Abstract
Objective
Risk factors beyond tobacco smoking associated with chronic bronchitis are not well understood. We sought to describe the prevalence and risk factors of chronic bronchitis across four distinct settings in Peru with overall low prevalence of tobacco smoking yet varying degrees of urbanization, daily exposure to biomass fuel smoke and living at high altitude.
Methods
We analyzed data of 2,947 participants from rural and urban Puno, Lima and Tumbes including spirometry, blood samples, anthropometry and administered questionnaires about respiratory symptoms. We used multivariable Poisson regression to assess biologic, socioeconomic and environmental risk factors associated with chronic bronchitis.
Results
Overall prevalence of chronic bronchitis was 5.9% (95%CI 5.1%–6.9%) with variation by setting: prevalence was lower in semi-urban Tumbes (1.3%) vs. highly urbanized Lima (8.9%), urban Puno (7.0%) and rural Puno (7.8%; p<0.001). Chronic bronchitis was more common among participants with vs. without COPD based on FEV1/FVC< LLN (12.1% vs 5.6%, p<0.01) and it was associated with increased reporting of dyspnea on exertion (p<0.001), hospitalization (p=0.003) and workdays missed due to respiratory symptoms (p<0.001). Older age (Prevalence ratio [PR]=1.23 for each 10-years of age, 95%CI 1.09–1.40) past history of asthma (PR=2.87, 95%CI 1.80–4.56), urbanization (PR=3.34, 95%CI 2.18–5.11) and daily exposure to biomass fuel smoke (PR=2.00, 95%CI 1.30–3.07) were all associated with chronic bronchitis.
Conclusions
We found important variations in the prevalence of chronic bronchitis across settings. Prevalence increased with both urbanization and with daily exposure to biomass fuel smoke. Having chronic bronchitis was also associated with worse patient-centered outcomes including dyspnea, hospitalization and missed workdays.
Keywords: Chronic bronchitis, biomass fuel, pollution, urbanization, Peru, COPD, respiratory disease, tobacco
INTRODUCTION
Chronic respiratory illness is a leading cause of disease burden in low- and middle-income countries (LMICs), with chronic obstructive pulmonary disease (COPD) as an important cause of death worldwide.1,2 Chronic bronchitis, defined as chronic phlegm production or productive cough for three consecutive months per year for at least two consecutive years,3 is considered to be a clinical disease phenotype of COPD and a possible predictor of worse morbidity and mortality.4,5 However, the clinical and pathologic understanding of COPD is evolving.6,7 There is evidence that different disease phenotypes (chronic bronchitis, emphysema and asthma) are associated with unique risk factors and necessitate targeted interventions and therapies.5,7,8 The chronic bronchitis phenotype is also described in populations without spirometric evidence of COPD and is thought to represent a population at risk for COPD.9 The presence of chronic bronchitis among patients with and without COPD has been associated with accelerated decline in lung function, increased COPD exacerbations, hospitalizations, comorbidities and mortality when compared those without chronic bronchitis.2,10
Although tobacco smoking is widely recognized as the most important risk factor for chronic bronchitis,10 it is unclear if the declining prevalence of tobacco smoking worldwide has had any effect on the prevalence of chronic bronchitis.5,11,12 Reports from population-based studies performed in the United States and Europe describe chronic bronchitis prevalence ranging from 3% to 22% in the general population of adults and from 14% to 74% among those with COPD.5,13,14 There is more limited data regarding the prevalence of chronic bronchitis in LMICs. The PLATINO study in Latin America15 and the work of Lu et al in China identified rates of chronic bronchitis similar to that found in the United States and Europe. Thus far, studies in LMICs have also demonstrated that tobacco smoking remains a major risk factor of chronic bronchitis; however, risk factors beyond tobacco smoking that may be associated with chronic bronchitis are not well characterized. If smoking rates are declining, but chronic bronchitis remains a public health burden, then risk factors beyond tobacco smoking must be further investigated.16 We conducted a large cohort study to describe the prevalence of chronic bronchitis and associated risk factors among individuals with and without COPD in four distinct settings with a low prevalence of tobacco smoking and varying degrees of urbanization and exposure to indoor air pollution.
METHODS
Design and setting
The CRONICAS study is a longitudinal, population-based study aimed to determine the prevalence and risk factors for chronic pulmonary and cardiovascular disease across four disparate regions in Peru. The study protocol was described elsewhere.17 The four settings under study varied based on degrees of urbanization, living at high altitude, prevalence of daily biomass fuel use and ambient air pollution levels (Table 1). Pampas de San Juan is a dense, urbanized community 25 kilometers south of central Lima and consists primarily of Andean immigrants. Tumbes is a semi-urban sea-level community in northern Peru within an agrarian setting and little to no vehicular traffic.17 Puno is a southwestern city in the Andes, located on the shores of Lake Titicaca at 3,825 meters above sea-level. Within Puno there are two separate sites: an urban setting located at the city center and a rural setting made up of inhabitants from surrounding communities.
Table 1.
Description of study site characteristics and environmental exposures.
| Altitude | Setting | Vehicular traffic | Development | Indoor pollution | Mean Outdoor PM2.5 concentrations (μg/m3) available from other sources | |
|---|---|---|---|---|---|---|
| Lima | Sea level | Dense shantytown outside center of Lima | Dense throughout the day (50) | Paved and dirt roads | Primarily influenced by outdoor air pollution | 37.5 (50) |
| Urban Puno | 3,825 m elevation | Dense city center | Dense between 7am–7pm, otherwise minimal | Primarily paved roads | Primarily influenced by outdoor air pollution | 23.0 (32) |
| Rural Puno | 3,825 m elevation | Farming communities far from city center | Little to none; bicycles and walking are the primary forms of transportation | All dirt roads | Indoor cookstoves | Not available |
| Tumbes | Sea level | Semi-urban setting with enrollment predominantly from farming communities far from city center | Little to none; bicycles and walking are the primary forms of transportation | Primarily dirt roads | Outdoor or semi-indoor cookstoves | Not available |
Study Participants
We identified a sex- and age-stratified random sample of adults aged ≥35 years. We aimed to enroll 1000 subjects per site. In Puno, we stratified recruitment to include 500 participants each from the urban and rural settings.17,18 Exclusion criteria were pregnancy, physical disability and active pulmonary tuberculosis. Fieldworkers obtained verbal consent from study participants prior to enrollment. The study was approved by the Institutional Review Boards at Universidad Peruana Cayetano Heredia and A.B. PRISMA in Lima, Peru, and the Johns Hopkins Bloomberg School of Public Health in Baltimore, USA.
