Introduction
Advances in clinical assessment, epidemiology, metabolomics, and genomics have provided new insights into the pathogenesis of obesity co-morbidities including insulin resistance, fatty liver disease, type 2 diabetes mellitus (T2DM), and cardiovascular disease. Yet we know very little about the factors causing people to become obese in the first place. In that regard, studies of genetic obesity models in humans and experimental animals are of critical value. Here we provide a review and update on Prader-Willi syndrome (PWS), a unique genetic model of obesity associated with hypotonia, sarcopenia, cognitive dysfunction, hyperphagia, progressive fat deposition, and varying degrees of hypopituitarism.
Genetics of PWS
Chromosomal localization
PWS is the most common syndromic obesity disorder, with a prevalence of 1 in 10,000 to 1 in 15,000 live births annually. Occurring equally in males and females and detected in all races1, it is characterized by infantile hypotonia and failure to thrive followed by progressive obesity and hyperphagia in childhood. PWS results from lack of expression of paternally inherited genes in the region of chromosome 15q11.2-q131. Seventy percent of patients have a deletion of the paternally inherited region, while twenty-five percent have inherited two copies of the critical region on chromosome 15 from the mother; the latter is called maternal uniparental disomy. Five percent have abnormal imprinting or methylation that silences paternal genes in the PWS region.
Candidate Genes
Chromosome 15q11.2-13 contains a number of genes that contribute to the PWS phenotype (Figure 1).
Figure 1.
Representation of chromosome 15q11-13
Targeted knockout of individual genes in mice recapitulates some but not all of the clinical and biochemical features of PWS; the complete clinical picture likely requires deletion or deficiencies or two or more of the candidate genes.
NDN
The NDN gene (Figure 1) encodes the protein necdin, which is preferentially expressed in the hypothalamus 2. Experimental studies in mice have demonstrated at least four different phenotypes of necdin-null mutations. These have included respiratory defects similar to those found in children and adults with PWS. One necdin-deficient mouse model had respiratory failure secondary to abnormal central respiratory drive with frequent apnea and decreased respiratory response to hypoxia2. Another necdin knockout model showed reduced hypothalamic neuron populations within oxytocin-expressing neurons in the paraventricular nuclei and GnRH neurons in the preoptic area2. It is theorized that the reduction in GnRH neurons might explain the reduced gonadal function in PWS3. Behavioral studies in these mouse models have identified increased skin scraping, similar to skin picking manifestations of PWS2. Lastly, necdin-deficient mouse models also had increased pain tolerance similar to PWS patients, possibly due to necdin promotion of nerve-growth factors within sensory neurons2.
MAGEL2
The MAGEL2 gene is located upstream of NDN(Figure 1), and encodes the protein MAGEL 2 protein, which like necdin is highly expressed in the hypothalamic supraoptic, paraventricular and suprachiasmatic nuclei 2. MAGEL2 knockout mice have decreased wakefulness as measured by decreased daytime running and motor activity that is associated with decreased levels of orexin, a sleep and appetite-regulating hormone 2. MAGEL2 knockout mice also have postnatal growth retardation until week 6 of life, and then accelerated postnatal catch up growth and adiposity 4. In contrast to young children with PWS, MAGEL2 knockout mice are hypophagic; thus their adiposity may result from diminished energy expenditure. Lastly, MAGEL2 knockout mouse models have demonstrated delayed pubertal onset and decreased fertility in females. The number of hypothalamic GnRH-neurons is normal but the number of corpus lutea is diminished, suggesting decreased ovulation 2. Male Magel2 knockouts have lower levels of testosterone but no defects in genital anatomy or sperm count2. Therefore, while the MAGEL2 knockout has similar wakefulness, growth, adiposity, and pubertal patterns to PWS patients, these are not as severe and do not explain the complete human phenotype.
SNURF
SNURF (SNRPN Upstream Reading Frame) is a small nuclear ribonucleoprotein complex involved in mRNA splicing in the brain. Mouse models lacking the SNRPN gene have variable phenotype with some exhibiting hypotonia and impaired feeding2. Early mortality has thus far precluded analysis of metabolic status and feeding behavior.
SNORD 116
The nucleolus contains diverse small RNAs (sno RNAs) that complex with small nucleolar ribonucleoproteins and mediate post-transcriptional, sequence-specific methylation that dictates mRNA folding and stability. Defects in certain snoRNAs have been implicated in neurologic, cardiovascular and oncologic diseases2. SNORD 116 is expressed at high levels in appetite-controlling centers of the hypothalamus including the arcuate, paraventricular, and ventromedial nuclei. SNORD 116 deletion in mice leads to anxiety, deficiency in motor learning, and growth retardation, mimicking in some ways the hypotonia and failure to thrive seen in infants with PWS2. Unlike other animal knockout models, SNORD 116 knockout mice are hyperghrelinemic and moderately hyperphagic 5; however, under standard feeding conditions, they do not become obese.
As no single gene knockout can fully explain the PWS phenotype, it is thought that PWS represents a contiguous gene disorder requiring loss of expression of several genes to cause the complete phenotype.
Clinical Manifestations of PWS
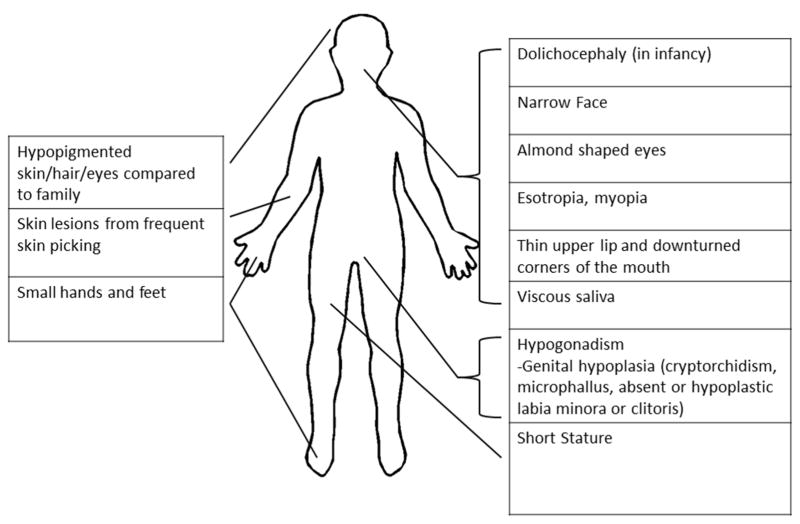
While the exact genetic mechanisms leading to Prader-Willi syndrome remain unclear, the phenotype is well-described 6. Clinical characteristics of PWS vary with age and minor clinical manifestations are often nonspecific (Table 1). Previous scoring symptoms quantifying number of major and minor clinical manifestations were used for diagnosis of PWS6. With improved sensitivity and availability of genetic testing, criteria for identifying patients needing prompt genetic testing have been developed 7. The most distinctive major clinical characteristics of PWS are infantile central hypotonia and failure to thrive, followed by progressive hyperphagia and fat deposition in early childhood. The infantile hypotonia and failure to thrive are so severe they generally (but not always) require assisted feedings with nasal gavage or gastrostomy tube. Minor criteria include developmental delay and hypothalamic dysfunction, manifest as temperature dysregulation, increased pain tolerance, and central as well as obstructive sleep apnea. Hypothalamic dysfunction can also result in central hypothyroidism, central adrenal insufficiency, growth hormone (GH) deficiency, and hypogonadotropic hypogonadism (discussed below). Many children with PWS also have behavioral disorders including anxiety and obsessive compulsive disorder that can be associated with chronic skin picking. The physical features and facies of PWS often become more pronounced with age (Figure 2).
