Abstract
Oxygen diffusion limitations within nascent tissue engineered (TE) grafts lead to the development of hypoxic regions, cell death, and graft failure. Previous efforts have been made to deliver oxygen within TE scaffolds, including peroxide-doping, perfluorocarbons, and hyperbaric oxygen therapy, to mitigate these effects and help maintain post transplantation cell viability, but these have suffered from significant drawbacks. Here we present a novel approach utilizing polymeric hollow-core microspheres that can be hyperbarically loaded with oxygen and subsequently provide prolonged oxygen delivery. These oxygen carriers are termed, microtanks. With an interest in orthopedic applications, we combined microtanks within polycaprolactone to form solid phase constructs with oxygen delivery capabilities. The mathematical laws governing oxygen delivery from microtank-loaded constructs are developed along with empirical validation. Constructs achieved periods of oxygen delivery out to 6 days, which was shown to prolong the survival of human adipose derived stem cells (hASCs) and human umbilical vein endothelial cells (HUVECs) as well as to enhance their cellular morphology under anoxic conditions. The results of this study suggest the microtank approach may be a feasible means of maintaining cell viability in TE scaffolds during the critical period of vascularization in vivo.
Keywords: Oxygen delivery, Microcapsule, Oxygen permeation, Polycaprolactone
INTRODUCTION
Tissue engineered approaches to regenerate bone, muscle, liver, fat, and blood vessels have all been demonstrated in small animal models with some measure of success. These successes are a bellwether of the promise of regenerative medicine to revolutionize healthcare by providing customized autologous grafts to replace damaged or failing tissues. These reports have helped elucidate the underlying mechanisms governing tissue formation and provided encouraging proofs-of-concept; however, they often overlook the challenges [1–4] associated with scaling the grafts from the cubic millimeter (mm3) domain of small rodents to the cubic centimeter (cm3) domain of humans.
Within small grafts the process of diffusion alone can accommodate sufficient transport of nutrients, oxygen, and waste to maintain the viability of cells, whereas in larger, diffusion-limited grafts, convection is required to meet metabolic needs. Unfortunately, convection requires functional vascular networks [5,6] and these can take several days to weeks to grow into the graft from surrounding tissues. During this period, cellular metabolic demand within the transplant exceeds supply leading to nutrient deficiency and hypoxia [1,7,8], which can ultimately result in cell death and graft failure if persistent.
Diffusion limitations apply to all metabolic molecules and can be estimated by comparing rates of delivery and/or generation to rates of consumption, as is captured in the Thiele modulus [9] (1D case): , with scaffold thickness (h), cell density (ρcell), maximum cellular oxygen uptake rate (Vmax), Michaelis constant (km), and oxygen diffusion (D). A large Thiele modulus indicates diffusion limitations and the presence of gradients. When considering cell metabolism both the deficit of nutrients as well as the accumulation of waste products can be problematic [10,11]. In glucose metabolism, aerobic cellular respiration is able to net around 30 ATP per glucose molecule plus the relatively fast-diffusing CO2 waste product. If oxygen is not available, anaerobic glycolysis nets 2 ATP plus the relatively slow-diffusing lactic acid waste product [7,12]. If the energy requirements of the cell are to be maintained under anaerobic conditions, Vmax, anaerobic needs to be fifteen times (15X) greater than Vmax, aerobic. Thus, the Thiele modulus of glucose under anaerobic conditions is approximately four times that of aerobic conditions, and diffusion-limitations occur over proportionally shorter distances. This is in addition to the more diffusion-limited and less tolerated waste product, lactic acid.
Given the deleterious consequences of hypoxia within diffusion-limited grafts, a number of approaches have been investigated to augment oxygen supply within nascent grafts to maintain cell viability during the period of vascularization. These have included: hyperbaric oxygen therapy where the patient and/or graft is placed in an oxygen enriched environment to enhance the oxygen content dissolved in the blood and interstitial fluid [13–16], perfluorocarbon emulsions which utilize the high oxygen solubility properties of these chemicals to enhance diffusion rates [2,17–20], and various peroxide compounds which generate oxygen during a decomposition reaction [21–23]. While each of these approaches has highlighted the benefits of oxygen augmentation, they each suffer from limitations in safety and/or efficacy that have prevented their widespread adoption. Thus, there remains a significant need for approaches that can deliver oxygen within grafts over extended periods of time.
In this paper, we describe a novel oxygen delivery strategy, which avoids many of the drawbacks of previous approaches. The approach uses microscopic hollow polymeric balloons that can be hyperbarically loaded with oxygen and subsequently release the gas back into the surrounding environment to nurture cells over an extended period of time. As the cellular analogue to a scuba tank we have termed these oxygen carriers, microtanks (Fig. 1). The polymeric shell of the microtank provides dual functions: mechanical confinement of the pressurized oxygen and a gas barrier to control the diffusion of oxygen out of the hollow core. Within this study, microtanks were melt-mixed into polycaprolactone to functionalize the polymer with oxygen delivery capability. PCL is frequently used in the structural phase of many tissue-engineering scaffolds, particularly in orthopedic applications, and thus represented an appropriate platform for proof-of-concept studies. In our system, oxygen diffuses out of the microtanks, through the bulk PCL phase, and ultimately into the hydrogel phase where it is consumed by cells. The aim of this paper is to lay the theoretical framework for the microtank approach as well as provide experimental evidence to validate the theory and demonstrate utility in extending cell viability under hypoxic conditions.
Figure 1. Schematic of microtank-loaded PCL constructs.
The microtanks contain a hollow core, which can be hyperbarically loaded with gaseous oxygen, surrounded by a polymeric shell composed of a structural, oxygen barrier layer to contain the pressurized oxygen. Scaffolding material composed of PCL is embedded with microtanks to create a syntactic foam with distributed closed-cells. The pressure differential across the microtank shell drives the outward diffusion of oxygen through the shell and into the bulk PCL where it continues to diffuse out to the periphery of the scaffolding and into the media/hydrogel to nourish cells. The release of oxygen from microtank-loaded constructs is regulated by the rate of permeation through the shell plus the rate of permeation through the bulk phase.
