Abstract
Polyglandular autoimmune inflammation accompanies Type 1 diabetes (T1D) in NOD mice affecting organs like thyroid and salivary glands. Whereas commensals are not required for T1D progression, germ-free (GF) mice had a very low degree of sialitis, which was restored by colonization with select microbial lineages. Moreover, unlike T1D, which is blocked in mice lacking MyD88-signaling adaptor under conventional but not under GF housing conditions, sialitis did not develop in MyD88-negative GF mice. Thus, microbes and MyD88-dependent signaling are critically required for sialitis development. The severity of sialitis did not correlate with the degree of insulitis in the same animal, was less sensitive to T1D-reducing diet, but was similar to T1D in microbiota-dependent sexual dimorphism. The unexpected distinction in requirements for the microbiota for different autoimmune pathologies within the same organism is crucial for understanding the nature of microbial involvement in complex autoimmune disorders including human autoimmune polyglandular syndromes.
Keywords: Akkermansia muciniphila, Autoimmune polyglandular syndrome, Gut microbiota, Hydrolyzed casein diet, MyD88, Sialitis, Type 1 diabetes
INTRODUCTION
Type 1 diabetes (T1D) develops spontaneously in humans and in rodents (NOD mice and bio-breeding, BB, rats). In addition to leukocytic infiltration in the pancreas, autoimmune responses against other glands: thyroid, adrenal, submandibular, and lacrimal, frequently occur. This makes NOD mice very similar to a subset of human patients with T1D developing autoimmune polyglandular syndrome (APS) types 2–4 (types are assigned dependent on the organs affected) (1–3). The rare APS type 1 and the more common type 2 are linked to AIRE (4) and MHC genes (5), respectively. Loss of tolerance and immunodeficiency in these patients results in autoimmune Addison’s disease typically accompanied by hypoparathyroidism (APS1) or hypothyroidism and/or T1D (APS2). The most prevalent APS type 3 is characterized by the same features as APS2, except that adrenal defects are lacking. Instead, it is often accompanied by non-organ-specific (systemic) lupus erythematosus (SLE), rheumatoid arthritis, or Sjögren syndrome, which is similarly evident in type 4 APS characterized by associations of autoimmune endocrine disorders that do not fulfill criteria for APS1–3 (6). Whether these organ-specific autoimmune manifestations have common (or unique) mechanisms of regulation by extrinsic factors is not known. Indirect evidence based on changes in pre-disease onset microbiota, use of antibiotics, and testing of germ-free (GF) rodent models of autoimmune diseases suggest that the disease onset and/or severity are influenced by gut microbiota (7,8). Whereas a GF state keeps the incidence of diabetes similar to the levels of T1D in SPF mice (9,10) or even enhances it (11,12), it clearly reduces inflammation in other autoimmune diseases (13). Here we used the NOD mouse model to compare microbial influences on development of diabetes and sialitis.
MATERIALS AND METHODS
Mice
NOD/ShiLtJ (The Jackson Laboratory, Bar Harbor, ME) mice were housed in specific-pathogen-free (SPF) and GF facility at The University of Chicago Animal Resource Center and used in accordance with institutional guidelines for animal welfare. The Biological Sciences Division Institutional Review Board at the University of Chicago approved all animal studies. GF status was monitored by aerobic and anaerobic fecal cultures and PCR amplification of bacterial 16S rRNA genes from fecal DNA. All mice were killed at 13 weeks of age or when diagnosed with diabetes by testing for glycosuria using urine dipstick (Diastix, Bayer, Elkhart, IN) as indicated in the text. Littermates were used where possible.
Surgery
Gonads were excised from 5 week old males anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) combination with the Change-A-Tip handheld cauterizer (Bovie Medical Corporation, Clearwater, FL) and the incision was sealed with EZ Clips (Stoelting Co., Wood Dale, IL). Sham-operated mice were anesthetized followed by scrotal incision and sealed by application of wound clips.
Diet
Mice were fed JL Rat and Mouse/Auto 6F 5K67 chow diet (LabDiet, St. Louis, MO) or AIN-93G modified diet with 20% hydrolyzed casein (HC) (Harlan Laboratories, Madison, WI). Diets were autoclaved and microbiologically tested before GF use.
