Abstract
Introduction
Intravesical therapy is a valuable option in the clinical management of urinary tract disorders such as interstitial cystitis/ painful bladder syndrome (IC/PBS) and refractory overactive bladder. This review will cover the latest advances in this field using polymer and liposomes as delivery platform for drugs, protein and nucleic acids.
Areas covered
This review summarizes the significance of intravesical therapy for lower urinary tract disorders. The recent advancement of liposomes as a drug delivery platform for botulinum toxin, tacrolimus and small interfering RNA is discussed. The importance of polymers forming indwelling devices and hydrogels are also discussed, where all preparations improved efficacy parameters in rodent models. Clinical experience of treating IC/PBS with indwelling devices and liposomes are summarized and preclinical evidence about the downregulation of target gene expression in rodent bladder with liposomes complexed with siRNA is also reviewed.
Expert opinion
There have been several advances in the field of intravesical therapy for improving clinical outcomes. One of the most promising research avenues is the repurposing of drugs, given previously by other routes of administration, such as tacrolimus. Intravesical therapy also opens up novel therapeutic targets with improved efficacy and safety for underactive bladder.
Keywords: DMSO, Intravesical, Lidocaine, liposomes, NGF, Tacrolimus, onabotulinum toxin
1. Introduction
In simplest terms, intravesical therapy describes drug instillation directly into the bladder following insertion of catheter into urethra. This approach is widely used in eradication of bladder cancer and for preventing recurrences and cancer progression1. Moreover, in recent decades, intravesical therapy has also garnered attention in clinical management of non-cancerous lower urinary tract disorder such as IC/PBS2, radiation cystitis3 and refractory overactive bladder4. Intravesical therapy is frequently used as an adjunct to oral treatment regimens or as second line treatment5 in clinical management. The lining of urinary bladder also known as urothelium is highly impermeable6, 7, which offers unique opportunities as well as challenges in drug delivery. Impermeability of urothelium for large molecular weight drugs typically limits the opportunity for systemic distribution after instillation and leads to lower side effects (8 Fig. 1). Gene and protein delivery, are all relatively new areas of research in intravesical therapy that have recently gained momentum using physical (e.g., iontophoresis) or pharmaceutical techniques (e.g., liposomes, hydrogel, polymers and nanoparticles).
Figure 1.

Instillation of liposomes tagged with near infrared (NIR) dye into mouse bladder via transurethral catheter demonstrates that impermeability barrier restricts the systemic distribution of large molecular weight instilled agents. The mouse in the picture was instilled with 20 picomoles (pmoles) of NIR lipophilic dye (carbocyanine dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide and 25 minutes after instillation, 50% of instilled dose is lost in first urine void after instillation. Epifluorescence emanating from NIR dye allows sequential non-invasive assessment of the bio-distribution and demonstrates the advantage of localized drug effect with intravesical therapy. The bladder uptake of instilled agents is dependent on molecular weight and physicochemical characteristics. Incidentally, biodistribution of a small molecular weight tertiary amine drug, oxybutynin (Mol. wt. 357) after bladder instillation is in direct contrast to the distribution of large molecular weight NIR dye (Mol. wt. 1013). Tissue distribution area of the epifluorescence increases in the right picture, which indicates diffusion of instilled payload to the lamina propria and detrusor muscle.
2. Therapeutic significance
Mounting evidence has established that bladder urothelium expresses a host of receptors, which can be leveraged for exclusive therapeutic action in bladder via intravesical drug delivery. Examples of such receptors include muscarinic receptors9, cannabinoid receptors, growth factor receptors10. Moreover, mechanism of action for instilled drugs can be independent of orally administered drugs and an additive effect in certain cases can open up new commercial avenues11. Intravesical therapy permits administration of low doses to the desired diseased site and eliminate delivery to healthy tissues elsewhere. The only FDA approved use of DMSO, dimethyl sulphoxide12 is for IC/PBS, which illustrates that selective delivery of drugs to diseased site drastically increases the acceptability of potential risk from instilled agents. Since 1978, 50% DMSO is marketed as (Rimso-50) as an intravesical treatment of IC/PBS12. Symptomatic relief is a derivative of the anti-inflammatory and mast cell stabilizing properties of DMSO13. DMSO is also known to stimulate bladder afferent pathways and induce NO release from afferent neurons which can desensitize nociceptive pathways14. Ampipathic DMSO can disrupt hydrogen bonds, affect intercellular electrical uncoupler and scavenge hydroxyl radicals which can trigger inflammation15, 16. In a controlled crossover trial of 33 patients with biopsies suggestive of IC, DMSO was superior to placebo in objective and subjective improvement of patients17. Treatment was associated with a low frequency of serious adverse effects with only rare reports of systemic contact dermatitis and eosinophillic cystitis18, 19. DMSO is generally administered weekly for at least 6 weeks, either in the clinic or at home, by patients capable of self-catheterization20.
Intravesical instillation of prostaglandins has also been explored as a treatment of cyclophosphamide-induced cystitis21–23 and underactive bladder (UAB)24. A natural glycosaminoglycan (GAG), hyaluronic acid is instilled to replace the purportedly deficient GAG layer in IC/PBS25 and this approach is approved in Europe26 and Canada27. Encouraging results in IC/PBS patients prompted the recent exploration of hyaluronic acid as a treatment for recurrent bacterial cystitis28.
3. Major challenges to Intravesical therapy
Intravesical therapy faces challenges on several fronts including impermeability of the urothelium, and washout of instilled drugs by urine29 is linked to reduced duration of action of instilled drugs. First challenge is the variable and incomplete therapy response due to poor penetration of instilled drugs through the urothelium. A layer of GAG covalently attached to cell membrane proteins of urothelium30 is known to impede the permeability of instilled drugs.
3.1 Strategies to increase permeability
Several approaches have been attempted in order to overcome the bladder permeability barrier in the treatment of bladder cancer and various lower urinary tract disorders. One such approach is electromotive drug administration (EMDA), which combines the advantages of iontophoresis and electroporation. The procedure is performed under lidocaine anesthesia applied to the bladder. After transurethral delivery of drug in the bladder, a catheter electrode is inserted inside the bladder surface and another is placed on abdomen to deliver direct electrical current pulses. Intravesical electromotive administration increased the bladder uptake of mitomycin and improved its response rate in patients with high risk superficial bladder cancer31. The EMDA approach was recently tried for enhancing the delivery of hyaluronic acid in 31 IC/PBS patients. EMDA mediated delivery of hyaluronic acid was compared against bladder instillation of hyaluronic acid with follow-up at 12 and 24 months. EMDA group showed significant decrease in VAS score and 24h micturition frequency at 6 and 12 months compared to hyaluronic acid alone26, but the difference was not retained at the 24 month analysis.
The known effect of DMSO on cellular permeability architecture was exploited to enhance the paclitaxel penetration across the swine urothelium following intravesical delivery of cremophor micelles32. Recently, R11, a cell-permeable peptide enhanced the penetration of a fluorescent probe into mouse bladder33 and R11 was found to be localized in the lamina propria of the bladder wall 24h after instillation.
3.2 Strategies to prolong the duration of drug action
Since patients have to empty the bladder after every episode of drug instillation, a typical bladder residence time for instilled drugs is only of 2h34, 35. Frequent drug administration is therefore necessary to increase the duration of drug action and overcome the drug washout by voiding. Instilled drug also faces the risk of dilution from constant urine production. The problem of urine dilution can be reduced by complete bladder emptying just before dose administration and rate of urine production can be reduced by restricting fluid intake29.
Increased concentration in urine can improve the efficacy of drug by acting in the bladder without significant enhancement in toxicity29. Studies show that concentration gradient is an important variable in bladder uptake of instilled drugs as there is linear relationship between drug concentration in urine and the drug penetration into bladder tissue36. In a recent phase III trial, the concentration gradient driving the passive diffusion of mitomycin from urine to bladder urothelium was increased with reduced water intake of enrolled patients37. Consequently, the penetration of mitomycin C across the bladder urothelium was enhanced, which nearly doubled the recurrence-free rate in superficial bladder cancer patients. Enhanced penetration of mitomycin was associated with significantly higher dysuria, which did not cause treatment discontinuation29. There was no change in hematologic toxicity with enhanced penetration of mitomycin.
