Abstract
Characterization of cortisol production, regulation and function is of considerable interest and relevance given its ubiquitous role in virtually all aspects of physiology, health and disease risk. The quantification of cortisol concentration in hair has been proposed as a promising approach for the retrospective assessment of integrated, long-term cortisol production. However, human research is still needed to directly test and validate current assumptions about which aspects of cortisol production and regulation are reflected in hair cortisol concentrations (HCC). Here, we report findings from a validation study in a sample of 17 healthy adults (mean ± SD age: 34 ± 8.6 yrs). To determine the extent to which HCC captures cumulative cortisol production, we examined the correspondence of HCC, obtained from the first 1cm scalp-near hair segment, assumed to retrospectively reflect 1-month integrated cortisol secretion, with 30-day average salivary cortisol area-under-the curve (AUC) based on 3 samples collected per day (on awakening, +30 min, at bedtime) and the average of 4 weekly 24-hr urinary free cortisol (UFC) assessments. To further address which aspects of cortisol production and regulation are best reflected in the HCC measure, we also examined components of the salivary measures that represent: 1) production in response to the challenge of awakening (using the cortisol awakening response [CAR]), and 2) chronobiological regulation of cortisol production (using diurnal slope). Finally, we evaluated the test-retest stability of each cortisol measure. Results indicate that HCC was most strongly associated with the prior 30-day integrated cortisol production measure (average salivary cortisol AUC) (r = 0.61, p = 0.01). There were no significant associations between HCC and the 30-day summary measures using CAR or diurnal slope. The relationship between 1-month integrated 24-hr UFC and HCC did not reach statistical significance (r = 0.30, p = 0.28). Lastly, of all cortisol measures, test-retest correlations of serial measures were highest for HCC (month-to-month: r = 0.84, p < 0.001), followed by 24-hr UFC (week-to-week: r’s between 0.59 and 0.68, ps < 0.05) and then integrated salivary cortisol concentrations (week-to-week: r’s between 0.38 and 0.61, p’s between 0.13 and 0.01). These findings support the contention that HCC provides a reliable estimate of long-term integrated free cortisol production that is aligned with integrated salivary cortisol production measured over a corresponding one-month period.
Keywords: Hair cortisol, salivary cortisol, urinary free cortisol, methods comparison, stress
1. Introduction
Cortisol is a glucocorticoid hormone that is extensively examined in the context of psychoneuroendocrinological investigations because it plays a major role in regulating central and peripheral physiology and is implicated in many aspects of stress-related health and disease risk across a spectrum of physical and mental disorders. While acute cortisol reactivity comprises an important part of the adaptive response to challenge (Sapolsky, 2000), long-term changes in the secretion of cortisol (e.g., under conditions of chronic stress) may be detrimental and have been linked to an increased risk for physical and mental disease (Lupien et al., 2006; Chrousos, 2009). However, researchers aiming to understand the role of long-term cortisol changes as a mediator between chronic stress and disease often face methodological challenges when trying to obtain reliable estimates of long-term cortisol output. Specifically, common assessment strategies in blood or saliva only reflect acute circulating cortisol levels, which are highly volatile and influenced by a range of situational factors (e.g. Young and Breslau, 2004). Hence, to obtain reliable information on long-term secretion using these more invasive methods presents a large participant burden and likely requires a prohibitive number of repeated sampling. Another method to obtain information on longer-term cortisol secretion is urinary sampling, with urinary-free cortisol concentration (UFC) being assumed to provide an integrated index of cortisol secretion over the period of urinary sampling, usually 12 or 24 hours (Remer et al., 2008). However, assessment of UFC is associated with considerable problems, such as participant non-compliance (Yehuda et al., 2003), and there is still a debate about how well urinary cortisol reflects systemically circulating free cortisol levels (Murphy, 1999).