Gnotobiotic NOD mice
GF mice were colonized by introduction of specific microbial taxa via gastric gavage to the breeding pairs in separate isolators. Bacteria were transferred to the offspring naturally from the mother, and the colonization was confirmed by 16S rDNA PCR for genes specific for the lineages (14). The bacterial community in VSL#3 enriched for bifidobacteria, lactobacilli, and Streptococcus salivarius species and culturing and classification of a Proteobacterium “Similar to E.coli and Shigella“ (SECS) are previously described in Yurkovetskiy et al. (14,15). Akkermansia muciniphila was a generous gift from Dr. Dennis Sandris Nielsen (University of Copenhagen, Department of Food Science, Copenhagen, DK) and introduced by gavage of 2 × 108 CFUs.
Histology
Leukocytic inflammatory foci in salivary glands were scored in a blinded fashion on H&E stained 5 μm sections of wax embedded salivary glands. 8 sequential sections of each gland with 40 μm intervals were scored by counting the number of focal infiltrates larger than 50 cells (16) and measuring the mean size of all foci per section. Foci size was measured with a Leica graticule with 10 ×10 mm grid and the sialitis score was calculated as:
where N̄f is the mean number of foci per section and S̄f is the average size of the foci per section.
Insulitis was scored on at least 100 islets per pancreas on H&E stained 5 μm sections with 40 μm interval and graded as follows: 1, no visible infiltrations, 2, periinsulitis; 3, insulitis with less than 50% islet infiltration; 4, insulitis with more than 50% islet infiltration. Percent of islets with insulitis (score 3 and 4) was used for the analyses.
Statistical analysis
The results were analyzed for statistical probabilities of significance between two groups using two-tailed unpaired Student t-test. For analysis of more than two groups, significance was found by one-way ANOVA with Tukey’s post hoc test. Diabetes incidence at 30 weeks of age was analyzed by Fischer’s exact test. P values < 0.05 were considered significant. Graphs were generated and statistical analyses were performed by using GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA). Results are displayed as mean ± SEM.
RESULTS AND DISCUSSION
Sialitis development depends on microbes and does not correlate with insulitis
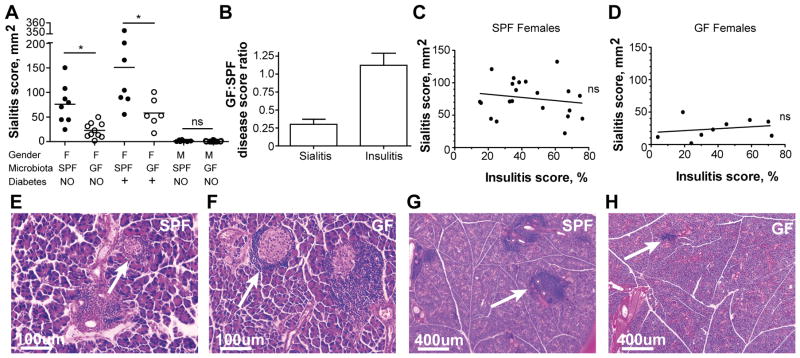
To test the contribution of intestinal microbes to the development of autoimmune sialitis in NOD mice, we examined the severity of inflammation (number and extent of inflammatory foci) in histological sections of the salivary glands in GF and SPF mice. The sialitis score (derived by multiplying the mean number of lesions per eight histological sections by their mean size) was significantly lower in GF females compared to SPF females at 13 weeks of age before the onset of overt diabetes (Figure 1A). In contrast, T1D in GF female NOD mice is either similar to incidence in SPF NOD mice (10) or augmented with the incidence close to 100% (11). The insulitis progresses similarly in our SPF and GF NOD mice and therefore has a GF:SPF ratio close to 1.0, whereas the sialitis score in GF NOD mice is only 0.33±0.06 of the score in SPF NOD mice (Figure 1B). In addition, non-diabetic 13 weeks old male mice had only very mild infiltrations if any in both SPF and GF conditions (Figure 1A). This is also in contrast to autoimmune diabetes development, where GF males loose the protection mediated by the gut microbiota (14).
Figure 1. Commensal microbes are required for development of sialitis but not insulitis.
A) Histopathology scores of salivary glands collected from specific pathogen free (SPF) and germ-free (GF) 13 weeks old non-diabetic female NOD mice (n = 8 and n = 10, respectively), diabetic females with glucosuria (n = 7 and n = 6 respectively), and 13 weeks old non-diabetic male NOD mice (n = 8 and n = 15 respectively). Sialitis score: leukocytic inflammatory foci were scored on 8–10 sequential sections of each gland by counting the number of focal infiltrates and measuring the mean size of all foci per section. Mean values are shown. * indicates P < 0.05. ns (non-significant) indicates P > 0.05. NO: non-diabetic, +: diabetic.
