Abstract
Osteoimmunology arose from the recognition that cytokines produced by lymphocytes can affect bone homeostasis. We have previously shown that osteoclasts, cells that resorb bone, act as antigen-presenting cells. Cross-presentation of antigens by osteoclasts leads to expression of CD25 and FoxP3, markers of regulatory T-cells in the CD8 T-cells. Osteoclast-induced FoxP3+ CD8 T-cells (OC-iTcREG) suppress priming of CD4 and CD8 T-cells by dendritic cells. OC-iTcREG also limit bone resorption by osteoclasts, forming a negative feedback loop. Here we show that OC-iTcREG express concurrently T-bet and Eomesodermin (Eomes) and IFN-γ. Pharmacological inhibition of IκK blocked IFN-γ, T-bet and Eomes production by TcREG. Furthermore, we show using chromatin immunoprecipitation, NF-κB enrichment in the T-bet and Eomes promoter. We demonstrate that IFN-γ produced by TcREG is required for suppression of osteoclastogenesis and for degradation of tumor necrosis factor receptor-associated factor 6 (TRAF6) in osteoclast precursors. The latter prevents signaling by receptor activator of NF-κB ligand (RANKL) needed for osteoclastogenesis. Knockout of IFN-γ rendered TcREG inefficient in preventing actin ring formation in osteoclasts, a process required for bone resorption. TcREG generated in vivo using IFN-γ−/− T-cells had impaired ability to protect mice from bone resorption and bone loss in response to high-dose RANKL. The results of this study demonstrate a novel link between NF-κB signaling and induction of IFN-γ in TcREG and establish important role for IFN-γ in TcREG-mediated protection from bone loss.
Introduction
Homeostasis describes the mechanisms by which biological systems maintain stability in response to environmental and internal perturbations. Homeostasis in the immune system, needed to minimize damage to healthy tissues, is achieved by a number of mechanisms. Such mechanisms include a balance between effector and regulatory cells. Disruption of this balance leads to autoimmune and inflammatory diseases (1, 2).
Bone homeostasis is maintained by a balance between bone-forming and bone-resorbing activities of osteoblasts and osteoclasts, respectively. This process is tightly regulated by a number of factors including hormonal changes, mechanical loading and diet. It has long been noticed that many chronic inflammatory diseases, e.g. rheumatoid arthritis, are accompanied by an increase in bone loss (3, 4). Pro-inflammatory cytokines such as TNF-α and IL-17 have been linked to the promotion of bone catabolism by increasing osteoclastogenesis via up-regulation of receptor activator of NF-κB ligand (RANKL) in stromal cells, a cytokine essential for the development and activation of osteoclasts (5–8). The observation that pro-inflammatory cytokines produced by activated lymphocytes can perturb bone-remodeling cycle gave rise to the study of Osteoimmunology, an emerging field studying the crosstalk between skeletal and immune systems (9–11).
We have previously identified a novel regulatory mechanism forming a feedback loop between osteoclasts and CD8 T-cells (12, 13). Primed by osteoclasts, CD8 T-cells differentiate into regulatory cells (TcREG) expressing CD25 and transcription factor FoxP3 (14), hallmarks of the regulatory cell phenotype (15). TcREG suppress bone resorption caused by osteoclasts in cell culture (16) and, by administration of high dose RANKL, in ovariectomized (OVX) mice, a murine model of postmenopausal induced osteoporosis (17, 18). T-cells produce RANKL upon activation thus directly inducing osteoclastogenesis and bone loss (19). In contrast, TcREG inhibit bone loss despite the fact that they also express RANKL (14). This discrepancy implies that other functions of TcREG overcome pro-resorptive effect of their activation shifting the balance toward bone protection. We, and others, have shown that similar to classical FoxP3+ CD4 T-cells (TREG), regulatory CD8 cells have immunosuppressive activity (20–23). Osteoclast-induced TcREG inhibit proliferation of effector T-cells in vitro (14, 17) and reduce the number of effector T-cells in the bone marrow of OVX mice (17, 18). Therefore TcREG directly regulate the levels of osteoclastogenic cytokine producing cells in the bone marrow, providing a mechanism for TcREG-mediated inhibition of the bone loss (24).
In addition to their immunoregulatory function, TcREG express IFN-γ (14). IFN-γ is usually viewed as a pro-inflammatory cytokine due to its strong macrophage-activating potential and the ability to drive differentiation of naïve CD4 T-cells toward a TH1 phenotype (25). However, accumulating evidence now reveals more complex, bidirectional, role for IFN-γ in autoimmune diseases (26). Anti-inflammatory properties of IFN-γ have been demonstrated in inflammatory disorders, such as collagen-induced arthritis (27), experimental autoimmune encephalomyelitis (28), asthma (29), autoimmune myocarditis (30) and others (26). In the context of bone remodeling, IFN-γ was reported to suppress osteoclastogenesis via degradation of RANK adapter protein tumor necrosis factor receptor-associated factor 6 (TRAF6) (31, 32) and induction of apoptosis by Fas - Fas ligand interaction (33). On the other hand, IFN-γ stimulates antigen-dependent T cell activation and production of osteoclastogenic factors RANKL and TNF-α (34, 35). It was concluded that in vivo the net effect of these two opposing qualities of IFN-γ is biased toward promoting bone resorption (34).
In this study we sought to characterize the role of IFN-γ in the context of its production by TcREG. We show that expression of the transcription factors T-bet and Eomes correlates with expression of IFN-γ in CD8 T-cells primed by osteoclasts. NF-κB was found to positively regulate expression of T-bet and, to a lesser degree, Eomes in TcREG. We demonstrate that IFN-γ contributes to TcREG mediated inhibition of osteoclastogenesis through a degradation of TRAF6 in osteoclast precursors and suppression of actin ring formation required for osteoclast resorbing activity. Upon withdrawal of TcREG, we show that actin ring formation is restored in osteoclasts. Finally, we demonstrate that IFN-γ−/− T-cells protect less significantly from RANKL induced bone loss as compared to wild type (WT) T-cells. Taken together, our results provide further characterization of TcREG population and demonstrate a significant role of IFN-γ in regulation of osteoclastogenesis and osteoclast resorption by these cells.
Materials and methods
Mice
Five- to 10-wk-old C57BL/6 mice from in-house breeding colonies were used for generation of osteoclasts and polyclonal CD8 T-cells. OT-I Rag−/− and OT-II Rag−/− mice were purchased from Taconic (model number 4175 and 1896, Germantown, NY, USA;). IFN-γ knockout (IFN-γ−/−) mice were from Jackson Laboratories (stock 002287). All animals were maintained in the Department of Comparative Medicine, Saint Louis University School of Medicine, in accordance with institutional and Public Health Service Guidelines. For adoptive transfer experiments, CD8 T-cells were isolated from donor mouse bone marrow and spleen (see below) and transferred to a recipient mouse at 1:1 ratio via tail vein in 100 μl of PBS.
Generation of osteoclasts
Osteoclast precursors were isolated as previously described (18). Briefly, femurs and tibia were harvested after mice were euthanized by CO2 asphyxiation. Each bone was placed in a 0.7 ml microcentrifuge tube that was pierced with a 22 G needle at the bottom. The 0.7 ml tube was placed inside a 1.5 ml microcentrifuge tube and spun for 30 seconds at 16,000 g. The bone marrow cells were resuspended and maintained in α-minimum essential medium (α-MEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), penicillin-streptomycin-glutamine (Invitrogen), recombinant murine M-CSF (eBioscience Inc, San Diego, CA) at 20 ng/ml and recombinant murine GST-RANKL (the expression system for GST-RANKL was a kind gift from Prof. Steven Teitelbaum, Washington University in St. Louis) at 50 ng/ml. Treatment with Versene (Gibco) for 10 minutes was used to harvest cells.