Questionnaire and clinical data
Data was collected using a modified version of WHO STEPS approach questionnaire for surveillance of non-communicable disease.14,19 Data collected included several factors potentially associated with chronic lung disease, such as age, sex, years of education, occupation, demographic information, other socioeconomic variables, smoking habits, biomass fuel use and self-reported medical conditions.17,20 Patients were asked specifically about presence of cough and sputum production. Trained technicians measured weight, height and blood pressure as previously described.17 Fasting blood samples were obtained and analyzed in a single facility using a standardized approach. Plasma glucose was measured using an enzymatic colorimetric method (GOD-PAP, Modular P-E/Roche-Cobas, Germany).
Spirometry
Methods for spirometry were described in detail elsewhere.21 Briefly, spirometry was conducted using the Easy-On-PC spirometer (ndd, Zurich, Switzerland) before and after 200 mcg of inhaled salbutamol via a spacer. Trained technicians measured pre- and post-bronchodilator spirometry in participants following joint American Thoracic Society and European Respiratory Society (ATS/ERS) guidelines.22 We adapted a standardized grading system for quality control, review, and interpretation.23 Participants with low quality spirometry were asked to repeat the test on another day for a total of three attempts. Overall, 96% of tests achieved an acceptable result and 95% met ATS/ERS criteria.
Household assessment of particulate matter
Indoor air pollution assessment was described in detail elsewhere.24 Briefly, we measured indoor particulate matter (PM) concentrations for a 24-hour period in one-minute intervals in a subset of 100 homes at each site using pDR-1000 (Thermo Fisher Scientific, Waltham, MA) in passive mode and adjusted for relative humidity. Empirical evidence suggests that the pDR-1000 detects particles in the size range of 0.3 to 2 μm more efficiently than those of 2 to 10 μm.26 Quintana et al reported a high degree of correlation between the PM determined by the pDR-1000 and PM2.5 (i.e., 2.5μm in size) measurements.25 Thus, PM concentrations measured with the pDR-1000 is an accurate approximation for PM2.5 concentrations. Particle monitors were placed 1.5–2.0 meters off the ground and 0.5–1.0 meters away from the stoves. For each study site, we calculated median values of the 24-hour average PM indoor concentrations using the one-minute intervals for each household; and, median values of the peak PM concentrations from the averages of 10-minute intervals for each household. We did not measure outdoor air pollution concentrations.
Definitions
We defined chronic bronchitis as having self-reported phlegm production for at least three months each year in two successive years.9 We repeated our analyses using a second definition that included both cough and phlegm production for at least three months each year in two successive years 26,27 given existing literature uses these different definitions such that our results could be compared concordantly (See Online Supplement). We defined COPD as a post-bronchodilator FEV1/FVC less than the lower limit of normal (LLN) for a given age, sex, and height using the GLI mixed ethnic population reference equation.28 Since there are no established reference equations for lung function in Peruvians, we utilized both the NHANES III Mexican-Americans29 and the Global Lung Function Initiative (GLI) mixed ethnic population reference equations28 to determine percent-predicted forced expiratory volumes. To compare our results to previous studies that also used a fixed threshold, we included our findings defining COPD as post-bronchodilator FEV1/FVC < 70% (Online Supplement).30,31 We defined reversibility as an improvement of greater than 12% and 200 mL in forced expiratory volumes.
We created a wealth index according to assets, household facilities, household income and occupation.20 We defined daily smoking as having at least 1 cigarette a day and pack-years as the reported number of packs (i.e., 20 cigarettes) per day multiplied by the number of years smoked; hypertension as having systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, self-report of a physician diagnosis or current use of anti-hypertensive medications; diabetes as a fasting glucose ≥126 mg/dL, self-report of physician diagnosis and current use of anti-hyperglycemic medications; and, metabolic syndrome based on region-specific cut-offs.15,32 Air pollution exposure was classified as daily biomass fuel use, urban dwelling, or neither.
Biostatistical methods
We calculated prevalence of chronic bronchitis and corresponding 95% confidence intervals for the entire cohort, and stratified by setting and COPD status. We used multivariable Poisson regression to model the prevalence of chronic bronchitis as a function of age, sex, hypertension, body mass index (BMI), history of asthma and post-treatment tuberculosis, pack-years of smoking, daily biomass fuel use, wealth index, living in an urban setting, and living at high altitude. Of 2,947 participants with complete spirometry data, 29 (<1%) participants were missing information on chronic bronchitis. We performed analyses in STATA 12 (StataCorp, College Station, Texas, USA) and R (www.r-project.org).
RESULTS
Participant characteristics
We enrolled 4,325 participants of which 3,601 completed a questionnaire. A total of 2,947 were considered to have complete data and included in further analyses. There were no significant differences in age (p=0.62), sex (p =0.41), daily smoking (p=0.53), biomass fuel use (p=0.73), and reported history of pulmonary tuberculosis (p=0.30) or asthma (p=0.28) between participants with and without complete data on cough, phlegm production, and spirometry.