B) Ratio of sialitis (n = 10) and insulitis (n = 13) scores in GF and SPF non-diabetic 13 weeks old female NOD mice. Mean values ± SEM.
C) Correlation between sialitis and insulitis scores from the same SPF non-diabetic 13 weeks old female NOD mice (n = 21). ns (non-significant) indicates P > 0.05.
D) Correlation between sialitis and insulitis scores in organs collected from the same GF non-diabetic 13 weeks old female NOD mice (n = 9). ns (non-significant) indicates P > 0.05.
E–F) Representative images of pancreatic islets (arrows) from SPF and GF 13 weeks old non-diabetic female mice are shown.
G–H) Representative images of foci of inflammation in salivary glands (arrows) from SPF and GF 13 weeks old non-diabetic female mice are shown.
About 55% of human patients with T1D have Sjögren syndrome, in particular during hyperglycaemic phases (17). As one of the most prevalent multiorgan autoimmune diseases, Sjögren syndrome is characterized (among other things) by the dry mouth (xerostomia) and dry eyes (keratoconjunctivitis) (18). NOD mice similarly manifest several features of Sjögren syndrome (lymphocytic infiltrations and progressive destruction of salivary and lachrymal glands) along with T1D, making them a good model for APS. The connection between overt autoimmune diabetes and development of salivary gland lesions was therefore investigated. As mice progressed towards overt diabetes (Figure 1A,E,F), the sialitis scores also grew. However, GF mice still lagged behind their SPF counterparts (Figure 1A,G,H). We then plotted insulitis vs. sialitis scores for SPF and GF NOD females. No correlation was found between these two processes in either condition, although sialitis was more pronounced in SPF mice as expected (Figure 1C,D). The two pathologies are thus developing independently of each other and are under different pressure from the microbiota.
Sexual hormones are required for microbial regulation of sialitis
T1D in NOD mice is known to be sexually dimorphic (1) similarly to the 3:1 female bias of T1D in APS2–4 patients (19) and unlike the 1:1 gender ratio of T1D in human patients with no other endocrine autoimmune manifestations (20). At the same time, the predominance of T1D in female NOD mice is lost in GF conditions (10–12). Protection of males depends on male sex hormones and on the microbiota: castrated males, which change their gut microbiota toward a female profile, developed insulitis and diabetes at a higher incidence comparable to female incidence (10,14). Several different microbial taxa have been proven protective for the males (14). Thus, we investigated the sexual dimorphism of sialitis and microbiota’s contribution to it. Sialitis was very mild in males in both SPF and GF conditions (Figure 1A). Castration significantly increased the sialitis score (Figure 2A). The fold change of the score for sialitis induced by castration (7.4±1.6) was even higher (P<0.001) than for insulitis (2.5±0.3), but it never reached the severity of sialitis in females (Figure 2B). Thus, sex hormones regulate both insulitis and sialitis in NOD mice and that could be related to the shaping of the microbiota by sex hormones (10,14). However, since male castration leads to only mild increase in the severity of inflammation of salivary glands, sialitis may be affected by other non-hormonal gender differences (21).
Figure 2. Sexual dimorphism of sialitis development.
