Abstract
Variation within the serotonin transporter gene-linked polymorphic region (5-HTTLPR) contributes to individual differences in trait neuroticism and increases risk for the development of psychopathology in the context of stressful life events. The underlying mechanisms may involve dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and the release of stress-related hormones. Yet, observed effects are small, possibly because they occur against the background of many other, mostly unknown, genetic and environmental variables. In this study, we removed much of the variance contributed by such background factors by including complex trait and behavioral measures in our analyses, to isolate the unique contributions of 5-HTTLPR genotype to cortisol baseline, reactivity, and recovery during the Trier Social Stress Test. We recruited 82 community-dwelling older adults (55 and older), an under-studied population, and measured salivary cortisol levels at baseline and following the TSST. As a comparison group we also recruited 88 younger adults (males only, 18–51 years old). Neuroticism, trait anxiety, perceived stress levels, and early childhood trauma experiences were measured using self-report questionnaires. An exploratory factor analysis revealed a latent anxiety trait. Cortisol baseline levels were significantly elevated in older adult S-allele carriers (but not in LL-homozygotes) who scored higher on the latent anxiety trait, relative to S-allele carriers. No such differences were found among younger adults, nor amongst measures obtained during the reactivity or recovery periods. These results highlight the utility of taking into account background variables that may otherwise obscure associations between genetic variables and endophenotypes.
Keywords: serotonin, cortisol, HPA axis, neuroticism, anxiety, stress
1. Introduction
One of the seminal foundations of “molecular psychology” (Canli, 2015) was the discovery of a link between a common variation within the serotonin transporter gene (SCL6A4), the serotonin-transporter-linked polymorphic region (5-HTTLPR), and complex personality traits (Lesch et al., 1996). Specifically, the 5-HTTLPR contains a 43 BP insertion/deletion polymorphism in which the short (“S”) allele is less transcribed than the long (“L”) allele (Heils et al., 1996), and it is the S-allele that is associated with elevated trait neuroticism (Lesch et al., 1996). Later work identified an independent single nucleotide A/G polymorphism (SNP; rs25531); L-alleles that also carry the G-allele (denoted by “LG”) are considered functionally equivalent to the S-allele (Hu et al., 2006; Wendland et al., 2006). An entire literature of replication and extension studies has since emerged based on these findings, which have been confirmed in multiple (Munafo et al., 2009b; Schinka et al., 2004; Sen et al., 2004) but not all (Munafo et al., 2005) meta-analyses.
Later work investigated the moderating effects of 5-HTTLPR genotype on the link between stressful life events and psychopathology, starting with the discovery from a large-scale longitudinal study that presence of the S-allele, in conjunction with stressful life events, predicted depressive symptoms (Caspi et al., 2003). Similarly, participants who were homozygous for the S-allele and had at least one traumatic life event exhibited a 6.7 fold increase in the odds of Major Depressive Disorder (MDD), compared to the 2.1 fold increase in those with at least one traumatic life event and the S/L or L/L allelic type (Kendler et al., 2005). These gene-by-environment (GxE) interactions appear to extend beyond depression, as other studies showed similar relationships in dissociative disorders (Pieper et al., 2011), post-traumatic stress disorder (Pietrzak et al., 2013), and a broad set of psychopathologies (Vinberg et al., 2014). These GxE interactions have again been supported in multiple (Karg et al., 2011; Uher and McGuffin, 2008, 2010; van Ijzendoorn et al., 2012) but not all (Munafo et al., 2009a; Risch et al., 2009) reviews and meta-analyses.
One plausible pathway by which 5-HTTLPR genotype may impose a stress-related toll is via the hypothalamic-pituitary-adrenal (HPA) axis and the release of stress-related hormones that can place an allostatic load on the body (McEwen, 1998). Indeed, several studies reported differential cortisol baseline or reactivity levels as a function of 5-HTTLPR genotype. For example, girls exposed to an acute laboratory stressor (backwards counting, followed by a social competency interview) who were homozygous for the S-allele exhibited significantly stronger cortisol reactivity, compared to girls who carried one or two copies of the L-allele (Gotlib et al., 2008). Differential levels of the cortisol awakening response as a function of 5-HTTLPR genotype have also been reported (Wust et al., 2009). A recent meta-analysis of the literature concluded that there was a small but significant association between 5-HTTLPR genotype and HPA-axis reactivity to acute psychosocial stress (Miller et al., 2013). However, this analysis also highlighted the need for additional studies, particularly in understudied populations such as older subjects, for which only one publication was available (Mueller et al., 2011).