As indicated by bladder retention of ~40% of instilled NIR dye 3h after instillation (Fig. 1), liposomes also have the potential to extend the residence time of instilled agents, beyond the first voiding event after instillation. The increased retention could be due to enhanced uptake and improved adherence of liposomes to the bladder surface. Other groups have used polymers to extend the bladder residence time of drugs and thereby duration of action. Polymeric devices or polymeric hydrogel38 can trap the drug and extend its delivery into the bladder. Indwelling bladder devices can act as a reservoir for drugs and circumvent the need for frequent instillations in patients. Polymers can allow fabrication of both resorbable and non-resorbable microdevices, which require cystoscope guided insertion and then removal from patients. Use of elastomeric polymers in fabrication of device can help sculpt a cylindrical shape that is streamlined for easy instillation, which after insertion reverses to a different retentive shape39. Several factors influence the diffusion mediated temporal release of the drug from device including aqueous permeability of the polymer, rate of polymer degradation and size of the orifice that releases the drug40.
In recent years, elastomeric polymers have been used to fabricate a continuous lidocaine-releasing intravesical system (LiRIS) for sustained delivery of lidocaine inside the bladder of 16 IC/PBS patients for a period of 2 weeks39. Lidocaine is contained in powdered form instead of solution in the small sized device41. Freely floating device in bladder was generally well tolerated by healthy volunteers and IC/BPS patients. 16 women meeting the National Institute of Diabetes and Digestive and Kidney Diseases criteria for IC/BPS consented for the insertion of device delivering total lidocaine dose of 200 mg or 650 mg39. At two weeks, treated patients reported clinically meaningful reductions in pain, urgency, voiding frequency, and disease questionnaires. Cystoscopic examinations showed resolution of Hunner’s lesions in five out of six subjects. Response rate of 64% measured by global response assessment were retained for 2 weeks after removal of the device. Further follow-up suggested that device responders retained the reduced pain response for longer durations.
Clearly, a mechanical bladder drug delivery device capable of sustained drug delivery has immense potential in clinical management of IC/PBS. Earlier attempts towards a bladder drug delivery device encountered the problem of encrustration, stone formation, infection, irritation, obstruction and hematuria after bladder insertion42. These adverse outcomes were probably related to the constant irritation from a foreign object inside the bladder. Recent advances in indwelling devices have overcome these drawbacks and further advances are necessary to improve the viability of indwelling devices for intravesical drug delivery.
Thermosensitive hydrogel offer another alternative for sustained drug delivery in bladder. A temperature sensitive biodegradable triblock polymer poly(ethylene glycol-b-[DL-lactic acid-co-glycolic acid]-b-ethylene glycol) (PEG-PLGA-PEG)43 was modified for bladder instillation44. The aqueous solution of polymer exhibits temperature dependent non-newtonian fluid behavior, which allows simple dispersion of drug at room temperature and serves the function of drug retentive device at body temperature, when instilled polymer solution converts into a gel44. The understood mechanism of gelation for block polymers is the concept of micelle packing and entanglement. The triblock copolymers form micelles at room temperature which equilibrate with unimers. However at body temperature there is tremendous increase in micelle volume fraction, which causes gelation due to micelle packing.
Recently, a thermosensitive biodegradable hydrogel capable of urine floatation was tested. Gel was composed of polyoxyethylene–polyoxypropylene–polyoxyethylene (PEOn–PPOn–PEOn) triblock copolymers called Poloxamer 407 and baking soda, which degrades in acidic pH of urine45. The gel was able to sustain the release of Adriamycin for 3 h after instillation without causing urinary obstruction. Poloxamer 407 has a molecular weight of 12,000 Da and at concentrations above 20% in urine it exhibits reversible thermal gelation. In a similar study, Pluronic F127 polymer formed a thermosensitive hydrogel for intravesical administration of liposomes and monomethoxy poly (ethylene glycol)-poly(ε-caprolactone) hybrid nanoparticles46. In another study, hydrogel was formed by co-polymerization of poly (N-isopropylacrylamide) with hyaluronic acid and gelatin47. Hydrogel formed by an 8% aqueous dispersion of co-polymer extended the dwell time of cisplatin in bladder. Hydrogel appear to be a preferred delivery platform for sustained intravesical drug delivery.
3.4 Strategies to improve bladder uptake of instilled drugs
Several drug delivery approaches have been tried to improve bladder uptake by increasing water solubility and bio-compatibility. Other approaches co-opted the physiologic process of endocytosis/ exocytosis occurring periodically in bladder. The trafficking occurs at the bladder surface for periodic expansion of bladder volume to accommodate more urine. It is known that expansion of bladder lining require addition of membrane surface by exocytosis of membrane vesicles in order to maintain impermeability48. It was earlier presumed that vesicle morphology of liposomes will allow them to co-opt the endocytosis process for bladder for drug penetration. Recently, ultrastructural and flow cytometry studies on cultured cells of human urothelium cell line demonstrated that clathrin mediated endocytosis49 is the predominant mechanism of large multi-lamellar liposomes into bladder (Fig. 2). In earlier studies, large multilamellar liposomes were reported to have greater affinity than sonicated small sized liposomes50, 51 for bladder cells. Liposomes can coopt the vesicular trafficking of urothelium and consequently aid in improving the delivery of drug cargo across the bladder permeability barrier.
Figure 2.
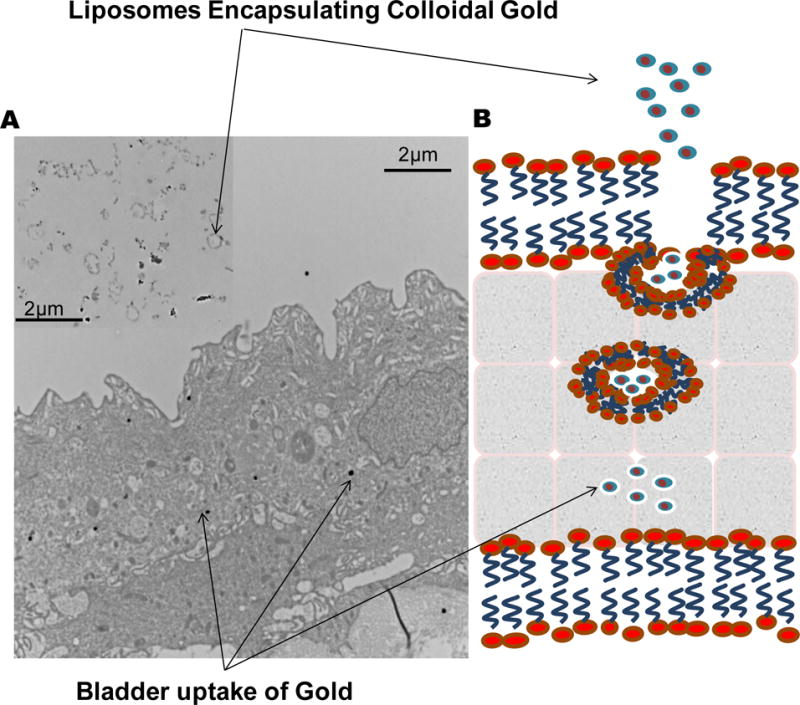
Liposomes are taken up by bladder via endocytosis as illustrated in panel B. Transmission Electron Microscope pictures of rat bladder after instillation of liposomes encapsulating gold support endocytosis as a mechanism in panel A. Gold appears as electron dense dark spots in TEM pictures. Instilled liposomes are shown in the inset for panel A. Endocytosis of liposomes in cell cultures was sensitive to treatment with chlorpromazine, suggesting predominant role of clathrin mediated endocytosis.
4. Empty Liposomes
Liposomes have been extensively studied as a drug delivery platform for altering pharmacokinetics and reducing toxicity of chemotherapeutic agents52–54. Liposomes composed entirely of endogenous bioactive lipids were shown to have therapeutic effect in dry eye55, 56 and in lower urinary tract disorders57.
4.1 Clinical Assessment of Liposomes
The safety and efficacy of intravesical empty liposomes was compared against orally administered pentosan polysulfate in an open label prospective study on 24 IC/PBS patients58. Patients were assigned to a 4 week treatment arm of either once weekly 40 mL instillation of 80mg liposomes or 3 times daily oral pentosan polysulfate (100 mg). Statistically significant decrease in urinary frequency and nocturia observed in intravesical liposome treatment arm was comparable to oral treatment of pentosan polysulfate. The therapeutic effect of liposomes was most profound on urgency with statistically significant decreases in pain, urgency and the O’Leary-Sant symptom score. A recent open label, single treatment arm study published by Peters et al corroborated the earlier results with significantly decreased pain, urgency and symptom scores in IC/PBS patients after 4 weekly instillations of liposomes57. Administration of endogenous phospholipids was also shown to be effective in patients with chronic active ulcerative colitis59.