A total of 175 participants had symptoms consistent with chronic bronchitis. Daily smoking was low at only 3.3% of all participants (Table 2). Participants with chronic bronchitis were slightly older, had a higher prevalence of hypertension, past history of asthma and post-treatment tuberculosis, and were more likely to report dyspnea on exertion, hospitalizations and missed workdays due to respiratory symptoms than those without chronic bronchitis. There were no differences in prevalence of chronic bronchitis by sex. The prevalence of diabetes and metabolic syndrome was low among those with chronic bronchitis compared to those without, however the prevalence of obesity was similar in both groups. We also analyzed characteristics of chronic bronchitis among participants with and participants without COPD and found similar trends (Table 3). Both among participants with and without COPD, those with chronic bronchitis were older, had a higher prevalence of asthma, and were more likely to report dyspnea on exertion and missed workdays due to respiratory symptoms versus those without chronic bronchitis. Participants without COPD who met criteria for chronic bronchitis were more likely to live at high altitude, live in a rural setting and were more likely to report increased hospitalization in the last year due to respiratory symptoms versus those who did not have chronic bronchitis. We also evaluated each site based on environmental exposures including indoor air pollution, biomass fuel use and smoking prevalence (Table 4).
Table 2.
Characteristics chronic bronchitis and non-chronic bronchitis in 2947 subjects.
| Total Cohort | ||||
|---|---|---|---|---|
| n (%) or Mean ± SD | Non-Chronic bronchitis (n=2,772) | Chronic bronchitis (n=175) | Total | P |
| COPD (FEV1/FVC < LLN) | 124 (4.5) | 17 (9.7) | 141 (4.8) | <0.01 |
| COPD (FEV1/FVC < 70%) | 155 (5.6) | 21 (12.0) | 176 (6.0) | <0.001 |
| Male | 1,358 (49.0) | 93 (53.1) | 1,451 (49.2) | 0.29 |
| Mean Age (SD), in years | 55.2 ± 12.3 | 59.3 ± 13.0 | 55.4 ± 12.4 | <0.001 |
| 35–44 years | 687 (24.8) | 30 (17.1) | 717 (24.3) | <0.001 |
| 45–54 years | 737 (26.6) | 34 (19.4) | 771 (26.2) | |
| 55–64 years | 708 (25.5) | 50 (28.6) | 758 (25.7) | |
| ≥65 years | 640 (23.1) | 61 (34.8) | 701 (34.9) | |
| Height (cm) | 156.5 ± 8.7 | 155.4 ± 8.4 | 156.4 ± 8.7 | 0.13 |
| Weight (kg) | 68 ± 12.8 | 65.7 ± 11.2 | 68.0 ± 12.7 | 0.01 |
| High Altitude | 929 (33.5) | 74 (42.3) | 1,003 (43.1) | 0.02 |
| Rural | 1,274 (46.0) | 105 (60.0) | 1,379 (46.9) | <0.001 |
| Current Daily Smoker | 95 (3.4) | 2 (1.1) | 97 (3.3) | 0.10 |
| Body Mass Index (BMI) | ||||
| Low (BMI < 18 kg/m2) | 14 (0.5) | 2 (1.1) | 2 (0.5) | 0.33 |
| Normal (BMI 18–24 kg/m2) | 761 (27.5) | 51 (29.1) | 51 (29.1) | |
| Overweight (BMI 25–29 kg/m2) | 1,228 (44.3) | 81 (46.3) | 81 (46.3) | |
| Obese (BMI ≥ 30 kg/m2) | 769 (27.7) | 41 (23.4) | 41 (23.4) | |
| Wealth Index | ||||
| Low | 842 (30.4) | 56 (32.0) | 898 (30.5) | 0.84 |
| Middle | 955 (34.5) | 61 (34.9) | 1,016 (34.5) | |
| Upper | 975 (35.2) | 58 (33.1) | 1,033 (35.0) | |
| Exposure | ||||
| Cooks Indoors | 1,837 (66.7) | 122 (69.7) | 1,959 (66.8) | 0.40 |
| Farmer | 101 (3.7) | 12 (6.9) | 113 (3.8) | 0.03 |
| Miner | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0.80 |
| Cook | 5 (0.2) | 1 (0.5) | 6 (0.2) | 0.27 |
| Worker | 98 (3.5) | 7 (4.0) | 105 (3.6) | 0.75 |
| Biomass Fuel for | 739 (26.7) | 51 (29.1) | 790 (26.8) | 0.47 |
| Cooking at least Daily | ||||
| Clinical Data | ||||
| hs-CRP log mg/L | 0.5 ± 1.1 | 0.9 ± 1.2 | 0.5 ± 1.1 | 0.74 |
| Diabetes | 188 (6.9) | 5 (2.9) | 193 (6.7) | 0.04 |
| Dyslipidemia (LDL ≥ 160 mg/dL) | 447 (16.4) | 22 (12.6) | 469 (16.2) | 0.19 |
| Metabolic syndrome | 1,292 (47.5) | 76 (43.7) | 1,368 (47.2) | 0.33 |
| Hypertension | 544 (19.6) | 42 (24.0) | 586 (19.9 | 0.16 |
| History of hypertension | 516 (18.6) | 44 (25.1) | 560 (19.0) | 0.03 |
| History of Heart disease | 105 (3.8) | 12 (6.9) | 117 (4.0) | 0.04 |
| History of Stroke | 12 (0.4) | 1 (0.6) | 13 (0.4) | 0.79 |
| History of Tuberculosis | 74 (2.7) | 12 (6.9) | 86 (2.9) | <0.001 |
| History of Asthma | 91 (3.3) | 23 (13.1) | 114 (3.9) | <0.001 |
| History of Chronic Bronchitis | 85 (3.1) | 32 (18.3) | 117 (4.0) | <0.001 |
| History of COPD (i.e., previous diagnosis by physician) | 4 (0.14) | 0 (0.0) | 4 (0.1) | 0.83 |
| History of Emphysema | 1 (0.04) | 0 (0.0) | 1 (0.0) | 0.94 |
| Symptoms | ||||
| Dyspnea on exertion | 212 (7.7) | 53 (30.3) | 265 (9.0) | <0.001 |
| Hospitalization in last 12 months due to respiratory symptoms | 14 (0.5) | 4 (2.3) | 18 (0.6) | <0.01 |
| Missed work days in the last 12 months due to respiratory symptoms | 54 (2.0) | 19 (10.9) | 73 (2.5) | <0.001 |
Table 3.
