Abstract
Importance
African American women living in urban, low-income environments are at high risk for poor nutrition during pregnancy and birth complications.
Objective
To test the effectiveness of prenatal docosahexaenoic acid (DHA) supplementation on birth outcomes and infant development in a sample of African American women with Medicaid insurance and living in the city of Pittsburgh.
Design
The Nutrition and Pregnancy Study (NAPS) is a double-blind, randomized controlled trial of prenatal DHA supplementation conducted between 2012 and 2014.
Setting
Participants were recruited from obstetric clinics at the University of Pittsburgh Medical Center.
Participants
Sixty-four pregnant, African American women were enrolled at 16–21 weeks of gestation and randomized to either 450 mg/day of DHA (22:6n-3)(n=43) or a soybean placebo (n=21). Four women (6.3%) withdrew from the study: two participants from each study arm; complete data were obtained for 49 infants (76.5%) at the 3-month assessment.
Interventions
Supplementation with DHA or placebo continued from the beginning of enrollment through delivery.
Main Outcome and Measures
Data on birth outcomes were collected from medical records. At approximately 3 months post-partum, mothers brought their infants to the laboratory where the Bayley Scales of Infant Development (BSID-III) were administered and cortisol response to the Face-to-Face Still-Face (FFSF) paradigm was assessed.
Results
Infants of mothers who received DHA supplementation had higher birth weight (3,174 grams versus 2,890 grams) than infants of mothers receiving placebo (F [2,40] = 6.09, p = .018, eta = .36), and were more likely to have a 1-minute Apgar score greater than 8 (OR = 5.99 [95% CI = 1.25–28.75], p = .025). Infants of mothers who received DHA compared with infants of mothers receiving placebo had lower levels of cortisol in response to the FFSF paradigm (F [1,32] = 5.36, p = .018, eta = .36). None of the scores on the BSID-III differed as a function of active supplement versus placebo.
Conclusions
Infants of women living in urban, low-income environments who received DHA supplementation had more optimal birth outcomes and more modulated cortisol response to a stressor. DHA supplementation may be effective in attenuating the negative effects of prenatal stress on offspring development.
Keywords: docosahexaenoic acid, supplementation, infant, cortisol, prenatal stress, birth outcomes
1. Introduction
In the U.S., high levels of acute and chronic stress are found among families living in low-income environments; neighborhood disorder, lack of safety and exposure to violence are all significantly higher in areas with lower per capita income (Evans, 2003; Ewart, 2002). More than a quarter of African Americans live in poverty (DeNavas-Walt and Proctor, 2014), and pregnant women living in poverty are at higher risk for poor nutrition (Fowles and Gabrielson, 2005), and are more likely to experience pregnancy and birth complications (Noble, McCandliss et al., 2007; Giscombe and Lobel, 2005).
Maternal and child health disparities among African Americans in the U.S. may emerge in part from differences in maternal psychosocial stress during pregnancy. According to the prenatal programming hypothesis (Weinstock, 2008; Seckl and Holmes, 2007), chronic exposure to stress leads to suboptimal modulation of maternal stress response and consequently, exposure of the fetus to high levels of glucocorticoids released by the mother. This exposure affects the development of the fetal stress architecture in part by adjusting the threshold at which a stress response is activated, and interfering with the feedback mechanisms involved in maintaining homeostasis. Although the postpartum environment continues to affect brain development, for a substantial number of children this initial insult may set the stage for a developmental trajectory that begins with a poorly modulated response to stress during infancy. Identifying factors that can potentially interrupt this cycle has significant implications for public health.