4.2 Liposomes + Botulinum Neurotoxin
Botulinum onabotulinumtoxinA (BoNT-A) is approved as a treatment for overactive bladder OAB by cystoscope guided injections into bladder. Injected BoNT-A is taken up by neurons after binding with synaptic vesicle glycoprotein 2 to cleave the synaptosomal-associated protein, 25 kDa (SNAP25) that is responsible for neuroexocytosis of acetylcholine and other neurotransmitter from nerves. OAB patients responding to BoNT show reduced detrusor overactivity and urgency60, 61, which is correlated with downregulation of purinergic receptor P2X3 and transient receptor potential vanilloid receptor subfamily 1 (TRPV1) on suburothelial sensory fibers.
Symptom relief with BoNT-A is also associated with increased postvoid residual urine volume and urinary tract infection UTI62. It is likely that adverse effects of BoNT-A share the same mechanism as its therapeutic effect and therefore could be reduced with a different delivery approach. Animal studies using a different isoform of botulinum toxin showed that liposomes were able to enhance the efficacy at lower doses63. Likewise, biochemical studies showed that metalloproteolytic activity of BoNT-A analogue was strongly enhanced by the presence of lipid membranes64. Since liposomes possess both aqueous and non-aqueous (lipid) environment, they can be used for delivery both fat-soluble and water soluble toxins. Therefore, liposomes were considered to develop a liquid instillation of BoNT-A for increasing the acceptability of risk from this treatment by reducing adverse effects.
Chuang et al formed a liquid instillation solution of BoNT-A by entrapping it with liposomes65. Liposome mediated transport of BoNT-A into urothelium was confirmed by immunohistochemical detection of its unique effect on neurotransmitters by proteolysis of synaptosomal-associated protein SNAP-2565. Liposomes limited the BoNT-A delivery to mucosa and restricted the delivery to detrusor muscle, which could potentially reduce the risk of incomplete bladder emptying in clinic. The lack of efficacy of BoNT-A instillation in absence of liposomes66 was presumed to be related to the action of proteases and proteinases in urine causing BoNT-A protein degradation, dilution of BoNT-A in urine or poor uptake of the BoNT-A into the urothelium.
Encouraged by animal results, Kuo et al tested the clinical efficacy of liposomal delivery of BoNT in 24 OAB patients4 in a randomized double-blind parallel controlled trial. The dose of empty liposomes was same as used earlier in patients by Chuang et al58 and empty liposomes were complexed with 200 IU of BoNT-A. Enrolled patients either received saline or liposome complexed with BoNT-A, which was held in bladder for 60 min. Patients were allowed to drink water and distend their bladders for another 30 min. Instillation of BoNT-A with liposomes significantly decreased the 3-day voiding frequency from baseline with median decrease of 6.5 voiding episodes from baseline. In contrast, saline instilled patients had no change from baseline. Significant decrease was also noted in urgency episodes, but not in urgency urinary incontinence episodes. There were no reports of large PVR, urinary retention, or UTI in treated patients during the follow-up period.
4.3 Liposomes +Tacrolimus
Hemorrhagic cystitis is a rare and potentially fatal disease that results from damage to the bladder transitional epithelium and blood vessels by specific chemotherapeutic agents and/or pelvic radiation therapy. Liposomes formulated with tacrolimus were recently tested as a potential treatment for hemorrhagic cystitis. Single dose application of liposomal tacrolimus significantly reduced the inflammation and voiding changes associated with chemotherapy-induced hemorrhagic cystitis67. Successful delivery of tacrolimus to the bladder also decreased the prostaglandin EP4 receptor expression in urothelium and infiltration of inflammatory cells in sub-urothelium region. Increased bladder tissue and urine levels of PGE2 and IL-2 were also suppressed by liposomal tacrolimus. Blood analysis of treated rats indicated that systemic levels of tacrolimus were below the detection limit of the (<1.5 ng/ml) clinically approved assay for tacrolimus68. Liposomal tacrolimus also showed promise in reducing inflammation and improving voiding outcomes in a recent study on rat model of radiation cystitis69. Studies show that bladder instillation of liposomal tacrolimus is a promising treatment of hemorrhagic cystitis without the risks associated with the systemic distribution of tacrolimus.
4.4 Liposomes +Nucleic acids
Unlike the approaches described earlier, this form of therapy is not intended to bind with a protein in a reversible or irreversible fashion, instead nucleic acids are delivered in bladder for blocking the synthesis of target protein by sequence-specific silencing of the respective mRNA using either antisense oligonucleotides (ODN) or small interfering siRNA therapeutics. ODN can be characterized as chemically modified stretches of single-strand DNA, which form RNA/DNA duplexes with complementary sequences of target mRNA and thereby inhibit translation. Research using this approach in urology has lagged behind the tremendous progress made in developing clinical therapies for oncology and ophthalmology70.
The primary hindrance in intravesical antisense therapeutics is the inefficient uptake of the ODN after bladder instillation. The bladder permeability for ODN is reduced in cancerous state, but still a local dose of 2.5 mg/kg was needed for the native ODN to enter the bladder wall71. It is known that tight junctions in urothelium are compromised in cancerous condition72–74 and therefore the strategy of relying on concentration gradient may not work in non-cancerous diseased condition.
Need for high dose illustrates that linear relationship between drug concentration in urine and the importance of concentration gradient in bladder tissue penetration36 is true for both small molecule drugs and large molecular weight nucleic acids. The ability of intravesical route to restrict systemic uptake shown in Fig. 1 by NIR dye was also shown by uptake of radioactive tag on instilled ODN. Radioactivity measurement showed that cancerous bladder wall accumulated up to 12 times higher amounts of ODN than the content in plasma71.
Several studies have shown that uptake of nucleic acids by non-cancerous bladder is inefficient at lower concentrations75. However, after complexation with liposomes, the bladder uptake of siRNA and ODN was significantly improved even at lower concentrations75, 76. As shown in Fig. 2, liposomes rely on endocytosis to improve bladder uptake of ODN76 and siRNA75 loaded liposomes in healthy bladder wall and downregulate the expression of nerve growth factor (NGF)76 and pannexin75. These two target proteins have pathophysiological relevance in voiding dysfunctions such as IC/PBS and UTI. In order to demonstrate functional efficacy of ODN and siRNA75, NGF and pannexin overexpression in bladder was induced by temporary exposure to acetic acid and lipopolysaccharide, respectively. Animals were pretreated with ODN76 and siRNA75 before the exposure to insults and treatment efficacy was confirmed by functional and molecular endpoints.
In an earlier study, small interfering siRNA complexed with liposomes successfully prevented the growth of bladder cancer in mouse model by reducing PLK-1 expression77. In contrast to siRNA, small activating RNAs (saRNAs) are a new class of double-stranded RNA molecules that can induce RNA expression by mechanisms including transcriptional activation. These agents are useful for overcoming the reduced protein expression in disease states. It is known that oncologic suppression of p53 gene leads to decreased expression of p21, a cyclin-dependent kinase inhibitor. Therefore, delivery of saRNA to p21 into mouse bladder using lipid nanoparticles achieved 40% tumor regression rate in the treated mice78.
4.5 Cell targeting with liposomes
As indicated earlier cellular uptake of liposomes is dependent on endocytosis and therefore uptake may differ in cell types differing in endocytosis machinery. Specific proteins and receptors expressed by different cell types in urinary tract can be targeted with liposomes attached with targeting ligands for respective receptors. Targeting diseased cells in urothelium can be achieved with intravesical delivery, but deeper tissue layers in bladder encounter additional barrier to entry before cell specific targeting can be achieved. Epidermal growth factor receptor expressed in bladder carcinoma was targeted with intravesical radioimmunotherapy with 213Bi-anti-EGFR-Mab and the approach holds promise in treatment of advanced bladder carcinoma79. Another potential target is ephrins are increasingly implicated in carcinogenesis as they mediate signals via tyrosine kinase activity that modulates diverse physiologic and developmental processes. Normal human urothelium typically expresses ephrin EphB2, but expression of EphB4 in predominant in cancerous epithelium80. The differential in expression induced by cancer can be leveraged for drug targeting. Glycan-specific targeting is another option for preferential binding of instilled agents to cancerous cells.