In this study, we examined baseline cortisol and cortisol reactivity/recovery to the Trier Social Stress Test (Kirschbaum et al., 1993) in two samples: a cohort of older (age 55 and over) adults, and a cohort of younger adults (18–51 years old). Based on an S-allele dominant model, we aimed to test whether presence of the S-allele (based on the functionally defined triallelic classification scheme: S, LA, LG) predicted baseline cortisol or cortisol response to an acute stressor, and whether the impact of 5-HTTLPR genotype on cortisol was further moderated by early life stress, or by trait-related or chronic levels of negative affect.
2. Materials and Methods
2.1 Participants
From an initial cohort of 94 adults aged 55 and older who were recruited from Stony Brook and the surrounding area via flyers and online postings, data from 82 older adults (Mage = 63.46, SDage=7.15; 46 women) were used in this study (data from 12 older adults were not used because they were run in the morning hours when cortisol levels are highly variable). Data from a subset individuals were previously reported in the context of a study on 5-HTTLPR gene methylation (Duman and Canli, 2015). The ethnic distribution included Caucasian (91.5 %), Asian (3.7%), Hispanic (3.7%), and African American (1.2%). A second cohort of 88 younger male adults was also recruited (Mage= 24.07, SDage= 7.74). This group was almost exclusively Caucasian with only 1.1% Asian and 3.4% identifying with multiple races, and all subjects were run midday (1100h–1500h) or in the evening (1500h–1900h). Upon calling in, potential participants were informed that during the 4-hour experiment they would provide blood and saliva samples and complete questionnaires, a life history interview and a mild stress task. Exclusion criteria included: being a smoker, experiencing substance or alcohol abuse, having a psychiatric diagnosis, taking psychiatric or hormonal medication, being diabetic, taking medication for cardiovascular disease, and currently being under immense stress. Additionally, all female subjects were post-menopausal; this condition addresses potential concerns regarding the effects of menstrual cycle status on cortisol measurements (Kirschbaum et al., 1999). The Committee on Research Involving Human Subjects (CORIHS) of Stony Brook University approved this research.
2.2 Measures
2.2.1 Questionnaires
Participants completed a number of questionnaires to assess for personality, childhood trauma and stress. We measured Neuroticism as a subscale of the NEO-FFI (Costa and McCrae, 1992). Sample questions included items such as I often feel inferior to others and I am not a worrier (reverse coded). Questions were answered on a 5 point Likert scale from "strongly disagree" to "strongly agree." Scores were then scaled into T scores based on normative samples in accordance with the professional manual to make them comparable across men and women (Costa and McCrae, 1992). To assess trait anxiety, participants answered the State Trait Anxiety Inventory (STAI; Spielberger, 1983) about how much they generally agree with 20 statements. Example statements include I am happy and I feel secure, and were assessed on a 4 point Likert scale ranging from "almost never" to "almost always." Early life stress was measured using the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994). The sum score of this questionnaire considers emotional, sexual and physical maltreatment. Questions include items such as I didn't have enough to eat and I felt someone in my family hated me. The 5 point Likert scale offered options from "never true" to "very often true."
Stress was assessed with two measures. One was the Perceived Stress Scale (PSS; Cohen et al., 1983), using 10 items to assess feelings on stress levels over the past month. It includes items such as in the last month, how often have you found that you could not cope with all the things that you had to do? , and, in the last month, how often have you been able to control irritations in your life? Participants answered using a 5 point Likert scale ranging from "never" to "very often." The second measure was the Trier Inventory for Chronic Stress Scale (TICS-CSSS; Schulz and Schlotz, 1999) and participants rated the frequency of various stressful experiences over the past 3 months from "never" to "very often." Items included I worry that something unpleasant will happen and I experience having to do too much.
2.2.2 5-HTTLPR and rs25531 Genotyping
Participants provided one blood sample. Peripheral blood mononuclear cells were extracted and stored in a −80°C freezer until analysis. We investigated a common length polymorphism due to an insertion/deletion (indel) in the promoter region of the 5-HTTLPR gene. We independently also genotyped for a second A/G Single Nucleotide Polymorphism (SNP) rs25531 (Wendland et al., 2006). Because there were no 5-HTTLPR short-allele carriers who also carried the rs25531 G-allele, we treated the sample as functionally tri-allelic (SA, LA or LG), with LG treated as functionally equivalent to S.