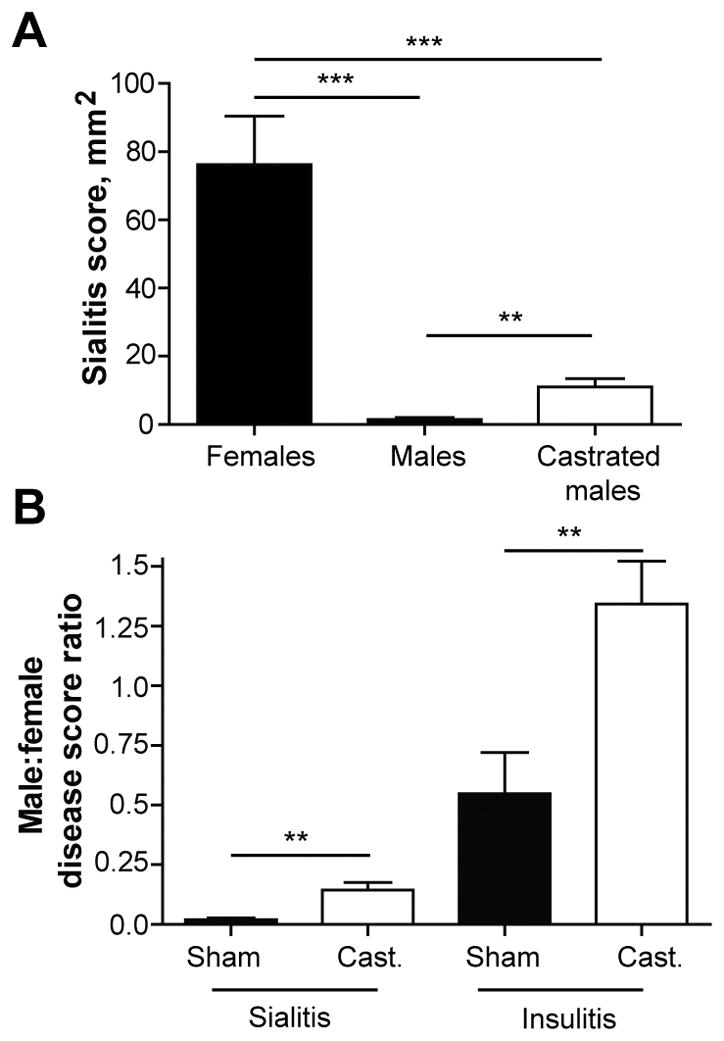
A) Histopathology scores of salivary glands from SPF 13 weeks old non-diabetic female (n = 10), intact male (n = 8), and castrated male (n = 5) NOD mice. Mean values ±SEM of one experiment are shown. ** indicate P < 0.01; *** indicate P < 0.001.
B) Ratio of sialitis and insulitis scores in SPF sham operated (n = 8 and n = 7, respectively) or castrated (Cast.) (n = 5 and n = 12, respectively) non-diabetic 13 weeks old male NOD mice compared to mean score in female NOD mice. Mean values ± SEM of one experiment are shown. ** indicate P < 0.01.
Sialitis is insensitive to diabetes-affecting dietary changes and can be induced by select microbial lineages
The microbiota is quite sensitive to dietary variations (22). Some dietary interventions lead to attenuation of autoimmunity (23–25). Semi-purified diets with modified protein fractions, e.g. by basing it solely on hydrolyzed casein (HC), can drastically reduce diabetes incidence in NOD mice and BB rats (26,27). HC intervention is associated with changes in the gut microbiota including increased Lactobacilli and decreased Bacteroides spp. levels (28). To test whether dietary changes would similarly affect sialitis to the same degree as it affects diabetes (29), we compared disease development in mice fed regular chow and HC diet. HC diet only showed a trend for reduction of the sialitis score in SPF mice and the scores in GF mice remained low irrespectively of the diet (Figure 3A). Similarly, hypoallergenic diet Pregestimil was not protective against salivary-infiltrating mononuclear cells in NOD mice (30). Thus, any changes in the microbiota associated with HC diet are not sufficient to reduce sialitis. The result raises a question of whether specific bacterial lineages affect sialitis, or can a rather broad range of microbes support salivary gland inflammation? The issue was addressed by using colonization of GF mice with defined microbial consortia. Previous reports suggested that Akkermansia muciniphila (in association with more abundant Proteobacteria) attenuated diabetes development in SPF NOD mice that were treated with antibiotics (8,24). However, colonization of GF mice with a combination of A. muciniphila and Proteobacterium SECS isolated from NOD mice (14) did not reduce the severity of histopathology in the pancreatic islets (Figure 3B). At the same time, salivary glands of gnotobiotic NOD mice colonized with these bacteria demonstrated an inflammation significantly higher in score than the GF NOD mice (Figure 3A). A very different mix of microbes, VSL3 probiotic (31), did not reduce insulitis development in colonized gnotobiotic mice (14). It also failed to increase the severity of sialitis in VSL3-colonized female NOD mice over sialitis observed in GF mice (Figure 3A). Thus, randomly chosen commensals can either promote sialitis or not. Moreover, the pro-inflammatory activity of A. muciniphila/proteobacteria combination was weaker than similar activity of the SPF microbiota. Thus, adding specific commensals supports sialitis in the GF mouse, but the effect of the tested colonizers was small suggesting that other bacteria are necessary to fully augment salivary gland inflammation.
