Abstract
The rise and emergence of resistance to antifungal drugs by diverse pathogenic fungal strains have resulted in an increase in demand for new antifungal agents. Various heterocyclic scaffolds with different mechanism of action against fungi have been investigated in the past. Herein, we report the synthesis and antifungal activities of 18 alkylated mono-, bis-, and trisbenzimidazole derivatives, their toxicities against mammalian cells, as well as their ability to induce reactive oxygen species (ROS) in yeast cells. Many of our bisbenzimidazole compounds exhibited moderate to excellent antifungal activities against all tested fungal strains, with MIC values ranging from 15.6–0.975 µg/mL The fungal activity profiles of our bisbenzimidazoles were found to be dependent on alkyl chain length. Our most potent compounds were found to display equal or superior antifungal activity when compared to the currently used agents amphotericin B, fluconazole, itraconazole, posaconazole, and voriconazole against many of the strains tested.
Keywords: Aspergillus, Candida, Cytotoxicity, Hoechst 33258, Reactive oxygen species (ROS)
Graphical abstract
1. Introduction
At present, there is an increased demand for novel antifungal drugs for both medical and agricultural purposes because of the rise in diverse pathogenic fungi and the emergence of resistant fungal strains.1 Fungal infections can vary in degree of pathogenicity and severity. For instance, some fungal infections are merely superficial and simple to treat, but others are systemic infections and are associated with high mortality rate. These therefore represent a major health concern worldwide, especially in immunocompromised patients.2 Aspergillus and Candida species are responsible for the majority of the documented infections, which may be endogenous (Candida infections) or environmentally acquired (Aspergillus and Cryptococcus infections).3 Candidiasis and aspergillosis account for 80 to 90% of systemic fungal infections.4 There are seven Candida spp. (C. tropicalis, C. glabrata, C. parapsilosis, C. stelltoidea, C. krusei, and C. kyfer) of medical importance, with the most important being C. albicans.5 The Aspergilli sp. are known to increasingly cause invasive diseases.6 In addition to A. niger, other species such as A. flavus, A. terreus, and A. nidulans are increasingly reported.7, 8 In addition to the known fungal infections, there are always new fungal pathogens that appear which can cause life-threatening infections in immunocompromised hosts.9
The currently widely used clinical antifungals are azoles (e.g., fluconazole (FLC), itraconazole (ITC), posaconazole (POS), and voriconazole (VOR)), polyenes (e.g., amphotericin B (AmB) and nystatin), allylamines (e.g., amorolfine, butenafine, and naftifine), and echinocandins (e.g., caspofungin and micafungin). These antifungal scaffolds are known to function by different mechanisms, including (i) inhibition of ergosterol biosynthesis by targeting the CYP450 14α-demethylase (azoles), (ii) inhibition of glucan synthase, which prevents the formation of β-glucan present in fungal cell wall, (iii) inhibition of chitin synthase causing cell lysis, (iv) ergosterol disruption by formation of polyene ergosterol complex, (v) inhibition of squalene epoxidase which converts lanosterol to ergosterol, (vi) interfering with pyrimidine metabolism, thus inhibiting nucleic acid synthesis, (vii) inhibiting fungal translation preventing protein synthesis, and (viii) inhibition of β-tubulin protein, which forms microtubule.10 The low efficacy, emerging resistance to standard therapy, significant side effects, and the toxicity associated with the existing antifungal drugs has resulted in a renewed interest in searching for newer antifungal scaffolds, which can be used either alone or as a part of combination therapy. In recent years, our group has been actively involved in developing novel small molecules as well as aminoglycoside-based antifungal agents to treat both topical and systemic fungal infections.11–13 Based on a recent report, a high-throughput screening of more than 100,000 individual compounds with heterocyclic cores, in the presence fungal pathogens, led to the identification of a promising benzimidazole scaffold.14
Benzimidazoles (mono-, bis-, and trisbenzimidazoles) are chemically and biologically relevant molecules, and have been studied extensively for their antimicrobial, anticancer, and DNA sequence recognition properties. The DNA-binding affinity of these benzimidazoles was explored by synthesizing various mono-, bis-, and trisbenzimidazoles, which were shown to bind AT-rich DNA minor groove.15–17 The DNA binding depended on alkyl chain spacers,18 orientation in case of bidentate ligands,19 as well as RNA duplex recognition by benzimidazole-aminoglycoside conjugates.20 Mono- and bisbenzimidazole analogues were tested for their efficacy as small molecule inhibitors for RNAs.21–23 Various trisubstituted benzimidazoles displayed good efficacy against Mycobacterium tuberculosis (Mtb) FtsZ, Francisella tularensis, methicillin-resistant Staphylococcus aureus (MRSA), and Acinetobacter baumannii.24–28 They also showed good activity against a range of cancer cell lines, such as non-small cell lung cancer, melanoma, leukemia, and breast cancer cells.29, 30 Various bisbenzimidazole analogues were found to be efficient EcTopo IA inhibitors, with potential antibacterial activity with low minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values against various Escherichia coli strains.31 Hoechst 33258-based bisbenzimidazoles containing terminal alkyne displayed selective inhibition of E. coli topoisomerase I over human topoisomerase I and II, and effectively inhibited bacterial growth.32 Hoechst 33258 was shown to bind fungal nucleic acid and can potentially be used against pneumocystis and possibly other fungi.33 These studies piqued our curiosity, and we decided to further synthesize and explore novel benzimidazole derivatives in a structure-activity-relationship study for their antifungal properties.
Herein, we report on the synthesis of 18 benzimidazole derivatives and their antifungal activities against a variety of Candida albicans and Aspergillus strains (C. albicans ATCC 10231 (A), C. albicans ATCC 64124 (B), C. albicans ATCC MYA-2876(S) (C), C. albicans ATCC 90819(R) (D), C. albicans ATCC MYA-2310(S) (E), C. albicans ATCC MYA-1237(R) (F), C. albicans ATCC MYA-1003(R) (G), C. glabrata. ATCC 2001 (H), C. krusei ATCC 6258 (I), C. parapsilosis ATCC 22019 (J), A. flavus ATCC MYA-3631 (K), A. nidulans ATCC 38163 (L), and A. terreus ATCC MYA-3633 (M)). We also present in vitro cytotoxicity studies of these compounds as well as their potential to induce reactive oxygen species (ROS) in fungi.