5-HTTLPR classification was determined through amplification by the polymerase chain reaction (PCR) with the following primers: LPR_L 5’-GGGGAGATCCTGGGAGAGGT-3’, LPR_R 5’-CGCTCTGAATGCCAGCACCTA-3’ and HotStarTaq Plus Master Mix Kit (Qiagen). These primers help to generate DNA fragments of 215 bp (S-allele) and 258 bp (L allele). PCR was carried out with 500 nM of each, forward and reverse primers and 20 ng of template in a 20 μl total reaction volume in Mastercycler PCR device (Eppendorf, Germany). The cycling conditions included heat activation at 95°C for 4 min, 41 cycles of 95°C for 20 sec, 68°C for 20 sec, and 72°C for 20 sec, followed by a final 2 min extension at 72°C and hold at 4°C. PCR products were run in 2% agarose gels stained with ethidium bromide and visualized using an InGenius gel documentation system (Syngene). Next, A/G SNP rs25531 status was determined, 7 μl of the 5-HTTLPRLPR PCR products were digested with 8 Units of MspI restriction enzyme (New England Biolabs, MA) for 2 hours at 37°C followed by an inactivation step of 20 min at 65°C. Digestion products were run in agarose gels. All genotyping work was done at the Genomics Core facility at Stony Brook University.
2.2.3 Cortisol Quantification
A total of 10 Salivettes® (Sarstedt, Germany) were collected: upon arrival, 2 minutes before the TSST, and then +2, +10, +20, +30, +45, +60, +90 and +105 minutes post stressor. Participants were instructed to chew on the cotton swab for about 1 minute. They then returned the cotton into a plastic tube. Salivettes were then frozen in a −20°C freezer until cortisol concentrations were measured in a contract laboratory (Rohleder, Brandeis University, Waltham, MA). Salivary cortisol concentrations were measured using a commercially available chemiluminescence immunoassay (CLIA; RE62019; IBL International, Toronto, Canada). Inter-assay variability was 3.3%, and intra-assay variability was 3.4%.
2.2.4 Stress Task
Participants completed the Trier Social Stress Task (TSST; Kirschbaum et al., 1993), a standard psychosocial stressor. In brief, participants were given 5 minutes to prepare notes for a speech on why they are qualified for their dream job. They were not permitted to use these notes during the task where they gave their 5-min speech to a panel of 2 unresponsive judges in white laboratory coats. Following the speech task, participants completed a 5-min mental arithmetic in front of the same panel.
2.3 Procedure
Upon arrival participants read and signed a consent form. Next, they provided their arrival saliva sample, followed by a blood draw conducted by a trained phlebotomist. Participants then completed questionnaires until they provided a baseline saliva sample taken between 11:00 and 19:00 (average time between blood draw and baseline saliva sample was 52 min for the older cohort and 47 minutes for the younger cohort) and were taken up to the TSST room. After the TSST, a structured life interview, more questionnaires and the remaining 8 saliva samples were administered at regular intervals. The study concluded with a debriefing and subject payment of $100 for their time.
2.4 Variable Assessment
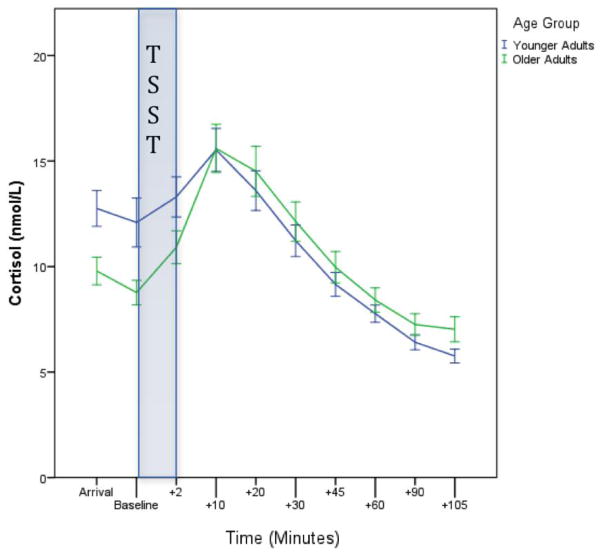
Data organization and statistics were done using SPSS v. 22 (Armonk, NY) and Mplus v. 7.3 (Muthén and Muthén, 1998–2012). Cortisol was assessed at baseline, in reaction to the acute stressor, and in recovery to the stressor. Baseline was the saliva sample taken directly before the TSST. Our samples had a strong positive skew and therefore were log transformed. Raw cortisol values are shown for each cohort in Figure 1†.
Figure 1.
Total Cortisol Values Over Time. Comparison of cortisol baseline measures and response to the TSST as a function of age groups.
In testing the correlations between our independent variables, experiencing early life stress, perception of current and chronic stress, neuroticism and trait anxiety across both cohorts, we found that they were generally significantly correlated (see Tables 1a and 1b).