Growing evidence from animal studies indicates that supplementation with polyunsaturated fatty acids (PUFAs), and with docosahexaenoic acid (DHA) specifically, improves maternal stress reactivity during pregnancy and protects neurodevelopment of the offspring, especially in the context of high levels of stress exposure (e.g., Feng et al., 2012; Pudell et al., 2014). DHA levels in human pregnancy are associated with immediate birth outcomes including birth weight, infant head circumference, and length of gestation (Carlson et al., 2013). An association between DHA consumption during pregnancy and later child developmental functioning also has been observed (Hibbeln et al., 2007; Kohlboeck et al., 2011). Among the few double-blinded, randomized controlled studies of DHA supplementation during pregnancy in humans, the results on offspring neurodevelopment have ranged from modest (Helland et al., 2003) to no effects (Makrides et al., 2010). One possible reason for the lack of consistent findings is that the effects of DHA supplementation may be most evident among vulnerable populations in terms high levels of stress exposure, and/or in terms of offspring functioning under conditions of stress. In the majority of experimental animal studies, effects of DHA supplementation on offspring functioning were observed under conditions of manipulated prenatal stress and/or manipulated stress exposure in the offspring as opposed to typical functioning (Keenan and Hipwell, 2015).
We recently completed a randomized controlled study of DHA supplementation in pregnant, African American women living in urban, low-income environments and observed significant differences in self-reported perceived stress and cortisol response to a controlled stressor at 30 weeks gestation (Keenan et al., 2014). In this report we extend those findings by testing the effects of prenatal DHA supplementation on birth outcomes and infant development at approximately 3 months of age in the same sample.
2. Methods
2.1 Study Design
We conducted a double blind, randomized controlled trial (NCT01158976) evaluating the effects of prenatal fatty acid supplementation on infant outcomes in a sample of African American women living in urban, low-income environments in Pittsburgh, Pennsylvania from 2010–2012.
2.2 Eligibility and Recruitment
Only demographically eligible women were approached for screening. Demographic eligibility included: Medicaid insurance or Medicaid eligible, African American race, age between 20 and 30 years, and 16–21 weeks of gestation. Women were recruited using two methods. First, research assistants attended obstetric clinics at the University of Pittsburgh Medical Center between 2010 and 2012 and provided fliers to patients that listed the demographic inclusion criteria, asking those eligible to complete the screening. Second, demographically eligible patients, identified through electronic medical records, were contacted by mail and phone to assess interest in the study. In person or telephone screenings of all demographically eligible patients were then conducted to assess whether sea fish consumption, an index of fatty acid intake, was less than 2 servings per week. Exclusion criteria consisted of known medical complications (gestational diabetes, pre-eclampsia), regular use of steroid medications, regular alcohol use, cigarettes or use of illegal substances (by maternal report), use of blood thinners or anticoagulants, use of psychotropic medications, body mass index >40, and allergy to iodine and or soy.
2.3 Participants
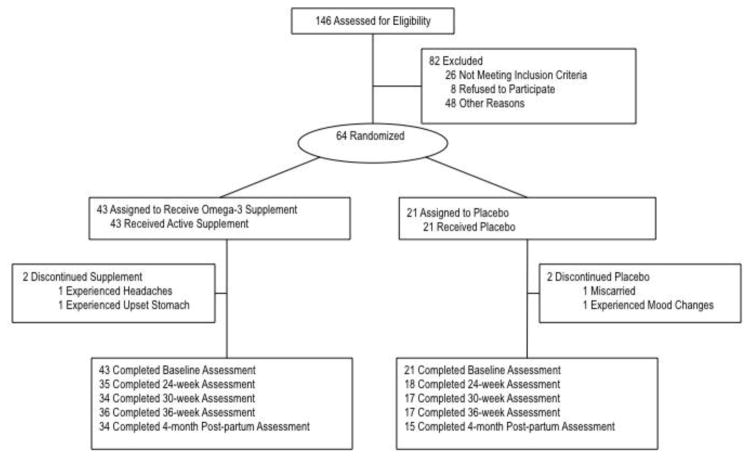
One hundred forty-six women were screened for eligibility. Of those screened, 26 were ineligible, 64 were eligible and enrolled, 48 were eligible at time of screening but could not be enrolled prior to the enrollment window (i.e., 16–21 weeks of gestation), and 8 refused to participate (see Figure 1). Participants were reimbursed on an accelerated schedule with $40 for their first visit and an increase in payments of $10 for each subsequent visit. The Institutional Review Board at the University of Chicago and the Human Research Protection Office at the University of Pittsburgh approved all study procedures.