MATERIALS & METHODS
Fabrication of Microtank-Loaded Constructs
Constructs containing microtanks were fabricated by melt mixing polycaprolactone (InstaMorph, USA) and commercially available polymer microtanks E030 (Henkel, CT, USA) at 100 °C. Different volume concentrations of microtanks in PCL were achieved by varying the amount of microtanks added during mixing. The final concentration of microtanks by volume was confirmed by casting a 200 µm sheet of the mixture, imaging using a bright field microscope, and using Image J to quantify the volume of microtanks within the known volume of the imaged material. For cell studies, a concentration of 7% V/V was used as this resulted in a consistency of PCL that was easy to process while still contributing significant oxygen capacity. The mixture was then cast into sheets of 1, 2, or 4 mm thicknesses and subsequently formed into disks using a hand punch (14 mm diameter).
Hyperbaric Oxygen Loading of Composite Microtank/PCL Disks
Disks were loaded with oxygen in a custom built hyperbaric chamber. The chamber was loaded to pressures of 10 – 20 atm with pure oxygen for periods equal to at least 3 times the theoretical periods of release for the disks. In the cell studies, disks were removed from the hyperbaric chamber following loading to pre-release for at least half of the time constant for outgassing , which corresponded to 30 hrs, to mitigate the effects of any burst release of oxygen occurring during this period. Different levels of oxygen delivery were achieved by allowing additional prerelease, the details of which are provided in each specific study.
Quantitative Measurement of Oxygen Release Profiles
A Seahorse XF24 Flux Analyzer (Seahorse Bioscience, USA) was used in conjunction with islet plates to provide quantitative measurement of oxygen release from the microtank-loaded PCL. Briefly, the polycarbonate XF24 Islet Capture rings (3mm internal diameter (ID) × 1mm height (H)) were filled with the microtank-loaded PCL, hyperbarically loaded with oxygen, and then placed within the 24-well microplate. The disks were covered with 675 µL of XF solution at pH 7.4. Individual oxygen probes monitored the oxygen delivery rates at increments of 20 minutes for the first 6 hours and every 30 minutes thereafter for 14 hours. Oxygen measurements were made by the XF24 Flux Analyzer by temporally sealing off a small volume of fluid surrounding the disk and monitoring the accumulation of oxygen; the rate of change of oxygen concentration within the known volume allows the delivery rate to be calculated. Oxygen is able to diffuse through the solution to atmosphere between readings.
Relative Measurement of Oxygen Release
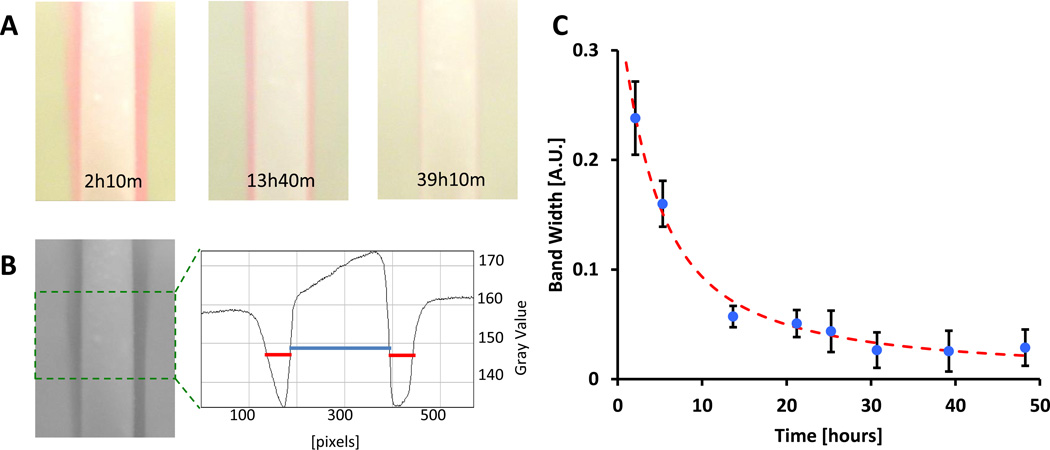
A colorimetric assay was developed to give quasi-quantitative readings of oxygen release from cylindrical constructs of different thicknesses. A solution of oxygen sensitive resazurin dye (3 mL of 0.1%), sodium hydroxide (1M), and glucose (0.133M) was prepared that turns pink in the presence of oxygen and colorless in the absence of oxygen. This occurs by the initial irreversible reduction of resazurin (blue) by glucose to resorufin (red) followed by reversible reduction of resorufin to dihydroresorufin (colorless) and the reversible oxidation of dihydroresorufin by oxygen to resorufin (red). Glucose is oxidized to gluconic acid. As the oxygen is consumed in the reaction, this solution enables the observation of quasi-steady state oxygen delivery rates from constructs, where the intensity and thickness of the pink band emanating from the construct surface is proportional to the flux of oxygen.
To measure the flux of oxygen, 14 mm diameter disks of various thicknesses composed of microtank-loaded PCL were formed, loaded with oxygen, and mounted vertically to a weighted support structure so their profile could be observed. The disks were placed in a glass enclosure, submerged in fresh resazurin solution, and allowed to reach quasi-steady state (~5 min). A digital camera was used to take images of the disk profile and resazurin bands.
The widths of the bands were quantified using a custom Matlab program. Briefly, the program aligns the disk to 90 degrees, crops the central 1/3 of the disk, extracts the green channel for maximum contrast, plots the average intensity (gray value) of the image from which the widths of the resazurin band and the disk are extracted. In the green channel the resazurin band shows up as a dark region in contrast to the white disk and background, producing a valley shape in the profile plot. For consistency, the width of the band is taken as the width at half-maximum of this valley. For each image, the pixel width of the bands from left and right faces are measured, averaged, and normalized by the pixel width of the disk to compensate for any variations in zoom of the image.