Over the past decade, the analysis of cortisol in hair has emerged as an alternative and promising strategy for the assessment of long-term cortisol secretion (reviews: Russell et al., 2012, Stalder and Kirschbaum, 2012; Staufenbiel et al., 2013). Hair grows on average 1cm/month (Wenning, 2000) and cortisol, like other steroid hormones, is maintained in hair at reliable levels for up to six months (Kirschbaum et al., 2009). Small amounts of cortisol are secreted by the hair follicle itself (Ito et al., 2005); however, the main proportion of cortisol found in hair is thought to be derived from the bloodstream via passive diffusion (Stalder and Kirschbaum, 2012). The overall validity of hair cortisol concentration (HCC) as an index of cumulative long-term systemic cortisol levels has been generally supported in human and animal research (Stalder and Kirschbaum, 2012). This includes indirect validation research, i.e. studies showing HCC profiles characteristic of conditions with well-described alterations of adrenocortical function, like Cushing’s or Addison’s Disease (e.g., Kirschbaum et al., 2009; Stalder et al., 2010; Thomson et al., 2010; D’Anna Hernandez et al., 2011; Skoluda et al., 2012). More direct validation data comes from research examining the relationship between HCC and cortisol levels in saliva, blood or urine (e.g., Davenport et al., 2006; Sauve et al., 2007). These studies generally revealed positive relationships between HCC and other well-established cortisol measures, yet the strength of correlations varied considerably between studies: medium to strong associations (rs between 0.48 and 0.90) emerged from animal research, in which other methods of cortisol sampling (e.g., feces or saliva) were often assessed repeatedly over long time periods (Davenport et al., 2006; Accorsi et al., 2008; Bennet and Hayssen, 2010). Research with humans however has revealed less strong relationships (rs between 0.06 and 0.57) (e.g., Sauve et al., 2007, D’Anna-Hernandez et al., 2011; see Stalder and Kirschbaum, 2012). One reason that a validation study is still needed is that most studies to date have not been specifically designed for the purpose of validating HCC measures (e.g., assessment of correspondence between cortisol measured in hair and cortisol measured in other standard sampling methods) nor elucidating which aspects of cortisol production and regulation are reflected with the HCC measure. Empirical findings used to support current assumptions about HCC as a measure of cumulative cortisol production come from studies that collected a small number of comparison cortisol samples that were obtained over a time span that was much shorter than the month-long duration that a 1 cm hair sample is thought to reflect (e.g., Steudte et al., 2011, Xie et al., 2011). Moreover, in other studies HCC and salivary cortisol concentrations were sampled concurrently (Sauve et al., 2007, Van Holland et al., 2012), which is problematic because these measures reflect cortisol concentrations across different time periods and durations.
The current study aims to address the need for an in-depth human validation study to directly test and validate current assumptions about which aspects of cortisol production are reflected in HCC by examining the relationship between HCC and cortisol measured with other sampling methods, collected repeatedly, over an extended and corresponding period of time. Our primary aim was to determine the extent to which the HCC measure captures cumulative cortisol production. Thus we examined the correspondence of HCC, obtained from the first 1cm scalp-near hair segment, assumed to retrospectively reflect 1-month integrated cortisol secretion, with 30-day average salivary cortisol area-under-the curve (AUC) (3 daily samples: on awakening, +30 min, at bedtime) and the average of 4 weekly 24-hr urinary free cortisol (UFC) assessments. Each of these cortisol indices is thought to represent the unbound, and thus biologically active, fraction of cortisol in the peripheral circulation (Beisel et al., 1964; Kirschbaum and Hellhammer, 1994; Stalder and Kirschbaum, 2012). To further address whether more dynamic aspects of cortisol production and regulation are also reflected in the HCC measure, we examined HCC associations with the cortisol awakening response (CAR) and the cortisol decline over the course of the day (diurnal cortisol slope). Lastly, we evaluated the test-retest stability of each cortisol measure from month-to-month for hair and from week-to-week for salivary and UFC.
2. Methods
2.1 Participants
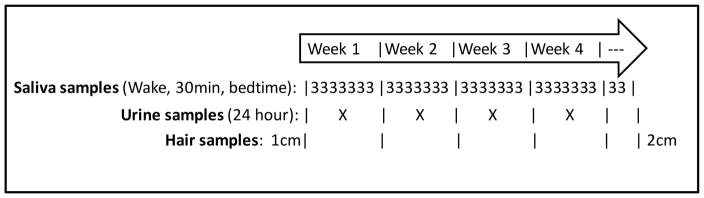
Twenty participants were recruited from two sites: University of California Irvine and the University of North Carolina at Chapel Hill. The samples of three individuals were excluded from the analyses because their sleep schedules were atypical (i.e., shift work) or they did not adequately adhere to the sample collection protocol. Participants were healthy adults, 10 men and 7 women aged 21 to 53 years (M = 34.06, SD = 8.60 years) who provided written informed consent to participate in this study. The Institutional Review Boards of the University of North Carolina School of Medicine and University of California, Irvine approved this study. Participants provided daily saliva samples, weekly 24-hour urine samples, and two hair samples, over the course of the 30-day study period (See Figure 1).
Figure 1. Summary of Study Design, Measures and Sample Collections.
A summary of the study design illustrates the measures included as well as the number and timing of cortisol samples collected during the month-long study period.