Figure 1.
Nutrition and pregnancy study participation rates
2.4 Randomization
Once enrolled, women were randomly assigned on a 2:1 ratio to receive an omega-3 nutritional supplement (n = 43) or a soybean oil placebo (n = 21) beginning at enrollment and through the end of pregnancy. We expected greater variability in the dependent measures (e.g., stress reactivity) among the experimental participants than the control patients. Thus, in order to optimize power to test the hypotheses, we enrolled a higher number of participants in the experimental group to adequately capture that variability.
The pharmacist at the University of Pittsburgh carried out a computer generated random assignment of identification (ID) numbers to active supplement or placebo in blocks of 18: six ID numbers were assigned to group A (placebo) and the remaining 12 were assigned equally to either group B or C, both of which received identical doses of active supplement. This approach allowed the pharmacist to randomize on a 2:1 ratio without having the unbalanced design break the blind. The pharmacist provided the staff with appropriate dosed bottles labeled with the ID and treatment letter (A, B or C), thus ensuring that participants and investigators were blinded to the groups to which the participants were assigned. Group assignment was not revealed until all data were collected after the 3-month follow-up.
2.5 Intervention and Procedures
Women received supplements via two strawberry flavored gel capsules providing: 450 mg of DHA (22:6n-3); 40 mg of DPA (docospentaenoic acid, 22:5n-6 and 22:5n-3) and ETA (eicosatetranoic acid, 20:4n-6); 90 mg EPA (eicosapentaenoic acid, 20:5n-3); and 10 mg Vitamin E (d-alpha tocopherol), supplied by Nordic Naturals. The strawberry flavored placebo contained 990 mg of soybean oil, 16.5 mg Vitamin E (d-alpha tocopherol), and 10 mg of EPA and DHA for flavor matching purposes. The supplement and placebo were identical in size, color, and smell. Each participant received a 6-week supply. Research assistants contacted participants by phone 3 times per week to ask the time of day that the supplement was taken, and gathered data on perception of taste, and possible gastrointestinal side effects to increase compliance.
Birth outcomes, including gestational age at delivery, birth weight, and 1 minute Apgar, were collected from the medical record. At approximately 3 months of age, mothers brought infants to the laboratory when the infant’s developmental level and stress reactivity were assessed. The Bayley Scales of Infant Development (Third Edition) (BSID-III) (Bayley, 2006) were administered in order to generate indices of communication and motor development.
Cortisol response to the Face-to-Face Still-Face paradigm (FFSF) (Tronick et al., 1978) was used to measure infant stress reactivity. The FFSF is a standard laboratory procedure comprising three 2-minute episodes: (1) mother playing typically with her seated infant; (2) mother maintaining a neutral expression with no vocalization; and (3) mother returning to typical play. Mothers were asked that their infants should not consume milk products (human or animal) for 1 hour prior to and during saliva collection.
Saliva was collected pre-FFSF, and 20 and 40 minutes post-FFSF by swabbing each infant’s mouth with an unflavored dental roll for several minutes. The dental roll was then placed in a labeled plastic salivette. Samples were immediately transferred to a freezer and stored at −20° C until assayed. On the day of testing, samples were thawed and centrifuged at 3,000 rpm for 10 minutes, allowing for a clear sample to be pipetted into appropriate test wells. All samples from each subject were assayed in the same batch to minimize variability, and assayed with reagents from the same lot. Samples with sufficient saliva were assayed in duplicate using the Salimetrics HS Salivary Cortisol EIA Kit for unbound cortisol. This assay has a lower limit of sensitivity from .007 to 1.2 μg/dL. The average between-assay variance is 3.9% and 7.1%, and the average within-assay variance is 6.7% and 6.9% for high and low concentrations, respectively. The correlation between saliva and serum as assessed by the Salimetrics HS Salivary Cortisol EIA Kit and the Coat-a-Count Serum Cortisol RIA kit is .96, p < .0001. Analyses were conducted with log10-transformed cortisol values, but are presented as untransformed μg/dL for ease of interpretation.
