Abstract
Chemosensory stimuli from conspecific and heterospecific animals, elicit categorically different immediate-early gene response-patterns in medial amygdala in male hamsters and mice. We previously showed that conspecific signals activate posterior (MeP) as well as anterior medial amygdala (MeA), and especially relevant heterospecific signals such as chemosensory stimuli from potential predators also activate MeP in mice. Other heterospecific chemosignals activate MeA, but not MeP. Here we show that male hamster amygdala responds significantly differentially to different conspecific signals, by activating different proportions of cells of different phenotype, possibly leading to differential activation of downstream circuits. Heterospecific signals that fail to activate MeP do activate GABA-immunoreactive cells in the adjacent caudal main intercalated nucleus (mICNc) and elicit selective suppression of MeP cells bearing GABA-Receptors, suggesting GABA inhibition in MeP by GABAergic cells in mICNc. Overall, work presented here suggests that medial amygdala may discriminate between important conspecific social signals, distinguish them from the social signals of other species and convey that information to brain circuits eliciting appropriate social behavior.
Keywords: Medial Amygdala, Circuit, GABA, Chemosignal, Vomeronasal, Olfactory
1
In many mammals chemosensory communication is important for regulating complex social behaviors, such as interaction among conspecifics prior to mating, and marking territory (Halpern, 1987; Meredith, 1998; Tirindelli et al., 1998; Keverne, 1999, Brennan, 2010). These signals must be distinguished from each other and from potentially similar signals of other species. In rodents, many conspecific and heterospecific chemical communication signals (including pheromones) are detected by the vomeronasal, or accessory-olfactory, system (Scalia and Winans, 1975; Johnston, 1998, Samuelsen and Meredith 2009b, Kaur et al 2014, Dey et al 2015) - but the main olfactory system can also detect some chemosignals used for communication (Meredith 1998, Schaal et al 2003, Baum and Kelliher 2009, Matsuo et al 2015 Govic and Paolini 2015, Perez-Gomez-et al 2015), possibly including some non-volatile stimuli (Spehr et al 2006). Both systems send afferent inputs to the amygdala which terminate mainly in separate, but adjacent nuclei (Cadiz-Moretti et al 2014, Perez-Gomez et al 2015). Vomeronasal sensory neurons and accessory-bulb neurons can both be highly selective in their responses to chemosensory stimuli (Leinders-Zufall et al 2000; He et al 2008; Kaur et al 2014; Ben-Shaul et al 2010) but all the nasal chemosensory information streams relevant for social and defensive behavior converge in the amygdala, the subject of the present study. The vomeronasal system projects via accessory olfactory bulb (AOB) to anterior (MeA) and posterior medial amygdala (MeP) (Scalia and Winans, 1975; Kevetter and Winans, 1981a, b; Cadiz-Moreti et al 2014). The olfactory system projects via main olfactory bulb (MOB) to regions of the olfactory amygdala and piriform cortex (Kevetter and Winans, 1981b; Coolen and Wood, 1998), with onward connections to medial amygdala (Coolen and Wood 1998; Keshavarzi et al 2015). There are also minor but potentially important direct projections from MOB to medial amygdala (Kang et al 2011, Thompson et al 2012). We previously reported categorical responses to several chemosensory stimuli in rodent medial amygdala (Me) in hamsters and mice. In male hamsters, exposure to conspecific stimuli, used as social signals by other male and female hamsters, increased immediate early gene (IEG) activity in MeA and MeP. In contrast, chemosensory stimuli from mice and other (heterospecific) species, less relevant to hamsters, only activated MeA, and not MeP; and no similar categorical pattern was seen in the IEG responses of the AOB (Meredith and Westberry, 2004). In mice, the categorical patterns of response were preserved but with the conspecific pattern now elicited by mouse stimuli (Samuelsen and Meredith 2009a). Olfactory input to MeA was not critical for this pattern of activation in hamster medial amygdala (Meredith and Westberry, 2004). The failure of heterospecific stimuli to significantly activate MeP coincided with activation of the caudal main intercalated nucleus cell-group (mICNc) adjacent to MeP (Meredith and Westberry, 2004). This is one of several dense clusters of small GABAergic cells in between the main amygdaloid nuclei. The paracapsular ICN cell clusters are important in stimulus evaluation by the amygdala fear-conditioning circuits in which they appear to inhibit output cells in their adjacent principal amygdala nuclei, the basolateral and central nuclei (Marowsky et al., 2005; Pape, 2005; Pape and Pare 2010; Busti et al., 2011). We have suggested a similar relationship between mICNc and the medial amygdala (Meredith and Westberry 2004) with which our findings here are consistent. We have preliminary brain-slice electrophysiological evidence for a functional inhibitory connection from mICNc to MeP (Biggs et al 2014). The similarity in the intra-amygdaloid circuits involved in fear conditioning and those explored here for chemosensory evaluation in medial amygdala, as well as their similar modulation by dopamine (Biggs et al 2014) suggests that the organization of amygdala circuits may involve two (or more) similar circuit modules which perform different functions by virtue of their different input and output connections.
Here, we investigate the phenotype of activated (IEG-expressing) cells in male hamster MeA and MeP for additional evidence that different ecologically-relevant chemosignals activate different patterns of responses in medial amygdala. We used double-label immunocytochemistry for Fos-related antigens (FRAs) and GABA, or for FRAs and GABAa-Receptor (GABA-R), to show differential activation of different cell-types within medial amygdala by different stimuli. We also show that activation of GABA-immunoreactive (-ir) cells in mICNc by heterospecific stimuli coincides with reduced activation of MeP and selective suppression of MeP cells that express GABA-R; suggesting that mICNc inhibits MeP contributing to the stimulus-characteristic patterns in medial amygdala.
These data suggest that GABA circuits, including mICNc, contribute to characteristic patterns of response to socially relevant stimuli in medial amygdala in hamsters and that the medial amygdala may be responsible for discrimination of critical chemical-communication signals.