Table 1.
Major and Minor Clinical Manifestations of PWS
| Age | Major Characteristics | Minor Characteristics |
|---|---|---|
| Birth to 2 years | Neonatal central hypotonia (i.e. floppy infant, poor suck) | Decreased fetal movement, weak cry |
| Failure to thrive in infancy | Infantile lethargy | |
| Global Developmental Delay | ||
| 2y–6y | Excess and rapid weight gain with central obesity | Behavioral problems (obsessive compulsive disorder, manipulative, perseverating, stealing) |
| Sleep Apnea | ||
| 12y - adulthood | Hyperphagia and obsession with food | Short stature |
| Hypogonadotropic gonadism with genital hypoplasia, delayed or incomplete pubertal progression | Behavioral Disorders (temper tantrums, obsessive compulsive disorder) | |
| Cognitive impairment, learning delays | Skin picking |
Figure 2.
Physical Findings in PWS
Control of Food Intake in Prader Willi Syndrome
Our group has published studies evaluating the developmental changes in appetite regulating hormones such as ghrelin in PWS versus control infants and children. In a cross-sectional study of 33 infants with PWS and 28 healthy controls, we determined that total serum ghrelin trended higher in the PWS group, but did not differ significantly from comparable controls of equivalent age, weight-for-age percentile and z-score and sex. However, one-third of young PWS (11/33) had ghrelin levels higher than the 95th percentile of ghrelin values in the normal controls; thus, hyperghrelinemia was detected in 1/3 of PWS subjects at an early age in the absence of reported hyperphagia8. More variability in ghrelin concentrations was also noted in PWS young children compared to controls. Interestingly, 6 of the 11 young PWS subjects with relatively high ghrelin concentrations had weight-for-age z-score <0. We speculate that hyperghrelinemia in young PWS children might be a response to failure to thrive or food restriction in infancy. A longitudinal assessment of total ghrelin in nine young PWS children showed no significant changes over 18 months. Total ghrelin in young PWS and control children was significantly higher than in older counterparts and the decline in ghrelin with age was more exaggerated in the control subjects than in the PWS subjects. Other researchers published similar findings to ours that there were no significant differences in plasma insulin or ghrelin levels between young PWS and controls 9, 10. Similar to our study, Goldstone also reports significantly higher plasma leptin levels in young PWS versus controls, suggesting a relative excess of fat to lean body mass9. No relationship was found between eating behavior in PWS subjects and concentrations of any hormones or insulin resistance, independent of age. It is postulated that changes in central pathways controlling appetite regulation and food reward might be responsible for the development of hyperphagia in PWS. Careful prospective longitudinal studies of infants and young children with PWS compared to controls are needed to examine further the relationship between changes in appetite regulating hormones, body composition and eating behaviors.
In older children with PWS compared to obese controls (OC), our group and others have demonstrated significantly higher ghrelin levels, total adiponectin, HMW adiponectin and HMW adiponectin:leptin ratio and lower fasting insulin levels and HOMA-IR 8,11,12,13. These findings are in the setting of equivalent leptin concentrations between PWS and OC, suggesting comparable degrees of adiposity. Lower levels of inflammatory cytokines such as IL-6 and c-reactive protein have also been reported in PWS compared to obese controls by our group 13. Interestingly, we also found sex differences with PWS females showing more striking hyperghrelinemia, hyperadiponectinemia and insulin sensitivity compared to PWS males12. The effects of sex hormones and treatment with growth hormone on these findings remain unclear.
Overall, these findings are consistent with a higher degree of insulin sensitivity in older PWS subjects. Moreover, high-density lipoprotein levels were lower and triglycerides higher in obese controls, but not PWS patients. No group differences in glucagon-like peptide-1 or aspartate aminotransferase or alanine aminotransferase were seen. Interestingly, we found a greater decline in ghrelin with age in control versus PWS subjects. A negative relationship between ghrelin and BMI-z score was also found in the older PWS and controls, but not in the younger cohort. Further studies are required to understand the pathophysiology of increased insulin sensitivity in PWS and to determine if their increased insulin sensitivity translates into protective clinical benefits long-term.
Finally, recent studies from our lab have assessed the acute effects of varying macronutrient content (high fat (HF) or high carbohydrate (HC) iso-caloric breakfast meals) in a randomized cross-over study of 14 PWS and 14 age and BMI-z matched obese controls (OC) 14. Relative to OC, PWS children had lower fasting insulin and higher fasting ghrelin and ghrelin/PYY. Ghrelin levels were higher in PWS across all postprandial time-points. Carbohydrate was more potent than fat in suppressing ghrelin concentrations in PWS (p=0.028); HC and HF were equipotent in OC, but less potent than in PWS (p=0.011). Interestingly, the rise in PYY following HF was attenuated in PWS (p=0.037); thus, postprandial ghrelin/PYY remained higher throughout. In summary, we found that PWS children had both fasting and postprandial hyperghrelinemia and an attenuated response to fat, yielding a high ghrelin/PYY ratio. We propose that the ratio of Ghrelin/PYY might be a novel marker of orexigenic drive in PWS and childhood obesity. Therapeutic approaches to either increase PYY or decrease ghrelin in PWS might prove successful. Future longer-term randomized controlled trials of varying macronutrient composition will facilitate the development of optimal dietary therapies to attenuate hyperphagia, prevent weight gain and promote weight loss in PWS.
Hypothalamic Pituitary Function in PWS
Growth hormone deficiency and short stature
Mild prenatal growth retardation occurs commonly in PWS; 41% of infants have a birth weight <2.5 kg; birth length is either normal or slightly below normal. Short stature is almost always present by one year of age and continues throughout childhood. The cause of the short stature is both growth hormone (GH) deficiency and the lack of a sufficient pubertal growth spurt 15,16. The average height of untreated PWS males is 155cm and of PWS females 145cm 17. The GH deficiency seen in PWS is independent of obesity and manifests in low spontaneous and pharmacologically stimulated GH secretion and low serum concentrations of insulin-like growth factor I (IGF-I) in both children and adults 18–29. This is unlike over-nutrition in common obesity, which is associated with normal or increased IGF-I levels. Body composition in PWS individuals resembles that of classic GH deficiency, with decreased lean mass and increased adipose tissue mass compared to age-matched controls 30.
Recombinant GH therapy for PWS was approved in the United States in 2000 and in Europe in 2001; GH is still not approved in some countries such as Canada. Generally agreed upon exclusion criteria for therapy include: severe obesity, uncontrolled diabetes mellitus, untreated severe obstructive sleep apnea, active cancer, or psychosis31. Recently, updated standardized growth charts for non-growth hormone treated subjects with PWS (3 to 18 years) were developed and can be used to monitor response to growth hormone therapy or to assess nutritional status32.