2.6 Statistical Analysis
All data were analyzed using SPSS version 22. Descriptive statistics were computed to examine the distribution of scores. Group differences (active versus placebo) in continuous, normally distributed scores were tested using analysis of variance. Group differences for dichotomous outcomes were tested using logistic regressions.
3. Results
As shown in Figure 1, 34 infants (79.1%) of mothers who received active supplement and 15 infants of mothers who received placebo (71.4%) completed the 4-month post-partum assessment. There was no significant difference in the distribution of sex of infant across the two groups: approximately half of the infants born to mothers receiving placebo were female (53.3%), and 41.2% of the infants born to mothers receiving active supplement were female. There were no effects of sex on birth weight or gestational age. There were no significant associations between infant and maternal cortisol reactivity. Descriptive statistics for all study variables are presented in Table 1.
Table 1.
Descriptive statistics
| Total Sample | Placebo | Active | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Birth Outcomes | |||||||||
| Birth weight (grams) | 3028.52 | 568.93 | 1065–4180 | 2919.56 | 537.74 | 1949–3700 | 3074.39 | 582.34 | 1065 – 4180 |
| Gestational age (weeks) | 38.61 | 2.45 | 27.60–41.60 | 38.84 | 2.06 | 33.9–41.0 | 38.85 | 2.00 | 32.3–41.4 |
| 1-minute Apgar | 8.07 | 1.48 | 4–9 | 7.82 | 1.55 | 4–9 | 8.18 | 1.45 | 4 – 9 |
| Bayley Scaled Scoresa | |||||||||
| Receptive Communication | 4.71 | 2.34 | 1–12 | 4.33 | 1.91 | 2–8 | 4.88 | 2.66 | 1 – 12 |
| Expressive Communication | 6.14 | 1.55 | 2–9 | 6.00 | 1.89 | 2–8 | 6.21 | 1.41 | 3 – 9 |
| Fine Motor | 2.37 | 1.93 | 1–8 | 2.33 | 2.13 | 1–7 | 2.38 | 1.88 | 1 – 8 |
| Gross Motor | 1.63 | 1.17 | 1–6 | 1.60 | 1.12 | 1–5 | 1.65 | 1.20 | 1 – 6 |
| Salivary Cortisol (ug/dL) | |||||||||
| Pre-stressor | 0.25 | 0.25 | 0.05–1.26 | 0.29 | 0.28 | 0.05–1.00 | 0.23 | 0.23 | 0.05–1.26 |
| 20 mins post-stressor | 0.26 | 0.19 | 0.06–1.17 | 0.32 | 0.27 | 0.08–1.17 | 0.22 | 0.15 | 0.06–0.75 |
| 45 mins post-stressor | 0.24 | 0.18 | 0.06–0.92 | 0.31 | 0.20 | 0.10–0.78 | 0.21 | 0.17 | 0.06–0.92 |
Bayley Scales of Infant and Toddler Development Screening Test, 3rd edition
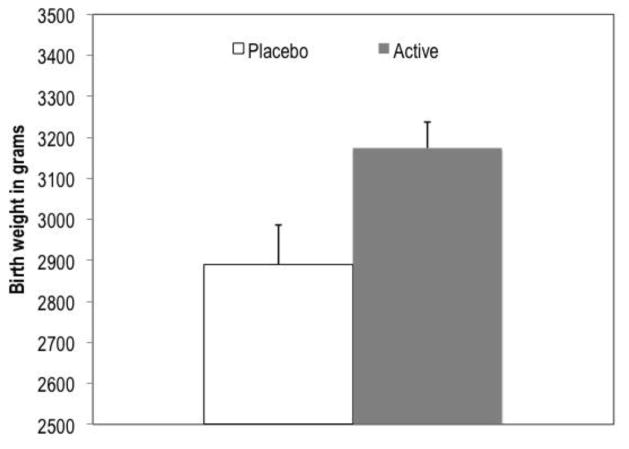
Regarding birth outcomes, there was no significant effect of supplementation on gestational age (M = 38.85, SE = 0.37 for active; M = 38.84, SE = 0.54 for placebo). There was an effect of supplementation on birth weight, with infants of mothers who received DHA supplementation weighing on average 3,174 grams (SE = 63.2), compared to 2,890 grams (SE = 96.9) for infants of mother receiving placebo (F [2,40] = 6.09, p = .018, eta = .36, controlling for gestational age) (Figure 2).