2. Results and discussion
2.1. Chemistry
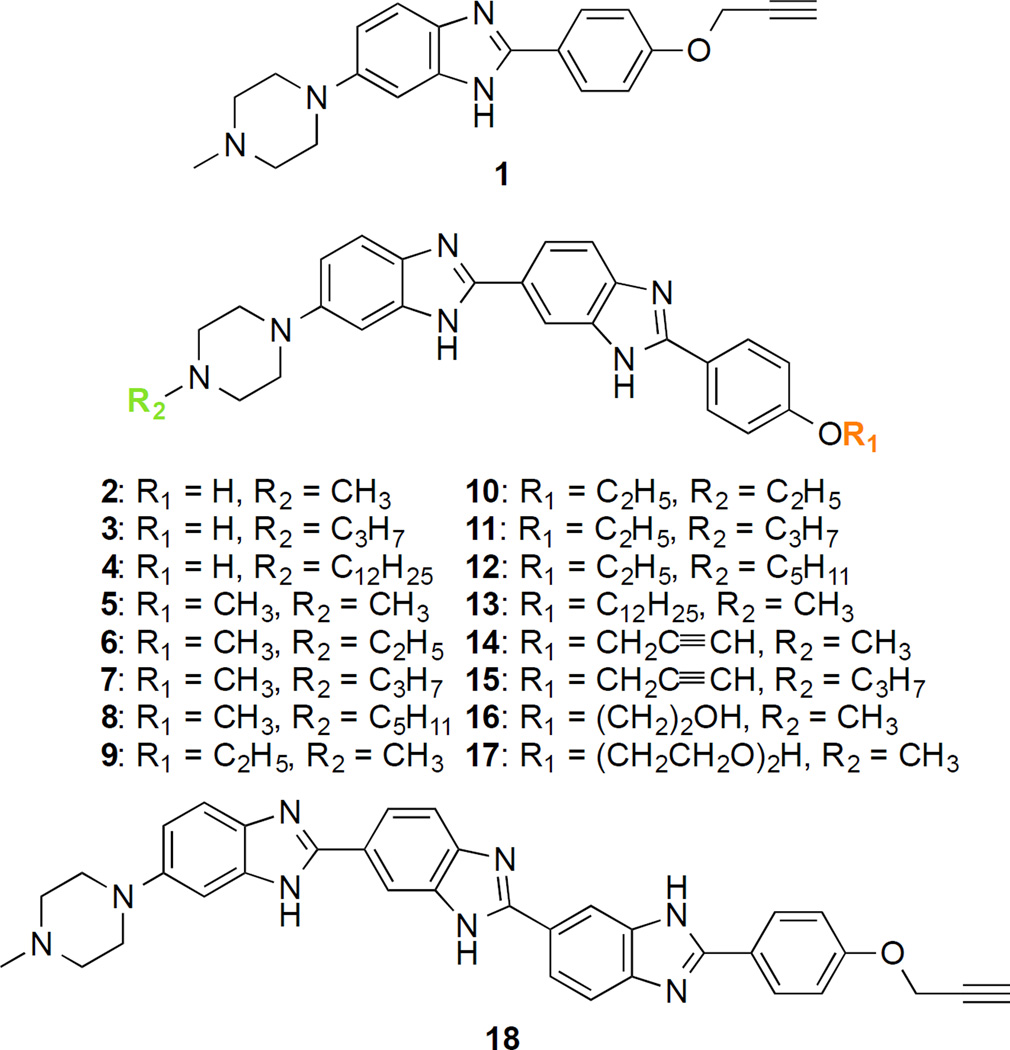
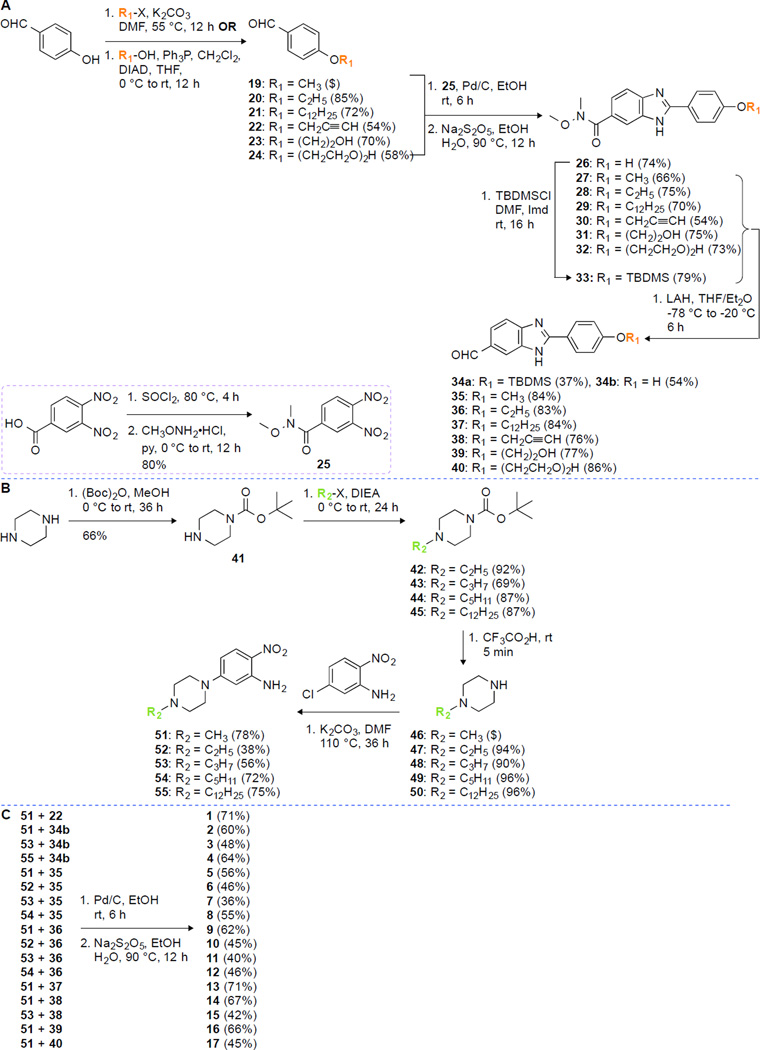
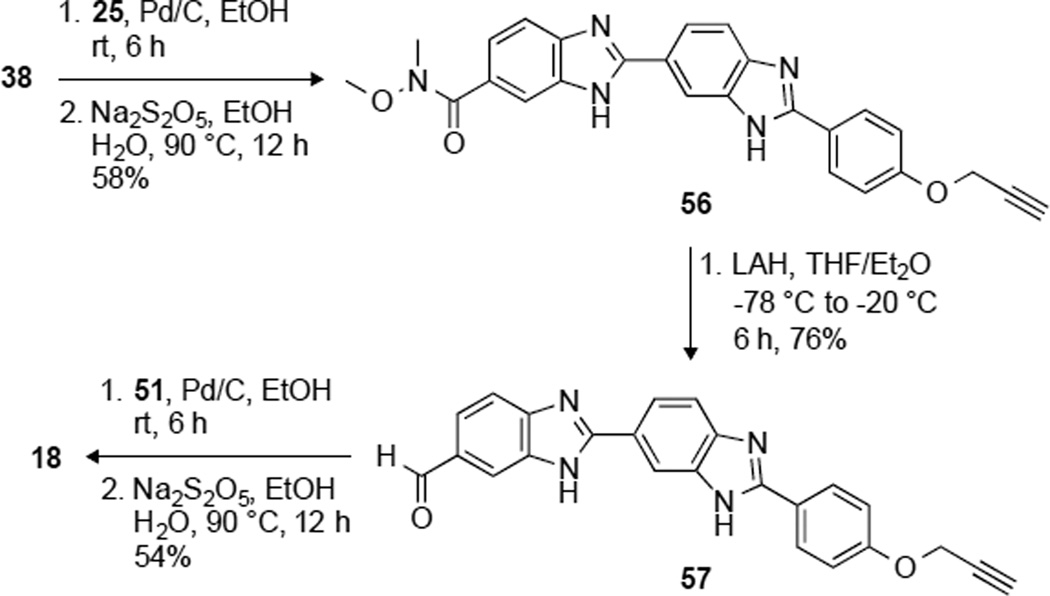
In order to study the antifungal activity of benzimidazoles, we synthesized 18 analogues to explore the correlation of antifungal activity with the number of imidazole rings as well as the identity of alkyl substituents. We used a divergent strategy to introduce structurally different linkers on either side of the benzimidazole moieties.16, 19 A total of 18 molecules, 1–18, were logically designed and synthesized, where compounds 1, 2–17, and 18 have one, two, and three benzimidazole rings, respectively, with various substitution patterns (Fig. 1). Analogues 1, 14, and 18 contain an alkyne functionality on the phenyl ring, whereas all of them have a methyl substitution on the piperazine ring. All the remaining analogues were designed with various alkyl substituents on either end of the molecules. The linkers were introduced on the 4-hydroxybenzaldehyde using a Mitsunobu reaction (propargyl alcohol (22), diethylene glycol (24)) and nucleophilic substitution reaction (ethyl iodide (20), 1-bromododecane (21), and 2-bromoethanol (23)) (Fig. 2). The 4-substituted benzaldehydes 19–24 were coupled with 3,4-diamine-N-methoxy-N-methylbenzamide in the presence of an oxidant (sodium metabisulfite) to yield the corresponding mono benzimidazole derivatives 26–32 in good yields (54–75%). Low temperature reaction of analogues 26–32 with lithium aluminum hydride (LiAlH4) resulted in the reduction of the Weinreb amide functionality to the corresponding aldehydes 34–40 in 54–86% yield. Because of the low solubility of compound 26 in tetrahydrofuran (THF), the hydroxyl group of 26 was protected by using tert-butyldimethylsilyl chloride (TBDMSCl) and the resulting analogue 33 was reduced with LiAlH4 to the corresponding aldehydes 34a,b with partial deprotection of the TBDMS group (Fig. 2A). The other end of the molecules was functionalized with various alkyl chains (ethyl, propyl, pentyl, and dodecyl), compounds 42–45 by substitution reaction of 4-Boc-piperazine (41). The acid mediated removal of the Boc-protecting group resulted in the corresponding secondary amines, which were used for SNAr with 5-chloro-2-nitro aniline to form compounds 51–55 in good yields (38–75%) (Fig. 2B). The monobenzimidazole analogue 1 was obtained in 71% yield by oxidative coupling of 1,2-benzene