Figure 3. Distinct effects of dietary and specific microbial challenges on sialitis and insulitis.
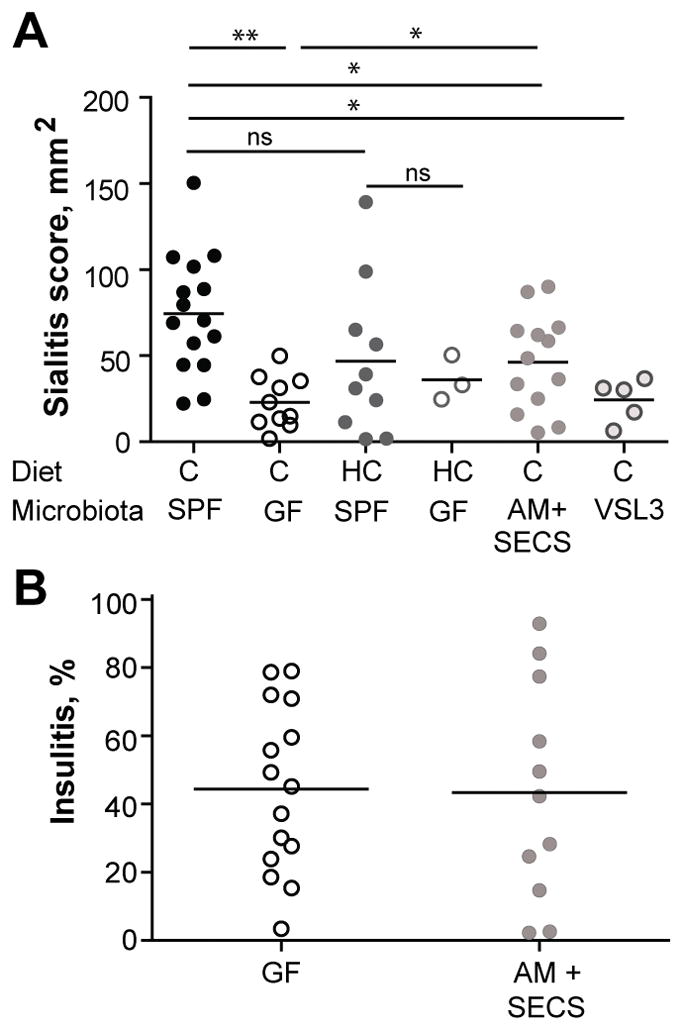
A) Histopathology of salivary glands from SPF and GF 13 weeks old non-diabetic female NOD mice on regular chow (n = 15 and n = 10 respectively) or 20% hydrolyzed casein (HC; n = 10 and n = 3 respectively) diet. GF mice colonized with VSL3 (n = 5), or Akkermansia muciniphila (AM) and SECS (Proteobacteria) colonized (n = 13) non-diabetic 13 weeks old female NOD mice Mean values of one experiment are shown. * indicates P < 0.05. ** indicate P < 0.01. ns (non-significant) indicates P > 0.05.
B) Histopathology of the pancreas from GF (n = 15) or GF mice colonized with AM and SECS (n = 11) colonized non-diabetic 13 weeks old female NOD mice. Mean values of one experiment are shown.
MyD88-dependent innate immune signaling pathway is required for sialitis development
Deletion of MyD88 adaptor protein in SPF NOD mice led to their complete protection from autoimmune diabetes, but did not affect T1D development in GF NOD mice (11). These findings led to the conclusion that in the absence of signaling through MyD88-dependent innate immune receptors, the microbiota induces negative signaling reducing both anti-commensal and autoimmune reactivity (11,32). To test whether the same rules apply to development of sialitis in the absence of MyD88, we examined salivary glands lesions in MyD88 KO NOD mice. Similarly to the effect of MyD88 KO on T1D, sialitis was significantly reduced in MyD88 SPF mice (Figure 4A, B). However, sialitis in MyD88 KO GF mice was minimal and even further reduced compared to WT GF NOD mice (Figure 4A,B).
Figure 4. MyD88-dependent signaling is required for sialitis development.