Figure 2.
Effect of supplementation on birth weight controlling for gestational age at birth
F (1,40) = 6.09, p = .018, cohen’s d = .77, controlling for gestational age; n for placebo = 13; n for active = 30; error bars represent standard error for the mean within each group
Supplementation was significantly associated with 1 minute Apgar score. Among the infants whose mothers received supplementation 75.8% had Apgar scores of 9 compared to 59.1% of the infants whose mothers received placebo (OR = 5.99 [95% CI = 1.25–28.75], p = .025, controlling for birth weight and gestational age) (Figure 3). A full distribution of the 1 minute Apgar scores across both groups is provided in Table 2.
Figure 3.
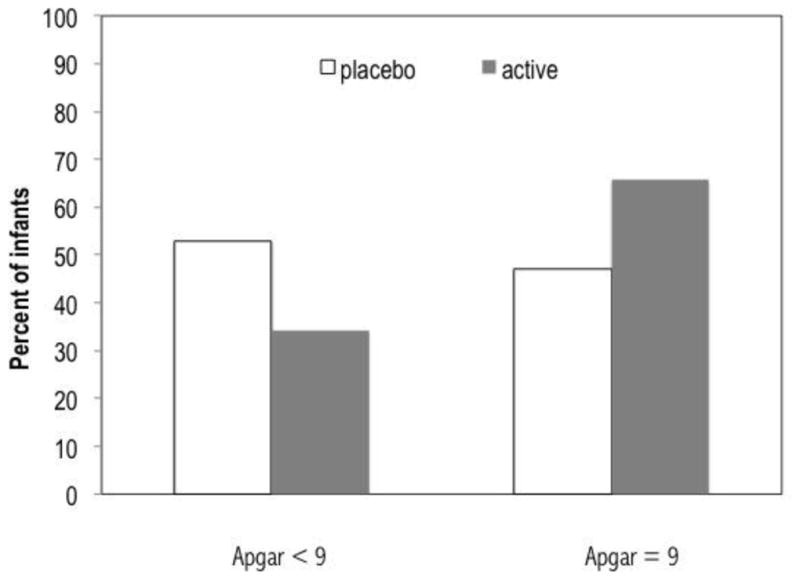
Effect of supplementation on one minute Apgar controlling for gestational age and birth weight
Odds ratio = 5.99 (95% CI = 1.25–28.75), p = .025, controlling for birth weight and gestational age; n for placebo = 13; n for active = 30
Table 2.