Table 1.
Tables 1a and 1b Correlations Between Phenotypic and Early Life Stress Scores
| 1a Older Adults | |||||
|---|---|---|---|---|---|
| CTQ | TICS | Neuroticism | PSS | STAI | |
| CTQ | — | ||||
| TICS | 0.055† | --- | |||
| Neuroticism | 0.19*,† | 0.53*** | --- | ||
| PSS | 0.16† | 0.7*** | 0.67*** | --- | |
| STAI | 0.15† | 0.71*** | 0.76*** | 0.75*** | --- |
| 1b Younger Adults | |||||
|---|---|---|---|---|---|
| CTQ | TICS | Neuroticism | PSS | STAI | |
| CTQ | --- | ||||
| TICS | 0.23**,† | --- | |||
| Neuroticism | 0.21**,† | 0.64*** | --- | ||
| PSS | 0.17*,† | 0.72*** | 0.7*** | --- | |
| STAI | 0.23**,† | 0.68*** | 0.78*** | 0.71*** | --- |
<0.05
<0.01
<0.001
Kendall's tau correlations due to non-normal distribution of CTQ; CTQ = Childhood Trauma Questionnaire, TICS = Trier Inventory for Chronic Stress, Neuroticism = Neuroticism Subscale of the NEO-FFI, PSS = Perceived Stress Scale, STAI = State Trait Anxiety Inventory.
Given the highly overlapping nature of these anxiety-related personality variables, an exploratory factor analysis (EFA) was conducted on the trait neuroticism, trait anxiety, chronic stress and perceived stress scores. A scree plot and parallel analysis revealed that a one-factor solution was a best fit with an additional unique correlation between the residuals of the Perceived Stress Scale and the Trier Inventory for Chronic Stress (RMSEA = 0.043, CFI/TLI=0.999/0.995, Χ2 (1)= 1.32, p = 0.25). These fit indices indicate a good fit (Hu and Bentler, 1999). Factor scores were extracted to use in subsequent analyses. The factor determinacy coefficient was 0.954, indicating the scores were highly correlated to the latent factor as they were above the traditional cut off of 0.8 (Gorsuch, 1983). We used a piecewise growth model (Muthén and Muthén, 1998–2012), with the latent factor for intercept representing baseline (i.e., pre-TSST) cortisol and two slope factors representing cortisol reactivity (i.e., change from pre-TSST to TSST+10m) and cortisol recovery (i.e., change from TSST+10m to TSST+105m). Individually-varying times of observation were used to account for any deviations from standardized sampling times. If a time between 2 samples was not recorded, the mean time for that sample within the group was used. Latent factors for intercept, reactivity slope, and recovery slope were regressed on the predictors of interest: S-allele, latent trait anxiety or childhood trauma and an interaction term between genetics and phenotype/life experience.
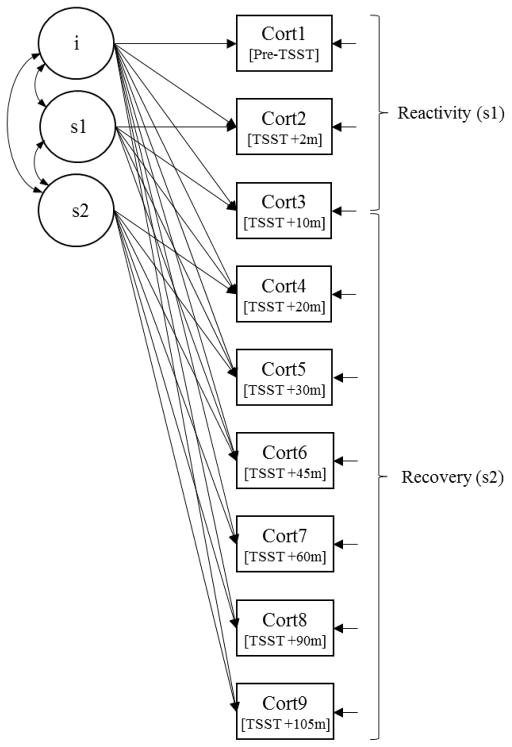
Given that cortisol may be affected by time of day (Weitzman et al., 1971), our participants were grouped based on the time of their baseline cortisol sample (Older adults: 49 participants "Midday": 1100h–1500h; 33 participants "Evening": 1500h–1900h; Younger adults: 70 participants "Midday" and 18 participants in the "Evening"). Age (in years), sex (female), and time of day (evening) were included as covariates in the piecewise growth models. All continuous independent variables were transformed into standardized scores to ensure interpretability of the coefficients. An overview of the model is shown in Figure 2.