diamine (51) with aldehyde 22. The bisbenzimidazoles 2–17 were synthesized with oxidative mismatched coupling of aldehydes 34b–40 with the 1,2-diamino derivatives 51–55 (Fig. 2C). In order to introduce a third benzimidazole ring, aldehyde 38 was coupled with 3,4-diamino-N-methoxy-N-methylbenzamide to yield 56, which was then reduced at lower temperature to form aldehyde 57 in good yield (76%). The 4-(4-methylpiperazin-1-yl)-benzene-1,2-diamine was then coupled with aldehyde 57 in presence of an oxidant to result in the formation the trisbenzimidazole analogue 18 (Fig. 3). All the intermediates and final target molecules were characterized using 1H and 13C NMR (Fig. S1–S111) as well as mass spectrometry, and were found to be at least 95% pure. The presence of an alkyne functionality on analogues 1, 14, and 18 gives an opportunity for future structural modifications using click chemistry.
Fig. 1.
Chemical structures of the 18 benzimidazole derivatives tested in this study.
Fig. 2.
Synthetic scheme for the preparation of A. benzimidazoles 34–40, B. 4-(4-substituted piperazin-1-yl) 2-nitroaniline intermediates 51–55, and C. mono- (1) and bisbenzimidazole (2–17) derivatives.
Fig. 3.
Synthetic scheme for the preparation of the trisbenzimidazole derivative 18.
2.2. In vitro antifungal assay
In order to investigate their potential as antifungal agents, we first determined the MIC values for benzimidazole analogues 1–18 along with those for five known antifungal drugs (AmB, FLC, ITC, POS, VOR) against 13 fungal strains (yeast and filamentous fungi, Table 1). We rapidly found that the mono- (1) and trisbenzimidazole (18) analogues displayed no activity (≥31.2 µg/mL) against the 13 fungal strains tested, and therefore decided to focus our efforts on derivatizing the bisbenzimidazole scaffold. We also observed that all of our bisbenzimidazole derivatives displayed no activity (≥31.2 µg/mL) against A. flavus ATCC MYA-3631 (strain K). As we and others previously discovered that the addition of long alkyl chains to aminoglycosides such as kanamycin A (KANA),34 kanamycin B (KANB),12, 35 and tobramycin (TOB)11, 13 results in killing of fungal cells through membrane perturbation, we decided to functionalize the bisbenzimidazole core with various alkyl chains hoping for an additive action against fungal growth. The divergent synthetic strategy allowed us to introduce various alkyl chains on either side of the molecule efficiently.
Table 1.