Distribution of 1-minute Apgar score for infants whose mothers received placebo or active supplement
| 1-minute Apgar | Placebo | Active | ||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| 4 | 1 | (5.9) | 2 | (5.3) |
| 5 | 1 | (5.9) | 2 | (5.3) |
| 6 | 1 | (5.9) | 0 | (0.0) |
| 7 | 2 | (11.8) | 4 | (10.5) |
| 8 | 4 | (23.5) | 5 | (13.2) |
| 9 | 8 | (47.1) | 25 | (65.8) |
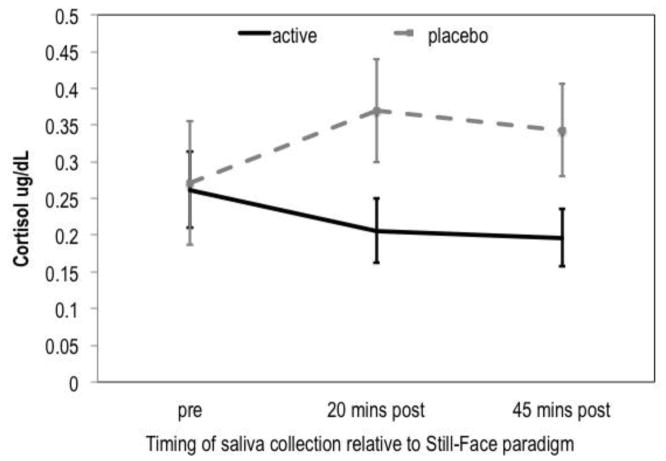
No group difference in pre-stressor cortisol levels was observed between infants whose mothers received placebo versus supplementation. Controlling for pre-stressor cortisol levels and time of collection, significant group differences were observed for levels measured at 20 and 45 minutes post-stressor, with infants of mothers taking placebo showing higher levels of cortisol than infants of mothers taking active supplement (F [1,32] = 5.36, p = .018, eta = .36, controlling for gestational age) (Figure 4). None of the scores on the Bayley Scales of Infant Development differed as a function of active supplement versus placebo.
Figure 4.
Effect of supplementation of infant cortisol response to the Still-Face Paradigm
F (1, 32) = 5.36, p = .027, cohen’s d = .82, Controlling for time of collection and pre-stressor cortisol levels; error bars represent standard error for the mean within each group at each time point
4. Discussion
To the best of our knowledge, this is the first study in which the effect of prenatal DHA supplementation on infant outcomes, including stress reactivity, has been tested in a U.S. population at high risk for adverse birth and infant outcomes. These findings are highly significant for maternal and child health as fatty acid supplementation led to improvements in birth weight, Apgar scores, and infant stress reactivity. To place the birth outcomes in a clinical context, we refer to epidemiological data published by the Center for Disease Control and Prevention. In 2013, the most recent year for which data are available, 16.3% of births to African American mothers were preterm and 13.1% of infants were low birth weight (i.e., less than 2500 grams) (Martin et al., 2015). In the present study, 13.6% of infants were born at less than 37 weeks gestation, a rate that was comparable across both the placebo and active supplement groups. The slightly lower than average rate of preterm delivery may be due to recruiting from a women’s hospital with a very high level of care. Access to prenatal services that prolong gestation across both groups may have led to difficulties in determining effects of fatty acid supplementation on gestation, an effect that has been fairly consistent in other studies (Keenan and Hipwell, 2015).
Nearly two thirds of infants of mothers receiving active supplement had 1-minute Apgar scores of 9, compared to less than half of infants of mothers receiving placebo. Although the 1-minute Apgar score is not predictive of later neurodevelopment, it is a standardized method for describing the status of the neonate immediately after birth (American College of Obstetricians and Gynecologists, 2015). Whereas, as a single predictor the clinical utility is likely to be negligible, in the context of other indices of the difference in Apgar scores provides additional support for the association between DHA supplementation and more optimal birth outcomes.
Prenatal supplementation was associated with increased birth weight when comparing average birth weight for both groups. Only 7 (13.0%) of infants in the present study weighed less than 2500 grams; 10.5% of infants of mothers who received active supplement and 18.8% of infants of mothers who received placebo were classified as low birth weight. Thus, the rate of low birth weight was reduced by almost 50% with DHA supplementation in the present study and by almost 20% compared to the national average for African American women. Although the small sample size calls into question the robustness of the findings, the effect sizes indicate that this is a preliminary finding worthy of replication efforts.