Increased angiogenesis at sites of inflammation and ulcer in IC/PBS can be targeted with ligands for Vascular endothelial growth factor receptor (VEGFR)10. The expression of VEGF receptors, VEGFR-1 and VEGFR-2, as well as VEGF coreceptors neuropilins (NRP) NRP1 and NRP2 is increased in diseased urothelium relative to normal urothelium. Bladder instillation of a targeted fluorescent tracer10, an engineered single-chain VEGF labeled with Cy5.5 dye (scVEGF/Cy) showed preferential accumulates at sites of bladder inflammation and ganglia.
5. Nanoparticles
Several recent reports highlight the efforts in application of nanotechnology for intravesical therapy. Nanocarriers of cytotoxic agents of favorable size and capable of adhering to urothelium can potentially prolong the duration of action and decrease toxicity. Nanoparticles made up of poly (lactide-co-glycolide) (PLGA) are frequently used for intravesical drug and gene delivery because of regulatory approval and biocompatibility and biodegradability of PLGA. Survivin is highly expressed in bladder cancer cells and therefore it was the target gene for siRNA mediated downregulation. The inefficient bladder uptake of native siRNA was overcome in a recent study by encapsulating the survivin siRNA in chitosan coated PLGA nanoparticles81. Double emulsion solvent evaporation technique was used for making nanoparticles and then coated with chitosan in order to improve the bioadhesion of nanoparticles. Instead of chitosan, poly(guanidinium oxanorbornene) was used for surface modification of PLGA nanoparticles in a separate study. Surface coated nanoparticles showed ten-fold higher bladder uptake compared to uncoated nanoparticles82.
As illustrated in Fig. 1, nanoparticles restricted the uptake of mitomycin into systemic circulation after bladder instillation. Chitosan coated poly-ε-caprolactone nanoparticles of mitomycin extended the survival of rats with bladder tumor83. In another study delivery of docetaxel to bladder tumor was enhanced by mucoadhesive nanoparticles formed by hyperbranched polyglycerols84. Nanoparticles increased the permeability of docetaxel across urothelium and also increased uptake into the animal tumor tissue. Albumin bound nanoparticles of paclitaxel were recently instilled in 18 refractory bladder cancer patients. Nanoparticles successfully eradicated any evidence of disease in 5 patients without inducing any toxicity85. Nanotechnology was recently applied in benign disorders for improving the delivery of anti-inflammatory quercetin in E. coli induced acute cystitis. Lipophilicity of quercetin for intravesical delivery was improved by its encapsulation in biodegradable monomethoxy poly(ethylene glycol)-poly(ε-caprolactone) micelles formed by self-assembly86. Quercetin loaded nanoparticles efficiently reduced the edema and inflammatory cell infiltration in infective cystitis.
6. Conclusions
There have been notable advancements in the application of drug delivery approaches for improving intravesical therapy of lower urinary tract disorders. The platform of liposomes and in dwelling devices has certainly improved clinical outcomes in IC/PBS and OAB patients. Various drug delivery platforms discussed here can be leveraged to improve intravesical drug delivery. The progress of nanotechnology in bladder cancer field can be instructive in advancing intravesical therapy for benign lower urinary tract disorders.
7. Expert opinion
The current mainstay of clinical management of lower urinary tract disorders is oral drug therapy with antimuscarinics. However, their use is often compromised by bothersome side effect of dry mouth and CNS side effects. Furthermore, only a limited portion of parent drug often reaches the disease site of bladder due to extensive metabolism. Disposition of systemically administered drugs can therefore often explain the lack of efficacy in lower urinary tract disorders, and the consequent reduced patient adherence.
Therefore, the development of intravesical therapies is an attractive approach, which has the potential to directly target disease sites in lower urinary tract, while avoiding systemic side effects. Intravesical route utilizes the outside anatomical access available for drug delivery directly to the disease site in bladder and thereby avoid unwanted exposure of the instilled drug to healthy tissues elsewhere in the body. The anatomical accessibility as well as the ability of intravesical route to restrict drug effect (Fig. 1) has facilitated the development of lethal toxins and toxic solvents into pharmaceutical drugs such as resinferatoxin, botulinum toxin and DMSO. A very selective and limited endocytic activity of urothelial cells and unique structural and functional configuration of urothelium is responsible for the poor systemic uptake of instilled agents.
The urothelial permeability barrier limits the tissue penetration of drugs and is a major limiting factor in intravesical therapy. Depending on physicochemical characteristics of instilled drug, studies demonstrate that only 1–7% of instilled dose reaches the systemic circulation after instillation87, 88. Instilled drugs also avoid the first pass effect of orally administered drugs89. Avoidance of first pass effect significantly increased the bioavailability of intravesical oxybutynin relative to orally administered oxybutynin in a cross-over trial. Therefore, intravesical oxybutynin is a viable option for patients who cannot tolerate oral oxybutynin due to side effects89.
Few advanced delivery concepts have so far been evaluated to overcome the characteristic constraints of intravesical route. Bladder residence time of 2h is typical for instilled drugs due to the patient need to empty the bladder after instillation34, 35. Behavior modifications such as reduced water intake can be beneficial to intravesical drug delivery. Indwelling intravesical devices are a major advance in this field, which allow formation of a drug depot in bladder that resists expulsion during voiding. Therefore, polymeric devices offer the advantage of extending the drug exposure for up to weeks. In dwelling device certainly extended the duration of lidocaine effect when compared to lidocaine instillation alone or as part of drug cocktail90. Lidocaine blocks the conduction from sensory nerves in the bladder without affecting the underlying bladder inflammation. Therefore, pain relief from lidocaine delivery likely altered the central sensitization in nociceptive pathways emanating from bladder, which can explain the long term relief. Thermosensitive hydrogels, formed by block polymers in water, constitute an interesting class of materials that can be developed specifically for intravesical therapy.
The biocompatibility, stimuli responsiveness to various external factors, and functionalization capacity are important characteristics of polymers for designing intravesical therapy. Biocompatibility is central to the success of intravesical drug delivery as illustrated by the efficacy of empty liposomes composed of endogenous lipids in IC/PBS patients and the interest in PLGA for nanoparticles. In clinical studies, once a week instillation of liposomes in IC/PBS patients performed with efficacy similar to thrice daily oral administration of pentosan polysulfate58. Bladder instillation of liposomes may be a reasonable alternative for IC/PBS patients with poor treatment adherence and having trouble remembering medication schedule.
This review article examined the strategies that have been utilized to improve the bioavailability after intravesical administration. Compared to isolated use of empty liposomes as therapeutic agents in eye and bladder, liposomes are more generally used as a drug delivery platform. Endocytosis of liposomes is the primary mode of bladder uptake and different mechanisms of endocytosis can become operational depending on the size of the liposomes. Small sized liposomes may be taken up in bladder by a lipid raft-mediated pathway, which is sensitive to inhibition by methyl-beta-cyclodextrin. These endocytic pathways are also shared by cell permeable peptide to deliver the payload of drugs inside cell91.
Liposomes and cell permeable peptides utilize the transcellular approach for intravesical drug delivery and their reliance on endocytosis often traps the delivered drug in endosomes. Another alternative to transcellular approach is the paracellular delivery, which occurs when drug is delivered via the tight junctions and lateral intercellular spaces. Both transcellular and paracellular approach can benefit from improved bioadhesion of instilled agents. Future research in the field of intravesical gene silencing will be focused on improving the accessibility of nucleic acids to the cytoplasm by enhancing endosomal escape of nucleic acids and/or reducing exocytosis of nucleic acids to the external milieu.
Liposomes served as a delivery platform for BoNT-A in refractory OAB patients, where liposomes allowed BoNT-A to better penetrate the urothelial barrier due to their biophysical structure. The penetration of liposomal BoNT-A was compared against BoNT-A in saline in the study. There are no other reports of drug delivery platforms being used for BoNT-A, other than a clinical study using DMSO as a carrier which lacked a control group92. The action of BoNT-A on downregulation of TRPV1 is also shared by another neurotoxin called resinferatoxin (RTX). Recent meta-analysis of intravesical RTX treatment for IC/PBS and detrusor overactivity was performed through a comprehensive search of 2,332 records93. It was found that RTX could significantly reduce bladder pain and increase maximum cystometric capacity, but did not improve frequency, nocturia, incontinence or first involuntary detrusor contraction.