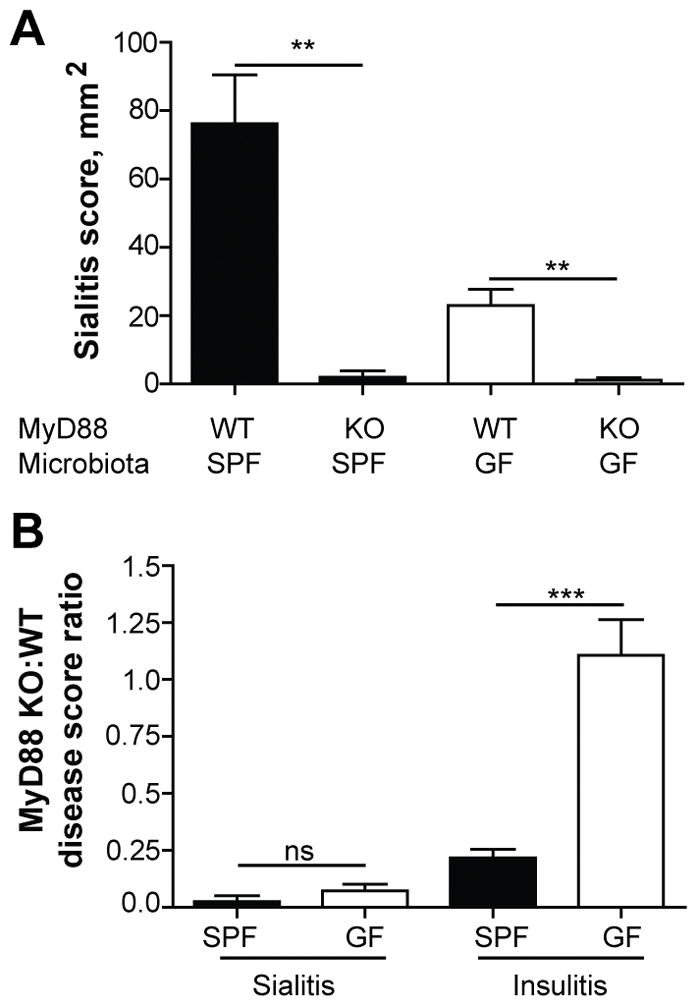
A) Histopathology scores of the inflammation of salivary glands from SPF and GF non-diabetic 13 weeks old MyD88-sufficient wild-type (WT; n = 8 and n = 10 respectively) and MyD88-deficient (KO; n = 4 and n = 5 respectively) female NOD mice. Mean values ± SEM of one experiment are shown. ** indicate P < 0.01.
B) Ratio of sialitis and insulitis scores in MyD88 deficient (KO) SPF (n = 4 and n = 8, respectively) or GF (n = 8 and n = 6, respectively) non-diabetic 13 weeks old female NOD mice compared to mean score in MyD88-sufficient (WT) NOD mice. Mean values ± SEM of one experiment are shown. *** indicate P < 0.001. ns (non-significant) indicates P > 0.05.
These findings show that microbe-induced sialitis is dependent on MyD88, and also that something besides live bacteria can induce a low grade sialitis in GF mice in MyD88-dependent manner. Such an effect may be mediated by the presence of autoclave-resistant pathogen-associated molecular patterns in the diet (33). Another possibility is that the presence of endogenous viruses contributes to activation of adaptive immune responses against self-antigens. GF animals are free of viruses except endogenous retroviruses, which have been shown to stimulate MyD88 pathway through toll-like receptor (TLR)-7 (34). In SPF mice other viruses may contribute to sialitis even in MyD88-independent manner: stimulation of MyD88-independent TLR3, which is highly expressed on the surface of salivary gland epithelial cells from Sjögren patients (35), by viral dsRNA is considered a likely contributor to Sjögren’s syndrome (36).
In sum, our study shows that in complex autoimmune diseases the microbiota can affect different manifestations of the pathological processes very differently. We find that gut microbiota is dispensable for the induction of T1D but not for development of severe sialitis. Autoimmune responses initiated by the innate sensing, antigen presentation, and co-stimulatory activation of adaptive immunity have previously been classified according to the regulatory effect of gut microbiota on disease outcome found in GF and gnotobiotic rodents (37). Development of diabetes in both NOD mice and BB rats belongs to the group, in which GF and conventional animals are equally susceptible to disease. However, attenuated development of sialitis in the same mouse model in GF isolators places this pathological manifestation in the group of diseases that develop independently of microbes, but where microbes amplify the disease. This group also includes models of other autoimmune disorders such as rheumatoid arthritis and SLE (7,38). The specific mechanisms that the microbiota uses to regulate different autoimmune diseases are not well understood. Although specific microbial lineages were shown to contribute to induction of autoimmunity (7), it is more likely that the salivary gland inflammation in NOD mice was induced by multiple members of the microbiota. Regardless, the basis for commensal involvement seems to lie in the communication between innate and adaptive immune responses, which are likely to be sensitive to regulation by the microbiota (37). Further inquiries into the nature of such regulation are warranted, but our findings point at additional and not yet appreciated complexity of the problem in multi-organ autoimmunity.