Characteristics of chronic bronchitis vs. non-chronic bronchitis among participants with chronic obstructive pulmonary disease (post-bronchodilator FEV1/FVC <LLN)
| non-COPD (FEV1/FVC ≥ LLN) | COPD (FEV1/FVC <LLN) | |||||
|---|---|---|---|---|---|---|
| non- Chronic bronchitis | Chronic bronchitis | P | non-Chronic bronchitis | Chronic bronchitis | P | |
| Sample size, n (%) | 2,648 (94.4) | 158 (5.6) | 124 (87.3) | 17 (12.1) | ||
| Demographics and Anthropometry | ||||||
| Male, n (%) | 1,368 (51.6) | 78 (49.3) | 0.58 | 78 (62.9) | 13 (76.5) | 0.27 |
| Mean age in years, ± SD | 55.1 ± 12.3 | 58.4 ± 12.7 | <0.01 | 56.5 ± 13.4 | 68.1 ± 13.1 | <0.01 |
| Mean height (cm), ± SD | 156.5 ± 8.7 | 155.2 ± 8.2 | 0.07 | 157.5 ± 8.7 | 158.0 ± 9.1 | 0.74 |
| Mean weight (kg), ± SD | 68.3 ± 12.8 | 66.1 ± 10.8 | 0.04 | 64.9 ± 12.1 | 61.7 ± 13.8 | 0.25 |
| High Altitude, n (%) | 870 (32.9) | 66 (42.0) | 0.02 | 60 (48.8) | 8 (47.1) | 0.89 |
| Rural, n (%) | 1,193 (45.1) | 94 (59.5) | <0.001 | 83 (66.9) | 11 (64.7) | 0.86 |
| Current daily smoker, n (%) | 94 (3.6) | 2 (1.3) | 0.13 | 1 (0.8) | 0 (0.00) | 0.71 |
| Body mass index categories, n (%) | ||||||
| Low (BMI < 18 kg/m2) | 12 (0.5) | 1 (0.7) | 0.64 | 2 (1.6) | 1 (5.9) | 0.53 |
| Normal (BMI 18–24 kg/m2) | 711 (26.8) | 43 (27.4) | 51 (41.1) | 8 (47.1) | ||
| Overweight (BMI 25–29 kg/m2) | 1,177 (44.4) | 75 (47.4) | 52 (41.9) | 6 (35.3) | ||
| Obese (BMI ≥ 30 kg/m2) | 750 (28.3) | 39 (24.8) | 19 (15.3) | 2 (11.8) | ||
| Wealth Index, n (%) | ||||||
| Low | 797 (30.1) | 47 (29.8) | 0.98 | 46 (37.1) | 9 (52.9) | 0.09 |
| Middle | 916 (34.6) | 54 (34.2) | 39 (31.5) | 7 (41.2) | ||
| Upper | 937 (35.4) | 57 (36.1) | 39 (31.5) | 1 (5.9) | ||
| Exposure, n (%) | ||||||
| Cooks Indoors | 1,750 (66.4) | 110 (70.1) | 0.41 | 89 (71.8) | 12 (70.6) | 0.92 |
| Farmer | 94 (3.6) | 10 (6.4) | 0.07 | 8 (6.5) | 2 (11.8) | 0.42 |
| Miner | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0.71 | |
| Cook | 4 (0.2) | 1 (0.7) | 0.16 | 1 (0.8) | 0 (0.0) | 0.71 |
| Worker | 90 (3.4) | 7 (4.4) | 0.49 | 8 (6.5) | 0 (0.0) | 0.28 |
| Biomass Fuel for Cooking at least Daily | 692 (26.1) | 43 (27.2) | 0.76 | 48 (38.7) | 8 (47.1) | 0.51 |
| Clinical data, n (%) or Mean ± SD | ||||||
| Mean hs-CRP (log mg/L) | 0.5 ± 1.1 | 0.5 ± 1.1 | 0.59 | 0.5 ± 1.1 | 1.0 ± 1.5 | 0.26 |
| Diabetes | 184 (7.1) | 5 (3.2) | 0.06 | 4 (3.3) | 0 (0.0) | 0.45 |
| Dyslipidemia (LDL ≥ 160 mg/dL) | 432 (16.6) | 21 (13.5) | 0.30 | 15 (12.5) | 1 (5.9) | 0.43 |
| Metabolic syndrome | 1,245 (47.9) | 69 (44.2) | 0.38 | 47 (39.2) | 7 (38.9) | 0.98 |
| Hypertension | 503 (19.0) | 40 (25.3) | 0.05 | 21 (16.9) | 4 (23.5) | 0.50 |
| History of hypertension | 341 (12.9) | 31 (19.8) | 0.02 | 13 (10.5) | 4 (23.5) | 0.12 |
| History of Heart disease | 103 (3.9) | 12 (7.6) | 0.02 | 2 (1.6) | 0 (0.0) | 0.60 |
| History of Stroke | 12 (0.5) | 1 (0.6) | 0.75 | 0 (0.0) | 0 (0.0) | |
| History of Tuberculosis | 58 (2.2) | 8 (5.1) | 0.02 | 16 (12.9) | 4 (23.5) | 0.24 |
| History of Asthma | 81 (3.1) | 16 (10.1) | <0.001 | 10 (8.1) | 7 (41.2) | <0.001 |
| History of Chronic Bronchitis | 74 (2.8) | 28 (17.7) | <0.001 | 11 (8.9) | 4 (23.5) | 0.07 |
| History of COPD (i.e., previous diagnosis by physician) | 1 (0.04) | 0 (0.0) | 0.91 | 3 (2.4) | 0 (0.0) | 0.52 |
| History of Emphysema | 1 (0.04) | 0 (0.0) | 0.94 | 0 (0.0) | 0 (0.0) | |
| Symptoms, n (%) | ||||||
| Dyspnea on exertion | 193 (7.3) | 41 (25.9) | <0.001 | 19 (15.3) | 12 (70.6) | <0.001 |
| Hospitalization in last 12 months due to respiratory symptoms | 10 (0.4) | 2 (1.3) | 0.10 | 4 (3.2) | 2 (1.1) | 0.10 |
| Missed work days in the last 12 months due to respiratory symptoms | 46 (1.7) | 14 (8.9) | <0.001 | 8 (3.5) | 5 (29.4) | <0.01 |
Table 4.