The results for infant stress reactivity are equally compelling. Although significant changes in behavioral distress and heart rate are typically observed in the infant’s response to the face-to-face-still-face paradigm in the first 6 months of life, a change in cortisol level is not typically observed (Grant et al. 2009; Lewis and Ramsay, 2005; Tollenaar et al., 2011). In a few studies, subgroups of infants were identified as demonstrating an increase in cortisol, including infants of mothers reporting prenatal anxiety (Tollenaar et al, 2011), mothers demonstrating less sensitive caregiving (Bosquet-Enlow et al., 2014), and infants of mothers who abused alcohol during pregnancy (Haley et al, 2006). In comparison to the significant increase in cortisol in response to the still face among infants whose mothers received placebo, the lack of a cortisol response among infants whose mothers received active supplement could be conceptualized as a normalizing effect: despite exposure to prenatal stress, fatty acid supplementation protects the integrity of the development of the HPA-axis resulting in a more typical infant cortisol response.
The lack of effects of supplementation on neurodevelopment test scores in the present study is consistent with previous research (e.g., Makrides et al., 2010). Even among studies reporting modest effects, the impact of prenatal fatty acid consumption on neurodevelopmental measures appears to emerge later in childhood as opposed to being reliably observed in infancy (Hibblen et al., 2007). This pattern of results may reflect lower sensitivity of neurodevelopmental tests in early infancy (Harris & Langkamp, 1994), especially among children from families living in low-income environments (Hess et al., 2004). Continued assessment into childhood is needed to further explore the potential impact of prenatal fatty acid consumption on neurodevelopment, including the possibility that deficits that emerge later in development may be partially mediated by early disruptions in HPA-axis functioning.
5. Conclusion
This is the first study to test the effect of prenatal supplementation with PUFA on birth outcomes, and infant stress reactivity, and developmental level in a sample of African American women living in urban poverty. Observed obstetric and neonatal health disparities among racial minorities and families living in poverty may be in part due to differential exposure to prenatal stress. We previously reported that DHA supplementation resulted in less perceived stress and more modulated cortisol response to stress during pregnancy (Keenan et al., 2014). The results from the present study add to those findings and provide further support for the hypothesis that nutritional moderators targeting prenatal stress among vulnerable populations could have a significant positive effect on the neurodevelopment of children. The fact that statistically significant differences in infant outcomes were observed in a small sample is encouraging, but also underscores the need for replication in a larger and more diverse sample with respect to sociodemographic factors and prenatal stress exposure. Future research also is needed to test whether the observed effects of fatty acid supplementation during pregnancy on infant outcomes are mediated by changes in prenatal stress.
Highlights.
The effectiveness of prenatal docosahexaenoic acid (DHA) supplementation on birth outcomes and infant development in a sample of African American women with Medicaid insurance is tested
Compared to infants of mothers who received placebo, infants of mothers who received DHA supplementation had more optimal birth outcomes and lower and more typical cortisol responses to a controlled stressor
DHA supplementation may be effective in attenuating the negative effects of prenatal stress on offspring development
Acknowledgments
Role of Funding Source
This study was supported by NIH grants R21 HD 058269 and R01 HD084586 to Dr. Keenan, with additional support from the University of Chicago Institute for Translational Medicine (UL1TR000430).