Isolation of splenocytes and generation of TcREG
Splenocytes were isolated as previously described (18). Briefly, single cell suspensions of spleens were prepared in PBS+1% FBS and filtered through 40μ cell strainer. CD8 T-cells were prepared by first enriching for T-cells using Pan-T-cell beads then purified by negative selection using appropriate magnetic beads (Miltenyi; Auburn, CA). To generate TcREG, day 3 osteoclasts were plated at 5×105 cells/well in 24-well tissue culture plates (Corning Inc., Corning, NY, USA). Next day, osteoclasts were pulsed with a peptide derived from residues 257–264 (SIINFEKL) of ovalbumin (5 μM; AnaSpec Inc.) for 3 hours. Freshly isolated, 2.5×105 cells/well OT-I splenic CD8 T-cells were then added in 2 ml of RPMI supplemented with 10% heat inactivated FBS, penicillin-streptomycin-glutamine, non-essential amino acids, 10 mM HEPES, and 55 μM β-mercaptoethanol. M-CSF and RANKL were added at 20 ng/ml and 100 ng/ml, respectively. For polyclonal CD8 T-cells from C57BL/6 mice α-CD3 antibody at 1 μg/ml was used instead of OVA peptide and was added to the T-cells before plating them on osteoclasts. In some experiments γ-secretase inhibitor DAPT and IKK inhibitor IKK-16 (both from Selleckchem) were added to the media at 10 μM concentration.
Western Blot
Whole cell lysates from TcREG were prepared according to manufacturer’s instructions (#9803, Cell Signaling, Danvers, MA, USA). Proteins were resolved on NuPAGE 4–12% Bis-Tris gel (Life Technologies, Carlsbad, CA, USA), transferred on a nitro-cellulose membrane and blocked in Tris-Buffered Saline (TBS) containing 5% bovine serum albumin (BSA) and 0.1% Tween-20. The membranes were probed overnight with primary antibody against TRAF-6 (Millipore) and β-actin (Cell Signaling). Membranes were washed and incubated for 1 hour with horseradish peroxidase (HRP) labeled secondary antibody (Cell Signaling).
Flow cytometry
Anti-mouse antibody used for cell staining were from BD Biosciences: CD45-BV711, FoxP3-e450, CD8-R700; BioLegend: CD3-FITC; and eBiosciences: Tbet-PE, EOMES-peCy7, IFNγ-APC, IL6-FITC, IL10-PE. Protein transport inhibitor (1 μg/ml; GolgiStop, BD Biosciences) was added to the medium 3 hrs prior to collecting the cells for staining to prevent IFN-γ secretion.
For FACS, cells were incubated with anti-mouse FcgRIII/IIR (Fc-block; BD Pharmingen) for 10 min and then stained for 45 min on ice with fluorophore-conjugated antibody. Stained cells were washed, fixed with 1% paraformaldehyde, and analyzed on LSRII (BD Biosciences) instrument. Data were analyzed using FlowJo software (version 8.73; TreeStar, Ashland, OR, USA).
Osteoclastogenesis and actin ring formation assays
For osteoclast differentiation experiment control T-cells and TcREG made from WT C57BL/6 or IFN-γ−/− mice were co-cultured with osteoclast precursors in the presence of M-CSF and RANKL. After five days the T-cells were removed and adherent cells were stained with a fluorescent substrate Elf-97 (Invitrogen) for tartrate-resistant acid phosphatase (TRAP) activity in accordance with the manufacturer’s instructions. In some experiments, recombinant IFN-γ (kindly provided by Prof. Mark Buller, Saint Louis University) was added to the medium at 20 or 100 U/ml at the time of adding T-cells.
For actin ring assay day 3 osteoclasts were plated on bovine bone slices at 3×103 cells/well in a 48-well plate. Control naïve T-cells and TcREG were added at 3×103 cells/well on day 5. After 24 hrs co-culture T-cells were removed by aspiration and adherent cells were fixed (4% para-formaldehyde (EMS), 0.2% Triton-X100 (Sigma) in PBS) for 10 min, washed 3 times with PBS and then stained with Alexa Fluor 488-conjugated phalloidin (ThermoFisher Scientific) for 15 min. The cells were photographed and actin ring numbers and sizes were blind-scored.
Assessment of bone resorption and volume density
Serum levels of C-terminal telopeptide of type I collagen (CTX) were measured as a marker of bone resorption (36) by ELISA according to the manufacturer’s instructions (Immunodiagnostic Systems, Plc.) Food was withdrawn for 6 to 10 h prior to collecting blood via sub-mandibular vein. After 1 h incubation at room temperature clotted blood was spun to obtain serum.
To measure bone volume density over total volume (BV/TV) the bones were scanned in μCT 40 (Scanco Medical) at 55 kVp, 145 μA, and a resolution of 16 μm. Gauss sigma of 1.2, Gauss support of 2, lower threshold of 237, and upper threshold of 1000 were used for all the analyses. Regions of interest were selected 50 slices below the growth plate of proximal tibia to evaluate the trabecular compartment. BV/TV was obtained by quantitative μCT using Scanco Phantoms for calibration (37).
ChIP-qPCR
Chromatin preparation and immunoprecipitation were done as previously described (38). Briefly, TcREG collected at 24 hr, or freshly isolated naïve CD8 T cells were fixed for 10 min at room temperature with 1% formaldehyde at 2×106 cells/ml in α-MEM. Fixation was stopped by addition of glycine to 0.125 M. Fixed cells were centrifuged at 1,000 g for 5 min at 4°C, washed twice with cold PBS, frozen on dry ice and stored at −80°C. For chromatin isolation 5×106 fixed cells were resuspended in 300 μl of sonication buffer (10 mM Tris HCl pH 7.6, 0.4% sodium dodecyl sulfate (SDS)), containing freshly added protease inhibitors (Pierce, EDTA-free) and incubated on ice for 20 min. The cell suspension was sonicated (Branson 250 Sonifier, tip 102) for 14–16 cycles (30 sec on, 30 sec off) at 4°C to shear the chromatin to an average DNA length of approximately 400 bp as determined by gel electrophoresis after reversing the crosslinks. A total of 900μl of adjustment buffer (10 mM Tris HCl pH 7.6, 1.3 mM EDTA, 0.133% sodium deoxycholate, 1.33% Triton X-100) was added to cell lysates followed by incubation on ice for 20 min and then clarified by centrifugation at 16,000 g for 10 min at 4°C. Glycerol was added to supernatant to 5% and snap-frozen and stored at −80°C.