MIC valuesa,b (in µg/mL) determined for compounds 1–18 and for five control antifungal agents (AmB, FLC, ITC, POS, and VOR) against various yeast strains and filamentous fungi.
| Yeast strains | Filamentous fungi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpd # | A | B | C | D | E | F | G | H | I | J | K | L | M |
| 1 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 |
| 2 | 7.8–15.6 | 7.8 | 7.8–15.6 | 7.8 | 3.9–7.8 | 3.9–7.8 | 7.8–15.6 | 0.975 | 7.8 | 0.975 | 31.2 | 1.95 | 7.8 |
| 3 | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 | 7.8 | 31.2 | 7.8 | >31.2 | 7.8 | 15.6 |
| 4 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 |
| 5 | 15.6 | 31.2 | 15.6 | 15.6 | 7.8 | 15.6 | >31.2 | 3.9 | 31.2 | 3.9 | 31.2 | 15.6 | 15.6 |
| 6 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 7.8 | 31.2 | 7.8 | >31.2 | >31.2 | >31.2 |
| 7 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 7.8 | 31.2 | >31.2 | >31.2 | >31.2 |
| 8 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 15.6 | >31.2 | >31.2 | >31.2 |
| 9 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 15.6 | 7.8 | 7.8 | 3.9 | 31.2 | 3.9 | 7.8 |
| 10 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | >31.2 | 31.2 | 15.6 | 3.9 | >31.2 | 15.6 | 15.6 |
| 11 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 15.6 | >31.2 | >31.2 | >31.2 | >31.2 |
| 12 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 |
| 13 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 |
| 14 | 7.8–31.2 | 31.2 | 7.8 | 15.6 | 7.8 | >31.2 | 15.6 | 7.8 | 7.8 | 1.95 | >31.2 | 15.6 | 15.6 |
| 15 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 15.6 | >31.2 | >31.2 | >31.2 | >31.2 |
| 16 | >31.2 | >31.2 | 31.2 | >31.2 | 31.2 | >31.2 | 31.2 | 31.2 | 7.8 | 15.6 | 31.2 | 15.6 | 15.6 |
| 17 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 7.8 | 31.2 | >31.2 | >31.2 | >31.2 |
| 18 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 31.2 | >31.2 | >31.2 | >31.2 | 31.2 | 31.2 |
| AmB | 3.9 | 3.9 | 1.95 | 0.975 | 1.95 | 3.9 | 3.9 | 1.95 | 3.9 | 1.95 | 15.6 | 15.6 | 3.9 |
| FLC | 62.5a | >125a | 15.6a | >125a | >125a | 62.5a | 62.5a | >31.2 | >31.2 | 1.95 | 62.5 | 62.5a | 62.5 |
| ITC | 0.5a | >62.5a | 7.8a | 31.2a | 31.2a | 31.2a | 31.2a | 7.8 | 0.48 | 0.12 | 0.48 | 0.195a | 0.975 |
| POS | 0.5a | >62.5a | 7.8a | 31.2a | 31.2a | 15.6a | 15.6a | 0.12 | 0.06 | <0.03 | 0.24 | 0.195a | 0.48 |
| VOR | 0.24 | 3.9 | 1.95 | 1.95 | 0.975 | 7.8 | 1.95 | 0.06 | 0.12 | <0.03 | 0.24 | 0.03 | 0.12 |
Yeast strains: A = C. albicans ATCC 10231, B = C. albicans ATCC 64124, C = C. albicans ATCC MYA-2876(S), D = C. albicans ATCC 90819(R), E = C. albicans ATCC MYA-2310(S), F = C. albicans ATCC MYA-1237(R), G = C. albicans ATCC MYA-1003(R), H = C. glabrata ATCC 2001, I = C. krusei ATCC 6258, J = C. parapsilosis ATCC 22019. NOTE: Here, the (S) and (R) indicate that ATCC reports these strains to be susceptible (S) and resistant (R) toITC and FLC.
Filamentous fungi: K = Aspergillus flavus ATCC MYA-3631, L = Aspergillus nidulans ATCC 38163, M = A. terreus ATCC MYA-3633.
Known antifungal agents: AmB = amphotericin B, FLC = fluconazole, ITC = itraconazole, POS = posaconazole, and VOR = voriconazole.
These values were previously reported in ref #13.
For yeast strains: MIC-0 values are reported for benzimidazole analogues 1–18 and AmB, whereas MIC-2 values are reported for azoles. For filamentous fungi, MIC-0 values are reported for all compounds.
We began our study with compound 2, which has a methyl group on the piperazine ring and a hydroxyl group at the para position of the phenyl ring. This compound was found to exhibit broad-spectrum activity against both yeast and filamentous fungi (MIC values ranging from 0.975 to 15.6 µg/mL). In order to evaluate the role of the alkyl chain, we modified both the piperazine and phenyl ends of compound 2 with different substitutions. At this point, it was unclear whether modifications at one end of the molecule would be more favorable than modifications at the other end. Therefore, we commenced by conducting modifications on the piperazine ring. We introduced linear alkyl chains of 3 and 12 carbons in length (from now on referred to as C3 and C12) on the piperazine end to form compounds 3 and 4, respectively. Analogue 4 exhibited no activity against all 13 yeast/fungal strains tested, whereas derivative 3 displayed good activity (7.8 µg/mL) against C. glabrata ATCC 2001 (strain H), C. parapsilosis ATCC 22019 (strain J), and A. nidulans ATCC 38163 (strain L), and low activity (15.6 µg/mL) against A. terreus ATCC MYA-3633 (strain M). This initial analysis provided us valuable information regarding the effect of substitution pattern, as addition of the C12 chain on the piperazine end (analogue 3) completely obliterated the activity of the scaffold. To further confirm the relationship of the regiospecificity of substitutions and their corresponding antifungal activities, we tested compound 13, which contains a C12 substituent on the phenyl end. We observed that addition of this long alkyl chain at this position also resulted in complete loss of antifungal activity. Collectively, these findings suggested that, unlike in the case of aminoglycosides, addition of long linear alkyl chains such as the C12 is detrimental for antifungal potency.
Based on our finding that addition of a short alkyl (methyl (C1) (2) and n-propyl (C3) (3)) substituent to the piperazine ring while having a hydroxyl moiety attached to the phenyl ring led to good activity against various fungal strains, we pondered if replacing the hydroxyl moiety of compounds 2 and 3 by a methoxy group (compounds 5 and 7) would have any effect on the antifungal activity of these compounds. We also wondered if increasing the length of the alkyl chain on the piperazine ring would correlate with a decrease in antifungal activity. To explore this latter idea, we additionally generated compounds 6 and 8 containing an ethyl (C2) and a pentyl (C5) chain, respectively. In general, we found that increasing the length of the chain from C1 to C5 on the piperazine ring while keeping the R1 group constant on the phenyl ring (compounds 5–8) resulted in a gradual decrease in antifungal activity against almost all strains tested. Compound 5 with a C1 side chain displayed good activity (3.9–7.8 µg/mL) against four of the tested strains (C. albicans ATCC MYA-2310(S) (strain E), C. glabrata ATCC 2001 (strain H), and C. parapsilosis ATCC 22019 (strain J)). As we increased the chain length we saw a significant drop in activity. Indeed, compound 6 with a C2 side chain displayed good activity (7.8 µg/mL) against two of the tested strains (C. glabrata ATCC 2001 (strain H) and C. parapsilosis ATCC 22019 (strain J)), whereas analogue 7 with a C3 side chain showed good activity (7.8 µg/mL) against only one of the tested strain (C. krusei ATCC 6258 (strain I)), and analogue 8 with a C5 side chain only displayed low activity (15.6 µg/mL) against one of the tested strain (C. parapsilosis ATCC 22019 (strain J)). When comparing the antifungal activity of compounds 2 and 5 as well as that of compounds 3 and 7, we discovered that the presence of a hydroxyl moiety on the phenyl ring was more beneficial than that of a methoxy group.