2.2 Design and Procedure
Salivary Cortisol
Each individual collected three saliva samples per day for 30 days: 1) immediately on waking, 2) 30 min post-awakening, and 3) at bedtime. Saliva sampling was conducted using Salivettes (Sarstedt, Nümbrecht, Germany). Times of sampling were both self-recorded by the participant and objectively verified by use of MEMS 6 Track Cap containers (MWV Healthcare, Switzerland Ltd). The use of such objective verification methods substantially increases validity of salivary cortisol data, particularly concerning the CAR (e.g., Kudielka et al., 2003; Broderick et al., 2004; review: Stalder et al., 2016). Participants were instructed not to go back to sleep during morning sampling period (30 min post-awakening) and to refrain from eating, drinking and/or brushing teeth for at least 1 hour before sampling. After collection, samples were stored in participants’ home refrigerators until the end of each week when they were collected and processed by research staff members. Saliva samples were spun at 2000g (3100 rpm) for 15 minutes and 1.5ml of saliva was transferred to a 2.0ml cryovial for storage until all participants’ samples were collected. Processed saliva samples were stored in a freezer at −80°C. Salivary cortisol was measured using a commercial enzyme immunoassay from Salimetrics (Carlsbad, CA, USA), with 10% of the samples measured in duplicate. The detection limit was .007μg/dL, and the intra- and interassay coefficients of variation were 6.7% and 5.0%, respectively. Cortisol values identified as outliers were winsorized to 3 SD of each individual’s mean.
An integral, total cortisol output measure for salivary cortisol was obtained by calculating the area under the curve with respect to ground (AUC) for each day (Pruessner et al., 2003) using the MEMS-derived saliva collection times (i.e., from MEMS caps data) and adjusting for the total number of hours the AUC spanned (e.g., time from awakening sample to the bedtime sample). On average, missing or invalid cortisol samples were observed on only 4.24 (range: 0–13) samples out of the 90 total samples for each participant. The AUC for each day was summed into a monthly integrated salivary AUC value for each individual. For this, missing AUC values were mean imputed from the valid AUC values for each individual before the AUC month-long sum score was created (i.e., if an individual was missing 3 days, their average AUC across days * 3 was added to their sum of their valid daily AUC scores).
The chronobiology of cortisol was assessed using two approaches. First, the cortisol awakening response (CAR) was assessed as the difference between the 30min and waking samples, adjusted for the time between the two samples (Bouma et al., 2009; Childa and Steptoe, 2009; Fries et al., 2009; Clow et al., 2010). This score was calculated for each day, then averaged to derive a month-long integrated score. The CAR represents a distinct aspect of circadian cortisol secretion and is frequently assessed as a measure in psychoneuroendocrinology (PNE) research. Second, the diurnal slope is the difference between the waking and bedtime samples, adjusted for the time between these two samples. The diurnal slope was calculated for each day and then averaged for a month-long score. The diurnal rhythm captures the overall change in cortisol across the daytime hours (Kertes and van Dulmen, 2012). Both of these chronobiology metrics are moderately heritable and distinct from cortisol levels alone (Wüst et al., 2000; Bartels et al., 2003; Van Hulle et al., 2012). While the month-long integrative scores are of primary interest, week-long sum scores were also calculated and used in follow-up analyses.
Urinary Free Cortisol
Participants collected a 24-hour urine sample once each week on the same day of each of the four weeks for consistency. Participants were allowed to choose which day was most convenient for them, as long as collection occurred on the same day each week across the sampling month. Participants were instructed not to collect the first void of the day on the morning that urine collection started, but to begin collecting each void thereafter for an entire 24 hour period (Yehuda et al., 2003). The 24-hour urine samples were refrigerated for 1–2 days before being processed and stored at −80°C. Total sample volumes were recorded by lab technicians and later used for correcting data prior to analyses. UFC was measured using a commercial enzyme-linked immunosorbent assay kit from IBL International (Hamburg, Germany). The detection limit was 2.95ng/ml, and the CVs were 7% for intraassay and 17%, for interassay, respectively. Volume adjusted UFC was calculated for each 24-hour urine sample (i.e., 24-hour urinary cortisol = (sample (μg/dL) x volume (mL))/100).
A monthly urinary AUC was computed using all four weekly samples. For individuals missing a single urinary collection (n = 3), the individual’s average was used in place of the missing UFC value for the monthly AUC calculation.
Hair Cortisol
Approximately 100 strands of hair were sampled from the posterior vertex as close as possible to the scalp using fine scissors. Hair samples were wrapped in aluminum foil with a marker to indicate which end was closest to the scalp. Then samples were stored at room temperature. Based on the assumption of an average hair growth rate of 1 cm/month (Wennig, 2000), we analyzed the proximal 1cm segment of hair from the baseline (prior month) and final (test month) hair samples to assess cortisol accumulation over a 1-month period. We also analyzed the second 1cm segment from the final hair sample (final hair segment 2) to determine how well it was correlated with the sample obtained at baseline. HCC was measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS), the current gold-standard approach for hair steroid analysis (Gao et al., in press), following our previously published protocol with a limit of quantification for cortisol of 0.09 pg/mg and intra- and interassay CVs between 3.7 and 8.8% (Gao et al., 2013). A single outlier was winsorized to 3 SD of the sample average. All scores were log transformed to normalize the distribution.
Hair Treatment
In the beginning and at the end of the study period, participants answered questions regarding different forms of hair treatment. Three questions were combined into a binary score indicating any kind of hair treatment (n = 7) vs. no treatment (n = 9, with n = 1 missing information). These questions were: “Do you dye/bleach your hair?” , “Do you perm your hair?” , and “Do you do any other hair treatments? (e.g., straightening, hair growth stimulators, hair-gel, etc.)” .