2. Experimental Procedures
2.1 Animal Care and Housing
All animals used in these experiments were sexually naive adult (2-3 month old) male golden hamsters (Mesocricetus auratus), bred in our laboratory or ordered from Charles River Laboratories (Wilmington, MA), and maintained on a long photoperiod (a partially reversed- 14L/ 10D light cycle). The animals were group-housed in clear plastic cages (44-cm × 21-cm × 18-cm) containing bedding with food and water ad libitum. On the day before stimulus exposure, each male hamster was separated from its cage mates and housed alone. All animal use was approved by the Institutional Animal Care and Use Committee of Florida State University
2.2 Collection of Stimuli
The stimuli described here were collected in the same manner for all of the experiments. Female hamster vaginal fluid (HVF) was collected from several (5-6) naturally cycling females on the day of behavioral estrus. For collection, the female was placed on a plexi-glass lid with holes on the top of a cage containing a stud male. The female hamster was allowed to run freely and sniff toward the holes for approximately 2 minutes. HVF was collected by gently scraping around the edge of the vagina with a blunt metal spatula. After collection, whole HVF was diluted 1:10 w/w with distilled water, centrifuged to remove solids and stored at −20°C. Flank Gland Secretion (FGS) was collected from both male and female hamsters. Male and female FGS (mFGS, fFGS) were transferred to clean cotton swabs by gently pinching up the loose skin around the flank gland and rubbing the swab on the secretion soaked fur at least 10 times up and down. FGS source animals were different for each exposure and males were not cage or litter mates of the test animals. Each stimulus swab had FGS from 3 different donors. Female FGS was collected from naturally cycling females on the day of behavioral estrous. Both male and female FGS was collected immediately before each test exposure. Male and female mouse urine (m/fMU) was collected from several (5-6) male or female mice placed in a metabolic cage overnight. Female donor mice were naturally cycling, but were not separated according to different estrous stages. Fresh urine was diluted 1:10 with distilled water and stored frozen. On the day of testing, frozen liquid stimuli were thawed and 200 μl was added to a cotton swab for each presentation. The test males in these experiments had no previous experience with or exposure to any of the heterospecific stimulus materials, no contact with female stimuli since weaning and no experience or exposure to the donors of any hamster stimuli.
2.3 Exposure and Behavior Testing
Five minutes before addition of a cotton swab containing the chemosensory stimulus, male hamsters were removed from their home cage and placed in a clean cage. Each swab was replaced every 3 minutes for a total of 15 minutes of stimulus (or clean swab) exposure. The animal was then returned to its home cage for an additional 30 minutes. During exposure, we observed and used a keypad and computer to record various aspects of the hamster's behavior such as: sniffing the swab, licking the swab, general investigation of the clean cage, grooming, escape behavior (scrabbling up the wall of the cage), flank marking and sleep. Stimulus investigation includes licking or other contact with the scented (or control) swab-tip and sniffing in close proximity to it.
2.4 Tissue Processing for Double-Label Fluorescent Immunocytochemistry
Brains were labeled for GABA or GABA-R and double labeled for FRAs immuno-reactivity to identify the phenotypes of activated cells. After stimulus exposure for 15 minutes, animals were returned to their home cage for a further 30 minutes, then deeply anesthetized with sodium pentobarbital (90 mg/Kg; Ovation Pharmaceuticals, Deerfield IL USA) and perfused through the heart with 0.1M phosphate-buffered saline (pH 7.4) followed by 4% paraformaldehyde. Brains were removed and post-fixed for 1-2 hours, cryo-protected in 30 % sucrose overnight, then sectioned serially on a freezing microtome at 25-μm thickness. After sectioning, free- floating coronal sections were washed for 1 hour in 0.1 M PBS (3 washes), incubated in 1% Hydrogen Peroxide for 30 minutes, then washed at least 3 times in 0.1 M PBS. All secondary antibodies were made in donkey so sections were blocked with 0.1 M PBS with 5% Normal Donkey Serum (NDS; Jackson Immunoresearch, West Grove PA USA) for 1 hour. Primary antibodies were: for FRAs (Santa Cruz sc253; 1:10,000; Santa Cruz Biotechnology, Dallas TX USA) and either a mouse monoclonal anti-GABA (Sigma A0130, clone GB-69; 1:10,000; Sigma Aldrich, St. Louis MO USA) or an affinity-purified goat polyclonal anti-GABAa-R (recognizing α1, α2, α3, α5 subunits; Santa Cruz sc7349; 1:500). Both primary antibodies were diluted in NDS solution and sections were incubated for 24 hours at room temperature. The following day, sections were washed in 0.1 M PBS (5 washes), and incubated in a solution containing NDS and both secondary antibodies for 2 hours at room temperature. The secondary antibodies, raised in donkey, were conjugated to Alexa 594 (red) for the FRA anti-rabbit secondary (Molecular Probes A-21209; 1:500; Life Technologies Corp., Grand Island NY USA) and Alexa 488 (green) for the GABA anti-mouse secondary (Molecular Probes A-21202; 1:500) or GABA-R, anti-goat secondary (Molecular Probes A-11055; 1:500). After secondary antibody incubation, sections were washed 3 times in 0.1 M PB, and mounted on Superfrost Plus slides (Fisher Brand) using Vectashield Hard Mount (Vector Laboratories, Burlingame CA USA).
2.5 Counting
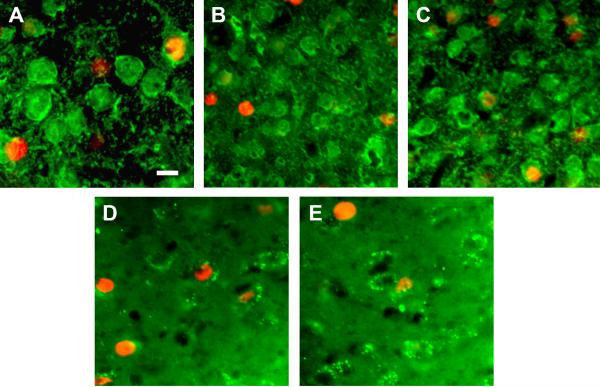
Sections were taken throughout the rostral/ caudal extent of the medial amygdala. For MeA, two alternate sections were processed for either GABA and FRAs or GABAa-Receptor (alpha 1-alpha 3) (GABA-R) and FRAs double-label immunofluorescence, such that 4 sections (100 μm) were averaged to represent total FRAs counts for MeA. For graphs showing double-label with FRAs and GABA, the 2 GABA labeled sections (separated by 25μm) were averaged together. For GABA-R data, counts for the other 2 sections were averaged. Similarly, for MeP, four sections, located approximately 300 microns caudal to the set of 4 sections in MeA were processed in the same way. The sections through MeP were also the sections in which we counted cells of the intercalated nucleus (mICNc). For GABA and GABA-R labeling, labeled cells were counted using Metamorph software (Molecular Devices, Sunnyvale CA USA) (courtesy of Dr. Marc Freeman) and a monochrome camera with filters of the appropriate wavelength for the two fluorophores. All densely FRA-labeled nuclei (red filter) and all densely labeled cell bodies (green filter) were counted to generate numbers of FRAs-activated cells and numbers of GABA-ir or GABA-R-ir cells within the outlines of each anatomical nucleus of interest (MeA, MeP, mICNc). For double-label counts merged (red plus green) images taken with a 20X power objective were closely inspected and all apparent double-labeled cells were recorded and marked. Each area of the nucleus of interest was then reexamined in the original section, using a 40X objective and focusing up and down to determine whether ambiguous double labeling was actually in the same cell or due to superimposition of more than one cell. Erroneous counts were removed from the total. Examples of images from sections processed for double immunofluorescence are in Figure 1 and show double and single labeled cells.