To get a full depiction of activity profile, we decided to also introduce an ethoxy group on the phenyl ring of these molecules while keeping the C1 (9), C2 (10), C3 (11), and C5 (12) side chains on the piperazine ring. As with the methoxy series (compounds 5–8), we found that increasing the length of the alkyl chain on the piperazine ring resulted in a decrease in antifungal activity, with compounds 11 (C3) and 12 (C5) being inactive and compound 9 being more active than 10. Interestingly, when comparing the MIC values for compounds 2, 5, and 9 as well as those of compound 3, 7, and 10, we discovered that the presence of an ethoxy group on the phenyl ring conferred equal or better antifungal activity than that of an hydroxyl group, which itself conferred better antifungal activity than the methoxy group, as stated above.
As compounds comprised of a methyl on the piperazine ring displayed the best antifungal activities, we next explored keeping this moiety constant while adding a proparyl group on the hydroxyl moiety of compounds 2 to afford derivative 14. Surprisingly, compound 14 displayed very similar antifungal activities to those of its counterparts with ethoxy (compound 9) and hydroxyl (compound 2) moieties. Analogue 14 showed excellent activity (1.95 µg/mL) against C. parapsilosis ATCC 22019 (strain J) and good activity (7.8 µg/mL) against four additional Candida strains tested (C, E, H, and I). Interestingly, replacing C1 with C3 on the piperazine ring (compound 15) significantly reduced its activity.
As compounds 2 and 9 were great antifungal agents, we thought that combining the structural features of these two R1 substituents, as in compound 16, could potentially be beneficial. However, the desired effect was not observed with compound 16, which only showed good activity (7.8 µg/mL) against C. krusei ATCC 6258 (strain I) and low activity against strains J, L, and M. Finally, to test the influence of the hydrophilicity of the substituent, we synthesized compound 17 with a diethylene glycol on the phenyl. Adding hydrophilicity did not improve the antifungal activity, as 17 was only active against C. krusei ATCC 6258 (strain I) (7.8 µg/mL).
Overall, all the fungal strains used in this study were more sensitive to analogues 2, 9, and 14 than FLC. Our compounds were even more potent against some strains when compared to the clinically potent antifungal agents AmB, ITC, POS, and VOR. Analogues 2, 9, and 14 displayed better activity against C. albicans ATCC 64124 (strain B), C. albicans ATCC 90819(R) (strain D), and C. albicans ATCC MYA-2310(S) (strain E) when compared to POS and ITC. Both compounds 2 and 9 showed better activity against C. albicans ATCC MYA-1237(R) (strain F) than POS and ITC. Finally, compound 2 displayed equal or better activity than AmB against C. albicans ATCC MYA-1237(R) (strain F), C. glabrata ATCC 2001 (strain H), C. parapsilosis ATCC 22019 (strain J), and A. nidulans ATCC 38163 (strain L).
2.3. Antifungal time-kill studies
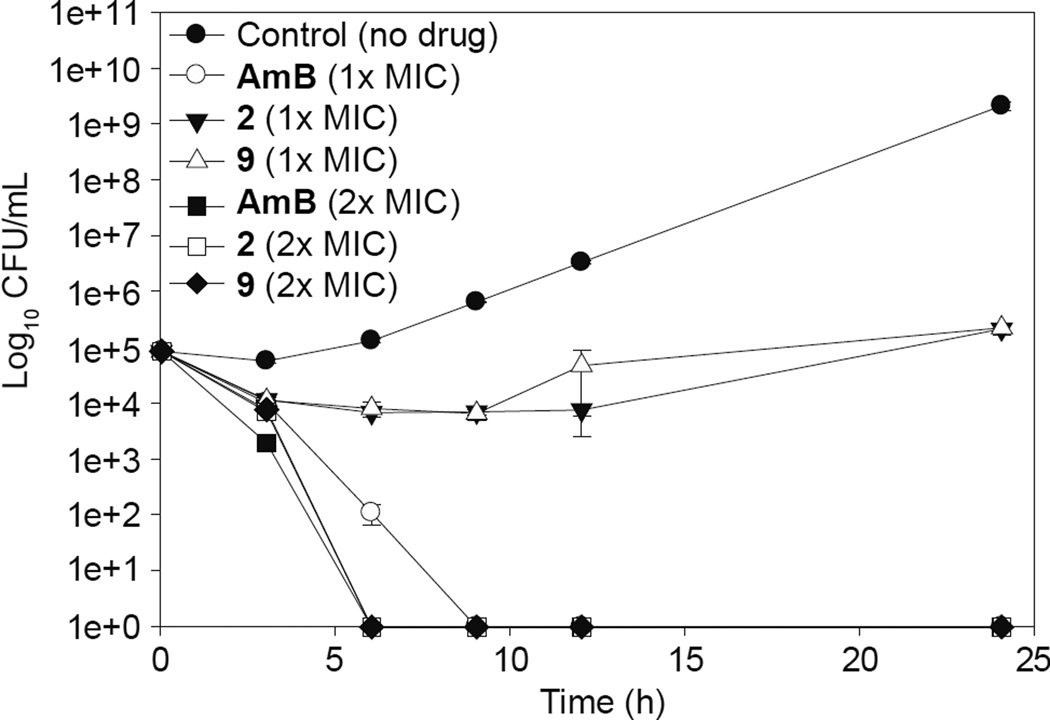
Data from time-kill studies can provide critical information regarding the rate and extend of fungicidal activity and other pharmacodynamics characteristics that are crucial for translation to in vivo models.36 In order to understand the rate of the antifungal activity, our best compounds 2 and 9, as well as AmB (positive control) were selected for time-kill assays against the azole-resistant fungal strain C. albicans ATCC 64124 (strain B) (Fig. 4). At 1× MIC, we observed fungistatic properties for both compounds 2 and 9, while our positive control AmB remained fungicidal and completely killed fungal cells after 9 h of drug treatment. Both compounds 2 and 9 displayed similar activities by reducing the fungal population by 1 log CFU and both compounds remained fungistatic up to 24 h. To evaluate a possible dose-response effect, we increased the doses of analogues 2 and 9 by 2 fold (2× MIC) and treated the fungal cells. Interestingly, at 2× MIC, our compounds 2 and 9 displayed fungicidal activity and reduced the cell population by 5 log CFUs, which were equivalent to one of the most potent FDA-approved antifungal agents, AmB (also at 2× MIC).