2.3 Statistics
We tested the correspondence between HCC, salivary cortisol measures, and UFC as well as the test-retest stability of these measures using correlation analyses. The primary focus was on the month-long interval. We also conducted secondary analyses to determine the week-to-week correspondence.
3. Results
3.1 Associations between Cortisol Concentrations in Hair, Saliva and Urine
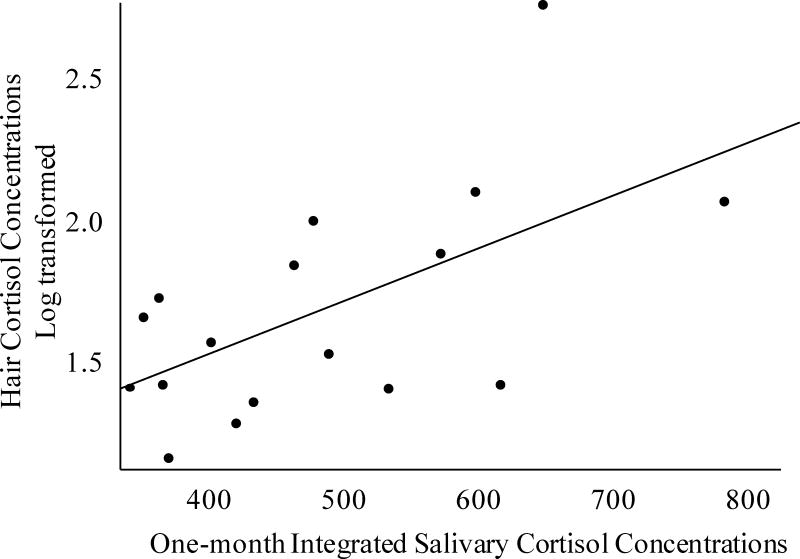
We first addressed the primary question of the correspondence between a 1cm HCC measure and the prior 30-day average salivary cortisol AUC score to determine the extent to which the HCC measure captures cumulative cortisol production over the prior 30-day period. Pearson’s correlations showed that HCC (first 1cm segment obtained at the end of the test month) was significantly correlated with 1-month integrated salivary cortisol AUC (r = 0.61, p = 0.01, see Figure 2) and accounted for 36% of the variation.
Figure 2. Association of Monthly Salivary Cortisol AUC and Hair Cortisol.
Associations between participant’s monthly salivary and hair cortisol concentrations. Hair cortisol concentration values were winsorized and then log transformed. Salivary AUC values were integrated across the sample day, adjusted for total hours between collecting the first and last sample of the day and then summed across the month. The correlation between hair and salivary cortisol concentrations were r = 0.61, p = 0.01.
More detailed examination of the cumulative nature of this relationship showed that the magnitude of associations with salivary cortisol AUCs steadily increased with more time. Using weekly average AUC scores, summed to reflect the accumulation of time, the magnitude of associations increases from the prior week (r = 0.38, p = 0.13), to the prior 2 weeks (r = 0.40 p = 0.11), to the prior 3 weeks (r = 0.50, p = 0.04), to the prior 4 weeks (r = 0.56, p = 0.02) to the whole 30-day period (r = 0.61, p = 0.01) as reported above.
To examine whether the HCC reflected more dynamic aspects of cortisol chronobiology, rather than cumulative cortisol production, analyses were conducted of HCC associations with the CAR and the diurnal slope. Table 1 shows no significant association between HCC and the monthly average CAR or diurnal slope, respectively. Associations between HCC and the CAR remained below significance when including data from the prior week (r = 0.17), prior 2 weeks (r = −0.01), prior 3 weeks (r = 0.30) and the prior 4 weeks (r = 0.27), ps > 0.20. Additional analyses, which included only samples those that adhered to a strict waking sample collection time (based on Stalder et al., 2016), did not improve the association between the CAR and HCC (N = 15: r = −0.11, p = 0.69). The magnitude of associations between HCC and salivary diurnal slopes remained below significance from the prior week (r = 0.25), prior 2 weeks (r = 0.33), the prior 3 weeks (r = 0.30) and the prior 4 weeks (r = 0.31), ps > 0.20.
Table 1.