Figure 1. Examples of labeled cells in medial amygdala and mICNc.
A-C. GABA-ir cell bodies and processes (green) + FRAs expressing nuclei (red). A. Anterior medial amygdala after exposure to HVF. Both GABA(+) (top right) and GABA(−) cells (bottom left) are activated. B, C. GABA + FRAs label in mICNc. Smaller almost uniformly GABA(+) cells but a few GABA(−) cells; B. Few FRAs-activated GABA(+) cells after conspecific fFGS exposure; C. More FRAs(+)/GABA(+) cells after heterospecific fMU exposure. D, E GABA-Receptor-ir outlining cells (green) + FRAs labeled nuclei (red) in posterior medial amygdala. D. Many activated GABA-R(+) cells after conspecific HVF exposure; E. Fewer GABA-R(+)/FRAs(+) cells after exposure to heterospecific mMU. Scale bar 10 um applies to all panels.
2.6 Statistical Analysis
Three types of ANOVA were used to analyze the data. For the initial comparison of responses to different stimuli across amygdala sub-areas, a two-way repeated-measures (RM) ANOVA compared numbers of densely FRAs-immunoreactve cells in each subarea (Area) for each stimulus (Exposure). For comparison of responses of cells of different phenotype within an area, a two-way RM ANOVA compared numbers of cells with dense FRAs-ir of each cell-type (Phenotype) for each stimulus (Exposure). RM ANOVAs were used for the analyses which include area or phenotype as a factor because all areas were present in each animal and both phenotypes were present in the same areas, so different areas or different phenotypes were not fully independent. Differences in FRAs expression between different brain areas in response to the same stimulus are not meaningful by themselves, because the areas differ in size and cell packing. However, the interaction between Exposure and Area indicates a difference between areas in the relative response to a given set of stimuli. Exposure was a factor for all analyses because we were primarily interested in differences in responses to the different stimuli – within and across areas or cell-phenotypes. Tukey posthoc tests were used to reveal significant differences between the response of a cell-type (or all FRAs(+) cells for Fig 2) in a specific brain-area to each stimulus, compared to the response to clean control-swabs in the same phenotype cells in the same brain area (asterisks in Figs 2-4,).
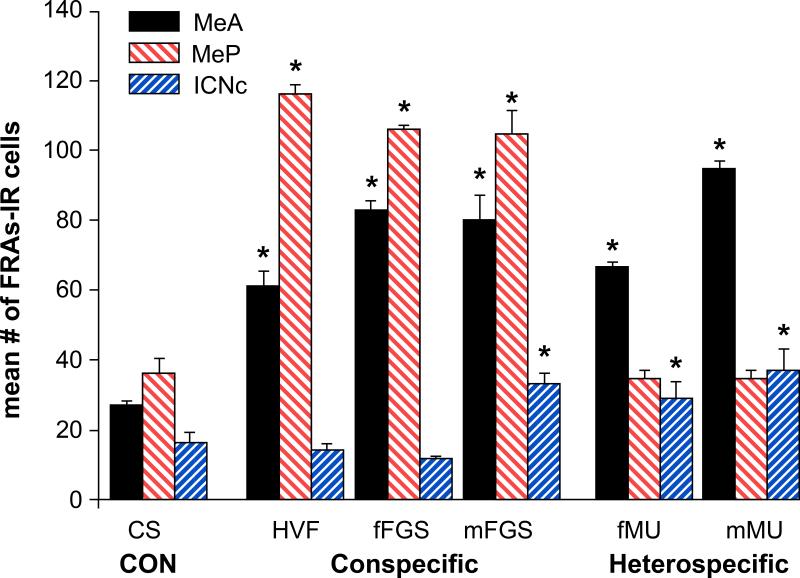
Figure 2. Total FRAs expression in medial amygdala and caudal intercalated nucleus (mICNc).
Conspecific stimuli significantly activated both MeA and MeP, while the heterospecific stimuli significantly activated only MeA and mICNc, with MeP activation not significant; possibly suppressed. One conspecific stimulus (mFGS) also activated mICNc, but in this case MeP was activated above clean swab (CS) control level. CS = Clean Swab; HVF = Hamster Vaginal Fluid; f/mFGS = female/male Flank-Gland Secretion; f/mMU = female/male Mouse Urine. Mean number + SE of FRAs positive cells averaged over 4 sections (both sides) for each anatomical nucleus. Asterisks indicate significant differences from CS controls (p< 0.01).
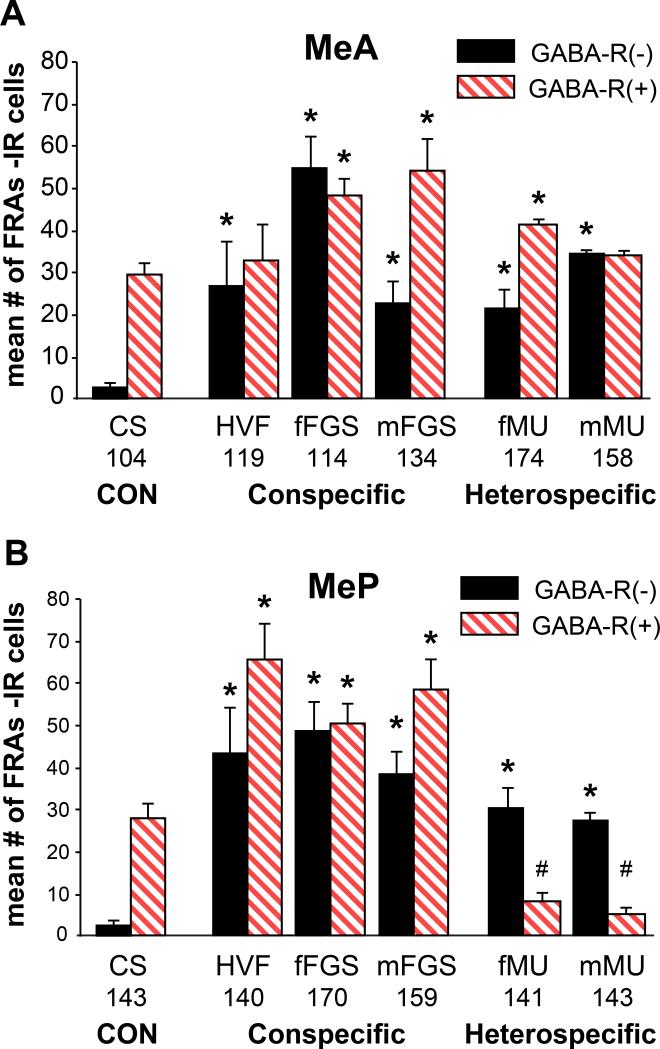
Figure 4. FRAs activation in cells that are GABA-Receptor (+/−) in Medial Amygdala.