Cell Source
Humans ASCs were a kind gift of Dr. Jeffrey Gimble. The hASCs were isolated under an Institutional Review Board approved protocol according to published methods [24]. Cells from pooled donors were used at passages 3 – 5.
A vial of pooled Human Umbilical Vein Endothelial Cells (HUVECs) (Lonza, USA) were expanded in tissue culture flasks up to Passage 5 using Endothelial Basal Medium 2 (EBM-2) culture medium (Lonza, USA) supplemented with the Endothelial Growth Medium-2 (EGM-2) Bullet Kit (Hydrocortisone, human fibroblast growth factor (hFGF)-2, vascular endothelial growth factor (VEGF), R3-insulin-lke growth factor (IGF)-1, ascorbic acid, human epidermal growth factor (hEGF), Gentamicin & Amphotericin (GA-1000), and Heparin) and 2% FBS. HUVECs were used for experimentation at Passage 6.
Time Course of Cell Metabolism
Human ASCs (Passage 3–5) were combined into fibrin gels (8 mg/mL bovine fibrinogen, 2 U/mL thrombin, in phosphate buffered saline (PBS)) and cast into 100 µL gels in polycarbonate 4mm ID molds at a cellular concentration of 2,500 cells/µL. The polycarbonate disks were positioned on top of microtank-loaded PCL disks (12 mm diameter × 4mm thick), which were glued to the bottom of 12-well plates. Three groups of microtank-loaded PCL disks were used (n = 4 per group): Neg: disks were purged with nitrogen; Pos: disks were equilibrated in atmosphere (21% O2, 79% N2), and µTank, in which the disks were loaded in 100% O2 at 15 atm and allowed to pre-release for 24 hrs before cell seeding.
Cells were fed with 2 mL of expansion medium (89 mL Low Glucose DMEM, 1 mL FBS, 1 mL P/S, 20 µL FGF) and then placed in anoxic chambers, which were purged for 15 min with anoxic gas mixture then sealed closed. The chambers contained oxygen-absorbing packs to quench any released oxygen. Chambers were incubated at 37 °C and contained a water reservoir to maintain humidity. Anoxic culture conditions (0% O2, 5% CO2, 95% N2) were used to simulate the worst-case scenario within diffusion limited grafts (Neg). Positive controls groups (Pos) were cultured under normoxic conditions (21% O2, 5% CO2, 74% N2) to provide a benchmark. Samples were assessed at Days 0, 2, 4, and 6.
Effect of Oxygen Delivery on Cellular Proliferation
Human ASCs and HUVECs were combined (ratio of 5:2) at 2,500 cells/µL into fibrin gels and cultured on disks similar to those used for the time course study with the following groups (n = 4): Neg: disks were purged with nitrogen; Pos: disks were equilibrated in atmosphere, and µTank (High, Med, Low), in which the disks were loaded in 100% O2 at 15 atm and allowed to pre-release for 30, 54, and 78 hrs, respectively, to achieve different levels of oxygen delivery before cell seeding. Cells were fed with 2 mL of expansion medium and then placed in anoxic chambers (Neg, µTank), which were purged for 15 min with anoxic gas mixture then sealed closed, or directly in the incubator (Pos). There were no further medium changes over the six-day period in all groups except for one set of Pos controls, which received media changes every other day to observe the effects of nutrient deprivation. The chambers contained oxygen-absorbing packs to quench any released oxygen. Chambers were incubated at 37 °C and contained a water reservoir to maintain humidity. Samples were removed at Day 6 for analysis.
Effect of Oxygen Delivery on Vascular Assembly
Human ASCs and HUVECs were combined at 2,500 cells/µL each into fibrin gels and cultured on disks similar to those used for the time course study with the following groups (n = 4): Neg: disks were purged with nitrogen; Pos: disks were equilibrated in atmosphere, and µTank, in which the disks were loaded in 100% O2 at 15 atm and allowed to pre-release for 30 hrs before cell seeding. Cells were fed with 5 mL of EGM-2 (Lonza, Switzerland) and then placed in anoxic chambers, which were purged for 15 min with anoxic gas mixture then sealed closed. The chambers contained oxygen-absorbing packs to quench any released oxygen. Chambers were incubated at 37 °C and contained a water reservoir to maintain humidity. Samples were removed at Day 4 for Live/Dead staining according to the manufacturer’s instructions (Life Technologies, California) and confocal imaging on a Zeiss LSM 510 confocal microscope.
PicoGreen DNA Assay
The PicoGreen assay was carried out according to the manufacturer’s instructions. Gels were transferred into 600 µL of TEX + Proteinase K and frozen at −20°C. Samples were subsequently heated at 55 °C overnight to digest proteins and lyse cells. The samples were combined with PicoGreen reagent (20 µL sample + 80 µL TEX + 100 µL PicoGreen) and transferred (200 µL) into opaque 96-well plate along with DNA standards. Fluorescence was measured at 485/528 nm using a plate reader.
Alamar Blue Assay
A solution of 0.001% resazurin in expansion media was prepared fresh and added at 2 mL/well to a 24-well plate. Gels were transferred individually into the wells and allowed to incubate for 2 hrs at 37 °C in the incubator. 200 µL samples of the solution were placed in opaque 96-well plates and measured using fluorescence spectrometry at 540/590 nm using a plate reader.
MATHEMATICAL MODEL
The proposed mathematical models describe the oxygen delivery from microtank-loaded polymer constructs as detailed in the Materials and Methods section. The geometry of the system is highlighted in Fig. 1. Briefly, polymeric hollow-core microspheres/microtanks are suspended in a bulk phase polymer. Oxygen stored in the microtanks must permeate across the polymeric shell and then diffuse out through the bulk phase polymer to be delivered at the construct surface.