Figure 2.
Caption. Piecewise growth model. Growth model illustrates the intercept (baseline) at Cort1, the reactivity slope (Cort1 through Cort3), and the recovery slope (Cort3 through Cort9); i = intercept, s1 = reactivity slope, s2 = recovery slope.
3. Results
3.1 Sample Characteristics
In our older adult sample, 23.2% (19 participants) were true LL (LA LA), 47.6% (39 participants) were classified as SL or LLG, and 29.3% (24 participants) were SS, SLG or LGLG; corresponding allele frequencies were: LA = 46.95%; LG=8.54%; S=44.51%. This distribution did not significantly differ from the Hardy-Weinberg Equilibrium (χ2 (3) = 6.98, p = 0.073). In the younger adult sample 35.2% (31 participants) were true L/L, 46.6% (41 participants) were classified as S/L or L/LG, and 18.2% (16 participants) were S/S, S/LG or LG/LG; corresponding allele frequencies were: LA = 58.52%; LG =2.84%;S=38.64%. Similarly, this distribution also did not significantly differ from the Hardy-Weinberg Equilibrium (χ2 (3) = 1.00, p = 0.80). Samples did not differ on anxiety scores (t(168)=1.27, p = 0.21); however, older adults reported significantly higher childhood trauma scores (M = 38.67, SD = 15.32) than did younger adults (M=33.92, SD = 8.9; t(111.16)= −2.31, p =0.023); full table of means, standard deviations and differences between older and younger adults trait and environmental factors can be found in Table 2. Furthermore, the older adult sample included men and women, whereas the younger sample included only men. Given this age-gender confound, we analyzed data for the older and younger cohorts in separate regression models.
Table 2.
Differences Amongst Scores for Older and Younger Adults
| Older Adults | Younger Adults | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | t value | p value | |
| CTQ | 38.67 | 15.32 | 72 | 33.92 | 8.9 | 83 | −2.31 | 0.023* |
| TICS | 14.1 | 8.39 | 80 | 18.6 | 9.38 | 88 | 3.27 | 0.001** |
| Neuroticism | 45.33 | 10.27 | 82 | 47.41 | 11.39 | 88 | 1.25 | 0.21 |
| PSS | 13.9 | 7.25 | 80 | 14.56 | 7.2 | 87 | 0.59 | 0.55 |
| STAI | 35.13 | 10.1 | 78 | 36.7 | 9.37 | 83 | 1.02 | 0.31 |
| † Latent Anxiety | −0.096 | 0.96 | 82 | 0.089 | 0.95 | 88 | 1.27 | 0.21 |
<0.05
<0.01
Latent Anxiety scores calculated by EFA including TICS, Neuroticism, PSS and STAI scores; CTQ = Childhood Trauma Questionnaire, TICS = Trier Inventory for Chronic Stress, Neuroticism = Neuroticism Subscale of the NEO-FFI, PSS = Perceived Stress Scale, STAI = State Trait Anxiety Inventory.
3.3 Unconditional Model
We tested the assumption that the TSST induced a stress response and subsequent recovery. Older adults exhibited a positive slope during the reactivity phase post-stressor (M= 0.006, p <0.001) and a negative slope in the recovery phase (M= −0.004, p <0.001). Younger adults exhibited a similar and significant pattern.
3.4 Underlying Relationship between Genotype and Phenotype
Next, we examined whether presence of the S-allele predicted the latent anxiety score. We conducted ordinary least squared (OLS) regressions accounting for the effects of age and sex and found that presence of S-allele did not significantly predict anxiety in either older adults (p = 0.26) or, when accounting for age, in younger adults (p = 0.41).
3.5 Genotype-by-Phenotype Interaction Predicting Cortisol
We then conducted piecewise growth modeling assessing whether there was a significant effect of presence of the S-allele controlling for age, sex, and time of day. There was no significant effect in the older adult group. Next, we considered the interaction between presence of S-allele and latent anxiety or childhood trauma‡. In older adults, the interaction term between S-allele presence and childhood trauma was non-significant; however, presence of S-allele interacted with anxiety interaction to predict higher levels of baseline cortisol (p = 0.032). Thus, older adults with an S-allele and higher anxiety scores exhibited higher baseline cortisol levels. This interaction was not seen in younger adults. Full regression coefficients for the S-allele x Anxiety model in older and younger adults provided in Tables 3a and 3b.
Table 3.