A. MeA: GABA-R(−) cells in MeA were activated by all stimuli. GABA-R(+) cells were activated by all but HVF and mMU. mFGS and fMU activated more GABA-R(+) cells than GABA-R(−).
B. MeP: FRAs expression in both GABA-R(+) and GABA-R(−) cells in MeP was significantly different than with CS controls for all stimuli. However: heterospecific stimuli significantly suppressed GABA-R(+) cells (#; suggesting GABA inhibition) - and significantly activated GABA-R(−) cells in MeP. Conspecific stimuli activated both phenotypes. Mean total numbers of GABA-R(+) cells are shown for each area below the bars in the graph.
3. RESULTS
3.1 Activation of cells of all phenotypes: (Total numbers of FRAs(+) cells)
Male hamsters were exposed to one of the following stimuli: Control clean swabs (n=13), HVF (n=12), fFGS (n= 6), mFGS (n=15), fMU (n=7) or mMU (n=12). All animals investigated the stimulus swab for at least 10% of the time available and mean stimulus-investigation time was higher for all stimuli than for clean control swabs (one-way ANOVA p<0.01). There were no significant differences between mean investigation time for the different stimuli so no indication that differences in investigation could account for differences in FRAs expression. Extra animals were incorporated in groups exposed to HVF, mFGS and mMU as these stimuli seemed likely to carry the most diverse social messages. Groups exposed to fFGS and fMU were smaller but limiting the number of animals did not result in more variable neural responses for these groups (see graphs).
To determine the number of activated cells regardless of cell phenotype (Fig 2), we counted the number of FRAs(+) cells in four 25μm sections through each area of interest; two processed for FRAs and GABA-ir (data in Fig. 3) and two processed for FRAs and GABA-R-ir (data in Fig. 4). The average of total FRAs expression summed for these 4 sections is represented in Fig.2. A Two-Way RM ANOVA comparing “Area” (MeA, MeP, and mICNc) and “Exposure” (CS, HVF, fFGS, mFGS, fMU, and mMU) revealed significant overall effects of both exposure (p< 0.001, F=84.668, df= 6, 50) and brain area (p< 0.001, F= 20.999, df= 3, 50), as well as a significant interaction (p< 0.001, F=117.356, df= 6, 50). The significant main effects and interaction indicate that responses to chemosensory stimuli differed, in part, because the pattern of cell-activation across brain areas differed for different stimuli. As we have previously demonstrated, male hamsters that were exposed to any of the chemosensory stimuli (conspecific or heterospecific) had significantly more FRAs expression in MeA than clean-swab controls (p< 0.001 for all groups: asterisks on the graph in Fig. 2). In contrast, in MeP, only males exposed to the conspecific stimuli: HVF (p< 0.001), fFGS (p< 0.001) and mFGS (p< 0.001) had significantly greater FRAs expression than clean swab controls. These results using FRAs-ir and double-labeled tissue are essentially the same as we reported previously, using FOS or FRAs activation (Meredith and Westberry, 2004).
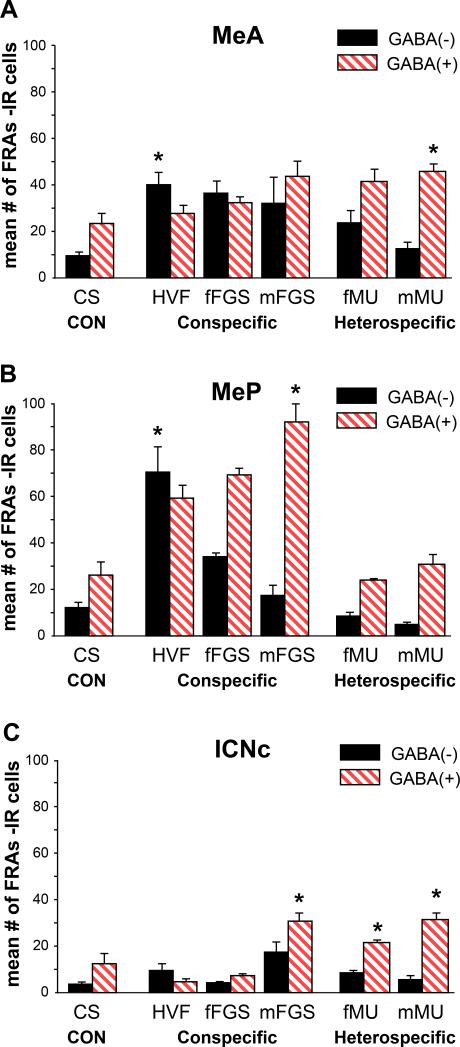
Figure 3. FRAs activation in cells that are GABA(+/−) in Medial Amygdala.
Patterns of activation of GABA(+/−) cells were different from patterns of total-FRAs response. Mean number +SE of FRAs(+) cells for 2 sections.
A. MeA: All biological stimuli significantly activated MeA (see fig 2). One conspecific stimulus, HVF, activated significantly more GABA (−) cells than in CS controls (asterisk) and both female stimuli activated more GABA(−) than GABA(+) cells. Heterospecific stimuli (fMU, mMU) activated GABA (+) cells significantly more than for CS controls, and more GABA(+) than GABA(−) cells.
B. MeP: HVF activated significantly more GABA(−) cells and mFGS more GABA (+) cells than CS controls (p< 0.001). Neither phenotype was significantly activated by heterospecific stimuli
C. mICNc: There was significant activation of GABA (+) cells above CS controls in mICNc for all of the heterospecific stimuli and one conspecific stimulus, mFGS.
Also as in the previous report, FRAs expression in the mICNc of males exposed to HVF or fFGS was slightly lower than in the mICNc of males exposed to clean swab controls (Fig. 2). However, exposure to either of the heterospecific stimuli significantly activated FRAs expression in the mICNc above the level in clean swab controls (p< 0.001 for both fMU and mMU), and in both cases associated with the suppression of response in MeP, compared to controls. One conspecific stimulus, mFGS, elicited significant mICNc activation (p=0.001) but was not associated with suppression of MeP (see further information below).
3.1.1 Activation of GABA(+/−) cells
As described above, male hamsters that were exposed to each of the chemosensory stimuli had significantly increased FRAs expression in MeA, but only those exposed to conspecific stimuli had increased FRAs expression in MeP. However, there were significant differences in the pattern of activation across cell phenotypes that distinguished responses to different conspecific stimuli. Both GABA-ir cells (GABA(+)) and non-GABA-ir cells (GABA(−)) had significantly increased FRAs activation in one or both areas with exposure to some stimuli, as did GABA(+) cells in the mICNc. To determine differences between exposure and phenotype, we ran separate two-way ANOVAs for each brain area (MeA, MeP or mICNc).