Correspondence of Cortisol Measures
| HCC | UFC | |
|---|---|---|
| Month-long Salivary AUC | 0.61* | 0.06 |
| Diurnal Slope (Wake to Bed) | 0.35 | 0.13 |
| CAR | 0.37 | 0.45 |
| UFC | 0.27 |
Correlations between hair cortisol concentration (HCC) with urinary free cortisol (UFC) and salivary cortisol measures: diurnal slope, from wake to bed, and cortisol awakening response (CAR), from wake to 30 minutes later. Significance indicated with * p-value < 0.05
HCC was not significantly correlated with 1-month integrated UFC (r = 0.27, p = 0.31), and correlations between HCC and weekly UFC were also not significant when including data from the prior week (r = 0.06), prior 2 weeks (r = 0.26), prior 3 weeks (r = 0.26) and the prior 4 weeks (r = 0.30), ps > 0.20. UFC was unrelated to 30-day integrated salivary AUC cortisol concentrations (r = 0.06, p = 0.83) and the diurnal slope (r = 0.13, p = 0.64), but showed a statistical trend for a correlation with the CAR (r = 0.45, p = 0.08). However, analyses which only included CAR samples collected within a strict time window (Stalder et al., 2016), showed no relation between the CAR and UFC (N = 15: r = 0.03, p = 0.91).
Adjusting the analyses for hair treatment did not significantly change any of the results reported above. Analyses stratified by sex or by study location yielded similar associations between HCC, salivary AUC, and UFC for both men and women and for participants from the University of California at Irvine and the University of North Carolina at Chapel Hill, respectively.
3.2 Stability of Cortisol Measures Across the Study Period
HCC from the three analyzed hair segments (baseline, final 1st segment, and final 2nd segment) were strongly positively inter-correlated. The baseline and final 1st cm segment showed strong month-to-month stability (r = 0.84, p < 0.0001). The baseline and final 2nd cm indicated strong correspondence segment (r = 0.90, p < 0.0001), these hair segments are thought to reflect the same time period. The final 1st and 2nd segments were similar to associations observed between baseline and final 1st segments (r = 0.91, p < 0.0001), as expected. Weekly integrated salivary cortisol AUC values were moderately associated across consecutive weeks (week 1 and 2: r = 0.38, p = 0.13; week 2 and 3: r = 0.57, p = 0.02; week 3 and 4: r = 0.61, p = 0.01) with correlation coefficients being slightly lower when assessing associations of measures with more than a 1-week lag, converging on a modest longer-term stability. Week-to-week associations of UFC were strong (r’s between 0.57 and 0.67, p’s < 0.05) and did not decrease with greater lags in time.
4. Discussion
The present study evaluated the general assumption that HCC reflects integrated long-term cortisol concentrations by examining the associations with 1-month integrated daily salivary cortisol concentrations and with 1-month integrated weekly 24-hour UFC. Our results generally support this assumption, particularly showing that HCC was significantly correlated with integrated salivary cortisol concentrations measured repeatedly over the same 1-month period (r = 0.61, p = 0.01). Correspondence of HCC with the integrated CAR or diurnal slope of salivary cortisol was not significant, suggesting that HCC correspondence is not driven by chronobiological cortisol metrics. Associations between HCC and UFC concentrations did not reach statistical significance in our relatively small sample, despite the fact that both HCC and UFC showed high intra-individual stability over time. UFC was not significantly correlated with integrated salivary cortisol concentrations beyond a trend leve1.
The present results suggest that HCC was most strongly associated with the 1-month integrated salivary cortisol concentrations compared to weekly salivary AUC scores, which may explain the somewhat lower correlations reported between HCC and salivary cortisol in other studies that employed a saliva collection protocol with fewer samples that did not span a whole month like the current study (e.g., Steudte et al., 2011, Xie et al., 2011). The strength of the observed association between hair and integrated salivary cortisol was in line with the highest correspondence reported in a prior human study (r = 0.57; D’Anna-Hernandez et al., 2011) and also agrees with the majority of animal studies, which typically revealed medium to strong associations between HCC and mean cortisol levels in saliva (Davenport et al., 2006; Bennet and Hayssen, 2010) or fecal samples (Accorsi et al., 2008). The salivary AUC scores that integrate across increasingly longer durations (2- 3- and 4-week accumulations of time) show increasingly stronger correlations with HCC and are significant by 3-weeks of time. This pattern reflects a reduced correspondence with shorter lengths of time, which would be expected given that salivary cortisol levels exhibit only modest intra-individual stability, as shown by the present data and by earlier work (e.g., Kirschbaum et al., 1990; Shirtcliff et al., 2005). The implication is that saliva samples must be measured repeatedly (and potentially across several weeks) to reduce the impact of state variability and obtain reliable trait estimates of cortisol production. The high participant demands and financial costs associated with numerous repeated salivary cortisol assessments can unfortunately be prohibitive (Hellhammer et al., 2007). Hence, for researchers interested in obtaining measures of long-term cortisol production, HCC may provide a reliable, easily obtainable and economic option.