Presented below is evidence supporting the use of GH in PWS in infants/toddlers, children and adults. There does not seem to be a differential GH response based on genetic subtype of PWS 33,34.
a. Studies in infants/toddlers
Body fat content is increased and lean body mass is reduced in young infants and toddlers with PWS, even before progressive weight gain ensues35,36. This finding provides a rationale for initiating GH therapy at a young age. In a large randomized controlled GH trial in 91 pre-pubertal PWS children including 42 infants and 49 children were randomized to GH treatment or no GH treatment37. During year 1, infants were randomized to GH (1mg/m2/day) or no treatment; all infants were treated in the second year. Children >3 years of age were randomized to GH treatment (1mg/m2/day) or no treatment for 2 years. The median height improved over 2 years in both the treated infant and childhood groups. Further, head circumference normalized during GH therapy. While body fat percentage, body proportions and lean body mass SDS improved in GH treated children, they did not normalize fully. Interestingly, it was noted that GH prevented the reduction in lean body mass found in the untreated controls37.
More recently, another randomized controlled GH trial was completed in eight-five PWS infants and pre-pubertal children over 24 months. At baseline, mean fat percentage was elevated and 63% of infants and 73% of pre-pubertal children exhibited cardiovascular risk factors such as hypertension or dyslipidemia. GH treatment improved height-SDS and BMI-SDS and reduced fat mass and % body fat, and increased the ratio of HDL:LDL38. There were no effects on glucose homeostasis or plasma acylation stimulation protein (ASP). ASP is a hormone produced by adipocytes that is important in maintaining lipid homeostasis by increasing triglyceride storage and glucose uptake within adipose cells and reducing triglyceride lipolysis by inhibiting hormone-sensitive lipase.
Unique to the infant/toddler age, some authors report that GH has benefits on cognition. GH prevented the deterioration of specific cognitive skills over a 2 year randomized controlled GH trial in fifty pre-pubertal PWS children33: over 4 years of GH treatment, an improvement in abstract reasoning and visuospatial abilities were noted. Further longer-term controlled GH studies are required to assess the benefits of GH on cognition in early development in PWS infants/toddlers.
While GH has demonstrated many beneficial effects, there are notable risks of treatment. Some clinicians consider scoliosis to be a contraindication for GH treatment in PWS. However, a multicenter, randomized controlled GH study in infants and pre- and pubertal children (median age 4.7 (2.1–7.4) years) over two years demonstrated that both the onset and curve progression of scoliosis were no different between those on treatment than in controls39. A higher baseline IGF-I SDS was associated with a lower severity of scoliosis, suggesting a protective effect of GH.
In smaller studies, GH therapy has been associated temporally with sudden death in PWS. The deaths have been concentrated in young children who have a history of respiratory obstruction/infection or severe obesity40–48. Deaths have generally occurred early in the course of GH therapy. The exact cause of the sudden deaths has not been determined. Possibilities include impaired ventilatory responsiveness to hypercapnia and hypoxia, increased lymphoid tissue or tonsillar hyperplasia, and/or adrenal insufficiency. It is nevertheless unclear if mortality in GH-treated PWS patients exceeds expected mortality from PWS per se. Indeed, other studies support an opposing view that postulates a beneficial role for GH therapy in improved ventilation responsiveness to carbon dioxide and improved sleep quality in children with PWS27,49,50.
b. Data in older children
In older PWS children, GH treatment is now FDA-approved and improves growth velocity and final height 51. Additionally, body composition and bone mineral density are improved and resting energy expenditure (REE) is increased as a consequence of increases in skeletal muscle mass and enhanced rates of fatty acid oxidation 23,27,50–55. Improvements in physical strength, respiratory muscle hypotonia, and peripheral chemoreceptor sensitivity to carbon dioxide have also been noted 27,28,50,51. One study has also reported that GH treatment may improve overall sleep quality, with reduction in number of hypopnea and apnea events 50. Finally, a few studies have found mild behavioral improvements, including reduction in depressive symptoms in those older than 11 years of age. In contrast, a long-term randomized controlled trial of GH in 42 PWS children (3.5–14 years) showed no improvement in behavior problems (measured by a Developmental Behavior Checklist and a Children’s Social Behavior Questionnaire).
Adaptive function (the ability to complete daily activities) was recently assessed in a 1–2 year randomized controlled trial which then extended into 7 years of continuous GH treatment (1 mg/m2/day). The authors showed a marked delay in adaptive functioning (Vineland Adaptive Behavior Scale) in PWS infants and children, the severity of which increased with age and correlated inversely with IQ56. Those who began GH treatment in infancy had significantly improved adaptive functioning. No significant change in intelligence quotient has been demonstrated when GH therapy has been initiated later in childhood 50,57.
Fewer studies have examined the efficacy and safety of longer-term (beyond 2 years) GH treatment in PWS children. The longest study to date followed 60 pre-pubertal children for 8 years on continuous GH treatment (1mg/m2/day)58. Lean body mass (LBM) increased significantly during the first year and stabilized thereafter at a level above pretreatment baseline values (p<0.0001). Percentage fat standard deviation score (SDS) and BMI SDS decreased significantly during the first year but then remained stable at values comparable to those at baseline (p=0.06). The BMI-SDS, however, was significantly reduced at 8 years compared to baseline (p<0.0001). Height SDS and head circumference SDS both completely normalized. IGF-1 SDS increased to +2.36 after the first year (p<0.0001) and remained stable thereafter. No adverse outcomes on glucose homeostasis, lipids, blood pressure or bone maturation were observed. Therefore, long-term GH therapy has positive effects on height and body composition that reduce the severity (but do not eliminate the risk) of childhood or adult obesity.
c. Data in adulthood
The use of growth hormone in adults with PWS has been studied on a limited basis; doses have ranged from 0.2 – 1.6mg daily. Sode-Carlsen et al., evaluated the effects and safety of two years of GH therapy (average dose 0.61 mg daily) in 43 adults with PWS59. Based on CT findings, thigh muscle volume and lean body mass increased (p<0.001) while abdominal subcutaneous fat volume and body fat mass decreased (p<0.01). Fifteen of the patients had either diabetes (n=4) or impaired glucose tolerance (IGT) (n=11) at baseline. Among the 11 subjects with IGT, three progressed to overt diabetes, while three reverted to normal glucose tolerance. Höybye et al., studied 10 men with PWS after more than 5 years of GH treatment60. The men who began treatment during childhood years were taller (178 ± 11 cm versus 156 ± 5 cm) and had lower fat mass and higher fat free mass. Four individuals had type 2 diabetes which remained in good metabolic control. Two of these were treated with GH in childhood for 6 to 7 years duration and two were treated in adulthood with GH duration 14 to 23 years. The authors concluded that the decision to use GH needs to weigh both the benefits and potential side-effects in each patient60.