Fig. 4.
Representative time-kill studies of AmB as well as compounds 2 and 9 against C. albicans ATCC 64124 (strain B). At 1× MIC, fungal cells were either treated with the positive control AmB (white circle) or compounds 2 (black inverted triangle) and 9 (white triangle). At 2× MIC, fungal cells were treated with the positive control AmB (black square) or compounds 2 (white square) and 9 (black diamond). The untreated cells or negative control are presented as black circle.
2.4. In vitro cytotoxicity assay
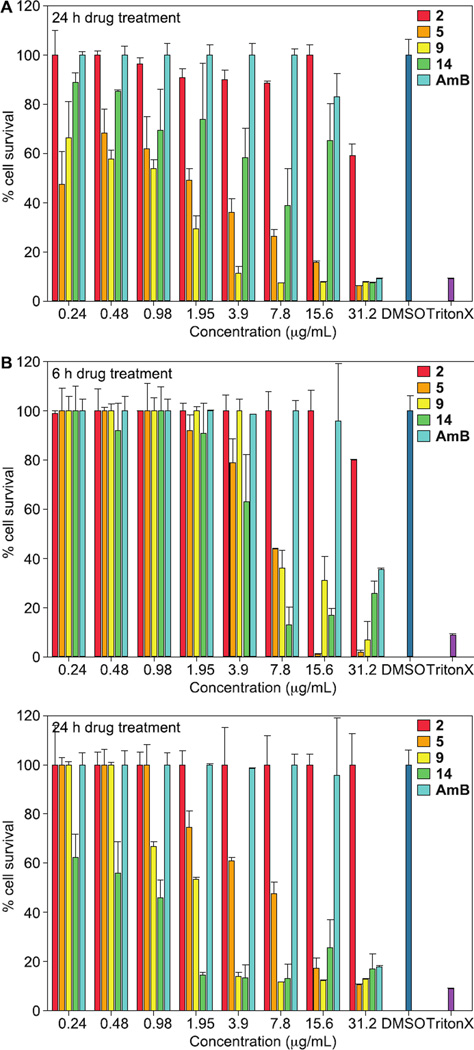
A key parameter for developing antifungal drugs is to selectively target fungal cells without killing mammalian cells. From our in vitro antifungal studies, we identified that compounds 2, 5, 9, and 14 were highly potent against pathogenic fungal strains. However, valuable antifungal compounds should have a large therapeutic index with high selectivity for fungal cells compared to mammalian cells since both are eukaryotic cells with similar biological properties. Thus, we opted to evaluate these four compounds against two mammalian cell lines, A549 and BEAS-2B, with the popular FDA-approved antifungal agent AmB as a positive control (Fig. 5). We observed that our compounds 5 and 9 displayed some toxic effect against A549 and BEAS 2B, with IC50 values ranging from 1.95–0.98 µg/mL, and 7.8–1.95 µg/mL, respectively. Similarly, compound 14 also exhibited toxicity against BEAS 2B (IC50 = 0.98 µg/mL), but showed only minimal toxicity against A549 (IC50 = 15.6 µg/mL), Intriguingly, we found that compound 2 showed less toxicity against both cell lines with the IC50 values of ≥31.2 µg/mL, which was better than our reference drug, AmB. Additionally, we evaluated the cytotoxic effect of these compounds on BEAS 2B cells at a 6 h time point and compared the results with those obtained at a 24 h time point (Fig. 5B). As expected, more BEAS 2B cells survived when treated for 6 h instead of 24 h. The only structural difference between these analogues is the substitution of the hydroxyl group by a methoxy, ethoxy, or propargyl group. Considering its low fungal MIC values, analogue 2 could potentially have a high therapeutic index and would be an attractive antifungal candidate for further safety evaluation in vivo.
Fig. 5.
Mammalian cell cytotoxicity of benzimidazole derivatives 2, 5, 9, and 14, as well as AmB against the A. A549 cell line after 24 h treatment, and B. BEAS 2B cell line after 6 h (top panel) and 24 h (bottom panel) treatment. Cells treated with DMSO (negative control) and Triton X (positive control).
2.5. ROS assays
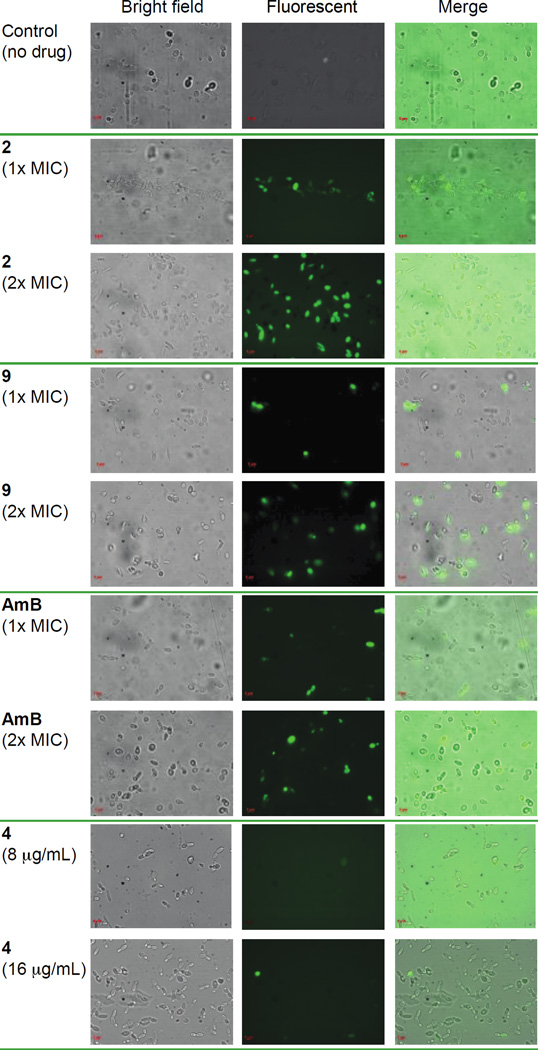
It has previously been reported that various fungicidal drugs (AmB, miconazole, and ciclopirox) induce ROS.37, 38 However, ROS induction by antifungal agents remains relatively unexplored. In order to determine if bisbenzimidazoles are capable of inducing ROS, we investigated the effect of two of our best compounds, 2 and 9, against C. albicans ATCC 10231 (strain A) in a dose depend manner at 1× and 2× MIC using a 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) dye.39 Generally, ROS is generated in mitochondria of eukaryotic cells as a byproduct during cellular metabolism. Cells neutralize the excess ROS production by activating their antioxidant functions. However, overproduction of ROS overwhelms the antioxidant capacity of cells, which in turn causes cell damage. When we treated yeast cells with our analogues 2 and 9 at their 1× and 2× MIC values, we observed a significant amount of ROS production in cells (Fig. 6). Similar results were observed for our control drug AmB (at 1× and 2× its MIC value). However, no ROS accumulation was observed for our untreated yeast cells. It is not clear whether yeast cells treated with our compounds undergo growth inhibition and cell death solely via ROS production. Nevertheless, it is one of the contributing factors for the cell death and it will be interesting in future studies to perform in-depth mechanistic analysis for these bisbenzimidazoles and potential additional drug conjugates comprising these molecules. In order to confirm our hypothesis that ROS production is a contributing factor for cell death, we also performed this type of assays with one of our inactive compounds, molecule 4. We observed no ROS accumulation with compound 4, confirming the role of ROS production in cell death.