The current study was designed to examine HCC correspondence with an integrative measure of salivary cortisol using the AUC. This afforded the opportunity to determine whether correspondence was truly due to cumulative cortisol exposure, or whether it was driven by other chronobiological metrics of salivary cortisol. Both the CAR and the diurnal rhythm are distinct from total cortisol output, have unique heritability properties from each other and total cortisol levels, and have predictive value for mental and physical health problems (Wüst et al., 2000; Bartels et al., 2003). Nonetheless, it was possible that HCC would correspond well with the CAR given that the awakening response represents the fastest change in cortisol within a given day and is thought to reflect the flexibility or elasticity of the adrenal axis (Chida and Steptoe, 2009). However, in order to make more confident assertions with regard to the CAR, a more rigid methodological approach would have to be employed (Stalder et al., 2016). Similarly, HCC may have corresponded with the diurnal rhythm as this metric reflects the longest change in cortisol across the day and the diurnal rhythm is highly stable (Shirtcliff et al., 2012), much like HCC. We found no empirical support that HCC is driven by the chronobiological metrics, as associations were not statistically significant and were especially low at the week-to-week level. Instead, we support a broader literature that suggests distinct influences on overall cortisol secretion versus changes (Klimes-Dougan et al., 2001; van Eekelen et al., 2003; Fairchild et al., 2008). While we did not collect a measure of cortisol reactivity (Dickerson and Kemeny, 2004; Michaud et al., 2008) or momentary fluctuations within the day (Adam et al., 2006), we suspect that here too HCC would correspond best with an integrated total hormone exposure metric as reactivity and recovery serves a distinct (and temporary) metabolic and regulatory function from cortisol levels. Total cortisol, more frequently, reflects preparative or permissive functions (Sapolsky et al., 2000) rather than the momentary stimulatory reactive functions (Brooke et al., 1994; van Leeuwen et al., 2011).
Integrated UFC did not significantly correlate with HCC or with integrated salivary cortisol concentrations at either the monthly or weekly levels. This finding is consistent with prior literature which has also reported low correspondence between UFC and salivary cortisol concentrations (Blackburn et al., 1987; Kathol et al., 1995; Putignano et al., 2001; Yehuda et al., 2003). We consider four possible explanations. First, urine was sampled less frequently (weekly) than saliva (daily), but this methodological detail is not a viable explanation for the lack of observed associations with HCC or integrated salivary cortisol measures. Given the relatively high intra-individual stability of UFC, the captured trait component should have been sufficient to yield significant correlations with HCC or 1-month integrated salivary cortisol. Second, in the current study, salivary samples only reflect the cortisol production during the day whereas the 24hr urinary samples also capture nighttime cortisol production. This is unlikely to explain the relatively poor correspondence as HCC would also include nighttime production and thus should correspond better with urinary samples. Third, given the self-selection of UFC sampling days, it is possible that participants chose the least busy and potentially less stressful day of the week to collect their urine samples, which in turn could have contributed to the poor correspondence of UFC with HCC and integrated salivary cortisol concentrations. In this regard, both UFC and salivary cortisol measures largely miss momentary fluctuations in cortisol (i.e., cortisol reactivity), whereas the HCC presumably includes acute cortisol changes along with other influences on cortisol functioning, such as basal levels and chronobiological fluctuations. Fourth, interassay CVs for the UFC analyses were also somewhat larger than those of saliva and hair cortisol analyses, which may have also reduced chances of detecting significant associations between UFC and hair or saliva cortisol concentrations. Fifth, although each of the cortisol indices measured in saliva, urine and hair are thought to reflect the unbound, biologically-active fraction of cortisol, the estimates of unbound cortisol derived with UFC may still vary due to differences in localized metabolism that occurs when cortisol enters the respective fluids or hair. Regarding UFC, approximately 20% of cortisol in the blood is abstracted by the kidney during passage through the organ, however, only a very small fraction is excreted as free cortisol in urine, whereas a large part of the cortisol is converted to cortisone in the kidney by the enzyme 11β-HSD2 (Hellman et al., 1971). Common immunoassays that measure free cortisol in urine may be confounded by cross-reactivity with cortisone or other metabolites (Murphy, 2002). 11β-HSD2 is also present in the salivary glands and converts approximately half of the free cortisol diffusing from the blood stream to cortisone (Meulenberg and Hofman, 1990; Smith et al., 1996). Regarding HCC, the hair follicle and sebaceous gland do not express 11β-HSD2, but the sweat gland and skin arterioles do (Smith et al., 1996) and higher concentrations of cortisone than of cortisol have been found in hair (Raul, et al., 2004; Stalder et al., 2013). Besides direct incorporation of cortisol from the blood circulation, it is conceivable that some conversion, presumably via the sweat glands may take place. Regardless of the mechanism, UFC did not show convergence with the other two cortisol metrics which raises questions about UFC despite the advantage of high stability.
In line with the notion that HCC reflects long-term integrated cortisol levels, the present study revealed high month-to-month stability for HCC. This corroborates earlier data showing high stability of HCC assessments across time periods between two and twelve months suggesting that a single HCC assessment captures between 59% and 82% of trait-specific variance (Stalder et al., 2012). Interestingly, the present data also revealed relatively high associations between weekly UFC measures, which also concurs with previous findings showing relatively high day-to-day stability (Remer et al., 2008). This suggests that HCC and UFC are relatively stable measures of cortisol production, which may reflect trait-like cortisol production whereas the lower weekly salivary cortisol correlations suggest that salivary cortisol is more suitable to capture momentary fluctuations of cortisol concentrations (Doane et al., 2015). Due to a lack of power, it was not possible to directly characterize state vs. trait components of cortisol production captured by the various measures of cortisol in the current study. It would be interesting in future studies (with larger sample sizes) to assess whether the cortisol trait component obtained from repeated ambulatory measures of cortisol in saliva is more strongly associated with HCC than the state component.