Thus far, no consensus exists as to age of initiation of GH treatment. Infants as young as 2–4 months of age have been treated with growth hormone, though no studies have systematically examined the risks and benefits of treatment in infancy. Currently, general consensus aims to treat before onset of obesity; thus many begin treatment before 2 years of age31. Treatment should be initiated by an experienced clinician. While further studies are still needed to determine optimal dosing in PWS, infants and children are generally started on a daily dose of 0.05–1mg/m2/day or 0.1–0.15mg/kg/week, with subsequent adjustments based on clinical response and IGF-1 levels (target +1-+2SDS). Adults can begin with a dose of 0.1–0.2 mg daily, with subsequent adjustments based on clinical response and IGF-1 levels (target 0 to +2 SDS). Until studies definitively concerns regarding sleep apnea, a pre-treatment airway and sleep evaluation is recommended prior to, and possibly during, GH therapy. The dose of GH should be adjusted to maintain IGF-I levels in the normal range (to prevent lymphoid or tonsillar hyperplasia). Children receiving GH therapy should also be monitored for potential side-effects of GH, which can include headaches, glucose intolerance, and worsening scoliosis. Our suggested protocol for initiation of GH and monitoring of infants/children with PWS on therapy is shown below in Figure 3.
Figure 3.
Algorithm for Initiation of GH Therapy in Infants and Children
Central Adrenal Insufficiency
Infants and children with PWS have hypothalamic dysfunction and, therefore, are at risk of central adrenal insufficiency. The prevalence of adrenal insufficiency remains unknown. Some studies that used metyrapone testing reported rates of central adrenal insufficiency as high as 60%; however, testing modalities vary considerably and more recent studies with low dose ACTH stimulation suggest prevalence as low as 14.3% 61. The identification and treatment of central adrenal insufficiency are critical, as it is suspected to be a potential cause of sudden death in some patients: small adrenal glands were found on autopsy in a subset of PWS patients who had died suddenly in childhood 62. Additionally, growth hormone may decrease conversion of cortisone to cortisol by inhibiting 11-beta hydroxysteroid dehydrogenase type 1. The impact of growth hormone therapy on baseline adrenal function and the adrenal response to stress in PWS remains unclear.
While no consensus guidelines exist, we recommend an evaluation of the corticoadrenal axis in PWS patients. To that end, the levels of ACTH and cortisol should be obtained at the time of diagnosis, prior to starting growth hormone therapy (Figure 3) and during times of acute stress (i.e. severe illness, prior to anesthesia or surgery) 63. Parents should be provided a vial of parenteral hydrocortisone that can be used at times of high fever, vomiting, or trauma. Prophylactic stress dose glucocorticoid treatment should be administered (37.5–50mg/m2/day) during times of acute illness or surgery 61,63,64.
Hypogonadism
Children with PWS classically have physical findings of hypogonadism. However, the degree of hypogonadism varies in severity: pubertal development is absent in some and delayed or arrested in others. Varying degrees of genital hypoplasia may be detected at birth. Females may have small labia or hypoplastic clitoris 63. Males commonly have either unilateral or bilateral cryptorchidism, under-developed scrotum, and/or microphallus63. Boys may require orchiopexy, but some studies suggest use of human chorionic gonadotropin (HCG) to promote testicular descent and increase scrotal and penile size 65. Long-term data regarding use of HCG in PWS infants is currently insufficient, but hormone treatment may prevent need for surgery and general anesthesia.
The cause of hypogonadism remains unclear. While it has generally been felt to be caused by hypogonadotropic hypogonadism, the evidence for this is conflicting and some argue there may be a component of primary gonadal insufficiency. Hirsch et al. have demonstrated increased gonadotropins with low inhibin B and low testosterone levels in teenage and adult males suggestive of primary gonadal dysfunction66. Females with PWS have delayed or absent maturation of ovarian follicles associated with delayed or arrested pubertal progression67. Despite this, they have normal or subnormal gonadotropin levels, suggesting central or hypothalamic hypogonadism. Breast development is highly variable with some studies demonstrating complete tanner V breast development and vaginal bleeding occurs in some but not all66. Interestingly, despite delayed or incomplete pubertal development, patients with PWS often have precocious adrenarche63. Compared to healthy children, children with PWS have higher serum DHEAS levels possibly reflecting early maturation of the adrenal zona reticularis68.
There are case reports of female patients with PWS giving birth to children with Angelman syndrome 69,70. Therefore, we recommend family planning counseling for teenage and adult women with PWS.
Hypothyroidism
Up to one third of children with PWS have been reported to have hypothyroidism due to hypothalamic pituitary dysfunction63, with low or low-normal Thyroid Stimulating Hormone (TSH) and low total or free thyroxine (fT4). Since thyroid hormone is essential for appropriate neurodevelopment during the first three years of life, we recommended that thyroid function be assessed at 3, 6 and 12 months of age and then at least annually63. Hypothyroidism can impede GH synthesis and secretion; thus growth hormone testing in PWS children should be conducted only after normal thyroid function is established. Conversely, growth hormone treatment may affect thyroid function; it is critical that thyroid function be monitored during GH therapy.
Glucose Homeostasis
Obesity is a strong risk factor for the development of type 2 diabetes (T2D). The prevalence of T2D in adults with PWS (7–20%) exceeds greatly the prevalence in the general population (5–7%)71. In one cohort of 66 adults with PWS, the mean onset of T2D was 20 years71. It is uncommon for pre-pubertal children with PWS to develop overt diabetes or glucose intolerance: a large population based PWS cohort in France showed no cases of T2D and only 4% incidence of glucose intolerance at a mean age of 10.2 years72.
In fact, fasting insulin and HOMA-IR in PWS are comparable to those in BMI-age matched controls before age 5 years9 and are significantly lower by age 1174. In several studies the lower fasting insulin and HOMA-IR were seen in adults with PWS73,74; however, a more recent smaller study failed to recapitulate these findings and suggested further investigation with hyperinsulinemic-euglycemic clamp75.
It has been assumed that T2D in PWS develops as a consequence of morbid obesity and concomitant insulin resistance. However, recent studies suggest the relationship between morbid obesity and development of T2D is more complex and appears to differ in PWS versus non-PWS individuals. For example, in PWS children compared to BMI-matched controls, the insulin response to both a mixed meal and an oral glucose load is lower14. Additionally, first and second phase insulin secretion were significantly lower in PWS adults compared to obese controls during an intravenous glucose tolerance test (IVGTT)76. Finally, normal or increased sensitivity to exogenous insulin has been observed in PWS individuals77,78. In a recent study by our group, PWS subjects had relatively lower fasting insulin levels and increased adiponectin levels compared to BMI-matched obese controls despite having similar levels of leptin12. These findings suggest that PWS children may be protected to some extent from obesity-associated insulin resistance13.
The incretin system has been inadequately investigated as a cause of the relative hypoinsulinemia seen in PWS. Several case reports suggest favorable outcomes using glucagon-like peptide 1 (GLP-1) agonists/analogs with regards to weight loss, glycemic control, ghrelin reduction and pre-prandial insulin secretion79. The paucity of side effects with GLP-1 pharmacotherapy in this group has led some to speculate those with PWS may have deficient GLP-1 signaling in the brain80. Fasting GLP-1 levels were no different between PWS and obese controls75,83, but levels have not been evaluated after stimulation with OGTT. Fasting glucose-dependent insulinotropic polypeptide (GIP) levels are higher in those with PWS compared to obese controls, but levels also have not been evaluated after enteral stimulation81.
The explanation for beta-cell dysfunction in PWS remains elusive, but one hypothesis is that a decrease in vagal parasympathetic tone to the pancreas in PWS individuals reduces insulin secretion82. Another hypothesis proposes that GH deficiency in PWS reduces beta cell insulin secretion and increases insulin sensitivity. We have also hypothesized that relative hyperadiponectinemia may promote fatty acid oxidation and thereby increase insulin sensitivity12. Exploration of the association among various genotypes in PWS and the presence or absence of glucose intolerance and diabetes has not been fully explored.