Oxygen Capacity
The oxygen capacity of the construct is determined by the concentration of microtanks, the solubility of oxygen in the bulk phase polymer, and the loading pressure. Upon hyperbaric loading, oxygen dissolves in the bulk polymer by Henry’s Law at a concentration, C = S(1-Φ)P, and enters the hollow core of the microtanks by the ideal gas law, , where Φ is the volume fraction of microtanks, P is the loading pressure, R is the ideal gas constant, T is temperature, and S is the solubility constant of oxygen in polymer at standard temperature and pressure (for PCL, ). Thus, at equilibrium, the concentration of oxygen is proportional to the loading pressure and the volume fraction of microtanks as: .
Kinetics of Delivery from Bulk Polymer Phase
In order for gas to be delivered from the construct it must diffuse from the microtanks through the PCL to the construct surface. For the simple case of outgassing from a slab of polymer with thickness 2d, an oxygen diffusion coefficient D, an initial dissolved concentration of oxygen in the polymer of C0, and surface concentration of oxygen C1, the oxygen delivery rate (ODR) from the surface can be calculated by solving the differential equation prescribed by Fick’s Law [26] as: .
The delivery of oxygen exhibits an exponential release profile and can be reasonably represented by the first term in the series after a period of .
Kinetics of Delivery from Microtank Phase
In addition to the diffusion of gas through the bulk polymer phase, oxygen must also permeate through the thin polymeric shell of the microtanks. The rate of transport, n, of a gas through a thin membrane is proportional to the pressure drop, ΔP across the membrane, the area, A, of the membrane, and the permeability, σ, of the membrane to the gas, which is the product of the solubility and diffusion of the gas within the polymer (σ = D × S), while inversely proportional to the thickness, d, of the membrane:. For a microtank of radius r loaded to a pressure P0 the oxygen delivery rate follows the exponential form:
Combined Kinetics of Delivery
Delivery of oxygen from microtank-loaded polymer constructs is thus the product of two phenomena: the permeation of gas out of the polymeric shell of the microtank into the bulk polymer and the diffusion of gas through the bulk polymer and out of the construct. Given that both phenomena are exponential and in series, as an approximation the time constant of oxygen delivery can be given as the sum of the time constants of the constitutive phenomena: τoutgass=τmicrotank+τbulk phase.
RESULTS
Microtanks Increase Oxygen Content and Delivery Period
To validate the theory governing oxygen delivery from microtank-loaded polymer constructs, PCL disks with and without microtanks were fabricated, loaded with oxygen, and then the oxygen delivery rate was monitored using a Seahorse XF24 Extracellular Flux Analyzer. The profiles were fitted to extract time constants (Fig. 2a) and integrated to calculate the total oxygen content within the disks (Fig. 2b). The total oxygen content in the microtank-loaded disks is approximately three times that of the disk alone, demonstrating the ability of microtanks to significantly increase the oxygen-carrying capacity of hyperbarically loaded polymers. The microtank-loaded disk is observed to have a significantly higher time constant than the PCL-only disk, suggesting the polymeric shell of the microtanks does contribute to slowing the release of gas from the disks.
Figure 2. MicroTanks enhance oxygen delivery capacity and duration.
(A) Oxygen delivery from hyperbarically loaded disks with microtanks (+µTanks, red) compared to without microtanks (−µTanks, blue), fitted with theoretical delivery profile to extract time constants of delivery. (B) Total oxygen delivered was calculated by integrating the delivery curves while time constants of delivery were calculated from the theoretical fit.
Visualization and Semi-quantitative Measurements of Oxygen Delivery
In scaling to larger constructs, the Seahorse XF24 Flux Analyzer was no longer suitable for monitoring oxygen release so we developed a semi-quantitative colorimetric assay to allow for visual monitoring of oxygen delivery as well as extraction of time constants. The assay utilizes the color changing properties of resazurin dye in the presence of oxygen to spatially indicate oxygen delivery. By imaging constructs successively over time and monitoring the intensity and widths of the resazurin bands emanating from the constructs it is possible to further characterize the oxygen delivery temporally (Fig. 3). This method allows for the characterization of oxygen delivery from arbitrarily sized and shaped constructs.
Figure 3. Resazurin assay provides colorimetric visualization and quantitation of oxygen delivery duration from microtank-loaded PCL constructs.
(A) Photograph of 2 mm thick disks in resazurin solution indicating oxygen release (pink) over a period of 2 days. (B) Green channel of photographs (left) are used to quantify the thickness of the resazurin bands emanating from the disks by plotting the average horizontal profile of the image (green box) and extracting the band widths (red) and disk thickness (blue) at half maximum. (C) Plotting the thickness of the resazurin band over time (blue circles) and fitting with the theoretical oxygen delivery equation (red) allows for the extraction of the time constant of delivery.
Period of Oxygen Delivery Increases with Construct Thickness
We sought to characterize the oxygen delivery in larger constructs as a function of construct thickness to validate the governing mathematical model. As shown in Figure 4, disks with thicknesses of 1, 2, and 4 mm were fabricated, loaded with oxygen, and then oxygen delivery was monitored by the resazurin assay. The profile obtained was fitted with the theoretical delivery profile to extract a time constant of delivery. It is observed that thicker disks exhibited significantly longer periods of release. This trend is consistent with the theory; however, the relationship is expected to be 2nd order where as empirically it was observed to be between 1st and 2nd order.
Figure 4. Time constant of oxygen delivery increases with polymer thickness.
Stacked bar graphs illustrate the contribution of the microtank (blue) vs. bulk phase (red) time constants to the total outgassing time constant. Microtank-loaded PCL disk with thicknesses of 1 mm, 2 mm, and 4 mm (pictured below in resazurin solution) were monitored over 1 week to extract time constants of delivery (above). n = 3. * indicates significant difference (p < 0.05 by one-way ANOVA and Tukey’s HSD).
Microtank Concentration Increase Oxygen Delivery
Additionally, the relationship between microtank concentration and oxygen delivery was investigated. Briefly, PCL disks containing 0%, 1.4%, or 7% V/V microtanks were fabricated, loaded with oxygen, and monitored by resazurin assay for oxygen release. The relative flux of oxygen from the disks was compared (Fig. 5), suggesting that oxygen delivery can be tuned by increasing the concentration of microtanks within the scaffold.