Acknowledgments
Funding: CHFH was supported by Carlsberg Foundation and Aase og Ejnar Danielsens Foundation, Denmark. LY was supported by T32 GM007183. AVC was supported by NIH/NIDDK Digestive diseases research core center grant DK42086, NIH grant AI0842418 and by JDRF grant 17-2011-519.
Abbreviations
- APS
autoimmune polyglandular syndrome
- BB
bio-breeding
- GF
germ-free
- HC
hydrolyzed casein
- SFB
segmented filamentous bacteria
- SLE
systemic lupus erythematosus
- SPF
specific-pathogen-free
Footnotes
Author contributions: CHFH and LY performed experiments. AVC designed and managed the project, and with CHFH wrote the manuscript.
Disclosure: The authors declared no conflict of interest.
References
- 1.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9:154–162. [PubMed] [Google Scholar]
- 3.Bernard NF, Ertug F, Margolese H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes. 1992;41:40–46. doi: 10.2337/diab.41.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Aaltonen J, Bjorses P, Sandkuijl L, Perheentupa J, Peltonen L. An autosomal locus causing autoimmune disease: autoimmune polyglandular disease type I assigned to chromosome 21. Nat Genet. 1994;8:83–87. doi: 10.1038/ng0994-83. [DOI] [PubMed] [Google Scholar]
- 5.Obermayer-Straub P, Manns MP. Autoimmune polyglandular syndromes. Baillieres Clin Gastroenterol. 1998;12:293–315. doi: 10.1016/s0950-3528(98)90136-1. [DOI] [PubMed] [Google Scholar]
- 6.Cutolo M. Autoimmune polyendocrine syndromes. Autoimmun Rev. 2014;13:85–89. doi: 10.1016/j.autrev.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Wu HJ, I, Ivanov I, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sorensen SJ, Buschard K, Hansen AK. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 9.Alam C, Bittoun E, Bhagwat D, Valkonen S, Saari A, Jaakkola U, Eerola E, Huovinen P, Hanninen A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia. 2011;54:1398–1406. doi: 10.1007/s00125-011-2097-5. [DOI] [PubMed] [Google Scholar]
- 10.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von BM, Mccoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 11.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Yamada T, Fujimura T, Kawamura E, Shimizu M, Yamashita R, Nomoto K. Diabetogenic Effects of Lymphocyte Transfusion on the NOD or NOD Nude Mouse. In: Rygaard, Brünner, Græm, Spang-Thomsen, editors. Immune-Deficient Animals in Biomedical Research. 5th Int. Workshop on Immune-Deficient Animals; Copenhagen. 1985; Basel: Karger; 1987. pp. 112–116. [Google Scholar]
- 13.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 14.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen CH, Holm TL, Krych L, Andresen L, Nielsen DS, Rune I, Hansen AK, Skov S. Gut microbiota regulates NKG2D ligand expression on intestinal epithelial cells. Eur J Immunol. 2013;43:447–457. doi: 10.1002/eji.201242462. [DOI] [PubMed] [Google Scholar]
- 16.Thompson C, Jacobsen H, Pomeranz KD, Nagai K, Cooke A. Non-depleting anti-CD4 antibody not only prevents onset but resolves sialadenitis in NOD mice. Autoimmunity. 2004;37:549–554. doi: 10.1080/08916930400021352. [DOI] [PubMed] [Google Scholar]
- 17.Binder A, Maddison PJ, Skinner P, Kurtz A, Isenberg DA. Sjogren’s syndrome: association with type-1 diabetes mellitus. Br J Rheumatol. 1989;28:518–520. doi: 10.1093/rheumatology/28.6.518. [DOI] [PubMed] [Google Scholar]
- 18.Delaleu N, Jonsson R, Koller MM. Sjogren’s syndrome. Eur J Oral Sci. 2005;113:101–113. doi: 10.1111/j.1600-0722.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 19.Forster G, Krummenauer F, Kuhn I, Beyer J, Kahaly G. Polyglandular autoimmune syndrome type II: epidemiology and forms of manifestation. Dtsch Med Wochenschr. 1999;124:1476–1481. doi: 10.1055/s-2008-1035684. [DOI] [PubMed] [Google Scholar]