Tables 3a and 3b Presence of S-allele x Latent Trait Anxiety Score Model
| 3a Younger Adults | |||
|---|---|---|---|
| B weight | S.E. | P Value | |
| Baseline (Intercept) | |||
| Age | −0.021 | 0.028 | 0.46 |
| Time of Day | −0.094 | 0.084 | 0.26 |
| S allele | −0.01 | 0.065 | 0.88 |
| Anxiety | −0.036 | 0.082 | 0.66 |
| S allele x Anxiety | 0.03 | 0.088 | 0.73 |
| Reactivity | |||
| Age | 0.002 | 0.001 | 0.014* |
| Time of Day | −0.002 | 0.003 | 0.58 |
| S allele | <0.001 | 0.002 | 0.92 |
| Anxiety | 0.001 | 0.002 | 0.43 |
| S allele x Anxiety | −0.001 | 0.002 | 0.46 |
| Recovery | |||
| Age | −0.001 | <0.001 | 0.023* |
| Time of Day | 0.002 | 0.001 | 0.094 |
| S allele | −0.001 | 0.001 | 0.15 |
| Anxiety | <0.001 | <0.001 | 0.7 |
| S allele x Anxiety | <0.001 | 0.001 | 0.69 |
| 3b Older Adults | |||
|---|---|---|---|
| B weight | S.E. | P Value | |
| Baseline (Intercept) | |||
| Age | 0.041 | 0.023 | 0.074 |
| Time of Day | −0.128 | 0.055 | 0.021* |
| Sex | −0.125 | 0.052 | 0.016* |
| S allele | −0.022 | 0.062 | 0.73 |
| Anxiety | −0.071 | 0.062 | 0.25 |
| S allele x Anxiety | 0.144 | 0.067 | 0.032* |
| Reactivity | |||
| Age | 0.001 | 0.001 | 0.54 |
| Time of Day | 0.001 | 0.002 | 0.72 |
| Sex | −0.002 | 0.002 | 0.2 |
| S allele | −0.001 | 0.002 | 0.64 |
| Anxiety | 0.002 | 0.003 | 0.41 |
| S allele x Anxiety | −0.004 | 0.003 | 0.17 |
| Recovery | |||
| Age | <0.001 | <0.001 | 0.13 |
| Time of Day | −0.001 | 0.001 | 0.056 |
| Sex | 0.002 | 0.001 | 0.001** |
| S allele | <0.001 | 0.001 | 0.77 |
| Anxiety | <0.001 | <0.001 | 0.64 |
| S allele x Anxiety | 0.001 | 0.001 | 0.13 |
<0.05
<0.01
3.6 Gender Effects in the Older Adult Sample
Women displayed lower baseline cortisol levels than men (B = −0.125, p = 0.016) and a weaker recovery slope (B = 0.002, p = 0.001) in the S-allele by anxiety model. However, men and women did not exhibit different reactivity slopes (p = 0.2).
4. Discussion
In this study, we examined social stress reactivity as a function of 5-HTTLPR genotype in a population that has received scant attention in the literature, older healthy adults. For comparison, we also examined the same question in a healthy younger adult sample. We were particularly interested in two aspects of their physiological response to a laboratory-based social stressor: first, whether presence of the S-allele (based on the triallelic classification scheme: S, LA, LG) predicted baseline cortisol or cortisol response to an acute stressor; second, whether the impact of 5-HTTLPR genotype on cortisol would be amplified by early life stress, or by latent trait anxiety.
We found that 5-HTTLPR genotype did not predict latent trait anxiety in the older or younger groups and that 5-HTTLPR genotype did not predict cortisol baseline in older adults, when we controlled for age, sex, and time of day. This finding is consistent with a meta-analysis which also found no evidence for a main effect of genotype on basal cortisol (Miller et al., 2013). However, we also found no significant effect of 5-HTTLPR genotype per se on cortisol response to, or recovery from, a social stressor. This result stands in contrast to the only other study (Mueller et al., 2011), which examined a cohort of comparable size (N = 99) and age (M = 61.1) as ours. In that study, LL-homozygotes showed an elevated cortisol response to the social stressor, relative to S-allele carriers. However, both studies may have been under-powered to make a definitive claim for or against a main effect of 5-HTTLPR genotype, because the estimated effect size of 5-HTTLPR genotype on HPA-reactivity is small (0.27) (Miller et al., 2013). Thus, significantly larger studies need to be conducted in the future to address what role, if any, 5-HTTLPR genotype plays in the social stress response and how this response may change over time when accounting for gene-by-environment or gene-by-phenotype interactions.