In MeA, there was a significant main effect of exposure (p<0.0001; F=6.329, df=5, 28) and a significant interaction (p=0.001; F=5.521,df= 5, 28), but no significant overall effect of phenotype (Fig. 3A). These results indicate that FRAs-expression responses to the range of stimuli were different in GABA(+) or GABA(−) cells, but that there was no overall difference in levels of activation of the two phenotypes. Tukey posthoc tests revealed that FRAs expression in males exposed to the conspecific stimulus, HVF, had dense FRAs expression in significantly more GABA(−) cells than GABA(+) cells (p>0.01; Fig 3A), as well as significantly more activated GABA(−) cells than in control males exposed to clean swabs (p=0.006; * in Fig 3A). On the other hand, male hamsters that were exposed to either heterospecific stimulus had significantly more FRAs activated GABA(+) cells than GABA(−) cells (p<0.001; Fig 3A). For animals exposed to male mouse urine (mMU) there were significantly more activated GABA(+) cells than in controls (p<0.002) (Fig. 3A).
In MeP, there were differences in the pattern of activation in cells of different phenotype, although there were no significant differences between total FRAs expression for the three conspecific stimuli (see Fig. 2). There were significant main effects of exposure (p< 0.001, F= 7.115, df= 5, 28) and phenotype (p< 0.001, F= 27.512, df= 1, 28), as well as a significant interaction (p= 0.006, F= 4.097, df= 5, 28), indicating that responses to different stimuli differed in the relative activation in cells of different phenotype as well as in the overall increase in FRAs compared to control. Males exposed to HVF had significant FRAs activation in GABA(−) cells in MeP (p<0.001 compared to CS), while the other conspecific stimuli activated more GABA(+) cells. mFGS activated GABA(+) cells almost exclusively and significantly more than control (p=0.03). The activated cells were predominantly in ventral MeP (see discussion). Exposure to the heterospecific chemosensory stimuli did not increase FRAs expression in MeP overall (Fig. 2), or in either the GABA(+) or GABA(−) sub-population (Fig. 3B).
In mICNc, as expected, there was significant FRAs activation in GABA(+) neurons in males exposed to either of the heterospecific stimuli (Fig. 3C). These were also the groups that did not have significant FRAs activation in the adjacent MeP. For the female conspecific stimuli (HVF and fFGS), FRAs activation in GABA(+) cells was lower than in clean swab controls, although not significantly so here. In mICNc, there were significant main effects of exposure (p< 0.001, F= 6.926, df= 5, 28) and phenotype (p< 0.001, F= 32.429, df= 1, 28), as well as a significant interaction (p= 0.001, F= 5.521, df= 5, 28). There were no significant effects among the few GABA(−) cells in mICNc. One conspecific stimulus, mFGS, also activated FRAs in GABA(+) cells in the mICNc without suppression of MeP, but the pattern of FRAs expression in MeP was different than with the other conspecific stimuli (see above and discussion).
3.1.2 Activation of GABA-Receptor (+/−) cells
Increased FRAs expression in cells bearing GABA-Receptors shows activation of cells that could have been suppressed by GABA inhibition but were not. Thus a decrease compared to control in the activation of GABAa-R (+) cells may be evidence of GABA inhibition. To examine the possibility that GABA inhibition shapes the pattern of medial amygdala activation by different stimuli, we analyzed FRAs expression in MeA and MeP in response to representative stimuli. The average total number of GABA-R (+) cells (activated or not) was very similar across all exposure groups for both MeA and MeP (not significantly different; p>0.05), as indicated by numbers on the graphs in Fig. 4. These graphs show activation of FRAs(+) cells of GABA-R(+) and GABA-R(−) phenotype in MeA (Fig. 4A) and MeP (Fig. 4B).
Within the MeA overall, there was a significant main effect of phenotype (p=0.0035; F= 9.405; df= 1, 25) and exposure (p=0.003; F= 5.768; df=5, 25) and a significant interaction (p=0.03; F=2.701; df=5, 25). All stimuli activated GABA-R(−) cells (p < 0.05), and fFGS (p= 0.01), mFGS (p< 0.001) and fMU (p= 0.03) also activated GABA-R(+) cells. HVF and mMU did not excite GABA-R(+) cells above control level but these potentially inhibitable cells were not suppressed below control level either so there is no clear evidence for GABA inhibition within MeA as part of the response pattern for any stimulus (Fig. 4A). GABA(+) cells were activated in MeA (see above) and their local action on GABA-R(+) cells may have reduced excitation by some or all stimuli but we cannot see that effect here. The relative levels of activation of GABA(+) and GABAR(+) cell activation within MeA does not show a reciprocal variation across stimuli that might suggest a predominant local interaction
Within the MeP, there were significant and dramatic differences in FRAs activation of GABA-R(+) cells between exposure groups. Male hamsters exposed to heterospecific stimuli had significantly fewer activated GABA-R(+) cells than males exposed to clean swab controls (p= 0.001 for both fMU and mMU; #). Males exposed to conspecific stimuli had significantly more FRAs activated GABA-R(+) cells in MeP (p< 0.001 for HVF and fFGS; p= 0.01 for mFGS) (Fig 4B). These cells had the potential to be inhibited but, with medial amygdala activation by conspecific stimuli, inhibitory circuits were apparently not engaged. These data suggest that the suppression of activation in MeP with heterospecific stimuli may be due to GABAergic inhibition; possibly from the adjacent mICNc, as described above.
In MeP, there was a significant main effect of exposure (p< 0.0001, F19.634, df= 5, 25) and a significant interaction (p< 0.0001, F= 6.251, df= 5, 25), but no significant main effect of phenotype (There was less activation of both types of cells by heterospecific, compared to conspecific stimuli). However, GABA-R(−) cells were activated significantly above the very low control (CS) baseline (p<0.05; Tukey posthoc tests). Thus, GABAR(+) cells in MeP were selectively suppressed by heterospecific stimuli, as would be expected if they were actively inhibited by GABA. However, the GABA-R(−) cells were activated by the same heterospecific stimuli. Neither of these responses made any distinction between the two heterospecific stimuli.
The relative numbers of activated cells of various types suggest many GABA-R(+) (and GABA-R(−)) cells are also GABAergic, but there was no increase from baseline in the numbers of MeP GABA (+) cells activated by heterospecific stimuli (Fig 3B) and, thus, no clear evidence for the selective inhibition of GABA-R(+) cells by the activation of local GABA(+) cells. Equally, as in MeA, the relative levels of FRAs expression of GABA (+) and GABA-R (+) cells across all the various stimuli, either within MeP or between MeA and MeP, does not show the reciprocal relationship that would suggest that the GABA (+) population had a predominant inhibitory action on the GABA-R (+) cells.