- 20.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 21.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emani R, Asghar MN, Toivonen R, Lauren L, Soderstrom M, Toivola DM, van Tol EA, Hanninen A. Casein hydrolysate diet controls intestinal T cell activation, free radical production and microbial colonisation in NOD mice. Diabetologia. 2013;56:1781–1791. doi: 10.1007/s00125-013-2941-x. [DOI] [PubMed] [Google Scholar]
- 24.Hansen CH, Krych L, Buschard K, Metzdorff SB, Nellemann C, Hansen LH, Nielsen DS, Frokiaer H, Skov S, Hansen AK. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes. 2014;63:2821–2832. doi: 10.2337/db13-1612. [DOI] [PubMed] [Google Scholar]
- 25.Toivonen RK, Emani R, Munukka E, Rintala A, Laiho A, Pietila S, Pursiheimo JP, Soidinsalo P, Linhala M, Eerola E, Huovinen P, Hanninen A. Fermentable fibres condition colon microbiota and promote diabetogenesis in NOD mice. Diabetologia. 2014;57:2183–2192. doi: 10.1007/s00125-014-3325-6. [DOI] [PubMed] [Google Scholar]
- 26.Hoorfar J, Buschard K, Dagnaes-Hansen F. Prophylactic nutritional modification of the incidence of diabetes in autoimmune non-obese diabetic (NOD) mice. Br J Nutr. 1993;69:597–607. doi: 10.1079/bjn19930059. [DOI] [PubMed] [Google Scholar]
- 27.Visser J, Brugman S, Klatter F, Vis L, Groen H, Strubbe J, Rozing J. Short-term dietary adjustment with a hydrolyzed casein-based diet postpones diabetes development in the diabetes-prone BB rat. Metabolism. 2003;52:333–337. doi: 10.1053/meta.2003.50052. [DOI] [PubMed] [Google Scholar]
- 28.Visser JT, Bos NA, Harthoorn LF, Stellaard F, Beijer-Liefers S, Rozing J, van Tol EA. Potential mechanisms explaining why hydrolyzed casein-based diets outclass single amino acid-based diets in the prevention of autoimmune diabetes in diabetes-prone BB rats. Diabetes Metab Res Rev. 2012;28:505–513. doi: 10.1002/dmrr.2311. [DOI] [PubMed] [Google Scholar]
- 29.Shehadeh N, Weis R, Teninboum G, Benderly A, Etzioni A, Shamir R. The influence of oral insulin on the development of autoimmune diabetes in NOD mice fed a hypoallergenic diet. Diabetes Nutr Metab. 2004;17:1–5. [PubMed] [Google Scholar]
- 30.Hermitte L, Atlan-Gepner C, Payan MJ, Mehelleb M, Vialettes B. Dietary protection against diabetes in NOD mice: lack of a major change in the immune system. Diabete Metab. 1995;21:261–268. [PubMed] [Google Scholar]
- 31.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, De SC, Di MU, Falorni A, Boirivant M, Dotta F. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 32.Burrows MP, Volchkov P, Kobayashi KS, Chervonsky AV. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc Natl Acad Sci U S A. 2015;112:9973–9977. doi: 10.1073/pnas.1508740112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina TV. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35:135–145. doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spachidou MP, Bourazopoulou E, Maratheftis CI, Kapsogeorgou EK, Moutsopoulos HM, Tzioufas AG, Manoussakis MN. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren’s syndrome. Clin Exp Immunol. 2007;147:497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyriakidis NC, Kapsogeorgou EK, Gourzi VC, Konsta OD, Baltatzis GE, Tzioufas AG. Toll-like receptor 3 stimulation promotes Ro52/TRIM21 synthesis and nuclear redistribution in salivary gland epithelial cells, partially via type I interferon pathway. Clin Exp Immunol. 2014;178:548–560. doi: 10.1111/cei.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado MA, Kakkanaiah V, MacDonald GC, Chen F, Reap EA, Balish E, Farkas WR, Jennette JC, Madaio MP, Kotzin BL, Cohen PL, Eisenberg RA. The role of environmental antigens in the spontaneous development of autoimmunity in MRL-lpr mice. J Immunol. 1999;162:6322–6330. [PubMed] [Google Scholar]