To perform ChIP, 900 μl of chromatin was precleared by incubating with 40 μl of protein G dynabeads (Life Technologies, pre-washed with PBS/0.5% BSA) for 1 hour at 4°C. Precleared chromatin was incubated with anti-NF-κB p65 (Millipore), anti-T-bet (Santa Cruz), or anti-Eomes (Abcam) antibody overnight with gentle rotation. Prewashed protein G dynabeads (40 μl) were added to the chromatin-antibody mixture and rotated for 1.5 hrs at 4°C. The beads were washed twice at 4°C in: RIPA buffer (10 mM Tris HCl pH 7.6, 1 mM EDTA pH 8.0, 0.1% sodium deoxycholate, 1% Triton X-100), RIPA plus 0.3 M NaCl, LiCl buffer (0.25 M LiCl, 0.5% NP-40, 0.5% sodium deoxycholate). The beads were further washed in TET (10 mM Tris HCl pH 7.6, 1mM EDTA pH 8.0, 0.2% Triton X-100), and finally resuspended in TE (10 mM Tris HCl pH 7.6, 1mM EDTA pH 8.0). To reverse the crosslinks, the beads (150 μl in TE buffer) or input chromatin were incubated at 65°C overnight after addition of 5 μl of 10% SDS and 7μl of proteinase K (20 mg/ml). The supernatant containing eluted DNA was collected, the beads washed once with 150 μl of TE containing 0.5 M NaCl, and pooled with the first supernatant. The DNA was extracted with phenol:chloroform, precipitated with 20 μg of glycogen, 0.5 volume of 7.5 M ammonium acetate and 2.5 volumes of ethanol. Precipitated DNA was dissolved in 30 μl of 3 mM Tris HCl pH 8.0 and stored at −20°C until further analysis. Enrichment of NF-κB p65-, T-bet-, and Eomes-binding sites containing DNA sequences in the eluted DNA was determined by quantitative real-time PCR using Power SYBR Green PCR Master Mix (#4367659; Applied Biosystems, Foster City, CA). Sequences of oligonucleotides and annealing temperatures used for qPCR are shown in Table 1. The average raw CT values are provided in supplementary materials and the relative fold enrichment was calculated as follows: 2^(Ct InputTcREG – Ct ChIPTcREG)/2^(Ct InputNaïve – Ct ChIPNaïve). The ΔΔCT was further normalized to ADP-ribosylation factor 2 (Arf2) enrichment levels (oligonucleotides for Arf2 promoter qPCR: forward, 5′-AGGCTTATGTACCTCCCCGT; reverse, 5′-CTGACTGACTGCAGCTCCAA).
Table I.
Primers used for chromatin immunoprecipitation (ChIP)-qPCR
| ChIP Antibody | Promoter | Site | Forward primer | Reverse primer | Ta (°C)1 |
|---|---|---|---|---|---|
| NFκB p65 | IFN-γ | 1 | AGTGTGTGTCTAGTGCTGGA | GTCAACGTGCCCAGAAAGAA | 58 |
| IL-10 | 1 | ATTATGACCTGGGAGTGCGT | TGTGGCTTTGGTAGTGCAAG | 56 | |
| T-bet | 1 | GAGTTTCAGGTGGCAGGTTG | TCCTGGGCTTTCTCTGCAG | 58 | |
| Eomes | 1 | AGTCTCCCTCCTCTTCATACCT | AGGCCATGAATTTGAGAGGGG | 56 | |
| 2 | TAAGCCACGGAAAACCAGGC | GGTGTCTCAGGCACACTTTAAAAT | 56 | ||
| 3 | GTCAGCCCGAGTTCTCTGAG | AAGCTTTCCAACCTGGGTCC | 56 | ||
| T-bet / Eomes | IFN-γ | CNS-22 | CCAGGACAGAGGTGTTAAGCCA | GCAACTTCTTTCTTCTCAGGGTG | 58 |
| CNS-34 | GGTATGCATCATCCCGGG | TGGCCTGTCTTCAGAAGTTTGC | 58 | ||
| IL-10 | 1 | GTTACACGTCTCCAAGGCTG | GCAGTTGGTCAGAGGAGAGT | 58 |
Ta is the annealing temperature used for PCR. CNS indicates conserved nucleotide sequence (across multiple species) in the interferon (IFN)-γ enhancer.
Statistical analysis
Statistical significance was assessed by paired two-tailed Mann-Whitney U test in GraphPad Prism 5. P values < 0.05 were considered significant.
Results
IFN-γ is required for TcREG-mediated suppression of osteoclastogenesis
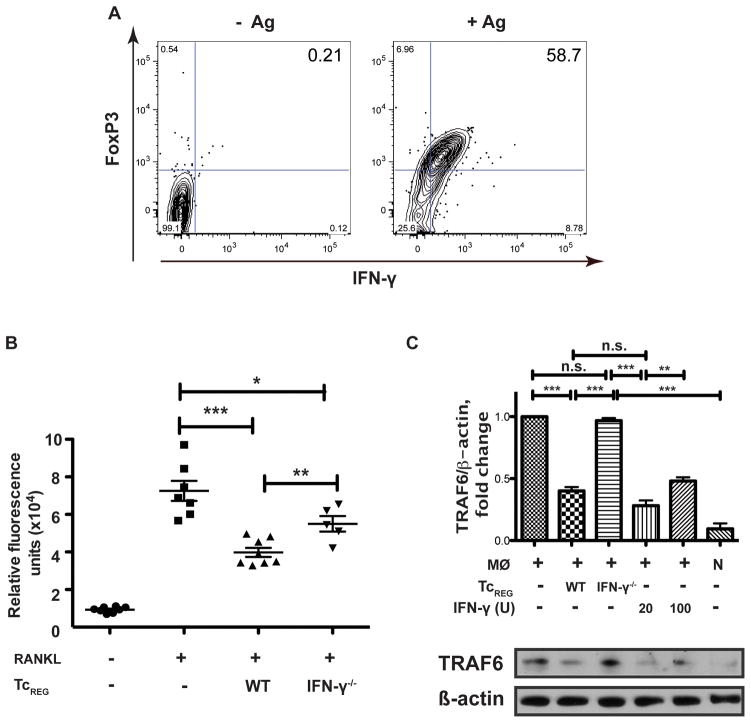
IFN-γ was reported to negatively regulate osteoclastogenesis when directly added to osteoclast precursor culture (34) or when secreted by activated γδ T-cells (39). CD8 T-cells primed by osteoclasts express FoxP3 and IFN-γ as measured by intracellular fluorescent staining of TcREG (Fig. 1A). Additionally, we have previously demonstrated the presence of IFN-γ in the medium conditioned by TcREG (14, 16). To test whether TcREG-mediated inhibition of osteoclast differentiation is dependent on IFN-γ we generated TcREG from WT or IFN-γ−/− mice and co-cultured them with osteoclast precursors for 5 days in the presence of M-CSF and RANKL. After removal of non-adherent T-cells the adherent cells were stained for osteoclast-specific TRAP activity (Fig. 1B). WT TcREG significantly reduced osteoclast formation whereas lack of IFN-γ partially rescued osteoclastogenesis significantly increasing TRAP-specific fluorescence. IFN-γ produced by T-cells was shown to mediate inhibition of osteoclastogenesis by causing degradation of RANK adapter protein TRAF6 (32). We found that TRAF6 was reduced in bone marrow macrophages 24 h after co-culture with WT TcREG (Fig. 1C). Treatment with 20 or 100 U/ml of recombinant IFN-γ resulted in similar levels of TRAF6 inhibition. In contrast, IFN-γ−/− TcREG did not affect TRAF6. Together, these results demonstrate that one of the mechanisms used by TcREG to inhibit osteoclastogenesis is IFN-γ mediated suppression of TRAF6 in osteoclast precursors.
Figure 1. TcREG inhibit osteoclastogenesis via IFN-γ.