4 Discussion
4.1 Summary
As previously reported, medial amygdala in male hamsters distinguished categorically between conspecific social chemosensory signals and heterospecific signals, used by other species for similar types of communication. Conspecific stimuli activated both MeA and MeP but heterospecific stimuli activated only MeA (and mICNc) but not MeP. Here we use double-label immunocytochemistry to show co-expression of immediate-early genes (FRAs) together with either GABA-ir(+/−) or GABA-Receptor-ir(+/−). The results reveal that subpopulations of medial amygdala cells distinguish in their responses between different ecologically-relevant conspecific signals carrying potentially different social messages. These differential responses could engage downstream circuits responsible for an appropriately differential behavioral response to different conspecific signals. Additionally, GABA-R(+) cells in MeP were selectively suppressed by heterospecific stimuli, which all also activated GABA(+) cells of the adjacent mICNc - as predicted by our hypothesis that MeP is generally suppressed by heterospecific stimuli via GABA inhibition from mICNc. Heterospecific stimuli appear to be detected and distinguished categorically from conspecific stimuli but appear not to be distinguished from each other. Thus, amygdala processing may be sufficient for the selection of appropriate behavioral response or to avoid inappropriate response to both conspecific and heterospecific stimuli.
4.2 Medial amygdala distinguishes between responses to conspecific stimuli
There were no differences in total FRAs expression in MeP between animals exposed to various unfamiliar conspecific stimuli, here or previously (Meredith and Westberry, 2004). However, when the responses of cells of different phenotype are separated, there are distinctive patterns for different conspecific stimuli among GABA(+/−) cells and among GABA-R(+/−) cells. For example, HVF significantly activated GABA(−) cells, whereas mFGS elicited significant FRAs activation in GABA(+) cells in MeP. The fFGS response was similar to mFGS here, although not significantly different from control, possibly due to smaller numbers animals in this group. In other populations and other brain areas mFGS and fFGS responses are not similar. Overall, the patterns of response of both GABA(+/−) cells and of GABA-R(+/−) cells in MeA or in MeP (or a combination of all 4 phenotypes) differ significantly between conspecific stimuli when comparing the average numbers of activated cells of each phenotype (relevant bars in Figs 3 and 4) in a Chi2 analysis. These numbers are all independent observations but we suspect that some categories overlap. Some GABA(+) cells may also be GABAR(+), for example (see below). A more unambiguous analysis combines GABA(+/−) cell responses in MeP with GABA-R(+/−) cells in MeA. There is no overlap in phenotype here and patterns of response to different conspecific stimuli are significantly different (Chi2 = 64.209; dF= 6; p<0.001). The heterospecific chemosensory stimuli produced no overall increase in FRAs in MeP, and also selectively suppressed GABA-R(+) cells. These same stimuli activated GABA-R(−) cells in MeP but not sufficiently to relieve the overall suppression of MeP and not differently for the stimuli tested. Test animals had no previous experience with the heterospecific species and no experience with the donors of conspecific stimuli. Thus, these appear to be responses to categories of stimuli, not to individuals. MeA, which has particularly strong chemosensory input (Maras and Petrulis 2010a,b), does respond to all stimuli so activation of subpopulations there might also initiate differential output to MeP However, MeA showed no clear differential responses within the GABA(+/−) cell-types and the level of activation of GABA(+) cells by heterospecific stimuli is similar to that for mFGS. Thus, there appear to be no selective increase of MeA GABAergic cell activation that might have suggested that heterospecific stimuli selectively suppress MeP by activating GABAergic cells in MeA.
Chemosensory responses in mouse medial amygdala are similarly organized (Samuelsen and Meredith, 2009a) but with “heterospecific” patterns elicited by hamster stimuli and “conspecific” patterns by mouse stimuli. However, there may be circuit or sensitivity differences between species. The principal female chemosignal for hamsters (HVF) activates predominantly GABA(−) cells in male hamster MeP (Fig 3), distributed between dorsal and ventral MeP. Choi et al, 2005, reported that the principal female chemosignal for mice (fMU) activates a group of putatively GABAergic cells in male mouse dorsal MeP that express the LHX6 (LIM-homeodomain) gene. These cells do connect to reproductive circuits in hypothalamus but it is not clear that they are the predominantly activated cell type. The same (fMU) stimulus activates both dorsal and ventral MeP in mice (Samuelsen and Meredith 2009, Carvalho et al 2015), although with a bias towards dorsal activation. For male-male communication, mMU activates predominantly ventral MeP in male mice (Samuelsen and Meredith, 2009a). In male hamsters, the male stimulus, mFGS, also activates predominantly ventral MeP (JM Westberry and M Meredith, unpublished observations). The male (mFGS) signal in a third species, mandarin voles, failed to significantly activate medial amygdala, possibly reflecting social structure differences from other rodents (He et al 2014). Threatening heterospecific (cat; predator) stimuli generally activate ventral MeP in mice (Samuelsen and Meredith 2009b, 2011; Perez-Gomez et al 2015) and rats and are avoided by both species (Dielenberg et al 2001; Govic and Paolini 2014). In mice, Carvalho et al did not find a predominant ventral activation in mice by predator stimuli, nor any general distinction between areas activated by conspecific stimuli as a class (pooled data for male and female stimuli) compared to heterospecific stimuli. In electrophysiological-unit recordings, Bergan et al 2014, found some overlap in responses to male, female and predator stimuli but with distinguishable overall patterns. Mice have a remarkable variety of receptors selective for stimuli from different types of predators, in the VNO (Isogai 2011; Papes et al 2010) and in other chemosensory pathways, converging in ventral MeP (Perez-Gomez et al 2015, Carvalho et al 2015). Interestingly, hamsters show neither avoidance of predator stimuli (cat collar, cat urine) nor activation of MeP (CB Blake and M Meredith, unpublished observations).
Our data suggest MeP could function to distinguish between conspecific stimuli and to discriminate between conspecific and heterospecific signals. Although there are no absolute differences between areas of medial amygdala activated by different stimuli, the differences in activation of different cell-types and the larger differences between MeA and MeP for heterospecific stimuli represent information important for behavioral response. Output from MeP together with MeA could thus, initiate appropriate behavioral and physiological responses via their selective connections to circuits in medial preoptic and hypothalamic areas (Choi et al 2005; Been and Petrulis 2011, 2012; Lin et al 2011).