Treatment of T2D in PWS individuals requires weight reduction by dietary modification and, sometimes, addition of an oral agent such as metformin, or insulin therapy. The role of GLP-1 agonists therapy is promising, but has not yet been fully elucidated.
Extra-hormonal comorbidities in PWS
Sleep Disorders
Sleep disturbances are common in children with PWS. This may manifest as obstructive sleep apnea, central sleep apnea, and hypoventilation syndromes. PWS children may have abnormal arousal and cardiorespiratory response to hypercapnia83. Central sleep apnea occurs more commonly in infants with PWS (less than 2 years of age), while obstructive sleep apnea is more frequent in children over 2 years of age84. Central sleep apnea may result from hypothalamic dysfunction and/or abnormal responses to hypoxia and hypercapnia by chemoreceptors; obstructive sleep apnea reflects the combined effects of obesity and hypotonia 84. Growth hormone may potentiate the risk of obstructive sleep apnea by increasing growth of adenotonsillar tissue (see more below).
The effects of chronic sleep disturbance and hypoventilation on long term neurocognitive outcomes remain unknown. Nevertheless, a sleep history should be obtained at each clinic visit; formal polysomnography should be performed if there is any evidence of sleep disturbance, including persistent snoring or excessive daytime sleepiness. Recent guidelines from the American Academy of Pediatrics recommend obtaining polysomnography prior to growth hormone therapy and within 2–3 months following initiation of growth hormone treatment with annual evaluation65.
Some centers recommend low flow nocturnal supplemental oxygen therapy for PWS infants with central sleep apnea84. Older children with obstructive sleep apnea may be respond to intranasal glucocorticoids or may require ENT referral for tonsillectomy/adenoidectomy 84 or initiation of CPAP.
Some children with PWS have narcolepsy 85,86. Narcolepsy is associated with a reduction in neurons expressing hypocretin (orexin), a neurohormone secreted by the lateral hypothalamus 87. Therefore, it has been theorized that sleep disturbances in PWS are caused by hypothalamic dysfunction. However, studies of the total number of hypocretin neurons in PWS have been conflicting87,88. Treatments of sleep disturbances, including narcolepsy, are discussed later in this review.
Behavioral Problems and Psychiatric Disturbances
PWS children can have several behavioral problems in addition to disordered feeding 1. In young children these range from mild tantrums and stubbornness to more extreme property destruction or physical attacks of rage 89,90. Older adolescents and adults may exhibit sadness, anxiety and depression leading to negative self-image and withdrawal 89,91. In addition, children and adolescents may develop a form of obsessive-compulsive disorder. This may manifest as hoarding (food and non-food items), repetitive rituals, and skin picking89.
The pathogenesis of the various behavioral concerns is currently unclear, but recent findings implicate a role for the hormone oxytocin. Oxytocin is an anorexigenic neuropeptide secreted by the hypothalamic paraventricular nucleus; it is also thought to play a role in social cognition and obsessive-compulsive disorder. Reports have published decreased expression of the oxytocin receptor and decreased oxytocin neurons within the hypothalamic paraventricular neurons, and recent data have shown elevated oxytocin levels in PWS compared to healthy controls92. The increased oxytocin levels may reflect oxytocin resistance and help explain the obsessive-compulsive tendencies in PWS, but further studies are needed to determine causality. Recent studies showing increased oxytocin levels in PWS may reflect loss of regulatory feedback due to decreased oxytocin receptor expression92.
Metabolic Studies
Body composition in PWS
Several studies looking at body composition in PWS children by various methods (skinfold measurements, total body water, bioelectrical impedance analysis (BIA), deuterium dilution method) have demonstrated higher amounts of adipose tissue in PWS children compared to obese non-PWS children 93,94. However, as each technique has its advantages and disadvantages 17, results have been inconsistent 18,19. Our group has recently 19 used an abdominal MRI technique to compare changes in body composition between children with PWS and non-PWS BMI-z and age-matched controls. Preliminary results indicate that children with PWS have lower skeletal muscle mass compared to controls, but similar total adipose tissue volume in the abdominal region. In addition, a greater ratio of intramuscular adipose tissue/skeletal muscle was found in the PWS group, suggesting the possibility that PWS represents a unique congenital model of sarcopenia. Further studies are required to determine if this altered body composition phenotype in PWS might be associated with poor motor function throughout development.
Energy balance in PWS
Evidence suggests that the distinctive weight gain in individuals with PWS may be caused by an alteration of the components of total energy expenditure (EE) such as resting energy expenditure (REE), activity energy expenditure (AEE) and diet-induced thermogenesis (DIT)95. To date, the critical underlying energy expenditure and energy intake factors that regulate energy balance in PWS are not fully understood. Resting energy expenditure (REE) is considered the main component of daily EE, accounting for 50–75% of total EE. Approximately 20–40% of EE is spent during AEE, and 10–15% on TEF 96. A major determinant of REE is the Fat Free Mass (FFM). Total energy expenditure, measured by doubly labeled water, has been found to be decreased in individuals with PWS; most, but not all, of the decrease has been attributed to their small fat free mass and reduced physical activity 94. Overall, studies also indicate that PWS individuals have lower absolute REE or basal metabolic rate (BMR) than matched-controls 94,97–101. However, when results were adjusted for the lower FFM in PWS (per unit of body surface area (function of weight and height) or per kg of lean body mass), differences were no longer apparent. Diet-induced thermogenesis (DIT) (the energy expended with the digestion and absorption of food) has been studied on a limited basis in individuals with PWS and the results have been inconclusive. Some studies showed lower values of DIT in obese and PWS individuals 102–105, while others showed increased values of DIT after a meal challenge 106 or no difference at all 75,107,108. Therefore, obese and PWS individuals may exhibit subtle changes in DIT and further controlled studies are required to assess for this defect which might have effects on metabolic health. Finally, the contribution of physical activity to total energy expenditure in PWS individuals has been examined. These studies suggest that PWS individuals are less active in general, but there is a wide-range of activity levels, which is a function of individual characteristics. Also, physical activity is as energy burning and as metabolically beneficial in PWS individuals as in normal individuals 109–111. Therefore, raising physical activity levels in individuals with PWS will increase their total energy expenditure, increase their lean body mass and help in maintenance of body weight in general 112.
Current Standard Therapies
Nutritional Phases and Hyperphagia
Three major nutritional phases with distinctive clinical characteristics have been defined in PWS (Figure 4)113. In phase 1 (0–9 months), newborns exhibit low muscle tone (hypotonia) with weak and uncoordinated suck, resulting in low caloric intake. Typically, infants need gavage feeding or the use of special nipples to maintain body weight. By the age of 9–25 months, infants no longer need assisted feeding. In phase 2 (2.1–4.5 years), there is an increase in body weight without obvious food seeking or a change in dietary intake. At this stage, infants are likely to become obese if their caloric intake is not restricted. By the age of 4.5–8 years during phase 2b, a significant increase in appetite and a marked interest in food are noted. Yet children may stop eating voluntarily at this stage. However, if food intake is not strictly supervised during Phase 3 (8 years-adulthood), which is characterized by chronic overeating (hyperphagia) and lack of satiety, individuals will gain excessive weight and manifest progressive obesity113.