Figure 5. Amount of oxygen delivery increases with microtank concentration.
Plot of relative amount of oxygen released at 1 hr time point from PCL disks containing 0, 1.4, or 7% V/V microtanks, normalized to pure PCL disk. n = 3. * indicates significant difference (p < 0.05 by one-way ANOVA and Tukey’s HSD) between the 7% and the 0% and 1.4% groups.
Storage of Constructs at Low Temperatures Maintains Oxygen Content
Given that the time constant of oxygen delivery is inversely proportional to the rate of diffusion within the polymer and that diffusion is highly temperature dependent, we sought to slow outgassing through storage of constructs at reduced temperatures.
Disks of PCL containing microtanks were loaded with oxygen and then stored for 1 week at ambient pressure at 20 °C, −20 °C, or −80 °C, or at loading pressure in the hyperbaric chamber. Following the week of storage, the magnitude of oxygen delivery from the disks was compared using the resazurin assay. The results revealed that disks stored at −20°C and −80°C maintained equivalent oxygen content to freshly loaded disks (i.e. those stored within the hyperbaric chamber). In contrast, disks stored at 20°C had significantly less oxygen content than all groups (Fig. 6).
Figure 6. Low temperatures enable storage of hyperbarically loaded constructs.
Plot of relative amount of oxygen delivered at 1 hr time point from hyperbarically loaded PCL disks containing microtanks that were stored for 1 week in ambient pressure at 20 °C, −20 °C, or −80 °C, normalized to disks stored under loading conditions in hyperbaric chamber (n = 2). * indicates significant difference (p < 0.05 by one-way ANOVA and Tukey’s HSD) from all other groups.
Microtank-Loaded PCL Constructs Prolong Cell Viability Under Anoxic Culture
We assessed the potential to extend cell viability in anoxic environments by monitoring cellular metabolism over time with and without microtank-loaded constructs. Briefly, an Alamar Blue assay was used to measure the metabolism of hASCs cultured on PCL disks in 100 µL fibrin gels under anoxic conditions out to 6 days. The 4 mm disks from the characterization experiments were used, as they possessed the largest time constant.
At each time point, the relative metabolism of the cells normalized to the positive control was measured (Fig. 7). The metabolism of the negative control group, which contained constructs without microtanks, dropped steadily under anoxia. In contrast, the group containing microtanks maintained appreciable metabolism out to Day 4 before dropping off at Day 6. Notably, at Day 4 the Pos and µTank groups were both statistically higher than the Neg group, and not statistically different from each other as determined by one-way ANOVA with Tukey’s HSD (p < 0.05). This suggests that the oxygen delivery from the microtank-loaded construct is beneficial to ASC viability under anoxic conditions.
Figure 7. Time course of ASC metabolism under anoxic conditions.
Results of Alamar Blue assay of ASCs cultured in 3D fibrin gels on top of PCL disks containing oxygen loaded microtanks (µTanks), nitrogen purged microtanks (Neg), or ambient microtanks (Pos). µTank and Neg groups were cultured under anoxia (0% O2, 5% CO2, 95% N2) while the Pos group was cultured under normoxia (21% O2, 5% CO2, 74% N2) (n = 3). * indicates significant difference (p < 0.05 by one-way ANOVA and Tukey’s HSD) between indicated pairs.
Oxygen Delivery Enhances Cellular Proliferation
We then investigated how the amount of oxygen delivered affected cell growth. Three different amounts of oxygen were selected, based upon predicted cellular demand, to be higher, lower, or equal to demand. We determined the ratio of over time based on the literature values for oxygen uptake rate for hASCs and HUVECs [27,28] and the previously measured oxygen delivery rate for the disks over time (Fig. 8a).
Figure 8. Amount of oxygen delivery influences cellular growth.
(A) Theoretical ratio of oxygen delivery from microtank loaded PCL disks to cellular demand over 6 day culture based on initial cell number, oxygen uptake rates from the literature, and measured delivery profiles. Disks were loaded with oxygen under the same conditions and pre-released for 3, 2, or 1 days before cell encapsulation in gel to achieve the low, medium, and high delivery profiles, respectively. Dashed line indicates threshold where delivery is equal to demand. (B) Change in DNA content by PicoGreen Assay within gels between Day 0 and 6 Days of anoxic culture (Neg, Low, Med, High with amount of oxygen delivered indicated) or normoxic culture (Pos). Black line indicates average growth in a positive control that received feedings every two days (dashed lines represent corresponding standard deviation); all other groups received only an initial 5mL of media (n = 4). All differences are statistically significant (p < 0.05 by one-way ANOVA and Tukey’s HSD), except Pos vs. Med and Neg vs. Day 0 (indicated by not significant (N.S.) markers).
The change in DNA content relative to Day 0 was assessed at Day 6 for all groups using the PicoGreen Assay (Fig. 8b). All microtank-containing constructs performed significantly better than the negative control. The DNA content in the microtank groups correlated positively with the amount of oxygen delivered (i.e. groups receiving more oxygen exhibited higher oxygen content) and the DNA content in the microtank groups was comparable to that of the positive control.
Oxygen Delivery Enhances Cellular Elongation
The rational for oxygen augmentation in nascent tissue engineered grafts is to maintain the cells during the period of vascular assembly and anastomosis. Therefore, we investigated whether oxygen delivery could enhance this process. Co-cultures of ASC:HUVECs demonstrated significant viability and elongation after 4 days in fibrin gels. Live/dead confocal imaging of the gels, Fig. 9a, demonstrates a higher proportion of live cells within the Pos and µTank groups compared to the Neg group. Quantification of the ratio of live/dead cells is similar between the Positive and µTank groups, where as the Negative group displayed fewer viable cells than the µTank group, highlighting the therapeutic benefit of oxygen delivery (Fig. 9b).
Figure 9. Microtanks enhance vascular morphogenesis under anoxic conditions.