(A) Representative flow cytometry plots showing that OT-I CD8 T-cells primed by osteoclasts pulsed with SIINFEKL express Foxp3 and IFN-γ. T-cells were collected for staining after 48 h co-culture with osteoclasts. Protein transport inhibitor GolgiStop was added to the cultures during last 3 h of incubation. Cells were gated on CD45+CD3+CD8+ population to determine FoxP3 and IFN-γ induction. (B) IFN-γ−/− TcREG have reduced anti-osteoclastogenic activity. Osteoclast-specific TRAP mean fluorescence intensity values are shown for osteoclast precursors after co-culturing them for 5 days with TcREG generated from WT or IFN-γ−/− splenic CD8 T-cells (N=5–8 wells/group). (C) TcREG induced TRAF6 degradation in macrophages via IFN-γ. Bone marrow macrophages were cultured in the presence of WT or IFN-γ−/− TcREG, or recombinant IFN-γ (20 or 100 U/ml) for 24 h. Adherent cells were removed and whole cell lysates of the macrophages were subjected to Western blotting for TRAF6 and β-actin. Band intensities were quantitated to show normalized TRAF6 levels. Data are representative of three independent experiments. MØ – macrophage, N – naïve CD8 T-cells. Statistical significance was assessed by non-parametric paired t-test: * p<0.05, ** p<0.01, *** p< 0.001.
INF-γ blocks actin ring formation in osteoclasts reversibly
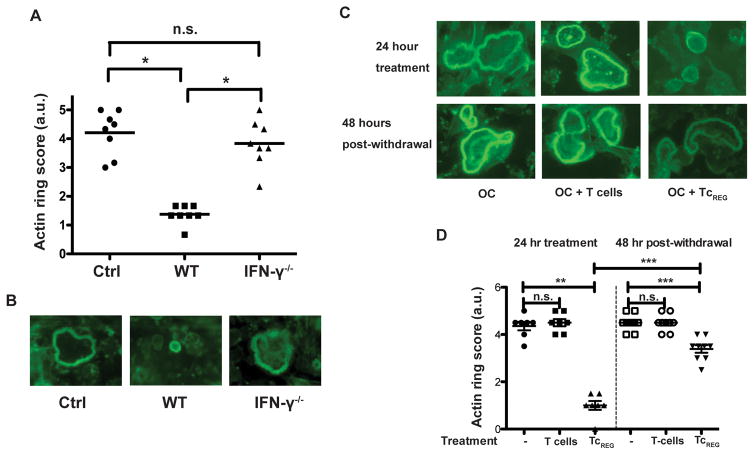
In order to resorb osteoclasts adhere to the bone surface and form sealing-zones composed of multiple actin ring structures (40–42). We tested whether IFN-γ is required for TcREG to inhibit actin ring formation in mature (day 5) osteoclasts. Coculturing day 5 osteoclasts (grown on bovine bone discs with WT TcREG dramatically reduced number and size of actin rings in the osteoclasts, whereas IFN-γ−/− TcREG did not affect actin ring formation (Fig. 2A, B). These data show that IFN-γ is a crucial mediator of anti-resorbing function of TcREG.
Figure 2. TcREG suppress actin ring formation via IFN-γ reversibly.
A) IFN-γ is required for TcREG to suppress actin ring formation. Osteoclasts grown on bovine bone slices were cultured for 24 h with WT or IFN-γ−/− TcREG followed by removal of T-cells and staining of osteoclasts with fluorophore-conjugated phalloidin to assay for actin ring formation. N=8 wells/group. (B) Representative images of the actin rings from (A) are shown. (C) Representative images showing actin ring structures in osteoclasts after co-culture with naïve T-cells or TcREG for 24 h (upper panel) or 48 h after removing T-cells (bottom panel). (D) Quantitative results from the experiment described in (C) were obtained by blind scoring of actin ring formation. Scoring in panels B and C are on a scale from 0 to 5, based on staining intensity, the number and thickness of rings per osteoclast considering all osteoclasts observed in a field (N=7–9 wells/group).. * p<0.05, ** p<0.01, *** p< 0.001.
We have previously reported that TcREG do not inhibit survival of osteoclasts (16). To investigate whether suppressive effect of TcREG could be reversed, we cocultured osteoclasts for 24 h with TcREG, removed them (by washing) and then continued culturing the osteoclasts for additional 48 h before staining with phalloidin to visualize actin rings. In contrast to naïve T-cells, TcREG significantly suppressed actin ring formation (Fig. 2C, D). Interestingly, when osteoclasts were allowed to grow for 48 h after removal of TcREG the actin ring formation was partially restored (Fig. 2C, D). These data suggest that suppression of actin ring formation in osteoclasts (and consequently their resorbing activity) requires sustained signaling by IFN-γ to degrade TRAF6.
Transcription factors T-bet and Eomes are coexpressed with IFN-γ in TcREG
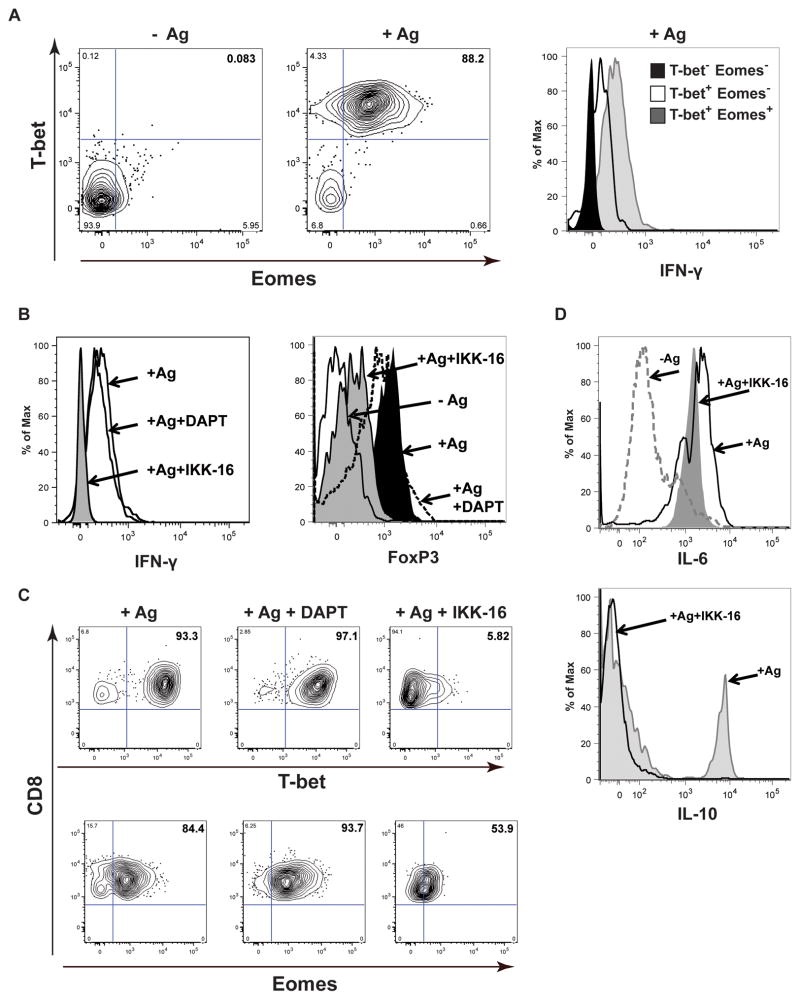
T-box transcription factor T-bet was originally described as a critical factor for TH1 polarization during viral infections and expression of IFN-γ in CD4 but not in CD8 T-cells (43). It was postulated that T-bet paralogue Eomesodermin (Eomes) controls CD8 T cell effector functions including IFN-γ production (44). Therefore, we measured T-bet and Eomes levels in osteoclast-induced TcREG. In the presence of Ag (SIINFEKL) OT-1 CD8 T-cells stained double positive for T-bet and Eomes (Fig. 3A, left). In contrast, naïve T-cells did not express substantial levels of either of the factors. IFN-γ was expressed in T-bet positive cells with the highest levels in T-bet+ Eomes+ double-positive CD8 T cells (Fig. 3A, right). These results suggest that T-bet and Eomes regulate IFN-γ production in osteoclast-primed CD8 T-cells.