Footnotes
Contributors
-
KeenanDepartment of Psychiatry and Behavioral NeuroscienceUniversity of Chicago
-
Alison HipwellDepartment of PsychiatryUniversity of Pittsburgh
-
Rose McAloonDepartment of Survey MethodologyUniversity of Maryland
-
Amy HoffmannDepartment of PsychologyUniversity of Central Florida
-
Arpita MohantyDepartment of PsychiatryUniversity of Pittsburgh
-
Kelsey MageeDepartment of PsychiatryUniversity of Pittsburgh
Conflict of Interest
None of the authors report conflicts of interest with the data presented in this paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Obstetricians and Gynecologists. The Apgar score. Committee Opinion No 644. Obstet Gynecol. 2015;126:e52–5. doi: 10.1097/AOG.0000000000001108. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Harcourt Assessment Inc; 2006. [Google Scholar]
- Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, Wright RJ. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev. 2014;90:377–85. doi: 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, et al. DHA supplementation and pregnancy outcomes. American Journal of Clinical Nutrition. 2013;97:808–15. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD. Income and Poverty in the United States: 2014. U.S. Government Printing Office; Washington, DC: 2015. U.S. Census Bureau, Current Population Reports, P60–252. [Google Scholar]
- Evans GW. The built environment and mental health. Journal of Urban Health. 2003;80:536–555. doi: 10.1093/jurban/jtg063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart CK, Suchday S. Discovering how urban poverty and violence affect health: Development and validation of a neighborhood stress index. Health Psychol. 2002;21:254–262. doi: 10.1037//0278-6133.21.3.254. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zou X, Jia H, Li X, Zhu Z, Liu X, Bucheli P, Ballevre O, Hou Y, Zhang W, Wang J, Chen Y, Liu J. Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: Possible role of neuronal mitochondria metabolism. Antiox Redox Signaling. 2012;16:275–89. doi: 10.1089/ars.2010.3750. [DOI] [PubMed] [Google Scholar]
- Fowles E, Gabrielson M. First trimester predictors of diet and infant birth weight in low-income pregnant women. J Community Health Nurs. 2005;22:117–30. doi: 10.1207/s15327655jchn2202_5. [DOI] [PubMed] [Google Scholar]
- Giscombé CL, Lobel M. Explaining disproportionately high rates of adverse birth outcomes among African Americans: The impact of stress, racism, and related factors in pregnancy. Psychol Bull. 2005;131:662–683. doi: 10.1037/0033-2909.131.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Austin MP, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants' cortisol responses to the still-face procedure. Dev Psychobiol. 2009;51:625–37. doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Harris SR, Langkamp DL. Predictive value of the Bayley mental scale in the early detection of cognitive delays in high-risk infants. J Perinatol. 1994;14:275–9. [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- Hess CR, Papas MA, Black MM. Use of the Bayley Infant Neurodevelopmental Screener with an environmental risk group. J Pediatr Psychol. 2004;29:321–30. doi: 10.1093/jpepsy/jsh036. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet. 2007;369:578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE. Modulation of prenatal stress via docosahexaenoic acid supplementation: Implications for child mental health. Nutrition Reviews. 2015;73:166–174. doi: 10.1093/nutrit/nuu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE, Bortner J, Hoffmann A, McAloon R. Association between fatty acid supplementation and prenatal stress in African Americans: A randomized controlled trial. Obstetrics Gynecology; 2014;124:1080–1087. doi: 10.1097/AOG.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlboeck G, Glaser C, Tiesler C, Demmelmair H, Standl M, Romanos M, Koletzko B, Lehmann I, Heinrich J LISAplus Study Group. Effect of fatty acid status in cord blood serum on children's behavioral difficulties at 10 y of age: Results from 245 the LISAplus Study. Am J Clin Nutr. 2011;94:1592–1599. doi: 10.3945/ajcn.111.015800. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Infant emotional and cortisol responses to goal blockage. Child Dev. 2005;76:518–530. doi: 10.1111/j.1467-8624.2005.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA. 2010;304:1675–83. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: Final data for 2013. National vital statistics reports. 2015;64(1):2015. [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–80. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Pudell C, Vicente BA, Delattre AM, Carabelli B, Mori MA, Suchecki D, Machado RB, Zanata SM, Visentainer JV, de Oliveira Santos O, Junior, Lima MM, Ferraz AC. Fish oil improves anxiety-like, depressive-like and cognitive behaviors in olfactory bulbectomised rats. European J Neurosci. 2014;39:266–74. doi: 10.1111/ejn.12406. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–88. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven JM, de Weerth C. Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress. 2011;14:53–65. doi: 10.3109/10253890.2010.499485. [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. Infants response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child and Adolescent Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–86. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]