(A) Confocal live (green) /dead (red) images (5X) of ASC:HUVECs in fibrin gels at Day 4. Representative images shown (n = 4). (B) Quantification of images using the ratio of areas of live and dead cells. * indicates significant difference by one-way ANOVA (p < 0.05).
DISCUSSION
Here, we present for the first time the governing principles, proof-of-concept, and experimental evidence to support the potential therapeutic benefit of the microtank approach. The delivery of oxygen from microtank-loaded PCL constructs is a completely novel approach to maintain cellular viability under anoxic conditions. The approach provides a number of advantages compared to previously described oxygen delivery technologies. Firstly, there are no cytotoxic byproducts produced in contrast to calcium peroxide-based approaches that release reactive oxygen species intermediates, alkali byproducts, and calcium ions stoichiometrically with each O2 molecule generated. Secondly, the system has a relatively high oxygen delivery capacity compared to perfluorocarbon-doped scaffolds, which are only able to contain approximately 50% v/v O2 at 1 atm and are unable to provide prolonged oxygen delivery. Thirdly, the approach is highly tunable: we can regulate the temporal kinetics of release by changing governing parameters related to microtanks size and loading pressures. In this study, we establish the oxygen delivery characteristics and relevant parameters.
As described above, the requirements for microtanks are: a low permeability shell and sufficient mechanical properties to withstand hyperbaric loading. To provide proof-of-concept of the microtank approach, we identified existing products that met these requirements. The microtanks used within this study have been repurposed from their intended use as lightweight fillers in composite resin systems, hence they exhibit high mechanical strength. The high oxygen barrier properties possessed by these particles, however, are a vestige of the methodology used in their manufacture and not an otherwise engineered or utilized property. By acknowledging the intended and dormant properties of these particles, we were able to utilize a commercial product and benefit from the associated reproducibility and accessibility. These unmodified microtanks demonstrated a time constant of oxygen delivery of approximately three hours, highlighting the utility of a polymeric shell to prolong oxygen release. By varying the thickness of the polymer shell possessed by the microtanks or forming them out of polymers with even lower oxygen permeability, it would be possible to greatly extend this period of release.
The oxygen delivery properties of microtank-loaded PCL constructs have been characterized within this study and are well described by the mathematical models presented. It has been shown that in these composite systems, the period of oxygen outgassing is influenced both by the contribution from the microtank and that of the bulk phase polymer. This provides multiple means of modulating the period of oxygen delivery from the construct, through manipulating construct geometry, microtank properties, or the bulk polymer composition. As predicted by theory, the period of outgassing increased with thicker constructs. This fact allowed us to achieve a time constant of release of over two days despite the microtanks themselves providing release with a time constant of roughly three hours. We have also shown that varying the concentration of microtanks within the bulk polymer can modulate the oxygen content stored within a polymer. The upper limit for microtank concentrations is on the order of 20% by volume, after which processing becomes prohibitively difficult (data not shown). The consequent limitation in oxygen carrying capacity can be mitigated, however, by increasing the pressure at which the construct is loaded since oxygen concentration is directly proportional to pressure. Due to mechanical considerations, the upper limit on the loading pressure is on the order of 100 – 1000 atm, well above the current needs of this study.
The loading and storage of constructs represents an important consideration for its ultimate application. Hyperbaric loading of a construct follows similar kinetics to the outgassing of the construct so it takes approximately 4 – 5 time constants to load the construct to 99% of its capacity. However, the diffusion and permeability of polymers are highly temperature dependent and the gas permeability, σ, has the form , where Ep is the activation energy for permeation. For polymers EP/R is commonly on the order of 5 × 103 K [29], so around room temperature an increase of 40 °C will approximately increase the permeability by an order of magnitude. Therefore, by loading the microtanks at elevated temperatures it will be possible to reduce loading times by orders of magnitude, depending on the thermal limits of the constituent polymers. This will require a hyperbaric chamber designed for operation at elevated temperatures and safety considerations given the use of pressurized oxygen but is well within the realm of technical feasibility. With respect to storage, we sought means to avoid the need to maintain constructs within hyperbaric chambers, to facilitate shipping and logistics, and have demonstrated that this can be easily achieved by storing the constructs at reduced temperatures. By theoretical estimates, the outgassing time constants can be reduced to 35 to 15,000 times the period of release at 37 °C by lowering the temperatures to −20 °C and −80 °C, respectively. The results presented within this study, while monitored over a shorter period, are consistent with these predictions. Together, these methods of increasing loading and facilitating storage improve the translational potential of the microtank approach.
The prolonged oxygen delivery from microtank-loaded PCL can significantly impact the transplantation of engineered tissue grafts by supporting cells under anoxic conditions. The oxygen delivery afforded by the microtank-loaded constructs was shown to maintain cellular metabolism, as measured by Alamar Blue assay, under otherwise anoxic conditions out past four days (i.e. two time constants). The beneficial effect can be quantified in comparison to the unloaded Neg control constructs, which exhibited an immediate and rapid decrease in metabolic activity under anoxia. The significant reduction in metabolism of cells in the µTank group by day 6 suggests that their capacities are exceeded by this time. The amount of oxygen delivered to cells was shown to positively correlate with proliferation as determined by changes in DNA content. Yet, too much oxygen can actually have a deleterious effect on cells due to oxygen toxicity. The positive correlation observed within this study suggests oxygen delivery was maintained within a therapeutic range. Over a 6 day period, the microtank-loaded constructs were able to achieve cellular proliferation comparable to constructs cultured under 20% O2 while the Neg unloaded constructs showed no significant proliferation. Thus, oxygen delivery from microtank-loaded constructs provides a means of maintaining cells under otherwise unfavorable anoxic conditions.
Finally, we found that oxygen delivery from microtanks-loaded constructs facilitated cellular elongation when endothelial cells were co-cultured with hASCs under anoxic conditions. While hypoxia is known to drive angiogenesis, vasculogenesis of endothelial cells is inhibited under severely hypoxic environments. One strategy for achieving vascularized constructs might envision utilizing an initial phase of oxygen delivery to enhance vascular morphogenesis and then as the oxygen delivery tapers off rely on the generation of hypoxic stress to drive angiogenesis and anastomosis. These results provide inital proofs-of-concept, though more optimization is necessary to facilitate full morphogenesis into capillary like structures.