Figure 3. Osteoclasts induce the transcription factors T-bet and Eomes in CD8 T-cells needed for IFN-γ production by TcREG.
(A) Flow cytometry plots showing stimulation of T-bet and Eomes expression in OT-I CD8 T-cells cultured under TcREG-inducing conditions (cocultured with osteoclasts for 48 h in the presence of the ovalbumin derived peptide antigen SIINFEKL). Cells were gated on CD45+CD3+CD8+ populations prior to determining T-bet and Eomes expression. Data are representative of five independent experiments. (B) Left panel: Inhibition of IKK, by IKK16, but not Notch signaling, by DAPT, blocks IFN-γ production by TcREG. OT-I CD8 T-cells were primed by osteoclasts for 48 h in the presence of 10 μM γ-secretase inhibitor DAPT or 10 μM IKK inhibitor IKK-16. GolgiStop was added to the cultures during last 3 h of incubation. T-cells were collected and stained for flow cytometry analysis. Right panel: Effect of the inhibitors on FoxP3 expression in CD8 T-cells. For both panels cells were gated on CD45+CD3+CD8+ population to determine IFN-γ or FoxP3 expression. (C) Reduction in IFN-γ production in TcREG by IKK-16 coincides with decrease in T-bet and Eomes expression levels. Flow cytometry plots showing cells from the experiment described in (B) and analyzed for T-bet and Eomes in CD45+CD3+CD8+ population. (D) Effect of IKK-16 on expression of IL-6 and IL-10 by TcREG. Flow cytometry plots showing that IKK inhibitor does not prevent induction of IL-6, whereas expression of IL-10 is blocked by IKK-16. Cells were gated on CD45+CD3+CD8+ population to determine expression of the cytokines.
Notch signaling was implicated in the regulation of IFN-γ expression in γδ T-cells (45), CD8 T-cells stimulated with anti-CD3 and anti-CD28 (46) and in peripheral T-cells (47, 48). Multiple cross-interactions have been described for Notch and NF-κB pathways in various experimental models (49). Therefore, we used the γ-secretase inhibitor DAPT and IKK inhibitor IKK-16 to measure the effect of Notch and NF-κB signaling, respectively, on IFN-γ expression. Treatment with DAPT had little to no effect on expression of IFN-γ, whereas IKK-16 completely blocked its production (Fig. 3B left). Additionally, we found that IKK16 significantly reduced FoxP3 expression in CD8 T-cells (Fig. 3B, right). While T-bet and Eomes levels were not affected by DAPT, they were drastically reduced in the presence of IKK-16 (Fig. 3C). Expression of T-bet was found to be more sensitive to NF-κB inhibition (93.8% vs 36.2% reduction in the levels of T-bet and Eomes, respectively, as compared to TcREG not treated with IKK-16). These results indicate that NF-κB positively regulates expression of T-bet and Eomes.
In addition to IFN-γ, TcREG also secrete IL-6 and IL-10 (14, 16). To test whether NF-κB signaling plays a role in induction of these cytokines, we examined the expression of IL-6 and IL-10 in TcREG in the presence of IKK-16. IL-6 expression was not significantly affected by IKK-16, whereas IL-10 expression was blocked by the inhibitor (Fig. 3D). Together, these data indicate that NF-κB signaling regulates expression of T-bet, Eomes, IFN-γ and IL-10.
NF-κB binds to T-bet, Eomes and IL-10 promoters and T-bet and Eomes bind to IFN-γ distal conserved sequences
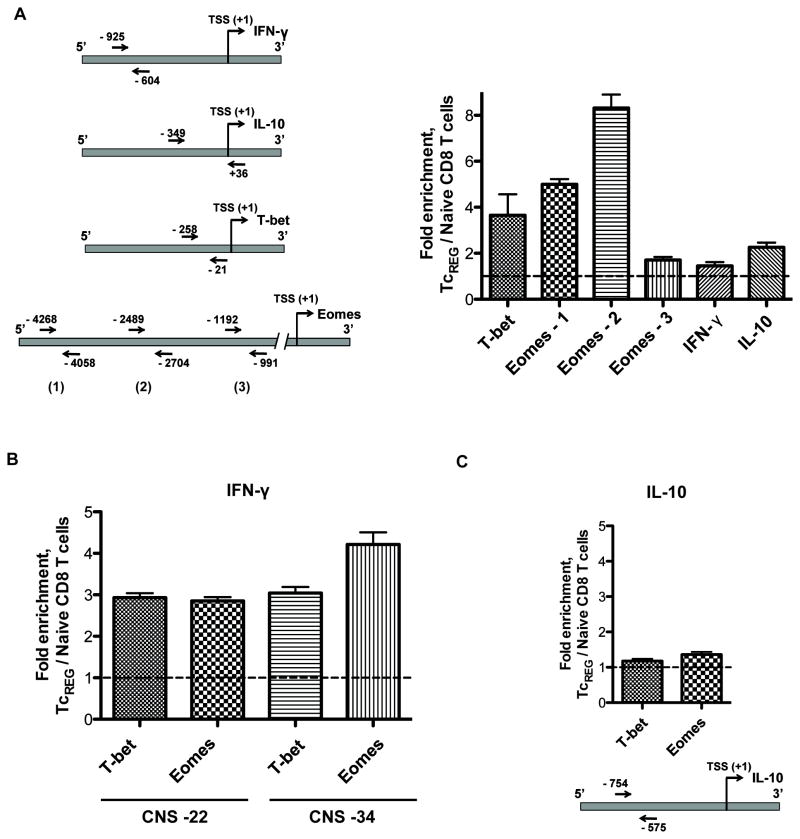
To determine whether NF-κB directly regulates expression of T-bet, Eomes, IFN-γ and IL-10, we performed ChIP using anti-p65 antibody followed by qPCR using osteoclast-induced TcREG and naïve CD8 T cells. We found enrichment of p65 on NF-κB-bindings sites in TcREG relative to naïve T-cells within T-bet, Eomes and IL-10 promoters, but not in IFN-γ promoter (Table S1 and Fig. 4A).
Figure 4. Gene regulatory network.
(A) Measurements of ccupancy of the p65 subunit of NF-κB on T-bet, Eomes, and IL-10 promoters in TcREG cells. Left panel: A diagram showing location of primers used for qPCR to detect enrichment of p65-binding sites. Right panel: Enrichment of p65-binding DNA sequences in the promoters of indicated genes of TcREG cells relative to naïve CD8 T cells. TcREG chromatin for use in ChIP was prepared after 24 h of incubation with osteoclasts. (B) T-bet and Eomes bind to distal conserved sequences CNS-22 and CNS-34 of IFN-γ gene in TcREG. Chromatin from naïve CD8 T cells (negative control) and from 24 h TcREG was immunoprecipitated with anti-T-bet or anti-Eomes antibody. Enrichment of T-bet and Eomes binding sites at −22k and −34K of IFN-γ gene was assessed by qPCR. (C) T-bet and Eomes do not bind IL-10 promoter. Chromatin prepared and immunoprecipitated as described in (B) was subjected to qPCR with primers, which flank putative T-bet/Eomes binding sites in IL-10 promoter. These results show no significant enrichment for these sites in TcREG cells relative to naïve CD8 T cells.