Figure 4.
Timeline of nutritional phases in PWS (adapted from Miller J, et al. 2011 Am J Med Genet Part A 155:1040–1049).
Food-related behaviors such as sneaking and hoarding food are serious problems for PWS individuals. When given unlimited access to food, children and teenagers with PWS can consume massive amounts of food. Bray et al., reported the average ad libitum energy intake of six unsupervised PWS age 16 to 25 years to be 5,167 ± 503 kcal/day 77. Consumption of food considered inappropriate such as dog and cat food, garbage, etc.) may be an additional concern. Families report that children with PWS may be obsessed with refrigerators and freezers, worry about food (if there is enough, where the next meal will come from) and are generally preoccupied with food and eating 114.
Nutritional Management
Data generated on preschool and school-age children with PWS indicate that an energy-restricted diet of 7 kcal/cm/day will induce weight loss in PWS at any age 114,115. In addition, diets providing 8–11 kcal/cm/day have been reported to allow for weight maintenance 114,115. These energy goals translate into daily intakes of approximately 600 to 800 kcal for young children with PWS and 800–1300 kcal for older children and adults with PWS 115. These energy goals are considerably lower than the caloric intake of normal children. Of note is that many group homes for PWS individuals provide a diet consisting of 1,000 kcal/day 116. It is important to note that early dietary restriction in children with PWS may limit growth velocity and, possibly, final height. In this regard, GH therapy may be useful in promoting linear growth and improving body composition (increase lean mass and reduce fat mass).
The optimal macronutrient composition of the diet for individuals with PWS has not been determined. Some authors suggest a diet consisting of approximately 25% protein, 50% carbohydrate and 25% fat 115. Based on limited data, others suggest that hunger may be attenuated by the use of a ketogenic diet or protein-sparing modified fast 117; however, findings remain inconclusive. Indeed, various dietary interventions have been studied in children with PWS; these include low calorie diets combined with specific behavior management 118, 119,120, hypocaloric diets 121,122, 123,114, hypocaloric-protein sparing diets 124,125, energy-restricted ketogenic diets 109,117, and balanced macronutrient diets devoid of simple sugar 126. Each of these dietary interventions required long-term intervention/adherence and had only limited success. The dietary composition of macronutrients optimal for metabolic control (e.g. maximizing degree and duration of ghrelin suppression and optimizing insulin sensitivity) in PWS remains to be determined and is the subject of our groups’ ongoing work127. Finally, an accurate pictorial assessment tool for appetite, satiety and degree of hyperphagia in PWS is also the subject of ongoing research in our group.
Due to potential for insufficient intake of essential vitamins and minerals during prolonged periods of caloric restriction, a daily multivitamin and calcium and vitamin D supplementation is often required.
Nutritional management should begin in toddlerhood to prevent excessive weight gain and obesity-related risks, such as type 2 diabetes and cardiovascular disease (3). Obesity typically emerges soon after the age of 2 years if dietary intake is not closely monitored. Early intervention may be helpful in preventing excessive weight gain 128; control of dietary intake through behavior and environmental modification (e.g. locking the refrigerator and cupboards) is required.
In general, nutrition education is essential to help individuals with PWS maintain healthy weights. Since PWS infants rarely wake to feed during nutritional phase 1, a regular feeding schedule should be one of the first strategies implemented by parents and caregivers. Monitoring of caloric intake and monthly monitoring of height, weight and head circumference for the first 6 months and quarterly for 12–24 months are advisable. Caloric intake should be increased gradually (by a maximum ~100–200 kcal/d) to avoid overshooting weight goals. Consistency with meal patterns and times for eating is important.
Care providers should be taught about portion sizes; these are best described using common measurements such as cups, teaspoons, and fluid ounces or grams. It may be useful initially to record measurements for all daily food intake. Individualized menus should account for different family lifestyles and be planned with a nutritionist on an ongoing basis. Consideration of food preferences is important; PWS individuals do have definite consistent preferences for sweets and in most studies have exhibited a preference for calorie-dense over lower calorie foods 129. The child should play an active role in meal planning when possible. Vitamin and mineral intake should also be monitored (especially calcium and vitamin D), and supplements given as needed.
Finally, daily exercise should be part of the regular routine in order to enhance aerobic fitness and energy expenditure and sustain weight loss 130. A reward system of points or verbal praise may provide motivation for weight loss. Additional nutritional education resources are available from the Prader-Willi Syndrome Association (USA).
Sex Hormone Replacement Therapy
PWS male and female adolescents classically have incomplete or absent pubertal development; together with sarcopenia and low muscle tone, sedentary lifestyle, and growth hormone deficiency 131, this places them at risk for osteopenia and fractures. Treatment with sex hormones may improve bone health, muscle mass and overall well-being. However, recent studies have determined GH treatment will improve bone size and strength independent of sex steroid replacement132. While experts agree that dosing and timing of sex hormone replacement should mirror normal pubertal timing, there is no consensus on a regimen or timing for pubertal induction 133. Therapy must be individualized; thus the management of sex hormone replacement therapy should be supervised by a pediatric endocrinologist.
Initial evaluation of teenage girls with incomplete or arrested puberty should include measurements of estradiol, gonadotropins, and inhibin B66. Those without contraindications may be treated with a low-dose estrogen patch; progesterone can be added after the onset of menses. Contraceptive counseling is warranted, as some PWS women have been reported to give birth134. There have been no documented cases of male PWS paternity63.
Testosterone has been reported to precipitate or exacerbate aggression in some adolescent PWS males 133. Teenagers should be treated initially with transdermal testosterone at low doses, with cautious dose titration as tolerated 133.
New Approaches to Therapy
Oxytocin for hyperphagia and behavioral disorders
Levels of the anorexigenic neuropeptide oxytocin have been shown to be higher in PWS subjects compared to healthy controls, suggesting dysregulated oxytocin feedback or responsiveness may play a role in hyperphagia and behavioral disturbances 92. Oxytocin has been shown to decrease aggressive behavior in animal studies, and improve recognition of facial emotions in adolescents with autism135. Clinical trials in PWS investigating the effects of intranasal oxytocin on hyperphagia, skin picking, obsessions and emotional states have yielded conflicting results, with single-dose trials showing improved trust in treated participants but multi-dose trials showing no benefits in clinical outcomes 135,136.
Co-enzyme Q10 (CoQ10) and Carnitine to increase energy expenditure
PWS is characterized by infantile hypotonia, sarcopenia, and decreased resting energy expenditure 63. Some of these features are found in other disorders with low levels of co-enzyme Q10 (CoQ10), an electron carrier and essential component of the mitochondrial respiratory chain 137. CoQ10 levels have been shown to be lower in PWS and obese children relative to healthy non-obese controls 138. While CoQ10 is often used as an adjunct treatment in PWS with no known adverse effects, its efficacy in improving motor development and metabolic function is inconclusive 139. However, individuals have reported improved daytime alertness with CoQ10 supplementation140.