One limitation of this study is that polyacrylonitrile, from which the microtanks are made, while biocompatible [30,31] and used in medical implants [33], is not biodegradable [32]. In future studies, PAN microtanks could be substituted for microtanks made of generally recognized as safe (GRAS) polymers, such as poly(lactic-co-glycolic acid) PLGA, polylactic acid (PLA), and polyvinyl alcohol (PVA) [34,35], which possess similar or better oxygen barrier properties compared to PAN. It is noted that PAN microtanks were used in this proof-of-concept study because they were commercially available, where as biodegradable microtanks were not. Furthermore, PCL may be substituted for other thermoplastics to achieve a significantly increased period of delivery (or thinner constructs) by using a bulk phase polymer with a significantly smaller Do2 than PCL. For example, polyethylene terephthalate (PET) has an oxygen diffusion coefficient approximately 1/10th that of PCL and therefore may be a suitable replacement. Yet, PCL allowed for the bench-top fabrication of constructs, given its ease-of-use and low melting point it, obviating the need for specialized processing equipment necessary for other thermoplastics. By utilizing a constant diameter filament/strut size, uniform oxygen delivery throughout a complex shaped construct could be accomplished through 3D printing methods thereby facilitating spatial as well as temporal control of oxygen delivery.
CONCLUSION
The delivery of oxygen by the outgassing of microtank-loaded polymers represents a novel approach to mitigate the negative effects of hypoxia within tissue-engineered grafts, especially where the graft contains a structural phase in addition to a hydrogel phase as in many orthopedic constructs. The approach can add oxygen delivery functionality in a facile, predictable, and tunable manner. The local delivery of oxygen may help overcome oxygen diffusion-limitations, enabling the scaling of tissue-engineered grafts from the diminutive to the clinically meaningful. The diverse applications and implementations of the microtanks approach together with its simplicity make it an exciting platform for future studies.
Acknowledgments
The authors thank Garrett Ma and Dr. Jennifer Elisseeff for use of and assistance in operating the SeaHorse Machine; Dr. Jeffrey Gimble for supplying the hASCs; the Microscopy and Imaging Core Module of the Wilmer Core Grant for use of the Zeiss LSM 510 confocal microscope; and the Johns Hopkins University Department of Biomedical Engineering and Center for Musculoskeletal Research for funding.
REFERENCES
- 1.De Groot M, Schuurs Ta, van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. [cited 2014 Aug 8];J Surg Res [Internet] 2004 Sep;121(1):141–150. doi: 10.1016/j.jss.2004.02.018. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15313388. [DOI] [PubMed] [Google Scholar]
- 2.Khattak SF, Chin K, Bhatia SR, Roberts SC. Enhancing Oxygen Tension and Cellular Function in Alginate Cell Encapsulation Devices Through the Use of Perfluorocarbons. 2007;96:156–166. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 3.Malda J, Rouwkema J, Martens DE, Le Comte EP, Kooy FK, Tramper J, van Blitterswijk Ca, Riesle J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. [cited 2014 Aug 8];Biotechnol Bioeng [Internet] 2004 Apr 5;86(1):9–18. doi: 10.1002/bit.20038. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15007836. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Wang C, Lü S, Wu J, Guo X, Duan C, Dong L, Song Y, Zhang J, Jing D, Wu L, Ding J, Li D. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. [cited 2014 Aug 8];Cell Tissue Res [Internet] 2005 Mar;319(3):429–438. doi: 10.1007/s00441-004-1025-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15672263. [DOI] [PubMed] [Google Scholar]
- 5.Moon JJ, West JL. Vascularization of Engineered Tissues - Approaches to Promote Angiogenesis in Biomaterials. NIH Public Access. 2014;8(4):300–310. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malda J, Klein TJ, Upton Z. The roles of hypoxia in the in vitro engineering of tissues. [cited 2014 Aug 8];Tissue Eng [Internet] 2007 Sep;13(9):2153–2162. doi: 10.1089/ten.2006.0417. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17516855. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. [cited 2014 Jul 30];Stem Cells [Internet] 2006 Mar;24(2):416–425. doi: 10.1634/stemcells.2005-0121. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16253984. [DOI] [PubMed] [Google Scholar]
- 8.Koh MY, Powis G. Passing the baton: the HIF switch. [cited 2013 Jun 4];Trends Biochem Sci [Internet]. Elsevier Ltd. 2012 Sep;37(9):364–372. doi: 10.1016/j.tibs.2012.06.004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22818162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehsan SM, George SC. Nonsteady State Oxygen Transport in Engineered Tissue? Implications for Design. 2013:19. doi: 10.1089/ten.tea.2012.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL. Regulation of mammalian oxygen homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 11.Stoppel WL, Roberts SC. In: Engineering Biomaterials for Regenerative Medicine [Internet] Bhatia SK, editor. New York, NY: Springer New York; 2012. [cited 2013 May 31]. Available from: http://www.springerlink.com/index/10.1007/978-1-4614-1080-5. [Google Scholar]
- 12.Croll TI, Gentz S, Mueller K, Davidson M, O’Connor AJ, Stevens GW, Cooper-White JJ. Modelling oxygen diffusion and cell growth in a porous, vascularising scaffold for soft tissue engineering applications. [cited 2014 Aug 8];Chem Eng Sci [Internet] 2005 Sep;60(17):4924–4934. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0009250905002666. [Google Scholar]