4.3 Overlap of GABA-ir and GABA-Receptor-ir cell types
We did not attempt triple-label immunocytochemistry for FRAs, GABA and GABA-R in the same section so we cannot say how many cells that expressed both GABA-ir and GABA-R-ir were activated by a given stimulus. However, an estimate can be made. If there were no overlap, the GABA(+) and GABA-R(+) cells would be equal to or less than the total number of FRAs-activated cells in a section (there may be activated cells that are neither). Assuming activation of GABA cells in the GABA-R-labeled sections is similar to that observed in the adjacent GABA-labeled sections, the degree of GABA/GABA-R overlap can be estimated by comparing the sum of GABA(+) and GABA-R(+) cells (per section) in the interleaved sets of sections with the total number of FRAs-activated cells (per section) in either set (the total numbers of FRAs-labeled cells is similar). Using this method, the sum of GABA(+) and GABA-R(+) cells exceeded the total number of FRAs activated cells in both sets of sections in several cases. For mFGS-exposed animals, this estimated overlap was 20-30% in both MeA and in MeP, and about 20% for both heterospecific stimuli in MeA only. Interestingly, there was more than 30% overlap for the smaller total numbers of cells activated in controls. There was essentially no overlap by this measure for HVF responses in either MeA or MeP, for both heterospecific stimuli in MeP and for fFGS responses in MeA. To the extent that these estimates reflect actual coexpression, this analysis is further evidence for differential response to the different stimuli.
4.4 Mechanisms for categorical response include a mICNc contribution
Male hamsters exposed to heterospecific chemosensory stimuli had no significant overall response in MeP, with increased expression in GABA(+) cells of the adjacent mICNc, as predicted. The null response in MeP was due to a significant suppression of GABA-R(+) cells. Unexpectedly, there was also activation of MeP GABA-R(−) cells. Thus, heterospecific stimuli, as well as different conspecific stimuli, may generate a distinctive output from MeP. mICNc is well placed to inhibit MeP in a manner similar to the action of the paracapsular ICN cells on lateral/basolateral and central amygdala nuclei of the fear conditioning circuit (Pape and Pare 2010). Here, as in previous studies (Meredith and Westberry 2004), mICNc may be suppressed compared to control by HVF (Fig 3C), so a bidirectional modulation of mICNc could contribute to patterns of MeP response for both conspecific and heterospecific stimuli. There are other possible inhibitory inputs to MeP besides mICNc that could be responsible for its suppression during heterospecific stimulation, including GABAergic projections from MeA (but see above), or from basomedial or other amygdala regions, if connections are similar to those in the rat (Pitkanen 2000). There are also potential modulatory influences from medial prefrontal cortex connections to medial amygdala (as well as to intercalated cells) (Pape and Pare 2010) and from dopamine (Marowsky et al 2005). The potential complexity of influences on MeP and mICNc means we should not expect a rigid reciprocal activation. However, the coincidence of a general increased activation of mICNc GABA(+) cells with suppression of GABA-R(+) cells in MeP during heterospecific stimulation is striking (and is also seen with male cat stimuli). Preliminary in vitro electrophysiological evidence suggests both excitatory and inhibitory functional connections from MeA to mICNc (and to MeP), as well as inhibitory connections from mICNc to MeP (Biggs et al 2014). There does seem to be a generally reciprocal relationship between mICNc activation and suppression of GABA-R(+) cells in MeP by heterospecific stimuli.
One conspecific stimulus, mFGS, activated both mICNc as well as MeP but overwhelmingly in MeP GABA(+) cells unlike MeP responses to other conspecific stimuli; and without suppression of GABA-R(+) cells, unlike responses to heterospecific stimuli. GABAergic cells may be projection neurons in MeP (Bian et al 2008; Keshavarzi et al 2014). Thus, a different set of MeP cells, less susceptible to mICNc inhibition, may be responsive to mFGS and some or all of these cells may be output cells. Male odors also activate mainly GAD(+) cells in rat MeP (Donato et al 2010) and these competitive, potentially threatening stimuli could activate different downstream circuits than other conspecific stimuli. Among the many GABA-ir or GAD-ir cells in MeP (and MeA), some are interneurons and may provide feedforward inhibition of principal cells (Keshavarzi et al 2014). However, within MeP (or within MeA) the activation of local GABA(+) cells does not seem to predict a general suppression of GABA-R(+) cells in the same subnucleus, so any contribution of local interneurons to the response patterns that distinguish conspecific stimuli is not evident in our data.
Overall, the responses in MeA and MeP in combination with mICNc indicate sensory processing in the amygdala that provides information important for the selection of appropriate behavioral response to social signals, and within amygdala areas that project to basal forebrain regions critical for those behaviors.
4.5 Conclusions
Overall, we show that categorically different response patterns elicited in medial amygdala by conspecific and heterospecific stimuli (Meredith and Westberry, 2004) (Samuelsen and Meredith, 2009a) are composed of separate patterns in GABA-ir and in GABA-Receptor-ir cells, especially in posterior medial amygdala (MeP). These separate patterns discriminate between different stimuli within the conspecific category and distinguish conspecific from heterospecific stimuli, likely in part, due to selective inhibition of GABA-R cells in MeP by the adjacent, largely GABAergic, Intercalated Nucleus (mICNc).
Highlights.
I. with 85 or fewer characters, as specified in the editor's letter:
Amygdala evaluates most innate or learned sensory stimuli, including social chemosignals.
Cell-types in hamster medial amygdala (Me) discriminate same-species (conspecific) signals.
Hetero- but not Con-specific stimuli selectively suppress posterior-Me GABA-receptor-ir cells.
Me may, thus, elicit stimulus-appropriate social behavior via its basal forebrain connections.
This amygdala chemosensory-circuit appears similar to that for fear-conditioning.
II. with 125 or fewer characters (including spaces), as specified in the current Neuroscience Guide for Authors:
Mammalian amygdala evaluates most innate or learned sensory stimuli, including olfactory/vomeronasal social chemosignals.
Response patterns of cell populations in hamster medial amygdala (Me) discriminate same-species (conspecific) stimuli.
Heterospecific stimuli selectively suppress GABA-receptor-ir cells in posterior Me, which conspecific stimuli activate.
Medial amygdala may, thus, elicit stimulus-appropriate patterns of social behavior via its basal forebrain connections.
The chemosensory circuit is similar to that for fear conditioning, suggesting a modular circuit organization for evaluation of diverse stimuli.
Highlights.
Amygdala evaluates innate/learned sensory stimuli, including social chemosignals.
Cell-types in hamster medial amygdala (Me) discriminate same-species signals.
Different-species stimuli selectively suppress posterior-Me GABA-receptor-ir cells.
Me may elicit stimulus-appropriate social behavior via basal forebrain connections.
This amygdala chemosensory-circuit appears similar to that for fear-conditioning.
Acknowledgements
Supported by NIDCD grants DC005813 (MM), F31DC05725 (JW), T32 DC000044 (MM). We thank Dr. Marc Freeman for use of his microscope and image analysis system for fluorescence double-labeled sections, Dr. Mike Sellix for his help with image analysis in the initial experiments and Charles Badland for expert assistance with the preparation of the figures. Funding sources had no involvement.