It is my opinion that FDA approval of cystoscope guided BoNT-A injection may also end up advancing the cause of gene therapy of lower urinary tract disorders. There are several diseases such as underactive bladder94, that manifest loss of function of key proteins in bladder. We predict that such diseases can be treated by injection of plasmid encoding key neurotrophin proteins in bladder. Bladder cancer is another disease that can benefit from gene insertion of p53, cytokines95 and tight junction proteins74 . IC/PBS can also potentially benefit from transfection of tight junction proteins96. Transfection of exogenous gene can be increased with help of liposomes or viral vectors. Size of transgene is an important determinant in the efficiency of gene transfection with a viral vector.
Intravesical therapy holds immense potential in meeting the unmet therapeutic need of underactive bladder (UAB)97. Most patients of UAB routinely perform clean intermittent catheterization and will find intravesical therapy more acceptable than patients of other conditions. UAB is detrusor contraction of inadequate strength and/or duration resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying in the absence of urethral obstruction. Definition of UAB may imply dysfunction of the detrusor muscle, but the underlying pathophysiologic abnormality could also be in the generation and integration of afferent input from bladder, which may explain the age dependent loss of bladder volume sensitivity98.
Afferent nerve fibers originating from bladder convey the stimulus of bladder distension, which initiates the activity in low threshold mechanoreceptive afferents during storage and also convey the magnitude of detrusor contractions during micturition. Therefore, the activation and maintenance of the micturition response are dependent upon normal relay of afferent information from the bladder to higher brain centers. Detrusor contraction is also potentiated by urethral afferents responding to urine flow and therefore bladder and urethral afferent dysfunction in diabetic patients can lead to UAB.
Since nociceptors play an important role in generating the afferent input from bladder, drugs that activate nociceptors such as TRPV1, TRPV499 and transient receptor potential ankyrin 1 (TRPA 1) have the potential to initiate or hasten voiding in UAB patients. Activation of TRP channels on afferent nerves in the bladder induces the release of neurokinins, which increase detrusor contractility. Drugs acting on neurotrophin receptors tyrosine kinase A, TrkA, TrkB and TrkC are another alternative to enhance the afferent input from bladder and neurotrophic activity100–102. Moreover, drugs activating TRP receptors are only likely to receive regulatory approval for topical use, as systemic administration of these agents is likely to induce pain. Therefore, there are several currently approved and unapproved drugs that have the potential103, 99 for repositioning as an intravesical treatment for UAB.
Since acetylcholine is an important neurotransmitter for both afferent and efferent signaling of micturition, therefore irreversible inhibitors of acetylcholinesterase enzyme have potential as intravesical treatment for UAB. Intravesical instillation of these agents in UAB can achieve the inverse of BoNT in terms of pharmacological action but with similar durability of action. Autonomous detrusor activity detected during storage phase of micturition facilitates the generation of bladder sensation and absence of spontaneous contractions can hinder the initiation of afferent signals, which can lead to neurogenic UAB from impaired afferent arm. The autonomous detrusor activity can be safely increased by instillation of drugs binding to HCN or KCNQ channels, whereas oral or systemic administration of these agents will encounter intolerable safety concerns.
Considering the drawbacks of existing therapies, newer advances are necessary for effective treatment of lower urinary tract disorders. One of the reasons intravesical therapy has been slow to advance is the lack of consensus on magnitude of treatment response in preclinical studies that would be clinically meaningful. Heterogeneity in study designs makes head to head comparison difficult for determining the superiority of one therapy versus the other. Other major problem is the difficulty faced in accruing patients for clinical trials to demonstrate the benefit of intravesical delivery over other delivery routes in terms of efficacy, toxicity and quality of life for patients with chronic diseases. The repeated catheterizations may be a cause of infection and irritation during voiding in certain treated patients.
Existing therapeutic outcome measures for intravesical therapies rely heavily on subjective impressions of patients leading to high heterogeneity in clinical response. Urine biomarkers and imaging methods can act as surrogates of treatment response and allow objective assessment of intravesical therapies. Objective assessment can not only provide the biological basis for the lack of response but also help in clinical decision-making. Availability of new tools can allow clinicians to propose a specific and tailored treatment to each patient with limiting systemic side effects.
Upregulated chemokine expression in bladder biopsy of IC/PBS patients104 indicates that lower urinary tract diseases are sustained by numerous cell surface receptors, and release of chemokines, cytokines. Cell culture studies have shown that cytokines and chemokines can reflect the ongoing autocrine, paracrine and endocrine signaling activity in basal and stressful state of urothelium and detrusor cells105. Correlation of with bladder tissue levels of chemokines with urine levels104 support the argument of urine as an easy to collect, information-rich biofluid that can provide surrogate markers for concurrent monitoring of lower urinary tract diseases106–108.
Lack of difference in tissue biopsy of responder and non-responders in a recent study4 illustrates the shortcomings of bladder biopsy in clinical trials. Tissue biopsy information is heavily dependent on site and wrong site selection can have undue influence on the findings. This fact is also borne out by lack of correlation between urothelial tissue NGF levels and OAB symptoms109, but evidence of correlation was found on urine measurement of NGF110–112. Differences in bladder biopsy and urine measurement highlight difficulty in selecting the right matrix for measuring surrogate markers. Urine levels of another neurotrophin, brain derived neurotrophic factor (BDNF) were also reported to be elevated in OAB patients113. Taken together114, 115 reported findings led us to postulate that proteins secreted into urine from lower urinary tract make them well suited for phenotyping patients and identify relationships among clinical features and outcomes.
We have recently seen intravesical therapy of hyaluronic acid being applied to indications other than interstitial cystitis, such as bacterial cystitis. The entry of bio-similars is likely to add incentive and renew interest in this kind of product differentiation for intravesical route. Intravesical therapy can also open up novel therapeutic targets with improved efficacy and safety for underactive bladder.
Article highlights.
Intravesical therapy is a valuable option in management of IC/PBS and refractory OAB.
Low systemic uptake of drugs given intravesically can allow potentially toxic solvents and drugs to be approved by the FDA.
Bioavailability of intravesically administered drugs is a function of molecular weight and physiochemical properties.
Recent advances in intravesical drug delivery aim to extend the duration of action and efficacy of drugs instilled in bladder.
In dwelling devices and liposomes have shown promise in clinical studies.
Liposomes offer the potential of cell specific drug delivery and realizing the potential of gene silencing in urology.