The present study is subject to some limitations, which should be acknowledged. Concerning the assessment of salivary cortisol, it has been suggested that objective verification of participants’ awakening time (e.g., by wrist actigraphy) is useful to ensure the accuracy of post-awakening cortisol assessments (e.g., Kupper et al., 2005; Griefahn and Robens, 2011; Smyth et al., 2013; Stalder et al., 2016). This was not feasible in the present study and may thus have increased the measurement error of time-adjusted salivary cortisol concentrations. We did, however, use electronic monitoring devices and were able to objectively verify sampling accuracy with MEMS caps (e.g., Kudielka et al., 2003; Broderick et al., 2004). UFC assessments are known to be sensitive to participant non-compliance and it has thus been suggested that regular compliance checks should be performed, e.g., by using body weight-corrected reference values of 24-hour urinary creatinine excretion (Remer et al., 2008). Although creatinine values were not measured in the present study, the urine samples were collected over a 24-hour period and we were able to correct for total sample volume. The strong stability of UFC across sampling weeks suggests that participant compliance was not the main problem with UFC in the current study. Finally, because of the highly intensive study protocol, the small participant sample size limited our statistical power for detecting smaller effects as well as our ability to conduct more sophisticated analyses to examine other questions of interest (e.g., the degree of state vs. trait components captured with each of these cortisol sampling methods).
In conclusion, these findings support the contention that HCC provides a reliable estimate of long-term integrated free cortisol production that is aligned with integrated salivary cortisol production measured over a corresponding one-month period.
Highlights.
Hair and saliva cortisol concentrations share significant variance.
Hair cortisol concentration is highly stable over time.
Urinary free cortisol is not associated with hair or salivary cortisol.
Acknowledgments
Supported, in part, by US PHS (NIH) grants R01 MH-091351 (to C.B.) and R01 HD-060628 (to P.D.W.) NC TraCS NIH 2KR381203 and K01 MH099411 (to S.J.S) and Iowa State University incentive-funding (to E.A.S), and K01 DA039288 (to K.P.M).
Role of Funding Sources:
Each funding source provided funds that allowed for data acquisition and analysis. The funding sources did not however play any role in any of the analyses, interpretation, or description the findings reported herein.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to declare.
Contributors:
All authors listed on this manuscript made substantial intellectual contributions to the initial study design, interpretation of results and the final manuscript write up.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Gamberoni M, Tamanini C, Seren E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen and Comp Endocrin. 2008;155:398–402. doi: 10.1016/j.ygcen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behav Genet. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Beisel WR, Cos JJ, Horton R, Chao PH, Forsham PH. Physiology of urinary cortisol excretion. J Clin Endocrinol Metab. 1964;24:887–893. doi: 10.1210/jcem-24-9-887. [DOI] [PubMed] [Google Scholar]
- Bennett A, Hayssen V. Measuring cortisol in hair and saliva from dogs: Coat color and pigment differences. Domest Anim Endocrinol. 2010;39:171–180. doi: 10.1016/j.domaniend.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Blackburn IM, Whalley LJ, Christie JE, Shering A, Foggo M, Bennie J, Farrer D, Watts G, Wilson H, Fink G. Mood, cognition and cortisol: their temporal relationships during recovery from depressive illness. J Affect Disord. 1987;13:31–43. doi: 10.1016/0165-0327(87)90071-1. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents' cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–50. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Brooke SM, de Haas-Johnson AM, Kaplan JR, Sapolsky RM. Characterization of mineralocorticoid and glucocorticoid receptors in primate brain. Brain Res. 1994;637:303–307. doi: 10.1016/0006-8993(94)91249-1. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiol Behav. 2011;104:348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen and Comp Endocrin. 2006;146:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, Granger DA. Latent trait cortisol (LTC) levels: reliability, validity, and stability. Psychoneuroendocrinology. 2015;55:21–35. doi: 10.1016/j.psyneuen.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gao W, Kirschbaum C, Grass J, Stalder T. LC–MS based analysis of endogenous steroid hormones in human hair. J Steroid Biochem Mol Biol. doi: 10.1016/j.jsbmb.2015.12.022. in press. [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC–APCI–MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:1–8. doi: 10.1016/j.jchromb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Robens S. Cortisol awakening response - Are sampling delays of 15 minutes acceptable? Int J Psychophysiol. 2011;82:202–205. doi: 10.1016/j.ijpsycho.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hellman L, Nakada F, Zumoff B, Fukushima D, Bradlow HL, Gallagher TF. Renal capture and oxidation of cortisol in man. J Clin Endocrinol Metab. 1971;33:52–62. doi: 10.1210/jcem-33-1-52. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal (HPA) axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Kathol RG, Poland RE, Stokes PE, Wade S. Relationship of 24-hour urinary free cortisol to 4-hour salivary morning and afternoon cortisol and cortisone as measured by a time- integrated oral diffusion sink. J Endocrin Invest. 1995;18:374–377. doi: 10.1007/BF03347841. [DOI] [PubMed] [Google Scholar]