It has been reported previously that T-bet and Eomes bind to distal conserved non-coding sequences (CNS) to regulate histone modification and expression of IFN-γ gene (50–52). Consistent with these results, we found that in TcREG cells T-bet and Eomes bind to CNS located at −22K and −34K from IFN-γ gene transcription start (Fig. 4B). In contrast, no enrichment by ChIP-qPCR was found for putative T-bet and Eomes binding site in IL-10 promoter (Fig. 4C). Our data demonstrate that in TcREG cells IFN-γ production is regulated by T-bet and Eomes; expression of these transcription factors is p65-dependent. In contrast, IL-10 is regulated by p65 and not by T-bet or Eomes.
IFN-γ is involved in anti-resorptive activity of TcREG in mice
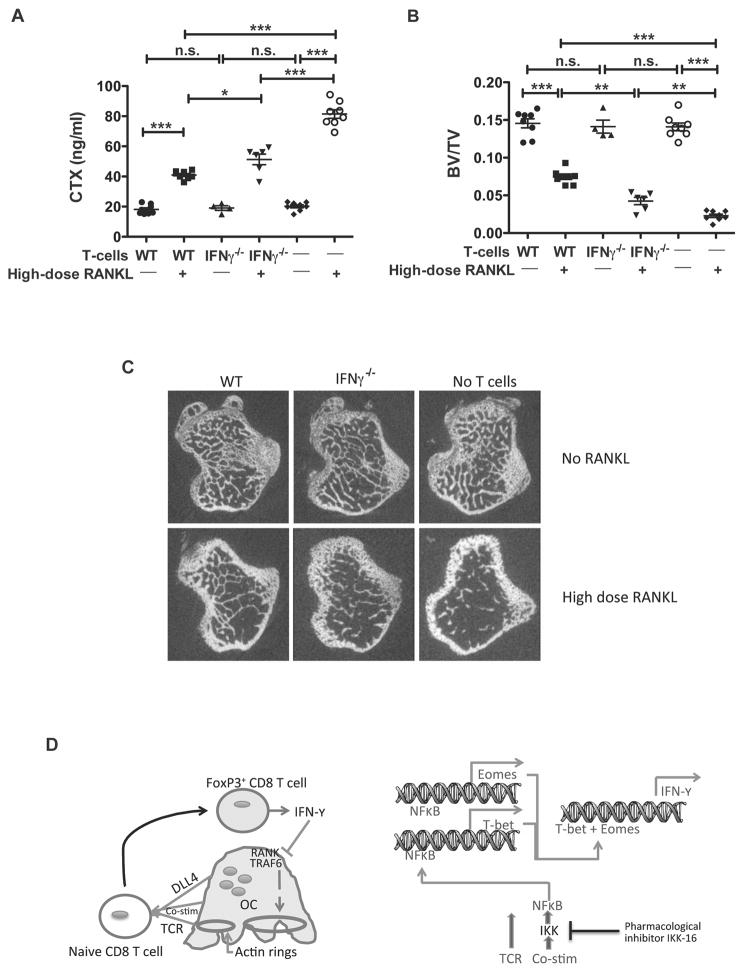
We have previously demonstrated that TcREG limit bone loss induced in mice by administration of high dose (1 mg/kg) of RANKL (17). As IFN-γ has pleiotropic effects in the context of other cell types, we tested the role of IFN-γ in TcREG-mediated reduction of bone loss in vivo. To this end, we adoptively transferred bone marrow and splenic CD8 T-cells from WT and IFN-γ−/− mice to OT-II Rag−/− mice followed by two daily doses of 1 mg/kg RANKL or buffer. OT-II Rag−/− mice were used as recipients because these mice lack endogenous TcREG, because they are not lymphopenic and because we could track the donor CD8 cells. Bone resorption levels were assessed by serum CTX levels 50 h after first injection of RANKL. Administration of WT CD8 T-cells significantly reduced high dose RANKL-induced bone resorption (Fig. 5A). This is consistent with our previous results demonstrating induction of TcREG in the bone marrow in response to RANKL administration (18). Interestingly, reconstitution of OT-II mice with IFN-γ−/− CD8 T-cells also decreased bone resorption as compared to control mice (which received only high dose RANKL) but significantly less efficient relative to WT T-cells (Fig. 5A). Similarly, mice injected with WT T-cells had increased bone volume as compared to control RANKL-treated animals (Fig. 5B). Adoptive transfer of IFN-γ−/− T-cells increased BV/TV but to lower extent, relative to WT T-cell treatment (Fig. 5B,C). Together, these results indicate that IFN-γ produced by TcREG in vivo is partially responsible for the bone protective effect of TcREG, and indicate that additional factors also contribute.
Figure 5. IFN-γ is important for the bone protective effect of TcREG in vivo.
(A) WT or IFN-γ−/− CD8 T-cells were adoptively transferred into OT-II mice (n=4–8 mice/group) followed 24 h later by administration of 1 mg/kg of RANKL daily for 2 days. Serum CTX levels at 50 h after first RANKL injections are shown to assess relative bone resorption levels. IFN-γ−/− CD8 T-cells have reduced ability to suppress RANKL-induced bone resorption as compared to WT CD8 T-cells. (B) μCT of proximal tibia of mice from the experiment described in (A). Reconstitution of OT-II mice with IFN-γ−/− CD8 T-cells partially protected mice, relative to WT T-cells, from losing bone volume in response to 1 mg/kg of RANKL resulting in intermediate levels of BV/TV. Consistent with these results, measurement of bone mineral density gave corresponding results. * p<0.05, ** p<0.01, *** p< 0.001. (C) Representative μCT images from the experiment described in (B). (D) A model summarizing the feedback loop between osteoclasts and CD8 T-cells: Left panel: Osteoclast are antigen-presenting cells that provide T cell receptor (TCR) activation, co-stimulation, and differentiation (Delta like ligand 4 [DLL4]) signals to naïve CD8 T-cells. This leads to induction of FoxP3 and IFN-γ in the CD8 T-cell. IFN-γ in turn causes degradation of TRAF6 and suppression of actin ring formation in osteoclasts inhibiting bone resorption. Right panel: A proposed model of events triggered in CD8 T-cells. Upon interaction with osteoclasts, NF-κB signaling is activated in the T-cells, leading to expression of transcription factors T-bet and Eomes, which then activate transcription of IFN-γ.
Discussion
An increasingly complex interaction between the skeletal and immune systems has been observed in multiple studies in recent years. This led to the development of a new interdisciplinary field of research termed osteoimmunology. The immune system plays an important role in regulating bone metabolism with various bone-affecting diseases spawning from immunologic imbalance. On the other hand, the immune system also protects bone and maintains homeostasis indicating that regulatory feedback loops must have evolved between the skeletal and immune compartments of the vertebrates. Here we extend our previous study of a negative feedback loop between osteoclasts and CD8 T lymphocytes, which not only limits bone resorption (Fig. 5D), but also limits activation of the immune system (13). In the present study we focused on the mechanism by which osteoclasts induce IFN-γ and the role of IFN-γ produced by TcREG in the anti-resorptive activity of these cells.