Measured exposures and respiratory disease prevalence by study site.
| Lima | Urban Puno | Rural Puno | Tumbes | P | |
|---|---|---|---|---|---|
| Participants, n (%) | 998 (33.9) | 503 (17.1) | 500 (17.0) | 946 (32.1) | |
| Age in years, Mean ± SD | 55.0 ± 11.8 | 55.2 ± 12.2 | 55.5 ± 12.5 | 55.8 ± 13.2 | 0.87 |
| Environmental exposures | |||||
| Median of average 24-hour indoor PM concentrations (μg/m3) using 1-minute intervals | 39 | 18 | 729 | 38 | <0.001 |
| Median of peak PM concentrations (μg/m3) for the averages of 1-minute intervals | 130 | 191 | 25,570 | 238 | <0.001 |
| Daily Smoking, n (%) | 32 (3.2) | 11 (2.2) | 1 (0.2) | 53 (5.6) | <0.001 |
| Prevalence of ≥ 10 pack- years smoking, n (%) | 28 (2.8) | 15 (3.0) | 6 (1.2) | 50 (5.3) | <0.001 |
| Biomass Fuel Use for Cooking, n (%) | 477 (48.1) | 65 (12.9) | 496 (99) | 582 (61.5) | <0.001 |
| Used biomass fuels daily for cooking, n (%) | 61 (6.2) | 25 (5.0) | 483 (96.6) | 221 (23.3) | <0.001 |
| Wealth Index, n (%) | |||||
| Low | 119 (11.9) | 120 (23.9) | 352 (70.4) | 307 (32.5) | <0.001 |
| Middle | 366 (36.7) | 129 (25.7) | 134 (26.8) | 387 (40.9) | |
| High | 513 (51.4) | 254 (50.5) | 14 (2.8) | 252 (26.7) | |
| Respiratory diseases | |||||
| COPD (FEV1/FVC < 70%), n (%) | 62 (6.2) | 31 (6.2) | 49 (9.8) | 34 (3.6) | <0.001 |
| COPD (FEV1/FVC < LLN), n (%) | 56 (5.6) | 25 (5.0) | 43 (8.6) | 17 (1.8) | <0.001 |
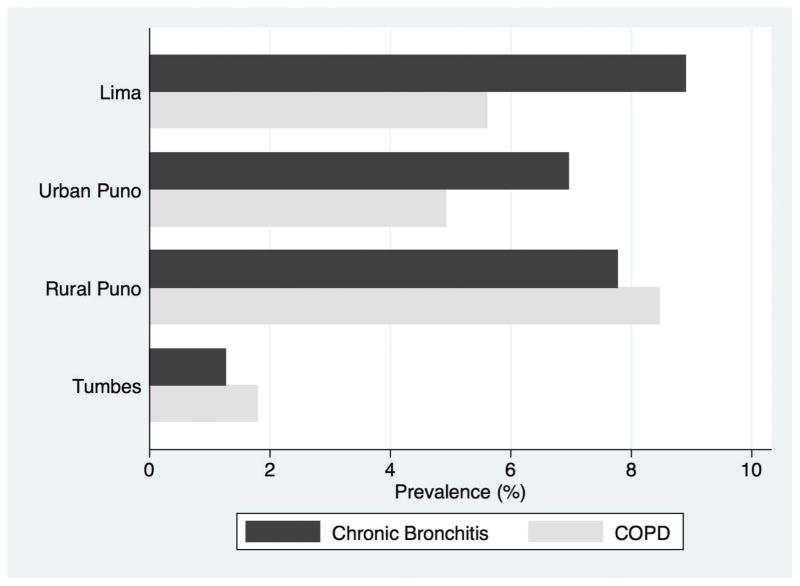
| Chronic bronchitis, n (%) | 89 (8.9) | 35 (7.0) | 39 (7.8) | 12 (1.3) | <0.001 |
| COPD (FEV1/FVC < LLN) among chronic bronchitis, n (%) | 7 (7.9) | 3 (8.6) | 5 (12.8) | 2 (16.7) | 0.55 |
| Chronic bronchitis among COPD group (FEV1/FVC < LLN), n (%) | 7 (12.5) | 3 (12.0) | 5 (11.6) | 2 (11.8) | 0.98 |
Daily PM measurements from rural Puno and Tumbes included homes that used biomass fuel stoves for cooking vs. Lima and urban Puno where the majority of homes used gas stoves or electricity for cooking (Table 4). Rural Puno had the highest daily variation in indoor PM concentrations as evidenced by high median peak concentrations and 24-hour average concentrations (Table 4) compared to low average mode concentrations (1.9 μg/m3), which likely reflected background PM levels during non-cooking periods. Although indoor PM concentrations in Tumbes varied to a lesser degree, differences in median peak concentrations and 24-hour average concentrations likely reflects the contribution of biomass fuel smoke to indoor air pollution. In contrast, there were more constant indoor daily PM concentrations in both Lima and urban Puno suggested by a smaller difference between median peak concentrations and median 24-hour average concentrations at these sites.
Prevalence of chronic bronchitis
Overall prevalence of chronic bronchitis was 5.9% (95% CI 5.1%–6.9%) (Table 2). Chronic bronchitis was more prevalent in Lima, rural Puno and urban Puno as compared to Tumbes (Table 4, Figure 1). Among participants with COPD (post-bronchodilator FEV1/FVC<LLN), 12.1% met criteria for chronic bronchitis when defined as phlegm production for at least three months each year in two successive years (Table 3).
Figure 1.
Differences in prevalence of COPD (defined as post-bronchodilator FEV1/FVC < LLN) and chronic bronchitis (defined as phlegm production for 3 months in two successive years) stratified by site.