Intravesical therapy can increase the acceptability of risk: benefit ratio for new therapies to meet the unmet therapeutic need for UAB
Efforts are needed to advance the treatment of IC/PBS and UAB
Acknowledgments
This work was partly supported by NIH grant DK088836
Footnotes
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Zargar H, Aning J, Ischia J, et al. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nature reviews Urology. 2014 Apr;11(4):220–30. doi: 10.1038/nrurol.2014.52. [DOI] [PubMed] [Google Scholar]
- 2.Tyagi P, Kashyap MP, Kawamorita N, et al. Intravesical Liposome and Antisense Treatment for Detrusor Overactivity and Interstitial Cystitis/Painful Bladder Syndrome. ISRN pharmacology. 2014;2014:601653. doi: 10.1155/2014/601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajaganapathy BR, Jayabalan N, Tyagi P, et al. Advances in Therapeutic Development for Radiation Cystitis. LUTS: Lower Urinary Tract Symptoms. 2014;6(1):1–10. doi: 10.1111/luts.12045. [DOI] [PubMed] [Google Scholar]
- 4.Kuo HC, Liu HT, Chuang YC, et al. Pilot study of liposome-encapsulated onabotulinumtoxina for patients with overactive bladder: a single-center study. Eur Urol. 2014 Jun;65(6):1117–24. doi: 10.1016/j.eururo.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis–a practical approach. Urology. 1997 May;49(5A Suppl):105–7. doi: 10.1016/s0090-4295(97)00181-7. [DOI] [PubMed] [Google Scholar]
- 6.Eldrup J, Thorup J, Nielsen SL, et al. Permeability and ultrastructure of human bladder epithelium. Br J Urol. 1983 Oct;55(5):488–92. doi: 10.1111/j.1464-410x.1983.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 7.Parsons CL, Boychuk D, Jones S, et al. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol. 1990 Jan;143(1):139–42. doi: 10.1016/s0022-5347(17)39897-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman J, Hensley H, Jacobs J, et al. Non-Invasive Imaging Of Near Infrafred Dye Labeled Liposomes Facilitates Evaluation Of Bioresidence Time. J Urol. 2010;183(4):e628. doi: 10.1016/j.juro.2010.02.1406. [DOI] [Google Scholar]
- 9.Tyagi S, Tyagi P, Van-le S, et al. Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol. 2006 Oct;176(4 Pt 1):1673–8. doi: 10.1016/j.juro.2006.06.088. S0022-5347(06)01533-3 [pii] 10.1016/j.juro.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 10.Saban MR, Backer JM, Backer MV, et al. VEGF receptors and neuropilins are expressed in the urothelial and neuronal cells in normal mouse urinary bladder and are upregulated in inflammation. Am J Physiol Renal Physiol. 2008 Jul;295(1):F60–72. doi: 10.1152/ajprenal.00618.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Yoshimura N, Masuda H, et al. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder-afferent pathways. Urology. 2005 Feb;65(2):238–42. doi: 10.1016/j.urology.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Shirley SW, Stewart BH, Mirelman S. Dimethyl sulfoxide in treatment of inflammatory genitourinary disorders. Urology. 1978 Mar;11(3):215–20. doi: 10.1016/0090-4295(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 13.Ghoniem GM, McBride D, Sood OP, et al. Clinical experience with multiagent intravesical therapy in interstitial cystitis patients unresponsive to single-agent therapy. World J Urol. 1993;11(3):178–82. doi: 10.1007/BF00211416. [DOI] [PubMed] [Google Scholar]
- 14.Birder LA, Kanai AJ, de Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol. 1997 Nov;158(5):1989–95. doi: 10.1016/s0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- 15.Childs SJ. Dimethyl sulfone (DMSO2) in the treatment of interstitial cystitis. Urol Clin North Am. 1994 Feb;21(1):85–8. [PubMed] [Google Scholar]
- 16.Santos NC, Figueira-Coelho J, Martins-Silva J, et al. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003 Apr 1;65(7):1035–41. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988 Jul;140(1):36–9. doi: 10.1016/s0022-5347(17)41478-9. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988 Feb;124(2):182–3. [PubMed] [Google Scholar]
- 19.Abramov Y, Goldberg RP, McGuire M, et al. Eosinophilic cystitis after bladder instillation with dimethyl sulfoxide. Urology. 2004 Jun;63(6):1182–3. doi: 10.1016/j.urology.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Biggers RD. Self-administration of dimethyl sulfoxide (DMSO) for interstitial cystitis. Urology. 1986 Jul;28(1):10–1. doi: 10.1016/0090-4295(86)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Shurafa M, Shumaker E, Cronin S. Prostaglandin F2-alpha bladder irrigation for control of intractable cyclophosphamide-induced hemorrhagic cystitis. J Urol. 1987 Jun;137(6):1230–1. doi: 10.1016/s0022-5347(17)44463-6. [DOI] [PubMed] [Google Scholar]
- 22.Trigg ME, O’Reilly J, Rumelhart S, et al. Prostaglandin E1 bladder instillations to control severe hemorrhagic cystitis. J Urol. 1990 Jan;143(1):92–4. doi: 10.1016/s0022-5347(17)39875-0. [DOI] [PubMed] [Google Scholar]
- 23.Miller LJ, Chandler SW, Ippoliti CM. Treatment of cyclophosphamide-induced hemorrhagic cystitis with prostaglandins. Ann Pharmacother. 1994 May;28(5):590–4. doi: 10.1177/106002809402800508. [DOI] [PubMed] [Google Scholar]
- 24.Hindley RG, Brierly RD, Thomas PJ. Prostaglandin E2 and bethanechol in combination for treating detrusor underactivity. BJU Int. 2004 Jan;93(1):89–92. doi: 10.1111/j.1464-410x.2004.04563.x. [DOI] [PubMed] [Google Scholar]
- 25.Lai MC, Kuo YC, Kuo HC. Intravesical hyaluronic acid for interstitial cystitis/painful bladder syndrome: a comparative randomized assessment of different regimens. Int J Urol. 2013 Feb;20(2):203–7. doi: 10.1111/j.1442-2042.2012.03135.x. [DOI] [PubMed] [Google Scholar]
- 26.Gulpinar O, Haliloglu AH, Gokce MI, et al. Instillation of Hyaluronic Acid via Electromotive Drug Administration Can Improve the Efficacy of Treatment in Patients With Interstitial Cystitis/Painful Bladder Syndrome: A Randomized Prospective Study. Korean journal of urology. 2014 May;55(5):354–9. doi: 10.4111/kju.2014.55.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulpinar O, Kayis A, Suer E, et al. Clinical comparision of intravesical hyaluronic acid and hyaluronic acid-chondroitin sulphate therapy for patients with bladder pain syndrome/interstitital cystitis. Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2014 Sep;8(9–10):E610–4. doi: 10.5489/cuaj.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zabkowski T, Jurkiewicz B, Saracyn M. Treatment of Recurrent Bacterial Cystitis by Intravesical Instillations of Hyaluronic Acid. Urology journal. 2015;12(3):2192–5. [PubMed] [Google Scholar]
- 29.Au JL, Badalament RA, Wientjes MG, et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst. 2001 Apr 18;93(8):597–604. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- 30.Hurst RE, Zebrowski R. Identification of proteoglycans present at high density on bovine and human bladder luminal surface. J Urol. 1994 Nov;152(5 Pt 1):1641–5. doi: 10.1016/s0022-5347(17)32495-3. [DOI] [PubMed] [Google Scholar]
- 31.Di Stasi SM, Giannantoni A, Stephen RL, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol. 2003 Sep;170(3):777–82. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Song D, Wientjes MG, et al. Effect of dimethyl sulfoxide on bladder tissue penetration of intravesical paclitaxel. Clin Cancer Res. 2003 Jan;9(1):363–9. [PubMed] [Google Scholar]
- 33.Hsieh JT, Zhou J, Gore C, et al. R11, a novel cell-permeable peptide, as an intravesical delivery vehicle. BJU Int. 2011 Nov;108(10):1666–71. doi: 10.1111/j.1464-410X.2011.10185.x. [DOI] [PubMed] [Google Scholar]
- 34.Gontero P, Cattel L, Paone TC, et al. Pharmacokinetic study to optimize the intravesical administration of gemcitabine. BJU Int. 2010 Dec;106(11):1652–6. doi: 10.1111/j.1464-410X.2010.09496.x. [DOI] [PubMed] [Google Scholar]
- 35.Palou J, Carcas A, Segarra J, et al. Phase I pharmacokinetic study of a single intravesical instillation of gemcitabine administered immediately after transurethral resection plus multiple random biopsies in patients with superficial bladder cancer. J Urol. 2004 Aug;172(2):485–8. doi: 10.1097/01.ju.0000131770.14409.7f. [DOI] [PubMed] [Google Scholar]
- 36.Gao X, Au JL, Badalament RA, et al. Bladder tissue uptake of mitomycin C during intravesical therapy is linear with drug concentration in urine. Clin Cancer Res. 1998 Jan;4(1):139–43. [PubMed] [Google Scholar]
- 37.Au JL, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. J Control Release. 2002 Jan 17;78(1–3):81–95. doi: 10.1016/s0168-3659(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 38.Elman NM, Patta Y, Scott AW, et al. The next generation of drug-delivery microdevices. Clin Pharmacol Ther. 2009 May;85(5):544–7. doi: 10.1038/clpt.2009.4. clpt20094 [pii] 10.1038/clpt.2009.4. [DOI] [PubMed] [Google Scholar]