Fig. 6.
Effect of benzimidazole derivatives on intracellular ROS production by C. albicans ATCC 10231 (strain A). Yeast cells were treated with no drug (negative control) or compounds 2 or 9, or AmB (positive control), at their 1× and 2× respective MIC values, or with compound 4 at 8 and 16 µg/mL for 1 h at 35 °C. These treated cells were further stained with DCFH-DA (20 µg/mL) for 30 min in the dark and were analyzed using a Zeiss Axovert 200M fluorescence microscope.
3. Conclusions
In sum, we have synthesized 18 benzimidazoles analogues (compounds 1–18) with alkyl chains of various lengths (C1, C2, C3, C5, and C12) on both ends of the molecules via a convenient convergent synthetic strategy. Interestingly, we did not observe any antifungal activities with our mono- and trisbenzimidazoles. However, we observed chain length dependent antifungal activities of our alkylated bisbenzimidazole analogues, with C1 displaying superior activity than (>) C2 > C3 > C5 > C12, a trend opposite to that previously observed with alkylated aminoglycoside (KANA, KANB, and TOB) derivatives. Compounds 2, 9, and 14 displayed broad-spectrum antifungal activities against both yeasts and filamentous fungi. In most cases, analogues 2, 9, and 14 displayed enhanced or comparable antifungal activity against fungal strains when compared to the commercial antifungal drugs FLC, ITC, POS, VOR, and AmB. We also observed the accumulation of ROS in yeast cells when treated with our compounds. It is very likely that these compounds may inhibit fungi by inducing ROS production in cells. It appears that compounds 2, 9, and 14 show promise as lead compounds for developing antifungal agents and their in vivo studies would be encouraging. From these new results, we can infer that the core structure plays a major role in the antifungal activity of the molecules, and that adding long alkyl substituents is not always beneficial for the production of antifungal agents. The facts that compound 14 displays significant antifungal activity and that it contains in its structure a chemical handle (alkyne group) are highly encouraging and set the stage for the future conjugation of this molecule to other drug scaffolds. Such studies are currently underway in our laboratory.
4. Experimental section / Supplementary material
The Supporting Information includes experimental procedures for all assays performed as well as 1H and 13C NMR spectra (Figs. S1–S111) for all molecules synthesized, which were all at least 95% pure.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of Health NIH AI90048 (to S.G.-T.) and by startup funds from the University of Kentucky (to S.G.-T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garibotto FM, Garro AD, Masman MF, Rodriguez AM, Luiten PG, Raimondi M, Zacchino SA, Somlai C, Penke B, Enriz RD. New small-size peptides possessing antifungal activity. Bioorg Med Chem. 2010;18:158–167. doi: 10.1016/j.bmc.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Onnis V, De Logu A, Cocco MT, Fadda R, Meleddu R, Congiu C. 2-Acylhydrazino-5-arylpyrrole derivatives: synthesis and antifungal activity evaluation. Eur J Med Chem. 2009;44:1288–1295. doi: 10.1016/j.ejmech.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 5.McCullough MJ, Ross BC, Reade PC. Candida albicans: a review of its history, taxonomy, epidemiology, virulence attributes, and methods of strain differentiation. Int J Oral Maxillofac Surg. 1996;25:136–144. doi: 10.1016/s0901-5027(96)80060-9. [DOI] [PubMed] [Google Scholar]
- 6.De Pauw BE, Picazo JJ. Present situation in the treatment of invasive fungal infection. Int. J. Antimicrob. Agents. 2008;32:167–171. doi: 10.1016/S0924-8579(08)70020-7. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett JE. Echinocandins for candidemia in adults without neutropenia. N Engl J Med. 2006;355:1154–1159. doi: 10.1056/NEJMct060052. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 10.Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Shrestha SK, Fosso MY, Garneau-Tsodikova S. A combination approach to treating fungal infections. Sci Rep. 2015;5:17070. doi: 10.1038/srep17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosso MY, Shrestha SK, Green KD, Garneau-Tsodikova S. Synthesis and Bioactivities of Kanamycin B-Derived Cationic Amphiphiles. J Med Chem. 2015;58:9124–9132. doi: 10.1021/acs.jmedchem.5b01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha SK, Fosso MY, Green KD, Garneau-Tsodikova S. Amphiphilic Tobramycin Analogues as Antibacterial and Antifungal Agents. Antimicrob Agents Chemother. 2015;59:4861–4869. doi: 10.1128/AAC.00229-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger-Kentischer A, Finkelmeier D, Keller P, Bauer J, Eickhoff H, Kleymann G, Abu Rayyan W, Singh A, Schroppel K, Lemuth K, Wiesmuller KH, Rupp S. A screening assay based on host-pathogen interaction models identifies a set of novel antifungal benzimidazole derivatives. Antimicrob Agents Chemother. 2011;55:4789–4801. doi: 10.1128/AAC.01657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puckowska A, Bielawski K, Bielawska A, Midura-Nowaczek K. Aromatic analogues of DNA minor groove binders--synthesis and biological evaluation. Eur J Med Chem. 2004;39:99–105. doi: 10.1016/j.ejmech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Ji YH, Bur D, Hasler W, Runtz Schmitt V, Dorn A, Bailly C, Waring MJ, Hochstrasser R, Leupin W. Trisbenzimidazole derivatives: design, synthesis and DNA sequence recognition. Bioorg Med Chem. 2001;9:2905–2919. doi: 10.1016/s0968-0896(01)00170-5. [DOI] [PubMed] [Google Scholar]
- 17.Behrens C, Harrit N, Nielsen PE. Synthesis of a Hoechst 32258 analogue amino acid building block for direct incorporation of a fluorescent, high-affinity DNA binding motif into peptides. Bioconjug Chem. 2001;12:1021–1027. doi: 10.1021/bc0100556. [DOI] [PubMed] [Google Scholar]