Lest for reporting only null results, we made some intriguing and statistically significant observations. First, we observed that cortisol baseline levels were significantly elevated in older adult S-allele carriers (but not LL-homozygotes) who scored higher on a latent measure of anxiety, relative to S-allele carriers who scored lower. These results resonate with the conclusion drawn by Miller et al. (2013) that the association between 5-HTTLPR genotype and HPA-axis reactivity may be strengthened against the background of a stressful environment. Our study suggests that such a “stressful environment” may include the perception of stress. Indeed, other work has shown that the perception of psychosocial stress, more than psychosocial stress per se, accelerates biological processes, particularly cellular aging as measured by telomere shortening (Epel et al., 2004). We saw this effect in older but not younger adults, although the two groups did not significantly differ on trait anxiety scores. This suggests that a cumulative effect of genotype and phenotype may accrue over time to affect physiology.
Secondly, we observed sex differences in the older cohort. Specifically, we found that women exhibited lower baseline cortisol levels and poorer recovery slopes than did age-matched men following an acute stressor, even after accounting for genetic and phenotypic confounds. Surprisingly, there are no other studies we are aware of comparing TSST responsiveness between postmenopausal women and age-matched men. Two TSST studies, using samples of 15 subjects or fewer per cell, have compared postmenopausal women without hormone replacement to postmenopausal women on hormone treatment or to premenopausal women. Neither study observed significant differences in TSST response (Kudielka et al., 1999; Newhouse et al., 2008), although one study reported lower baseline cortisol in placebo-treated compared to hormone-treated postmenopausal women (Newhouse et al., 2008). The small number of participants and studies conducted to date highlights the need for greater focus on this age cohort in future work.
Several studies have examined the interaction between early life stress, particularly childhood trauma, on cortisol. We did not observe such an interaction amongst older adults, consistent with another report (O'Hara et al., 2007). Likewise, Mueller et al. (2011) saw no significant genotype by early life stress interaction in the older cohort; however, this interaction was seen in the young adult cohort. Alexander et al. (2009) reported that presence of an S-allele, along with a history of high levels of traumatic life events, was associated with cortisol reactivity to an acute stressor in a younger adult male only cohort. The discrepancy between our results and these studies most likely reflects low endorsement frequencies of high trauma in our cohort, as well as the method that has been used for assessing life stress.
Differences in findings may be attributable to other reasons as well. For instance, we chose to analyze 5-HTTLPR as was proposed in an initial study examining neuroticism and anxiety (Lesch, et al., 1996). However, various meta-analyses and studies have argued for different groupings, presenting conflicting suggestions. For instance, Miller et al. (2013) discovered that a recessive model (S/S v. [S/L or L/L]) fits the available evidence better than a dominant model ([S/S or S/L] vs. L/L]). Our dataset had a small number of S/S (defined as well by S/LG or LG/LG) carriers, only 16 participants in the younger cohort and 24 in the older cohort, inhibiting our ability to investigate this issue further. Other research has suggested that simply grouping by S and L variants is too coarse, as there may be as many as 14 novel allelic types and that phenotypic differences may only be adequately seen if more precise classification of 5-HTTLPR is used (Nakamura et al., 2000). Thus, future studies should examine the complete set of polymorphic variants within the SCL6A4 gene, which will require much larger samples than had previously been studied.
Our study had several strengths. First, we compared the role of 5-HTTLPR genotype in two age cohorts of older and younger participants. One longitudinal study suggested that environmental effects are stronger in younger adulthood, whereas genetic variables are stronger in older adulthood (Tucker-Drob and Briley, 2014). Thus, the recruitment of older and younger adults of similar phenotypic and 5-HTTLPR genotype distributions may afford us a better opportunity to observe genetic contributions of 5-HTTLPR genotype over time, albeit in a cross-sectional design. Second, by using the TSST paradigm we captured three different measures of cortisol (baseline, reactivity and recovery), providing more information about potential mechanisms by which 5-HTTLPR and behavioral phenotypes may moderate stress physiology. Lastly, we considered a gene-by-phenotype interaction on the endocrine system. This model presents a novel view incorporating genetics, psychology and physiology.
We acknowledge that there are also some limitations and new avenues to explore. Research has suggested that there may be ethnic differences in the proportions of those with S/S, S/L and L/L genotypes (Taylor et al., 2006). While our sample was not completely Caucasian, the low ethnic minority representation prevented us from teasing apart any differences contributed by ethnicity. Additionally, we asked participants to report on experiences, such as childhood maltreatment, in a post-hoc manner. As with all self-report measures of this nature, responses are subject to biases and false memory. A longitudinal study design with concurrent, and objectively verifiable measures of life stress, would be ideal for studies of gene-by-environment interactions. Lastly, our younger cohort was all male while our older cohort was mixed sex. This prevented us from directly studying a three-way interaction (Age X S-allele X Latent Trait Anxiety) in our model.