- 39.Nickel JC, Jain P, Shore N, et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Science translational medicine. 2012 Jul 18;4(143):143ra00. doi: 10.1126/scitranslmed.3003804. [DOI] [PubMed] [Google Scholar]
- 40.Farokhzad OC, Dimitrakov JD, Karp JM, et al. Drug delivery systems in urology–getting “smarter”. Urology. 2006 Sep;68(3):463–9. doi: 10.1016/j.urology.2006.03.069. S0090-4295(06)00499-7 [pii]10.1016/j.urology.2006.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Cima MJ. An intravesical device for the sustained delivery of lidocaine to the bladder. J Control Release. 2011 Jan 20;149(2):133–9. doi: 10.1016/j.jconrel.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Knudsen BE, Chew BH, Denstedt JD. Drug-eluting biomaterials in urology: the time is ripe. BJU Int. 2005 Apr;95(6):726–7. doi: 10.1111/j.1464-410X.2005.05388.x. [DOI] [PubMed] [Google Scholar]
- 43.Jeong B, Bae YH, Kim SW. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res. 2000 May;50(2):171–7. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Tyagi P, Li Z, Chancellor M, et al. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharmaceutical research. 2004 May;21(5):832–7. doi: 10.1023/b:pham.0000026436.62869.9c. [DOI] [PubMed] [Google Scholar]
- 45.Lin T, Zhang Y, Wu J, et al. A floating hydrogel system capable of generating CO2 bubbles to diminish urinary obstruction after intravesical instillation. Pharm Res. 2014 Oct;31(10):2655–63. doi: 10.1007/s11095-014-1362-y. [DOI] [PubMed] [Google Scholar]
- 46.Men K, Liu W, Li L, et al. Delivering instilled hydrophobic drug to the bladder by a cationic nanoparticle and thermo-sensitive hydrogel composite system. Nanoscale. 2012 Oct 21;4(20):6425–33. doi: 10.1039/c2nr31592k. [DOI] [PubMed] [Google Scholar]
- 47.Chen JP, Leu YL, Fang CL, et al. Thermosensitive hydrogels composed of hyaluronic acid and gelatin as carriers for the intravesical administration of cisplatin. J Pharm Sci. 2011 Feb;100(2):655–66. doi: 10.1002/jps.22309. [DOI] [PubMed] [Google Scholar]
- 48.Truschel ST, Wang E, Ruiz WG, et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002 Mar;13(3):830–46. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajaganapathy BR, Chancellor MB, Nirmal J, et al. Bladder uptake of liposomes after intravesical administration occurs by endocytosis. PloS one. 2015;10(3):e0122766. doi: 10.1371/journal.pone.0122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frangos DN, Killion JJ, Fan D, et al. The development of liposomes containing interferon alpha for the intravesical therapy of human superficial bladder cancer. J Urol. 1990 Jun;143(6):1252–6. doi: 10.1016/s0022-5347(17)40248-5. [DOI] [PubMed] [Google Scholar]
- 51.Johnson JW, Nayar R, Killion JJ, et al. Binding of liposomes to human bladder tumor epithelial cell lines: implications for an intravesical drug delivery system for the treatment of bladder cancer. Sel Cancer Ther. 1989 Winter;5(4):147–55. doi: 10.1089/sct.1989.5.147. [DOI] [PubMed] [Google Scholar]
- 52.Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005 Oct;2(4):369–81. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 53.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995 Dec;13(12):527–37. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 54.Gregoriadis G, Allison AC. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett. 1974 Sep 1;45(1):71–4. doi: 10.1016/0014-5793(74)80813-6. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Dausch S, Maierhofer G, et al. A new therapy concept for the treatment of dry eye–the usefulness of phospholipid liposomes. Klin Monatsbl Augenheilkd. 2004 Oct;221(10):825–36. doi: 10.1055/s-2004-813715. [DOI] [PubMed] [Google Scholar]
- 56.Dausch D, Lee S, Dausch S, et al. [Comparative study of treatment of the dry eye syndrome due to disturbances of the tear film lipid layer with lipid-containing tear substitutes] Klin Monatsbl Augenheilkd. 2006 Dec;223(12):974–83. doi: 10.1055/s-2006-927266. [DOI] [PubMed] [Google Scholar]
- 57.Peters KM, Hasenau D, Killinger KA, et al. Liposomal bladder instillations for IC/BPS: an open-label clinical evaluation. International urology and nephrology. 2014 Dec;46(12):2291–5. doi: 10.1007/s11255-014-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chuang YC, Lee WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol. 2009 Oct;182(4):1393–400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 59.Stremmel W, Merle U, Zahn A, et al. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005 Jul;54(7):966–71. doi: 10.1136/gut.2004.052316. 54/7/966 [pii] 10.1136/gut.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006 Apr;49(4):644–50. doi: 10.1016/j.eururo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005 Sep;174(3):977–82. doi: 10.1097/01.ju.0000169481.42259.54. discussion 82–3. [DOI] [PubMed] [Google Scholar]
- 62.Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008 Jul;180(1):217–22. doi: 10.1016/j.juro.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandal M, Lee KD. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta. 2002 Jun 13;1563(1–2):7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 64.Caccin P, Rossetto O, Rigoni M, et al. VAMP/synaptobrevin cleavage by tetanus and botulinum neurotoxins is strongly enhanced by acidic liposomes. FEBS Lett. 2003 May 8;542(1–3):132–6. doi: 10.1016/s0014-5793(03)00365-x. [DOI] [PubMed] [Google Scholar]
- 65.Chuang YC, Tyagi P, Huang CC, et al. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J Urol. 2009 Aug;182(2):786–92. doi: 10.1016/j.juro.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 66.Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004 Oct;172(4 Pt 1):1529–32. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- 67.Chuang YC, Tyagi P, Huang HY, et al. Intravesical immune suppression by liposomal tacrolimus in cyclophosphamide-induced inflammatory cystitis. Neurourol Urodyn. 2011 Mar;30(3):421–7. doi: 10.1002/nau.20981. [DOI] [PubMed] [Google Scholar]
- 68.Nirmal J, Tyagi P, Chancellor MB, et al. Development of potential orphan drug therapy of intravesical liposomal tacrolimus for hemorrhagic cystitis due to increased local drug exposure. J Urol. 2013 Apr;189(4):1553–8. doi: 10.1016/j.juro.2012.10.123. [DOI] [PubMed] [Google Scholar]
- 69.Rajaganapathy BR, Janicki JJ, Levanovich P, et al. Intravesical Liposomal Tacrolimus Protects against Radiation Cystitis Induced by 3-Beam Targeted Bladder Radiation. J Urol. 2015 Apr 1; doi: 10.1016/j.juro.2015.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dadgostar H, Waheed N. The evolving role of vascular endothelial growth factor inhibitors in the treatment of neovascular age-related macular degeneration. Eye. 2008 Jun;22(6):761–7. doi: 10.1038/eye.2008.86. eye200886 [pii] 10.1038/eye.2008.86. [DOI] [PubMed] [Google Scholar]
- 71.Blietz CE, Thode B, Hauses M, et al. In vivo studies on the availability and toxicity of antisense oligonucleotides in bladder cancer. In vivo. 2009 Jan-Feb;23(1):13–9. [PubMed] [Google Scholar]
- 72.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009 Apr;1788(4):872–91. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Huygens A, Kamuhabwa AR, Roskams T, et al. Permeation of hypericin in spheroids composed of different grade transitional cell carcinoma cell lines and normal human urothelial cells. J Urol. 2005 Jul;174(1):69–72. doi: 10.1097/01.ju.0000162037.49102.56. [DOI] [PubMed] [Google Scholar]
- 74.Shorning BY, Griffiths D, Clarke AR. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PloS one. 2011;6(1):e16209. doi: 10.1371/journal.pone.0016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beckel JM, Daugherty SL, Tyagi P, et al. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. The Journal of physiology. 2015 Apr 15;593(8):1857–71. doi: 10.1113/jphysiol.2014.283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kashyap M, Kawamorita N, Tyagi V, et al. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013 Aug;190(2):757–64. doi: 10.1016/j.juro.2013.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nogawa M, Yuasa T, Kimura S, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005 Apr;115(4):978–85. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang MR, Yang G, Place RF, et al. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Res. 2012 Oct 1;72(19):5069–79. doi: 10.1158/0008-5472.CAN-12-1871. [DOI] [PubMed] [Google Scholar]