- 18.Kamal A, Ramulu P, Srinivas O, Ramesh G, Kumar PP. Synthesis of C8-linked pyrrolo[2,1-c][1,4]benzodiazepine-benzimidazole conjugates with remarkable DNA-binding affinity. Bioorg Med Chem Lett. 2004;14:4791–4794. doi: 10.1016/j.bmcl.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 19.Tanada M, Tsujita S, Sasaki S. Design of new bidentate ligands constructed of two Hoechst 33258 units for discrimination of the length of two A3T3 binding motifs. J Org Chem. 2006;71:125–134. doi: 10.1021/jo051836t. [DOI] [PubMed] [Google Scholar]
- 20.Willis B, Arya DP. Recognition of RNA duplex by a neomycin-Hoechst 33258 conjugate. Bioorg Med Chem. 2014;22:2327–2332. doi: 10.1016/j.bmc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Haga CL, Velagapudi SP, Strivelli JR, Yang WY, Disney MD, Phinney DG. Small molecule inhibition of miR-544 biogenesis disrupts adaptive responses to hypoxia by modulating ATM-mTOR signaling. ACS Chem. Biol. 2015;10:2267–2276. doi: 10.1021/acschembio.5b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rzuczek SG, Southern MR, Disney MD. Studying a drug-like, RNA-focused small molecule library identifies compounds that inhibit RNA toxicity in myotonic dystrophy. ACS Chem. Biol. 2015;10:2706–2715. doi: 10.1021/acschembio.5b00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velagapudi SP, Gallo SM, Disney MD. Sequence-based design of bioactive small molecules that target precursor microRNAs. Nat. Chem. Biol. 2014;10:291–297. doi: 10.1038/nchembio.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon YK, Ali MA, Wei AC, Choon TS, Ismail R. Synthesis and evaluation of antimycobacterial activity of new benzimidazole aminoesters. Eur J Med Chem. 2015;93:614–624. doi: 10.1016/j.ejmech.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Knudson SE, Awasthi D, Kumar K, Carreau A, Goullieux L, Lagrange S, Vermet H, Ojima I, Slayden RA. A trisubstituted benzimidazole cell division inhibitor with efficacy against Mycobacterium tuberculosis. PLoS One. 2014;9:e93953. doi: 10.1371/journal.pone.0093953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awasthi D, Kumar K, Knudson SE, Slayden RA, Ojima I. SAR studies on trisubstituted benzimidazoles as inhibitors of Mtb FtsZ for the development of novel antitubercular agents. J Med Chem. 2013;56:9756–9770. doi: 10.1021/jm401468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar K, Awasthi D, Lee SY, Cummings JE, Knudson SE, Slayden RA, Ojima I. Benzimidazole-based antibacterial agents against Francisella tularensis. Bioorg Med Chem. 2013;21:3318–3326. doi: 10.1016/j.bmc.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huigens RW, 3rd, Reyes S, Reed CS, Bunders C, Rogers SA, Steinhauer AT, Melander C. The chemical synthesis and antibiotic activity of a diverse library of 2-aminobenzimidazole small molecules against MRSA and multidrug-resistant A. baumannii. Bioorg Med Chem. 2010;18:663–674. doi: 10.1016/j.bmc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Tandon V. Synthesis and biological activity of novel inhibitors of topoisomerase I: 2-aryl-substituted 2-bis-1H-benzimidazoles. Eur J Med Chem. 2011;46:659–669. doi: 10.1016/j.ejmech.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Thimmegowda NR, Nanjunda Swamy S, Kumar CS, Kumar YC, Chandrappa S, Yip GW, Rangappa KS. Synthesis, characterization and evaluation of benzimidazole derivative and its precursors as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Bioorg Med Chem Lett. 2008;18:432–435. doi: 10.1016/j.bmcl.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 31.Nimesh H, Sur S, Sinha D, Yadav P, Anand P, Bajaj P, Virdi JS, Tandon V. Synthesis and biological evaluation of novel bisbenzimidazoles as Escherichia coli topoisomerase IA inhibitors and potential antibacterial agents. J Med Chem. 2014;57:5238–5257. doi: 10.1021/jm5003028. [DOI] [PubMed] [Google Scholar]
- 32.Ranjan N, Fulcrand G, King A, Brown J, Jiang Z, Leng F, Arya DP. Selective inhibition of bacterial topoisomerase I by alkynyl-bisbenzimidazoles. MedChemComm. 2014;5:816–825. doi: 10.1039/C4MD00140K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Disney MD, Stephenson R, Wright TW, Haidaris CG, Turner DH, Gigliotti F. Activity of Hoechst 33258 against Pneumocystis carinii f. sp. muris, Candida albicans, and Candida dubliniensis. Antimicrob Agents Chemother. 2005;49:1326–1330. doi: 10.1128/AAC.49.4.1326-1330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrestha SK, Grilley M, Anderson T, Dhiman C, Oblad J, Chang CW, Sorensen KN, Takemoto JY. In vitro antifungal synergy between amphiphilic aminoglycoside K20 and azoles against Candida species and Cryptococcus neoformans. Med Mycol. 2015;53:837–844. doi: 10.1093/mmy/myv063. [DOI] [PubMed] [Google Scholar]
- 35.Fosso M, AlFindee MN, Zhang Q, Nziko Vde P, Kawasaki Y, Shrestha SK, Bearss J, Gregory R, Takemoto JY, Chang CW. Structure-activity relationships for antibacterial to antifungal conversion of kanamycin to amphiphilic analogues. J Org Chem. 2015;80:4398–4411. doi: 10.1021/acs.joc.5b00248. [DOI] [PubMed] [Google Scholar]
- 36.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42:1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350–358. doi: 10.1016/j.celrep.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem. 2014;6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Chang W, Zhang M, Li X, Jiao Y, Lou H. Diorcinol D Exerts Fungicidal Action against Candida albicans through Cytoplasm Membrane Destruction and ROS Accumulation. PLoS One. 2015;10:e0128693. doi: 10.1371/journal.pone.0128693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.