We demonstrate that TcREG generated from IFN-γ−/− mice have reduced ability to suppress osteoclastogenesis and are unable to cause degradation of TRAF6 in osteoclast precursors relative to their WT counterparts. Further, we show that IFN-γ−/− TcREG fail to suppress the formation of actin rings in osteoclasts, which is in contrast to the effect produced by WT TcREG (Fig. 5D). These results suggest that TcREG regulate actin reorganization in osteoclasts via IFN-γ. Withdrawal of WT TcREG from osteoclast culture for 48 h led to a significant restoration of actin ring formation. Actin ring structures reflect the establishment of sealing zones between osteoclasts and bone that are necessary for resorption (53). Signals provided by αvβ3 integrins are required for inducing actin reorganization and attachment of osteoclasts to the bone matrix (54, 55). Mice lacking the β3 integrin have dysfunctional osteoclasts and show protection from bone loss induced by ovariectomy (56, 57). Interestingly, IFN-γ has been implicated in a suppression of αvβ3 expression and adhesion properties of various cell types (58–61). It is likely therefore, that TcREG directly inhibit osteoclast resorbing through a blockade of αvβ3 signaling by IFN-γ.
We show that expression of transcription factors T-bet and Eomes accompany production of IFN-γ by TcREG. Inhibition of Notch signaling by DAPT did not affect either IFN-γ or T-bet and Eomes expression. In contrast, we found that inhibition of IKK completely blocked IFN-γ production that was associated with 93.8% and 36.2% reduction in the levels of T-bet and Eomes, respectively. The IKK kinase complex is the core element of the NF-κB signaling cascade, which regulates the expression of genes critical for immune development and immune responses, cell survival and proliferation (62). Our data indicate that IFN-γ production by TcREG is controlled by NF-κB signaling. Based on our data and published studies (52, 63, 64), we interpret these results to indicate that NF-κB contributes (or regulates) to the expression of T-bet and Eomes in TcREG, which then leads to IFN-γ production (Fig. 5D).
Several studies have reported opposing effects of recombinant IFN-γ on bone development and homeostasis (34, 65, 66). IFN-γ directly targets osteoclast precursors thereby suppressing osteoclast differentiation but indirectly promotes bone resorption by stimulating T-cell activation and secretion of pro-osteoclastogenic cytokines RANKL and TNF-α (34). The net effect of IFN-γ treatment on the bone therefore appears to be highly dependent on the context and dose. We show here that adoptive transfer of WT CD8 T cells into OT-II mice (to generate TcREG in vivo) inhibits RANKL-induced bone loss, whereas lack of IFN-γ renders TcREG less protective. However, the absence of IFN-γ did not completely block the anti-resorptive effects of the treatment. These results indicate that IFN-γ is not the sole mediator of the bone-protecting activity of TcREG, but nevertheless a contributing factor. We have previously shown that in addition to IFN-γ, TcREG secrete IL-10 and IL-6 (14). IL-10 is a potent anti-inflammatory cytokine that inhibits production of cytokines by TH1 cells and is involved in regulation of bone metabolism (67). IL-6 is a pleiotropic cytokine that has both pro- and anti-inflammatory activities depending on a particular environment. Several studies have demonstrated IL-6 mediated inhibition of osteoclastogenesis through a number of mechanisms including suppression of RANKL signaling pathway and downregulation of IL-10 expression (68, 69). Together, our results indicate that TcREG limit bone resorption via multiple cytokines.
In summary, we have characterized the induction of IFN-γ expression by osteoclast-induced regulatory CD8 T-cells and demonstrated its important role in bone protection. The results of this study add to our understanding of how TcREG function to prevent excessive bone catabolism and maintain a healthy homeostasis. By exhibiting two separate functions that include direct inhibition of osteoclastogenesis via IFN-γ and regulation of then immune system TcREG are able to shift the balance toward bone anabolic environment.
Supplementary Material
Acknowledgments
Grant support: Research reported in this study was partially supported by National Institute of Arthritis and Musculoskeletal and Skin Disease of the NIH under Award Number RO1AR064821 and RO1AR068438. Washington University Musculoskeletal Research Core (NIH P30 AR057235) also partially supported this study.
We thank Dr. Ryan Teague for helpful discussions and Dr. R. Mark Buller for providing recombinant IFN-γ. We also thank Sheri Koehm and Joy Eslick in the flow cytometry core.
References
- 1.Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65:26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- 2.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 3.Hardy R, Cooper MS. Bone loss in inflammatory disorders. J Endocrinol. 2009;201:309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 4.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 5.Schett G, Teitelbaum SL. Osteoclasts and Arthritis. J Bone Miner Res. 2009;24:1142–1146. doi: 10.1359/jbmr.090533. [DOI] [PubMed] [Google Scholar]
- 6.Karmakar S, Kay J, Gravallese EM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 2010;36:385–404. doi: 10.1016/j.rdc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenblatt MB, Shim JH. Osteoimmunology: a brief introduction. Immune Netw. 2013;13:111–115. doi: 10.4110/in.2013.13.4.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol. 2012;8:684–689. doi: 10.1038/nrrheum.2012.167. [DOI] [PubMed] [Google Scholar]
- 11.Mori G, D’Amelio P, Faccio R, Brunetti G. The Interplay between the bone and the immune system. Clin Dev Immunol. 2013;2013:720504. doi: 10.1155/2013/720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiesel J, Miller C, Abu-Amer Y, Aurora R. Systems level analysis of osteoclastogenesis reveals intrinsic and extrinsic regulatory interactions. Dev Dyn. 2007;236:2181–2197. doi: 10.1002/dvdy.21206. [DOI] [PubMed] [Google Scholar]
- 13.Buchwald ZS, Aurora R. Osteoclasts and CD8 T Cells Form a Negative Feedback Loop That Contributes to Homeostasis of Both the Skeletal and Immune Systems. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/429373. Article ID 429373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiesel JR, Buchwald ZS, Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J Immunol. 2009;182:5477–5487. doi: 10.4049/jimmunol.0803897. [DOI] [PubMed] [Google Scholar]
- 15.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald ZS, Kiesel JR, DiPaolo R, Pagadala MS, Aurora R. Osteoclast Activated FoxP3(+) CD8(+) T-Cells Suppress Bone Resorption in vitro. PLoS ONE. 2012;7:e38199. doi: 10.1371/journal.pone.0038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchwald ZS, Kiesel JR, Yang C, DiPaolo R, Novack DV, Aurora R. Osteoclast-induced Foxp3+ CD8 T-cells limit bone loss in mice. Bone. 2013;56:163–173. doi: 10.1016/j.bone.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchwald ZS, Yang C, Nellore S, Shashkova EV, Davis JL, Cline A, Ko J, Novack DV, DiPaolo R, Aurora R. A Bone Anabolic Effect of RANKL in a Murine Model of Osteoporosis Mediated Through FoxP3+ CD8 T Cells. J Bone Miner Res. 2015;30:1508–1522. doi: 10.1002/jbmr.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 20.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 21.Correale J, Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol. 2010;67:625–638. doi: 10.1002/ana.21944. [DOI] [PubMed] [Google Scholar]
- 22.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 23.Niederkorn JY. Emerging concepts in CD8(+) T regulatory cells. Curr Opin Immunol. 2008;20:327–331. doi: 10.1016/j.coi.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacifici R. Role of T cells in ovariectomy induced bone loss--revisited. J Bone Miner Res. 2012;27:231–239. doi: 10.1002/jbmr.1500. [DOI] [PubMed] [Google Scholar]