- 79.Fazel J, Rotzer S, Seidl C, et al. Fractionated intravesical radioimmunotherpy with Bi-anti-EGFR-MAb is effective without toxic side-effects in a nude mouse model of advanced human bladder carcinoma. Cancer Biol Ther. 2015 Jul 15;:0. doi: 10.1080/15384047.2015.1071735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X, Choi WW, Yan R, et al. The differential expression of EphB2 and EphB4 receptor kinases in normal bladder and in transitional cell carcinoma of the bladder. PloS one. 2014;9(8):e105326. doi: 10.1371/journal.pone.0105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin DT, Steinbach JM, Liu J, et al. Surface-modified nanoparticles enhance transurothelial penetration and delivery of survivin siRNA in treating bladder cancer. Molecular cancer therapeutics. 2014 Jan;13(1):71–81. doi: 10.1158/1535-7163.MCT-13-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin DT, Hoimes CJ, Kaimakliotis HZ, et al. Nanoparticles for urothelium penetration and delivery of the histone deacetylase inhibitor belinostat for treatment of bladder cancer. Nanomedicine. 2013 Nov;9(8):1124–34. doi: 10.1016/j.nano.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erdogar N, Iskit AB, Eroglu H, et al. Cationic core-shell nanoparticles for intravesical chemotherapy in tumor-induced rat model: safety and efficacy. Int J Pharm. 2014 Aug 25;471(1–2):1–9. doi: 10.1016/j.ijpharm.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 84.Mugabe C, Matsui Y, So AI, et al. In vivo evaluation of mucoadhesive nanoparticulate docetaxel for intravesical treatment of non-muscle-invasive bladder cancer. Clin Cancer Res. 2011 May 1;17(9):2788–98. doi: 10.1158/1078-0432.CCR-10-2981. [DOI] [PubMed] [Google Scholar]
- 85.McKiernan JM, Barlow LJ, Laudano MA, et al. A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guerin refractory nonmuscle invasive bladder cancer. J Urol. 2011 Aug;186(2):448–51. doi: 10.1016/j.juro.2011.03.129. [DOI] [PubMed] [Google Scholar]
- 86.Wang BL, Gao X, Men K, et al. Treating acute cystitis with biodegradable micelle-encapsulated quercetin. International journal of nanomedicine. 2012;7:2239–47. doi: 10.2147/IJN.S29416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klem B, Lappin G, Nicholson S, et al. Determination of the bioavailability of [14C]-hexaminolevulinate using accelerator mass spectrometry after intravesical administration to human volunteers. Journal of clinical pharmacology. 2006 Apr;46(4):456–60. doi: 10.1177/0091270006286849. [DOI] [PubMed] [Google Scholar]
- 88.Walter P, Grosse J, Bihr AM, et al. Bioavailability of trospium chloride after intravesical instillation in patients with neurogenic lower urinary tract dysfunction: A pilot study. Neurourol Urodyn. 1999;18(5):447–53. doi: 10.1002/(sici)1520-6777(1999)18:5<447::aid-nau6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 89.Krause P, Fuhr U, Schnitker J, et al. Pharmacokinetics of intravesical versus oral oxybutynin in healthy adults: results of an open label, randomized, prospective clinical study. J Urol. 2013 Nov;190(5):1791–7. doi: 10.1016/j.juro.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Parsons CL. Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis. Urology. 2005 Jan;65(1):45–8. doi: 10.1016/j.urology.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 91.Lee JE, Lim HJ. LDP12, a novel cell-permeable peptide derived from L1 capsid protein of the human papillomavirus. Mol Biol Rep. 2012 Feb;39(2):1079–86. doi: 10.1007/s11033-011-0834-y. [DOI] [PubMed] [Google Scholar]
- 92.Petrou SP, Parker AS, Crook JE, et al. Botulinum a toxin/dimethyl sulfoxide bladder instillations for women with refractory idiopathic detrusor overactivity: a phase 1/2 study. Mayo Clinic proceedings. 2009 Aug;84(8):702–6. doi: 10.1016/S0025-6196(11)60520-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo C, Yang B, Gu W, et al. Intravesical resiniferatoxin for the treatment of storage lower urinary tract symptoms in patients with either interstitial cystitis or detrusor overactivity: a meta-analysis. PloS one. 2013;8(12):e82591. doi: 10.1371/journal.pone.0082591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim JC, Park EY, Hong SH, et al. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol. 2005 Oct;12(10):875–80. doi: 10.1111/j.1442-2042.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 95.Kuball J, Wen SF, Leissner J, et al. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. Journal of Clinical Oncology. 2002;20(4):957–65. doi: 10.1200/JCO.2002.20.4.957. 2002/2/15. [DOI] [PubMed] [Google Scholar]
- 96.Slobodov G, Feloney M, Gran C, et al. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004 Apr;171(4):1554–8. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 97.Tyagi P, Smith PP, Kuchel GA, et al. Pathophysiology and animal modeling of underactive bladder. Int Urol Nephrol. 2014 Sep;46(Suppl 1):S11–21. doi: 10.1007/s11255-014-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith PP. Aging and the underactive detrusor: a failure of activity or activation? Neurourology and urodynamics. Mar;29(3):408–12. doi: 10.1002/nau.20765. [DOI] [PubMed] [Google Scholar]
- 99.Aizawa N, Wyndaele JJ, Homma Y, et al. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourology and urodynamics. 2012 Jan;31(1):148–55. doi: 10.1002/nau.21212. [DOI] [PubMed] [Google Scholar]
- 100.Jang SW, Liu X, Chan CB, et al. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS One. 2010;5(7):e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jang SW, Liu X, Chan CB, et al. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chemistry & biology. 2009 Jun 26;16(6):644–56. doi: 10.1016/j.chembiol.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Praveen Kumar V, Gajendra Reddy R, Vo DD, et al. Synthesis and neurite growth evaluation of new analogues of honokiol, a neolignan with potent neurotrophic activity. Bioorganic & medicinal chemistry letters. 2012 Feb 1;22(3):1439–44. doi: 10.1016/j.bmcl.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Cardenas DD, Ditunno JF, Graziani V, et al. Two phase 3, multicenter, randomized, placebo-controlled clinical trials of fampridine-SR for treatment of spasticity in chronic spinal cord injury. Spinal cord. 2014 Jan;52(1):70–6. doi: 10.1038/sc.2013.137. [DOI] [PubMed] [Google Scholar]
- 104.Corcoran AT, Yoshimura N, Tyagi V, et al. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013 Feb;31(1):241–6. doi: 10.1007/s00345-012-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bouchelouche K, Alvarez S, Horn T, et al. Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha. Urology. 2006 Jan;67(1):214–9. doi: 10.1016/j.urology.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 106.Jacobs BL, Smaldone MC, Tyagi V, et al. Increased nerve growth factor in neurogenic overactive bladder and interstitial cystitis patients. Can J Urol. 2010 Feb;17(1):4989–94. [PubMed] [Google Scholar]
- 107.Tyagi P, Barclay D, Zamora R, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010 Sep 26;42(3):629–35. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 108.Tyagi P, Killinger K, Tyagi V, et al. Urinary chemokines as noninvasive predictors of ulcerative interstitial cystitis. J Urol. 2012 Jun;187(6):2243–8. doi: 10.1016/j.juro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Birder LA, Wolf-Johnston A, Griffiths D, et al. Role of urothelial nerve growth factor in human bladder function. Neurourol Urodyn. 2007;26(3):405–9. doi: 10.1002/nau.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu HT, Chancellor MB, Kuo HC. Decrease of urinary nerve growth factor levels after antimuscarinic therapy in patients with overactive bladder. BJU Int. 2009 Jun;103(12):1668–72. doi: 10.1111/j.1464-410X.2009.08380.x. [DOI] [PubMed] [Google Scholar]
- 111.Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008 Jul;72(1):104–8. doi: 10.1016/j.urology.2008.01.069. discussion 08. [DOI] [PubMed] [Google Scholar]
- 112.Liu HT, Tyagi P, Chancellor MB, et al. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009 Jun 12; doi: 10.1111/j.1464-410X.2009.08675.x. BJU8675 [pii] 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- 113.Antunes-Lopes T, Carvalho-Barros S, Cruz CD, et al. Biomarkers in overactive bladder: a new objective and noninvasive tool? Advances in urology. 2011:382431. doi: 10.1155/2011/382431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Desireddi NV, Campbell PL, Stern JA, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008 May;179(5):1857–61. doi: 10.1016/j.juro.2008.01.028. discussion 61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agarwal M, He C, Siddiqui J, et al. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013 May;73(6):573–81. doi: 10.1002/pros.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]