Lung function in chronic bronchitis
We compared post-bronchodilator lung function between participants with and without chronic bronchitis stratified by COPD status using both GLI and NHANES III equations (Table 5). Among participants with COPD, the presence of chronic bronchitis was associated with a lower post-bronchodilator FEV1/FVC (p<0.01 after adjusting for site differences) but was not associated with lower absolute or percent predicted forced expiratory volumes (all p>0.33 after adjusting for site differences). Among participants without COPD, chronic bronchitis was also associated with lower unadjusted post-bronchodilator FEV1/FVC and higher unadjusted percent predicted forced expiratory volumes (Table 5); however, these differences were no longer significant after adjusting for site differences (all p>0.10).
Table 5.
Post-bronchodilator lung function values stratified by disease phenotype.
| non-COPD (FEV1/FVC ≥ LLN) | COPD (FEV1/FVC < LLN) | |||||
|---|---|---|---|---|---|---|
| Spirometry (Mean ± SD) | non-Chronic bronchitis | Chronic bronchitis | P | non-Chronic bronchitis | Chronic bronchitis | P |
| FEV1 (L) | 2.8 ± 0.8 | 2.7 ± 0.8 | 0.38 | 2.3 ± 0.8 | 2.0 ± 0.8 | 0.22 |
| FEV1/height2 (L/m2) | 1.1 ± 0.2 | 1.1 ± 0.3 | 0.83 | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.12 |
| Percent predicted FEV1 (%) GLI | 113.4 ± 17.1 | 116.0 ± 18.5 | 0.10 | 94.0 ± 21.8 | 89.2 ± 25.6 | 0.44 |
| Percent predicted FEV1 (%)NHANES III Mexican-Americans | 108 ± 17.0 | 111.4 ± 18.4 | 0.04 | 89.9 ± 20.7 | 87.3 ± 24.4 | 0.60 |
| FVC (L) | 3.5 ± 1.0 | 3.5 ± 1.0 | 0.84 | 3.6 ± 1.1 | 3.4 ± 1.2 | 0.55 |
| FVC/height2 (L/m2) | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.21 | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.33 |
| Percent predicted FVC (%) GLI mixed ethnic population | 113.5 ± 17.6 | 118.7 ± 19.8 | <0.001 | 115.8 ± 22.4 | 115.4 ± 27.2 | 0.83 |
| Percent predicted FVC (%)NHANES III Mexican-Americans | 104.5 ± 16.3 | 109.6 ± 18.4 | <0.001 | 106.6 ± 20.5 | 108.4 ± 24.4 | 0.99 |
| FEV1/FVC (%) | 81.0 ± 5.2 | 79.0 ± 5.9 | <0.001 | 64.9 ± 6.3 | 60.1 ± 8.5 | <0.04 |
Factors associated with chronic bronchitis
We performed single variable analysis and identified potential demographic, environmental, behavioral and clinical factors (Table 6). We considered the major components that made each site unique (Tables 1 and 4) and included those components as risk factors for chronic bronchitis in our analyses. In multivariable analysis, risk factors that remained important were older age, daily biomass fuel use, living in an urban setting, history of asthma and living at high altitude (Table 6).
Table 6.
Single and multivariable regression of factors associated with prevalence of chronic bronchitis in Peru.
| Chronic bronchitis vs. non-Chronic bronchitis (n=2,974) | ||||||
|---|---|---|---|---|---|---|
| Single variable analysis | Multivariable analysis | |||||
| Prevalence ratio | 95% CI | P | Prevalence ratio | 95% CI | P | |
| Age per 10 years | 1.31 | 1.14–1.44 | <0.001 | 1.23 | 1.09–1.40 | 0.001 |
| Gender male vs. female | 1.20 | 0.87–1.57 | 0.30 | 1.28 | 0.94–1.75 | 0.13 |
| Hypertension | 1.29 | 0.90–1.85 | 0.16 | 1.15 | 0.79–1.69 | 0.45 |
| Overweight/Obese (BMI ≥25 kg/m2) | 0.90 | 0.65–1.24 | 0.52 | 1.02 | 0.72–1.44 | 0.91 |
| History of asthma | 3.76 | 2.43–5.83 | <0.001 | 2.987 | 1.81–4.56 | <0.001 |
| History of post-treatment tuberculosis | 2.45 | 1.36–4.40 | <0.01 | 1.74 | 0.95–3.20 | 0.08 |
| Smoking per 10 pack years | 0.86 | 0.60–1.21 | 0.38 | 0.86 | 0.61–1.23 | 0.42 |
| Air pollution exposure | ||||||
| Daily biomass fuel use | 2.38 | 1.60–5.53 | <0.001 | 2.00 | 1.30–3.07 | <0.01 |
| Urban dwelling | 3.63 | 2.44–5.39 | <0.001 | 3.34 | 2.18–5.11 | <0.001 |
| High Wealth Index | 0.92 | 0.67–1.26 | 0.60 | 0.83 | 0.59–1.17 | 0.29 |
| High altitude | 1.41 | 1.04–1.91 | 0.02 | 1.39 | 1.00–1.92 | 0.05 |
DISCUSSION
In this multi-center, population-based study in Peru, we found marked variation in prevalence of chronic bronchitis by setting, and this variation differed from that we observed by setting in COPD prevalence. Moreover, low prevalence of daily smoking in our population further enabled us to investigate other risk factors that were associated with chronic bronchitis. Specifically, our results suggest that chronic bronchitis appears to be as much of a problem in urban centers as it is in rural settings with chronic exposure to biomass fuel smoke. This suggests that factors related to urbanization such as traffic-related pollution are associated with increased prevalence of chronic bronchitis. The health and societal impact on participants with chronic bronchitis was evident, with a higher likelihood to report dyspnea on exertion, increased number of annual hospitalizations, and increased number of workdays missed due to respiratory symptoms. All of these findings held true independent of COPD status and in the absence of smoking. Our data supports the view that even in populations with low prevalence of cigarette smoking, chronic bronchitis is an important cause of respiratory illness, and identifies biomass fuel smoke and urbanization and associated pollution as potentially modifiable risk factors in addressing disease burden.