Carnitine deficiency, similar to CoQ10 deficiency, is associated with hypotonia, poor growth, and easy fatigability. Interestingly, unlike CoQ10, carnitine levels have been shown to be higher in PWS than in healthy controls suggesting impaired carnitine utilization in PWS140. Data remains inconclusive as to any positive effects of carnitine supplementation. In a recent study, twenty subjects were treated with carnitine 25mg/kg twice daily; thirteen subjects reported improved exercise tolerance and daytime alertness and seven subjects reported no benefits 140.
Modafinil for narcolepsy and daytime sleepiness
Excessive daytime sleepiness and increased napping can have a negative impact on quality of life85. Modafinil is a central stimulant that has been used in adults with narcolepsy and has recently been studied in PWS adolescents with hypersomnia. In a pilot study modafinil was well tolerated and reduced daytime sleepiness without serious side effects such as headache, insomnia, anxiety, or nausea 85.
N-acetylcysteine for obsessive picking of the skin
As previously discussed, PWS children and teens may have self-mutilation behaviors such as skin-picking. This has been related to obsessive-compulsive disorder and can be severe, leading to skin infections and scarring. Areas most commonly affected are the face, hands and legs and may be increased in areas of bug bites, scabs or eczematous lesions. Animal models have implicated glutamate neurotransmission in obsessive-compulsive behaviors141. N-acetylcysteine acts on the NMDA glutamate receptors to increase glutathione and has had potential efficacy in reducing nicotine-seeking and obsessive-compulsive behaviors in rat studies 142. A recent pilot study evaluated the effect of N-acetylcysteine (NAC) effect on skin-picking behavior in PWS 143. Thirty-five PWS children with skin-picking received an oral dose of 450–1200mg NAC daily and were then followed for 12 weeks. In this pilot study, results showed complete resolution of skin picking in about seventy percent and reduction in skin picking in almost thirty percent. Future studies with long term follow up are still needed to determine efficacy. While NAC is generally well-tolerated, side effects have included abdominal cramping, flatulence and diarrhea.
Beloranib for hyperphagia
Of great interest to the PWS research community is a medical agent that can reduce hyperphagia. Beloranib is an irreversible inhibitor of methionine aminopeptidase 2 (MetAP2), an enzyme which removes N-terminal methionine residues from newly synthesized proteins. MetAP2 inhibitors were previously used in cancer therapy because of their ability to slow endothelial cell growth and reduce angiogenesis; they were found to reduce food intake, lower body weight, and decrease adipose tissue mass at doses lower than those needed to inhibit angiogenesis and tumor growth144. While the exact mechanism of weight loss and decreased appetite is unclear, the MetAP2 inhibitors result in triglyceride lipolysis, fatty acid oxidation, ketogenesis and suppression of food intake related to alterations in signaling of the extracellular signal-related kinase (ERK) stress kinase pathway144. Small randomized, double-blind, placebo-controlled 4 week trials of beloranib as a novel treatment for PWS (n=17) have shown dose-dependent (placebo, 1.2mg, 1.8mg beloranib twice weekly) body weight/mass reductions despite concurrent 50% increase in total caloric intake during the study. Elevation in HDL and adiponectin and reduction in leptin, LDL and hsCRP were also observed during treatment 145. A similar randomized, double-blind placebo-controlled 4 week trial of beloranib (1.8 mg twice weekly) in patients with hypothalamic obesity also showed beneficial weight loss and improvements in cardiometabolic profile and hyperphagia 146. Future longer term clinical trials of beloranib are warranted to assess for changes in body weight, hyperphagia-related behaviors and overall longer term side-effect profile. As of December 2015, beloranib PWS trials have been placed on a partial clinical hold due to the death of 2 patients receiving the drug. The causes of death in each case were pulmonary emboli, but it is not known if these events were caused by beloranib. Prior trials using the medication had reported thromboembolic events, and clinical research participants will be screened for thromboembolic disease.
Bariatric surgery
While a medical cure for hyperphagia remains elusive both in PWS and the general population, bariatric surgery has found increasing success in the treatment of morbid obesity. Concerns were initially raised about the safety and long-term efficacy of surgical procedures including truncal vagotomy, gastroplasty, and endoscopic balloon placement and malabsorptive procedures such as Roux-en-Y gastric bypass and biliopancreatic diversion (BPD)) in the PWS population97. However, more recent data from several groups report beneficial effects of bariatric procedures in PWS. One group from Saudi Arabia reported findings in 24 subjects with PWS (4.9–18 years; pre-operative BMI= 46.2 ± 12.2 kg/m2) who underwent laparoscopic sleeve gastrectomy (LSG)147. BMI (kg/M2) changes in yearly visits during 5 years of follow-up were: −14.7 (year1; n=22); −15.0 (year2; n=18); −12.2 (year3; n=13); −12.7 (year4; n=11) and −10.7 (year5; n=7). Most subjects lost the majority of their weight within the first 2 years after surgery, with a plateau thereafter for another year, following by subsequent weight regain. The PWS group had 95% of comorbidities in remission or improved. No significant differences were seen in BMI change or growth rate or remission in obesity related co-morbidities between PWS and a non-PWS control group that underwent similar surgical procedures. No mortality or major morbidity was reported over the 5 year follow-up period. Interestingly, the families reported better control of hyperphagia and food-seeking behaviors post-operatively.
Another group from China reported their data on three children with PWS (15–23 years; mean BMI=46.7 kg/m2)148. Two patients underwent sleeve gastrectomy, while one underwent laparoscopic mini-gastric bypass (LMGBP). The mean weight loss and percentage of excessive weight loss at 2 years was 32.5 and 63.2%, respectively. The mean level of fasting acylated ghrelin decreased from 1134.2 pg/mL pre-operatively to 519.8 pg/mL 1 year post-operatively. No major peri-operative complications or mortality occurred. These authors also noted all patients had subjectively reduced food seeking behaviors and hyperphagia.
Removal of >75% of the stomach by sleeve gastrectomy is associated with favorable metabolic changes (reduction in ghrelin and increase in GLP-1) with minimal nutrient malabsorption. Future long- term studies of the use of LSG in PWS patients are warranted. Careful analysis of degree of weight loss, resolution of co-morbidities, and safety will be required to assess the overall success of bariatric procedures in the PWS population.
Long term care and Lifespan
While much is known about PWS in childhood and early adulthood, less is known about PWS in later adulthood. There are limited survival data beyond the fifth to sixth decade, but recent surveillance data estimate that mortality rates have declined to less than 3% annually due to improved medical care149. Given the increasing lifespan of PWS, it is generally advised to provide routine health surveillance for earlier detection of adult illness 150. The more frequently reported illnesses in older adults with PWS have included diabetes, cardiovascular disease with hypertension, osteoporosis and sleep disorders 150. The major causes of death are secondary to obesity and include respiratory failure with cor pulmonale, type 2 diabetes mellitus and arteriosclerosis. Due to autonomic dysfunction manifesting as a high pain threshold and abnormal temperature regulation, subjects may not complain of pain, fever or other signs of infection or other illnesses. Other health issues seen in adults with PWS include scoliosis, hypoventilation, recurrent respiratory infections/aspiration, choking, sleep apnea, hypertension, osteoporosis and leg ulceration 151.
