueller N, Moreno-Duarte I, Lin H, Ayata C, Cusin C, Cotten JF, Eikermann M. Etomidate and Ketamine. Residual Motor and Adrenal Dysfunction that Persist beyond Recovery from Loss of Righting Reflex in Rats. Pharmaceuticals (Basel) 2014;8:21–37. doi: 10.3390/ph8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Combs DJ, D’Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke. 1987;18:503–11. doi: 10.1161/01.str.18.2.503. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein LB, Davis JN. Beam-walking in rats: studies towards developing an animal model of functional recovery after brain injury. J Neurosci Methods. 1990;31:101–7. doi: 10.1016/0165-0270(90)90154-8. [DOI] [PubMed] [Google Scholar]
- 26.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–18. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118:30–9. doi: 10.1097/ALN.0b013e318278c896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121:311–9. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–75. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 34.Vijayan S, Ching S, Purdon PL, Brown EN, Kopell NJ. Thalamocortical mechanisms for the anteriorization of alpha rhythms during propofol-induced unconsciousness. J Neurosci. 2013;33:11070–5. doi: 10.1523/JNEUROSCI.5670-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–70. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A. 2012;109:3095–100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008;4:91–3. doi: 10.1038/nchembio0208-91. [DOI] [PubMed] [Google Scholar]
- 39.Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma. 2010;27:1091–9. doi: 10.1089/neu.2010.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, Bertrand L, Beltramini A, Gamand P, Albizzati S, Perdrizet D, Lebail G, Chollet-Xemard C, Maxime V, Brun-Buisson C, Lefrant JY, Bollaert PE, Megarbane B, Ricard JD, Anguel N, Vicaut E, Adnet F, Group KCS. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300. doi: 10.1016/S0140-6736(09)60949-1. [DOI] [PubMed] [Google Scholar]
- 41.Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46:10–20. [PMC free article] [PubMed] [Google Scholar]
- 42.Priestley SJ, Taylor J, McAdam CM, Francis P. Ketamine sedation for children in the emergency department. Emerg Med (Fremantle) 2001;13:82–90. doi: 10.1046/j.1442-2026.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- 43.Center A. Ketamine Addiction and Abuse - Abused by many young people at clubs and raves, ketamine is a hallucinogenic drug. [last accessed on 04/28/2016];It is sometimes used as a tranquilizer for humans and animals. https://www.addictioncenter.com/drugs/hallucinogens/ketamine/
- 44.He L, Hospital HP. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. The Application of Target Controlled Infusion of Etomidate Combined With Propofol in the Maintenance of Anesthesia During Brain Surgeries. [Google Scholar]
- 45.Cohen ML, Chan SL, Way WL, Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39:370–6. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–97. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 47.Fragen RJ, Avram MJ, Henthorn TK, Caldwell NJ. A pharmacokinetically designed etomidate infusion regimen for hypnosis. Anesth Analg. 1983;62:654–60. [PubMed] [Google Scholar]
- 48.De Paepe P, Van Hoey G, Belpaire FM, Rosseel MT, Boon PA, Buylaert WA. Relationship between etomidate plasma concentration and EEG effect in the rat. Pharm Res. 1999;16:924–9. doi: 10.1023/a:1018894523734. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann U, Grosse-Sundrup M, Eikermann-Haerter K, Zaremba S, Ayata C, Zhang B, Ma D, Isaacs L, Eikermann M. Calabadion: A new agent to reverse the effects of benzylisoquinoline and steroidal neuromuscular-blocking agents. Anesthesiology. 2013;119:317–25. doi: 10.1097/ALN.0b013e3182910213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.