The prevalence of chronic bronchitis in our study was similar to that found in some studies 9,27 but lower than in others.15,26 The PREPOCOL study in Colombia reported a prevalence of chronic bronchitis (defined as phlegm production and cough) across urban communities at varying altitudes of 2.7%,30 similar to the 3.4% in our study when using the same definition (supplement). The PLATINO study in five Latin American countries 9 found that among people with COPD (defined as a post-bronchodilator FEV1/FVC < 70%), 13% met criteria for chronic bronchitis, which was similar to the 12% we identified in our study when using the same definition. This differs from prevalence studies from Brazil and China. A study performed in an urban setting in Brazil 26 found a higher overall prevalence of chronic bronchitis (12.7%) among people with and without COPD. A study in rural and urban areas of China found chronic bronchitis to be as high as 30% in participants with COPD.15 A possible reason for these differences is the low prevalence of daily smoking in our population (3.3%) compared to higher prevalence of heavy smoking in Brazil (16.2%) 26 and China (48.4%).15 However, even in the PLATINO and PREPOCOL studies, in which the prevalence of chronic bronchitis was similar to what we observed, smoking rates were higher than that found in our study in Peru vis-à-vis a higher prevalence of daily biomass fuel use in our rural settings and components of urbanization including vehicular traffic in our urban settings.33
The prevalence pattern of chronic bronchitis was different from that of COPD. Specifically, COPD was more prevalent in rural settings where there was daily exposure to biomass fuel smoke than in either urban or semi-urban settings, whereas chronic bronchitis was equally prevalent in urban and rural settings and almost non-existent in a semi-urban setting. This discrepancy suggests that chronic bronchitis may develop through disease processes independent from other forms of COPD, and points to factors in urban and rural settings that contribute to this difference in prevalence. Multiple studies have demonstrated a strong association between the use of biomass fuels for cooking and chronic bronchitis.34 Exposure to biomass smoke is the most likely component in rural communities contributing to increased chronic bronchitis observed. This is seen in the elevated rates of chronic bronchitis in rural Puno as compared to Tumbes. In Tumbes, a semi-urban setting, daily biomass fuel utilization was lower than that of rural Puno (23% vs. 97%) and cooking in Tumbes is often performed outdoors leading to decreased exposures to high levels of household air pollution.35 This was confirmed by peak PM concentrations in Tumbes about one-hundredth of that observed in rural Puno.
The association between dense urbanization and chronic bronchitis is reflected in the higher prevalence of chronic bronchitis in Lima and in urban Puno as compared to semi-urban Tumbes. This trend was not seen in COPD. It is likely that, among our cohort, outdoor air pollution from vehicular traffic in urban areas plays an equally important role in the development of chronic bronchitis. Although Tumbes had a higher median indoor particulate matter concentrations than did urban Puno, the majority of indoor measurements from urban Puno were obtained in homes that use gas stoves vs. biomass fuel stoves in Tumbes. Thus, most of the indoor pollution is urban Puno likely reflected outdoor pollution from vehicular traffic. The link between outdoor air pollution and chronic respiratory disease is well substantiated in the literature.36,37 We also confirmed relationships between asthma and chronic bronchitis which have been previously reported by others.38 This relationship may reflect an association between asthma and increased susceptibility to similar environmental risk factors like outdoor air pollution or may be merely a reflection of progression of disease.
Obesity, diabetes and cardiovascular diseases have all be linked to both urbanization 39 and chronic bronchitis.40 Despite the varying degrees of urbanization in our study, a significant relationship between chronic bronchitis and cardiometabolic diseases was not observed. This may be due to the strength of the influence of other risk factors and warrants further attention to the topic. Pulmonary tuberculosis is also a known to cause lung damage through direct parenchymal injury and bronchial stenosis.41 In our study, there was a trend for a higher prevalence of chronic bronchitis among those with a diagnosis of post-treatment pulmonary tuberculosis. Our results do not lead to definitive evidence regarding the relationship between living at high altitude and propensity for chronic bronchitis. With current conflicting literature regarding the role of high altitude in lung disease,19,27 further investigation is warranted.
The variation in the study sites and low prevalence of tobacco smoking were strengths of the study allowing us to assess risk factors associated with the presence of lung disease in Peru. We chose to define COPD based on the LLN 42 utilizing GLI mixed ethnic population and percent-predicted FEV1 and FVC 19,20 utilizing both GLI mixed ethnic population and NHANES III Mexican-Americans populations as reference equations. This is limited by the fact that these populations may not be applicable to Peru. Given the prevalence of chronic bronchitis in our population, the sample size of the cohort does prove to be a limitation and further sub-analysis to analyze some of the more infrequently found risk factors including diabetes, hypertension and metabolic syndrome. We are also heavily reliant of self-reporting for obtaining history of previous illnesses, which may lead underestimation given the poor access to care, especially in the rural sites included in the study.
CONCLUSIONS
In both COPD and non-COPD subjects, those with chronic bronchitis had significantly worsened respiratory symptoms, more hospitalizations, and more workdays missed due to respiratory illness than those without chronic bronchitis. We did not find daily smoking to be related to chronic bronchitis, likely due to the extremely low prevalence of tobacco smoking. In populations with dense urbanization as well as those with high exposure to biomass fuel smoke, we found similarly high prevalence of chronic bronchitis. Controversy remains regarding urbanization-related factors beyond outdoor air pollution that contributes to chronic bronchitis including obesity, diabetes, and metabolic syndrome. Our study confirms the significant negative health and societal impact of chronic bronchitis and identifies urbanization and indoor air pollution from biomass fuel smoke as unique areas for future interventions.
Supplementary Material
Acknowledgments
Funding
This project was funded in whole with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900033C. Catherine H Miele was further supported by National institute of Health Fogarty International Center (5R25TW009340) and the University North Carolina Center for AIDS Research. William Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute.
The authors are indebted to all participants who kindly agreed to participate in the study. Special thanks to all field teams for their commitment and hard work, especially to Lilia Cabrera, Rosa Salirrosas, Viterbo Aybar, Sergio Mimbela, and David Danz for their leadership in each of the study sites, as well as Marco Varela for data coordination.