- 25.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 26.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Lee J, Park MK, Lim MA, Park EM, Kim EK, Yang EJ, Lee SY, Jhun JY, Park SH, Kim HY, Cho ML. Interferon gamma suppresses collagen-induced arthritis by regulation of Th17 through the induction of indoleamine-2,3-deoxygenase. PLoS One. 2013;8:e60900. doi: 10.1371/journal.pone.0060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller NM, Wang J, Tan Y, Dittel BN. Anti-inflammatory mechanisms of IFN-gamma studied in experimental autoimmune encephalomyelitis reveal neutrophils as a potential target in multiple sclerosis. Front Neurosci. 2015;9:287. doi: 10.3389/fnins.2015.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaishon L, Topilski I, Shoseyov D, Hershkoviz R, Fireman E, Levo Y, Marmor S, Shachar I. Cutting edge: anti-inflammatory properties of low levels of IFN-gamma. J Immunol. 2002;168:3707–3711. doi: 10.4049/jimmunol.168.8.3707. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001;167:5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- 31.Fox SW, Chambers TJ. Interferon-gamma directly inhibits TRANCE-induced osteoclastogenesis. Biochem Biophys Res Commun. 2000;276:868–872. doi: 10.1006/bbrc.2000.3577. [DOI] [PubMed] [Google Scholar]
- 32.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 33.Kohara H, Kitaura H, Fujimura Y, Yoshimatsu M, Morita Y, Eguchi T, Masuyama R, Yoshida N. IFN-gamma directly inhibits TNF-alpha-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol Lett. 2011;137:53–61. doi: 10.1016/j.imlet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, Yago T, Kobashigawa T, Togari A, Kamatani N. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35:3353–3363. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 36.Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000;66:100–103. doi: 10.1007/pl00005830. [DOI] [PubMed] [Google Scholar]
- 37.Nazarian A, Snyder BD, Zurakowski D, Muller R. Quantitative micro-computed tomography: a non-invasive method to assess equivalent bone mineral density. Bone. 2008;43:302–311. doi: 10.1016/j.bone.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappalardo A, Thompson K. Activated gammadelta T cells inhibit osteoclast differentiation and resorptive activity in vitro. Clin Exp Immunol. 2013;174:281–291. doi: 10.1111/cei.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novack DV, Faccio R. Osteoclast motility: Putting the brakes on bone resorption. Ageing Res Rev. 2009 doi: 10.1016/j.arr.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soysa NS, Alles N, Aoki K, Ohya K. Three-dimensional characterization of osteoclast bone-resorbing activity in the resorption lacunae. J Med Dent Sci. 2009;56:107–112. [PubMed] [Google Scholar]
- 42.Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 44.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 45.Gogoi D, Dar AA, Chiplunkar SV. Involvement of Notch in activation and effector functions of gammadelta T cells. J Immunol. 2014;192:2054–2062. doi: 10.4049/jimmunol.1300369. [DOI] [PubMed] [Google Scholar]
- 46.Cho OH, Shin HM, Miele L, Golde TE, Fauq A, Minter LM, Osborne BA. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol. 2009;182:3380–3389. doi: 10.4049/jimmunol.0802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palaga T, Miele L, Golde TE, Osborne BA. TCR-mediated Notch signaling regulates proliferation and IFN-gamma production in peripheral T cells. J Immunol. 2003;171:3019–3024. doi: 10.4049/jimmunol.171.6.3019. [DOI] [PubMed] [Google Scholar]
- 48.Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci U S A. 2008;105:3497–3502. doi: 10.1073/pnas.0712102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osipo C, Golde TE, Osborne BA, Miele LA. Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest. 2008;88:11–17. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- 50.Hatton RD, Harrington LE, Luther RJ, Wakefield T, Janowski KM, Oliver JR, Lallone RL, Murphy KM, Weaver CT. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Chang S, Collins PL, Aune TM. T-bet dependent removal of Sin3A-histone deacetylase complexes at the Ifng locus drives Th1 differentiation. J Immunol. 2008;181:8372–8381. doi: 10.4049/jimmunol.181.12.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuoka N, Harada M, Nishida A, Ito Y, Shiota H, Kataoka T. Eomesodermin promotes interferon-gamma expression and binds to multiple conserved noncoding sequences across the Ifng locus in mouse thymoma cell lines. Genes Cells. 2016;21:146–162. doi: 10.1111/gtc.12328. [DOI] [PubMed] [Google Scholar]
- 53.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 54.Zou W, Teitelbaum SL. Integrins, growth factors, and the osteoclast cytoskeleton. Ann N Y Acad Sci. 2010;1192:27–31. doi: 10.1111/j.1749-6632.2009.05245.x. [DOI] [PubMed] [Google Scholar]
- 55.Xu F, Teitelbaum SL. Osteoclasts: New Insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H, Kitaura H, Sands MS, Ross FP, Teitelbaum SL, Novack DV. Critical role of beta3 integrin in experimental postmenopausal osteoporosis. J Bone Miner Res. 2005;20:2116–2123. doi: 10.1359/JBMR.050724. [DOI] [PubMed] [Google Scholar]
- 57.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong W, Zhang GM, Liu Y, Lei Z, Li D, Yuan Y, Huang B, Feng ZH. IFN-gamma withdrawal after immunotherapy potentiates B16 melanoma invasion and metastasis by intensifying tumor integrin alphavbeta3 signaling. Int J Cancer. 2008;123:702–708. doi: 10.1002/ijc.23553. [DOI] [PubMed] [Google Scholar]
- 59.Tenaud I, Leroy S, Chebassier N, Dreno B. Modulation in vitro of keratinocyte integrins by interferon-alpha and interferon-gamma. Int J Dermatol. 2002;41:836–840. doi: 10.1046/j.1365-4362.2002.01598.x. [DOI] [PubMed] [Google Scholar]
- 60.Salzano M, Russo E, Postiglione L, Guerra A, Marotta V, Esposito S, Vitale M. Interferon-gamma inhibits integrin-mediated adhesion to fibronectin and survival signaling in thyroid cells. J Endocrinol. 2012;215:439–444. doi: 10.1530/JOE-12-0335. [DOI] [PubMed] [Google Scholar]
- 61.Russo E, Salzano M, Postiglione L, Guerra A, Marotta V, Vitale M. Interferon-gamma inhibits integrin-mediated extracellular signal-regulated kinase activation stimulated by fibronectin binding in thyroid cells. J Endocrinol Invest. 2013;36:375–378. doi: 10.3275/8649. [DOI] [PubMed] [Google Scholar]
- 62.Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popescu I, Pipeling MR, Shah PD, Orens JB, McDyer JF. T-bet:Eomes balance, effector function, and proliferation of cytomegalovirus-specific CD8+ T cells during primary infection differentiates the capacity for durable immune control. J Immunol. 2014;193:5709–5722. doi: 10.4049/jimmunol.1401436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol. 2008;181:8700–8710. doi: 10.4049/jimmunol.181.12.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–193. doi: 10.1111/j.0105-2896.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 66.Duque G, Huang DC, Dion N, Macoritto M, Rivas D, Li W, Yang XF, Li J, Lian J, Marino FT, Barralet J, Lascau V, Deschenes C, Ste-Marie LG, Kremer R. Interferon-gamma plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J Bone Miner Res. 2011;26:1472–1483. doi: 10.1002/jbmr.350. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, Yang W. Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed Res Int. 2014;2014:284836. doi: 10.1155/2014/284836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283:11535–11540. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]
- 69.Balto K, Sasaki H, Stashenko P. Interleukin-6 deficiency increases inflammatory bone destruction. Infect Immun. 2001;69:744–750. doi: 10.1128/IAI.69.2.744-750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.