Abbreviations
- AOB
Accessory Olfactory Bulb
- CS
Clean Swab (control stimulus)
- fFGS
female Flank-Gland Secretion
- fMU
female Mouse Urine
- FRAs
Fos-Related Antigens (IEG protein product)
- GABA(+/−)
Gamma Amino Butyric Acid (ir-positive or ir-negative)
- GABA-R(+/−)
Gamma Amino Butyric Acid(a)-Receptor (ir-positive or ir-negative)
- GAD
Glutamic Acid Decarboxylase
- HVF
Hamster Vaginal Fluid
- IEG
Immediate Early Gene
- -ir
immunoreactive
- MeA
Medial Amygdala, Anterior
- MeP
Medial Amygdala, Posterior
- mFGS
male Flank-Gland Secretion
- mICNc
main InterCalated Nucleus, caudal part
- mMU
male Mouse Urine
- MOB
Main Olfactory Bulb
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial or other interests.
Author Contributions
JMW and MM designed the research; JMW performed the research; JMW and MM analyzed the data and wrote the paper.
References
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Ann Rev Physiol. 2009;71:141–60. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm. Behav. 2011;59:536–48. doi: 10.1016/j.yhbeh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. Dissociated functional pathways for appetitive and consumatory reproductive behaviors in male Syrian hamsters. Horm. Behav. 2012;61:204–11. doi: 10.1016/j.yhbeh.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan JF, BenShaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. eLife. 2014;2014;3:e02743. doi: 10.7554/eLife.02743. DOI: 10.7554/elife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaul Y, Katz LC, Mooney R, Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci U S A. 2010;107:5172–5177. doi: 10.1073/pnas.0915147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X, Yanagawa Y, Chen WR, Luo M. Cortical-like functional organization of the pheromone-processing circuits in the medial amygdala. J Neurophysiol. 2008;99:77–86. doi: 10.1152/jn.00902.2007. [DOI] [PubMed] [Google Scholar]
- Biggs LM, Simonton AR, Meredith M. Modulation of amygdalar circuits important for chemosensory signal processing 2014 Abstract Viewer/Itinerary Planner. Soc Neurosci. 2014 [Google Scholar]
- Brennan PA. Pheromones and Animal Behavior. In: Menini A, editor. The Neurobiology of Olfaction. CRC Press; Boca Raton (FL): 2010. Ch. 6. [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Satzler K, Singewald N, Capogna M, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31:5131–5144. doi: 10.1523/JNEUROSCI.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiz-Moretti B, Otero-Garcia M, Martinez-Garcia F, Lanuza E. Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0954-y. DOI: 10.1007/s00429-014-0954y. [DOI] [PubMed] [Google Scholar]
- Carvalho VMA, Nakahara TS, Cardozo LM, Souza MAA, Camargo AP, Trintinalia GZ, Ferraz Papes F. Lack of spatial segregation in the representation of pheromones and kairomones in the mouse medial amygdala Front. 9. Neurosci. 2015:283doi. doi: 10.3389/fnins.2015.00283. 10.3389/fnins.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien M-S, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, Stowers L. Cyclic Regulation of sensory perception by a female hormone alters behavior. Cell. 2015;161:1334–1344. doi: 10.1016/j.cell.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–97. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Donato J, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol. behav. 2010;99:67–77. doi: 10.1016/j.physbeh.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Govic A, Paolini AG. In vivo electrophysiological recordings in amygdala submuclei reveal selective and distinct responses to a behaviorally identified predator odor. J. Neurophysiol. 2015;113:1423–36. doi: 10.1152/jn.00373.2014. [DOI] [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu Rev Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- He F, Wu R, Yu P. Study of Fos, androgen receptor and testosterone expression in the suberegions of medial amygdala, bed nucleus of stria terminalis and medial preoptic area in male mandarin voles in response to chemosensory stimulation. Beh. Brain Res. 2014;258:65–74. doi: 10.1016/j.bbr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim SS, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–538. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Pheromones, the vomeronasal system, and communication. From hormonal responses to individual recognition. Ann N Y Acad Sci. 1998;855:333–348. doi: 10.1111/j.1749-6632.1998.tb10592.x. [DOI] [PubMed] [Google Scholar]
- Kang N, Mccarthy EA, Cherry JA, Baum MJ. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. Neuroscience. 2011;172:196–204. doi: 10.1016/j.neuroscience.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AW, Ackels T, Kuo TH, Cichy A, Dey S, Hays C, Katewri M, Logan DW, Marton TF, Spehr M Stowers L. Murine Pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell. 2014;157:676–688. doi: 10.1016/j.cell.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi S, Power JM, Albers EHH, Sullivan RKP, Sah P. Dendritic organization of olfactory inputs to medial amygdala neurons. J. Neurosci. 2015;35:13020–13028. doi: 10.1523/JNEUROSCI.0627-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi S, Sullivan RKP, Ianno DJ, Sah P. Functional properties and projections of neurons in the medial amygdala. J.Neuroscience. 2014;34:8699–8715. doi: 10.1523/JNEUROSCI.1176-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. The vomeronasal organ. Science. 1999;286:716–720. doi: 10.1126/science.286.5440.716. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J Comp Neurol. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuyclei. Eur. J. Neurosci. 2010a;32:469–82. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neuroscience. 2010b;170:610–22. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Hattori T, Asaba A, Inoue N, Kanomat N, Kikusui T, Kobayakawa R, Kobayakawa K. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. PNAS. 2015;112:E311–20. doi: 10.1073/pnas.1416723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. GABAergic neurons: gate masters of the amygdala, mastered by dopamine. Neuron. 2005;48:877–879. doi: 10.1016/j.neuron.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for acquisition, expression and extinction of conditioned fear. Physiol. Rev. 2010;90:419–63. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gomez A, Bleymehl K, Stein BM, Pyrski M, Birnbaumer L, Munger SD, Leinders-Zufall T, Zufall F, Chamero P. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Current Biology. 2015 doi: 10.1016/j.cub.2015.03.026. http://dx.doi.org/10.1016/j.cub.2015.03.026. [DOI] [PMC free article] [PubMed]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. 2nd Ed Oxford University Press; New York: 2000. pp. 31–115. [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009a;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neuroscience. 2009b;164:1468–1476. doi: 10.1016/j.neuroscience.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 2011;180:96–104. doi: 10.1016/j.neuroscience.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schaal B, Couread G, Langlois D, Ginies C, Semon E, Perrier G. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of main olfactory system in social recognition of complex peptide ligands. J. neurosci. 2006;26:1961–70. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirindelli R, Mucignat-Caretta C, Ryba NJ. Molecular aspects of pheromonal communication via the vomeronasal organ of mammals. Trends Neurosci. 1998;21:482–486. doi: 10.1016/s0166-2236(98)01274-0. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 2012;520:1819–30. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]