List of abbreviations
- FEV1
Forced expiratory volume at 1 second
- FVC
Forced vital capacity
- COPD
Chronic obstructive pulmonary disease
- BMI
Body mass index
- PM
Particulate matter
- GLI
Global Lung Function Initiative
- LMIC
Low- and middle-income country
- PR
Prevalence ratio
Footnotes
Contributorship
William Checkley, Jaime Miranda and Antonio Bernabe conceived, designed and supervised the overall study. William Checkley, Jaime Miranda and Antonio Bernabe coordinated and supervised fieldwork activities in Lima, Tumbes and Puno. Catherine H Miele and William Checkley developed the idea for this manuscript, led the statistical analysis, and wrote the first draft. Devan Jaganath, Jaime Miranda, Antonio Bernabe, Robert Gilman, Gregory Diette and Robert Wise participated in writing of manuscript, provided important intellectual content and gave their final approval of the version submitted for publication. William Checkley had ultimate oversight over study conduct, analysis plan and writing of manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgel PR. Cough and Sputum Production Are Associated With Frequent Exacerbations and Hospitalizations in COPD Subjects. Chest. 2009;135:975. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 3.Medical Research Council. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet. 1965;1:775–9. [PubMed] [Google Scholar]
- 4.Guerra S, Sherrill DL, Venker C, et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64:894–900. doi: 10.1136/thx.2008.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim V, Criner GJ. Chronic Bronchitis and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2013;187:228–37. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:77–121. [PubMed] [Google Scholar]
- 7.Lee J, Cho MH, Hersh CP, et al. Genetic susceptibility for chronic bronchitis in chronic obstructive pulmonary disease. Respir Res. 2014;15:113. doi: 10.1186/s12931-014-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim V, Davey A, Comellas AP, et al. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40:28–36. doi: 10.1183/09031936.00141611. [DOI] [PubMed] [Google Scholar]
- 10.Pelkonen M, Notkola IL, Nissinen A, et al. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130:1129–37. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 11.Pelkonen MK, Notkola IL, Laatikainen TK, et al. Twenty-five year trends in prevalence of chronic bronchitis and the trends in relation to smoking. Respir Med. 2014;108:1633–40. doi: 10.1016/j.rmed.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Accordini S, Corsico AG, Cerveri I, et al. Diverging trends of chronic bronchitis and smoking habits between 1998 and 2010. Respir Res. 2013;14:16. doi: 10.1186/1465-9921-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. [accessed 31 May 2014];WHO STEPwise Approach to Survalance (STEPS) http://www.who.int/chp/steps/manual/en/index.html. http://www.who.int/chp/steps/manual/en/index.html.
- 15.LUM, Yao W, Zhong N, et al. Chronic obstructive pulmonary disease in the absence of chronic bronchitis in China. Respirology. 2010;15:1072–8. doi: 10.1111/j.1440-1843.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Prioritized Research Agenda for Prevention and Control of Noncommunicable Diseases. World Health Organization; 2011. [Google Scholar]
- 17.Miranda JJ, Bernabe-Ortiz A, Smeeth L, et al. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610–0. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Menezes AM, Perez-Padilla R, Jardim JB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 20.Howe LD, Galobardes B, Matijasevich A, et al. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–86. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaganath D, Miranda JJ, Gilman RH, et al. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015;16:40. doi: 10.1186/s12931-015-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson GT, Enright PL, Buist AS, et al. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–61. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 24.Pollard SL, Williams DAL, Breysse PN, et al. A cross-sectional study of determinants of indoorenvironmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health. 2014;13:1–12. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quintana PJ, Samimi BS, Kleinman MT, et al. Evaluation of a real-time passive personal particle monitor in fixed site residential indoor and ambient measurements. J Expo Anal Environ Epidemiol. 2000;10:437–45. doi: 10.1038/sj.jea.7500105. [DOI] [PubMed] [Google Scholar]
- 26.Menezes AM, Victora CG, Rigatto M. Prevalence and risk factors for chronic bronchitis in Pelotas, RS, Brazil: a population-based study. Thorax. 1994;49:1217–21. doi: 10.1136/thx.49.12.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of COPD in Five Colombian Cities Situated at Low, Medium, and High Altitude (PREPOCOL Study) Chest. 2008;133:343. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- 28.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi–ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hankinson JL, Odencrantz KR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:79–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 30.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530–5. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 31.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 32.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 33.Rivera RM, Cosio MG, Ghezzo H, et al. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12:972–7. [PubMed] [Google Scholar]
- 34.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–9. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Zou Y, Li X, et al. Lung Function and Incidence of Chronic Obstructive Pulmonary Disease after Improved Cooking Fuels and Kitchen Ventilation: A 9-Year Prospective Cohort Study. PLoS Med. 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumann LM, Robinson CL, Combe JM, et al. Effects of distance from a heavily transited avenue on asthma and atopy in a periurban shantytown in Lima, Peru. J Allergy Clin Immunol. 2011;127:875–82. doi: 10.1016/j.jaci.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindgren A, Stroh E, Montnémery P, et al. Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern Sweden. Int J Health Geogr. 2009;8:2. doi: 10.1186/1476-072X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirtcliffe P, Marsh S, Travers J, et al. Childhood asthma and GOLD-defined chronic obstructive pulmonary disease. Internal Medicine Journal. 2010;42:83–8. doi: 10.1111/j.1445-5994.2010.02238.x. [DOI] [PubMed] [Google Scholar]
- 39.Allender S, Foster C, Hutchinson L, et al. Quantification of Urbanization in Relation to Chronic Diseases in Developing Countries: A Systematic Review. J Urban Health. 2008;85:938–51. doi: 10.1007/s11524-008-9325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izquierdo-Alonso JL, Rodriguez-GonzálezMoro JM, de Lucas-Ramos P, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD) Respir Med. 2013;107:724–31. doi: 10.1016/j.rmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–8. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brusasco V. Spirometric definition of COPD: exercise in futility or factual debate? Thorax. 2012;67(7):569–570. doi: 10.1136/thoraxjnl-2012-201720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.