5. Conclusions
The contributions of individual candidate gene polymorphisms (such as 5-HTTLPR) to endophenotypes (such as cortisol) compete for our attention against the background of many other, mostly unknown, genetic and environmental variables. By including complex trait and behavioral measures in our analyses, we removed much of the variance contributed by such background factors to reveal contributions of 5-HTTLPR genotype to cortisol baseline in a cohort of older adults. This approach may be fruitful for other studies that examine gene-environment interactions in complex behavioral phenotypes.
Supplementary Material
Highlights.
82 older (ages 55+) and 88 younger (ages 18–51) adults participated in the Trier Social Stress Test.
Participants were tested for serotonin transporter gene-linked polymorphic region (5-HTTLPR) genotype.
Cortisol baseline was significantly elevated in older adult S-allele carriers who scored higher on the latent anxiety trait.
Older women displayed lower baseline cortisol levels than men and a weaker recovery slope in the S-allele by anxiety model.
Acknowledgments
Role of the funding source
This work was funded by the National Institute on Aging (National Institutes of Health) 1 R01 AG034578-01 and NSF BCS-0843346 awarded to TC.
We thank the many former members of the Canli Lab for their help in running the experimental sessions, data entry, and genotyping.
Footnotes
Because the older cohort was comprised of both males and females, whereas the younger cohort was comprised of males only, we also conducted an additional analysis on older and younger males only (see supplementary materials).
The models examining presence of S-allele and presence of S-allele x childhood trauma in younger adults did not converge and were therefore not included.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Canli T. The Oxford Handbook of Molecular Psychology. Oxford University Press; New York, Oxford: 2015. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO personality inventory (NEO PI-R) and NEP five-factor inventory (NEO-FFI): professional manual. Psychological Assessment Resources 1992 [Google Scholar]
- Duman EA, Canli T. Influence of life stress, 5-HTTLPR genotype, and SLC6A4 methylation on gene expression and stress response in healthy Caucasian males. Biol Mood Anxiety Disord. 2015;5:2. doi: 10.1186/s13587-015-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsuch RL. Factor Analysis. L. Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70:422–430. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: a meta–analysis. Mol Psychiatry. 2013;18:1018–1024. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Mueller A, Armbruster D, Moser DA, Canli T, Lesch KP, Brocke B, Kirschbaum C. Interaction of serotonin transporter gene-linked polymorphic region and stressful life events predicts cortisol stress response. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1332–1339. doi: 10.1038/npp.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Mol Psychiatry. 2005;10:415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009a;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, Jarvelin MR, Taanila A, Flint J. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009b;150B:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Dumas J, Hancur-Bucci C, Naylor M, Sites CK, Benkelfat C, Young SN. Estrogen administration negatively alters mood following monoaminergic depletion and psychosocial stress in postmenopausal women. Neuropsychopharmacology. 2008;33:1514–1527. doi: 10.1038/sj.npp.1301530. [DOI] [PubMed] [Google Scholar]
- O'Hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, Weiner M, Kraemer HC, Noda A, Lin X, Gray HL, Hallmayer JF. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry. 2007;12:544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Behavioral and molecular genetics of dissociation: the role of the serotonin transporter gene promoter polymorphism (5-HTTLPR) J Trauma Stress. 2011;24:373–380. doi: 10.1002/jts.20659. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Galea S, Southwick SM, Gelernter J. Examining the relation between the serotonin transporter 5-HTTPLR genotype x trauma exposure interaction on a contemporary phenotypic model of posttraumatic stress symptomatology: a pilot study. J Affect Disord. 2013;148:123–128. doi: 10.1016/j.jad.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Schulz P, Schlotz W. The Trier Inventory for the Assessment of Chronic Stress (TICS). Scale construction, statistical testing, and validation of the scale work overload. Diagnostica. 1999;45:8–19. [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) ("Self-Evaluation Questionnaire") Consulting Psychologists; Palo Alto, CA: 1983. [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA. Continuity of genetic and environmental influences on cognition across the life span: a meta-analysis of longitudinal twin and adoption studies. Psychol Bull. 2014;140:949–979. doi: 10.1037/a0035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinberg M, Miskowiak K, Kessing LV. Serotonin transporter genotype, salivary cortisol, neuroticism and life events: impact on subsequent psychopathology in healthy twins at high and low risk for affective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:193–198. doi: 10.1016/j.pnpbp.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four Hour Pattern of the Episodic Secretion of Cortisol in Normal Subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wust S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.