- 13.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg [Internet] 2007 Jun;(45 Suppl A):A39–A47. doi: 10.1016/j.jvs.2007.02.068. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2706093&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh a Y, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg [Internet] 2000 Nov;135(11):1293–1297. doi: 10.1001/archsurg.135.11.1293. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11074883. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh AY, Rollins MD, Hopf HW, Hunt TK. Hyperoxia improves microvascular perfusion in a murine wound model. Wound Repair Regen [Internet] 2005;13(3):303–308. doi: 10.1111/j.1067-1927.2005.130313.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15953050. [DOI] [PubMed] [Google Scholar]
- 16.Löndahl M. Hyperbaric oxygen therapy as adjunctive treatment of diabetic foot ulcers. [cited 2014 Aug 8];Med Clin North Am [Internet]. Elsevier Inc. 2013 Sep;97(5):957–980. doi: 10.1016/j.mcna.2013.04.004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23992903. [DOI] [PubMed] [Google Scholar]
- 17.Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. [cited 2013 Jun 4];Artif Organs [Internet] 2010 Aug;34(8):622–634. doi: 10.1111/j.1525-1594.2009.00944.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20698841. [DOI] [PubMed] [Google Scholar]
- 18.Tamimi F, Comeau P, Le Nihouannen D, Zhang YL, Bassett DC, Khalili S, Gbureck U, Tran SD, Komarova S, Barralet JE. Perfluorodecalin and bone regeneration. Eur Cell Mater [Internet] 2013 Jan;25:22–36. doi: 10.22203/ecm.v025a02. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23283637. [DOI] [PubMed] [Google Scholar]
- 19.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng [Internet] 2006 Aug;12(8):2077–2091. doi: 10.1089/ten.2006.12.2077. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16968150. [DOI] [PubMed] [Google Scholar]
- 20.Kimelman-Bleich N, Pelled G, Sheyn D, Kallai I, Zilberman Y, Mizrahi O, Tal Y, Tawackoli W, Gazit Z, Gazit D. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. [cited 2013 Jun 4];Biomaterials [Internet] 2009 Sep;30(27):4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19540585. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Zhang B, Zhao L, Guo G, Lin J. Study on the generation mechanism of reactive oxygen species on calcium peroxide by chemiluminescence and UV-visible spectra. 2007:575–580. doi: 10.1002/bio.1003. [DOI] [PubMed] [Google Scholar]
- 22.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. [cited 2013 May 22];Biomaterials [Internet]. Elsevier Ltd. 2009 Feb;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19019425. [DOI] [PubMed] [Google Scholar]
- 23.Camci-Unal G, Alemdar N, Annabi N, Khademhosseini A. Oxygen Releasing Biomaterials for Tissue Engineering. [cited 2014 Feb 24];Polym Int [Internet] 2013 Jun 1;62(6):843–848. doi: 10.1002/pi.4502. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23853426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, Halvorsen YDC, Ravussin E, Gimble JM. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. [cited 2014 Sep 15];Methods Mol Biol [Internet] 2008 Jan;449:69–79. doi: 10.1007/978-1-60327-169-1_5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18370084. [DOI] [PubMed] [Google Scholar]
- 25.Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. [cited 2014 Sep 15];J Tissue Eng Regen Med [Internet] 1(4):322–324. doi: 10.1002/term.35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18038424. [DOI] [PubMed] [Google Scholar]
- 26.Crank J. The Mathematics of Diffusion. Second. Oxford: University Press; 1975. [Google Scholar]
- 27.Von Heimburg D, Hemmrich K, Zachariah S, Staiger H, Pallua N. Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. [cited 2014 Jul 31];Respir Physiol Neurobiol [Internet] 2005 May 15;146(2–3):107–116. doi: 10.1016/j.resp.2004.12.013. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15766899. [DOI] [PubMed] [Google Scholar]
- 28.Eberl T, Gnaiger E. Oxygen dependence of respiration and uncoupled endothelial cells in coupled. (4 mM) doi: 10.1152/ajpcell.1996.271.6.C2053. [DOI] [PubMed] [Google Scholar]
- 29.Kuraray EVAL ™ , a unique Kuraray technology. Technical Brochure. 2013 [Google Scholar]
- 30.Lin W-C, Liu T-Y, Yang M-C. Hemocompatibility of polyacrylonitrile dialysis membrane immobilized with chitosan and heparin conjugate. [cited 2014 Dec 24];Biomaterials [Internet] 2004 May;25(10):1947–1957. doi: 10.1016/j.biomaterials.2003.08.027. Available from: http://www.sciencedirect.com/science/article/pii/S0142961203006872. [DOI] [PubMed] [Google Scholar]
- 31.Harrison JH. Synthetic materials as vascular prostheses. [cited 2015 Jan 12];Am J Surg [Internet] 1958 Jan;95(1):3–15. doi: 10.1016/0002-9610(58)90735-9. Available from: http://www.sciencedirect.com/science/article/pii/0002961058907359. [DOI] [PubMed] [Google Scholar]
- 32.Kawabata N, Ohashi K, Nishiyama T. Releasing polyacrylonitrile from poor biodegradability by insertion of a highly biodegradable chemical structure into the main chain. [cited 2015 Jan 12];J Appl Polym Sci [Internet] 2006 Feb 5;99(3):852–857. Available from: http://doi.wiley.com/10.1002/app.22591. [Google Scholar]
- 33.Ray CD. The PDN prosthetic disc-nucleus device. [cited 2015 Jan 12];Eur Spine J [Internet] 2002 Oct;(11 Suppl 2):S137–S142. doi: 10.1007/s00586-002-0425-7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3611571&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubo U. Development of polyvinyl alcohol shells overcoated with polystyrene layer for inertial confinement fusion experiments. [cited 2014 Feb 24];J Vac Sci Technol A Vacuum, Surfaces, Film [Internet] 1987 Jul;5(4):2778. Available from: http://link.aip.org/link/?JVA/5/2778/1&Agg=doi. [Google Scholar]
- 35.Tzvetkov G, Paradossi G, Tortora M, Fernandes P, Fery A, Graf-Zeiler B, Fink RH. Water-dispersible PVA-based dry microballoons with potential for biomedical applications. [cited 2013 Sep 24];Mater Sci Eng C [Internet]. Elsevier B.V. 2010 Apr;30(3):412–416. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0928493109003257. [Google Scholar]