- Kertes DA, van Dulmen M. Latent state trait modeling of children's cortisol at two points of the diurnal cycle. Psychoneuroendocrinology. 2012;37:249–255. doi: 10.1016/j.psyneuen.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, Hellhammer DH. Cortisol and behavior: Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology. 1990;15:297–307. doi: 10.1016/0306-4530(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kupper N, De Geus EJC, Van Den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, Pruessner J, McEwen BS. Beyond the stress concept: Allostatic Load--A developmental biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. Vol. 2. Hoboken, NJ: John Wiley & Sons; 2006. pp. 578–628. [Google Scholar]
- Meulenberg PMM, Hofman JA. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clin Chem. 1990;36:70–75. [PubMed] [Google Scholar]
- Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress. 2008;11:177–197. doi: 10.1080/10253890701727874. [DOI] [PubMed] [Google Scholar]
- Murphy BEP. Lack of specificity of urinary free cortisol determinations: why does it continue? J Clin Endocrinol Metab. 1999;6:2258–2259. doi: 10.1210/jcem.84.6.5809-2. [DOI] [PubMed] [Google Scholar]
- Murphy BEP. Urinary free cortisol determinations: what they measure. The Endocrinologist. 2002;12:143–150. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, … Cavagnini F. Salivary cortisol measurement in normal-weight, obese and anorexic women: Comparison with plasma cortisol. Eur J Endocrinol. 2001;145:165–171. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37:1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Remer T, Maser-Gluth C, Wudy SA. Glucocorticoid measurements in health and disease – metabolic implications and the potential of 24-h urine analyses. Mini Rev Med Chem. 2008;8:153–170. doi: 10.2174/138955708783498096. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30:E183–191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012;37:611–617. doi: 10.1016/j.psyneuen.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Smith RE, Maguire JA, Stein-Oakley AN, Sasano H, Takahashi K, Fukushima K, Krozowski ZS. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues. J Clin Endocrinol Metab. 1996;81:3244–3248. doi: 10.1210/jcem.81.9.8784076. [DOI] [PubMed] [Google Scholar]
- Smyth N, Clow A, Thorn L, Hucklebridge F, Evans P. Delays of 5–15min between awakening and the start of saliva sampling matter in assessment of the cortisol awakening response. Psychoneuroendocrinology. 2013;38:1476–1483. doi: 10.1016/j.psyneuen.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C. Analysis of cortisol in hair – State of the art and future directions. Brain Behav Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, Stark S, Bosch JA, Fischer JE. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:2573–2580. doi: 10.1210/jc.2013-1056. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Bio Psychol. 2010;85:357–360. doi: 10.1016/j.biopsycho.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum, Kudielka BM, Adam EK, Pruessner JC, Wust, StDockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien S, Clow A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability in hair cortisol concentrations. Psychoneuroendocrinology. 2012;37:602–610. doi: 10.1016/j.psyneuen.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BWJH, Spijker AT, Elzinga BM, van Rossum EFC. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology. 2013;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011;186:310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, van Uum SHM. Hair Analysis provides a historical record of cortisol levels in cushing’s syndrome. Exp Clin Endocrinol Diabetes. 2010;118:133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen AP, Kerkhof GA, van Amsterdam JG. Circadian variation in cortisol reactivity to an acute stressor. Chronobiol Int. 2003;20:863–878. doi: 10.1081/cbi-120024212. [DOI] [PubMed] [Google Scholar]
- van Holland BJ, Frings-Dresen MHW, Sluiter JK. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. Int Arch Occup Environ Health. 2012;85:849–52. doi: 10.1007/s00420-011-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Horm Behav. 2012;62:36–42. doi: 10.1016/j.yhbeh.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen N, Bellingrath S, de Kloet ER, Zitman FG, DeRijk RH, Kudielka BM, Wüst S. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology. 2011;36:699–709. doi: 10.1016/j.psyneuen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Intl. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, Lu Z. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clin Chem Lab Med. 2011;49:2013–2019. doi: 10.1515/CCLM.2011.706. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Yang RK, Guo LS, Makotkine I, Singh B, Pickholtz D. Relationship between 24-hour urinary-free cortisol excretion and salivary cortisol levels sampled from awakening to bedtime in healthy subjects. Life Sci. 2003;73:349–358. doi: 10.1016/s0024-3205(03)00286-8. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]