Adults with PWS have early functional decline, making activities of daily living more difficult150. Psychiatric symptoms or psychosis may emerge, necessitating psychologic evaluation and/or medical treatment. Tantrums and stubbornness may prevent PWS adults from living in the community at large and retaining jobs. Group homes that operate with resources of a multidisciplinary team and emphasize control of diet and behavior management tend to be successful 17. Additionally, most PWS adults who are successfully employed are in sheltered workshops that provide a structured environment that is free from all sources of potentially edible food items. Many PWS individuals have good fine motor skills. Thus, it is often beneficial for families to work with an attorney specializing in working with families of the disabled. Community resources for families are available through the Prader-Willi Syndrome Association in the United States.
Future Studies and Therapies
A lack of detailed understanding of the neuroendocrine control of appetite and obesity in PWS currently hampers progress into design of anti-obesity drugs. However, some strides have been made. It is possible that specific ghrelin antagonists may assist in reducing PWS-related obesity. In addition, recent evidence suggests that central melanocortin agonists may block ghrelin-mediated feeding. Therefore, potential combinatorial therapy with a ghrelin antagonist and melanocortin agonist may ameliorate hyperghrelinemia effects in PWS.
These studies provide some hope, but many questions still remain unanswered. Future studies may aim at understanding genetic mechanisms of PWS obesity and relative insulin sensitivity, the regulation of ghrelin receptors and effect on appetite and weight, the cause for hyperghrelinemia, and the risks and benefits of therapies aimed at controlling appetite-regulating hormones.
Summary
PWS has a unique phenotypic profile and remains the most common cause of syndromic obesity. Diagnostic advances have resulted in early detection of PWS in infants and young children, allowing for early treatment and improved outcomes. Growth hormone therapy has been shown to have therapeutic benefits in PWS, and requires close monitoring for adverse events. Several top priority areas for GH research in PWS include: determination of optimal timing and dosage of GH treatment initiation in early life; longer term data on safety and efficacy of GH in PWS populations across international databases and registries; further evaluation of GH effects on behavior and cognition across development; longer-term data on appropriate monitoring for sleep disordered breathing post-GH initiation; and randomized controlled trials to evaluate the effects of GH therapy in concert with other novel therapeutic strategies including bariatric surgery.
Learning Objectives.
Identify physical, hormonal and biochemical features of Prader Willi Syndrome.
Discuss the use of growth hormone therapy and risks and benefits of this and other treatments in PWS.
Review advances in therapeutic strategies for common comorbid conditions in PWS including sleep disturbance, hyperphagia, and skin picking.
Acknowledgments
Funding: The authors are supported in part by a grant from the Foundation for Prader-Willi Research (AMH, MF), grant from the Canadian Institutes of Health Research (AMH) and an NIH 5T32HD043029-12 (KAI) to the Duke Department of Pediatrics
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Krystal A. Irizarry, Email: Krystal.Irizarry@duke.edu, Medical Instructor, Division of Pediatric Endocrinology, Duke University Medical Center; 3000 Erwin Rd Ste 200, Durham, NC 27705.
Mark Miller, Email: Mark.Miller@duke.edu, Fellow, Division of Pediatric Endocrinology, Duke University Medical Center; 3000 Erwin Rd Ste 200, Durham, NC 27705.
Michael Freemark, Email: Michael.Freemark@duke.edu, Atkins Professor and Chief, Division of Pediatric Endocrinology, Duke University Medical Center. 3000 Erwin Rd Ste 200, Durham, NC 27705.
Andrea M. Haqq, Email: haqq@ualberta.ca, Associate Professor, Division of Pediatric Endocrinology, University of Alberta 1C4 Walter C. Mackenzie Health Sciences Center, 8440 - 112 St NW, Edmonton, AB Canada T6G 2R7.
References
- 1.Cassidy SB, Dykens E, Williams CA. Prader-Willi and Angelman syndromes: Sister imprinted disorders. American Journal of Medical Genetics. 2000;97(2):136–146. doi: 10.1002/1096-8628(200022)97:2<136::aid-ajmg5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Bervini S, Herzog H. Mouse models of Prader–Willi Syndrome: A systematic review. Front Neuroendocrinol. 2013;34(2):107–119. doi: 10.1016/j.yfrne.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Miller NLG, Wevrick R, Mellon PL. Necdin, a Prader–Willi syndrome candidate gene, regulates gonadotropin-releasing hormone neurons during development. Hum Mol Genet. 2009;18(2):248–260. doi: 10.1093/hmg/ddn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischof JM, Stewart CL, Wevrick R. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum Mol Genet. 2007;16(22):2713–2719. doi: 10.1093/hmg/ddm225. [DOI] [PubMed] [Google Scholar]
- 5.Ding F, Li HH, Zhang S, et al. SnoRNA Snord116 Deletion Causes Growth Deficiency and Hyperphagia in Mice. PLoS ONE. 2008;3(3):e1709. doi: 10.1371/journal.pone.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398–402. [PMC free article] [PubMed] [Google Scholar]
- 7.Gunay-Aygun M, Schwartz S, Heeger S, O’Riordan MA, Cassidy SB. The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics. 2001;108(5):E92. doi: 10.1542/peds.108.5.e92. [DOI] [PubMed] [Google Scholar]
- 8.Haqq AM, Grambow SC, Muehlbauer M, et al. Ghrelin concentrations in Prader-Willi syndrome (PWS) infants and children: changes during development. Clin Endocrinol (Oxf) 2008;69(6):911–920. doi: 10.1111/j.1365-2265.2008.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstone AP, Holland AJ, Butler JV, Whittington JE. Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int J Obes (Lond) 2012;36(12):1564–1570. doi: 10.1038/ijo.2011.274. [DOI] [PubMed] [Google Scholar]
- 10.Erdie-Lalena CR, Holm VA, Kelly PC, Frayo RS, Cummings DE. Ghrelin levels in young children with Prader-Willi syndrome. J Pediatr. 2006;149(2):199–204. doi: 10.1016/j.jpeds.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 12.Irizarry KA, Bain J, Butler MG, et al. Metabolic profiling in Prader–Willi syndrome and nonsyndromic obesity: sex differences and the role of growth hormone. Clinical Endocrinology. 2015;83(6):797–805. doi: 10.1111/cen.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011;96(1):E225–232. doi: 10.1210/jc.2010-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumus Balikcioglu P, Balikcioglu M, Muehlbauer MJ, et al. Macronutrient Regulation of Ghrelin and Peptide YY in Pediatric Obesity and Prader-Willi Syndrome. J Clin Endocrinol Metab. 2015;100(10):3822–3831. doi: 10.1210/jc.2015-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler MG, Meaney FJ. Standards for selected anthropometric measurements in Prader-Willi syndrome. Pediatrics. 1991;88(4):853–860. [PMC free article] [PubMed] [Google Scholar]
- 16.Wollmann HA, Schultz U, Grauer ML, Ranke MB. Reference values for height and weight in Prader-Willi syndrome based on 315 patients. Eur J Pediatr. 1998;157(8):634–642. doi: 10.1007/s004310050901. [DOI] [PubMed] [Google Scholar]
- 17.Greenswag LR. Adults with Prader-Willi syndrome: a survey of 232 cases. Dev Med Child Neurol. 1987;29(2):145–152. doi: 10.1111/j.1469-8749.1987.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 18.Costeff H, Holm VA, Ruvalcaba R, Shaver J. Growth hormone secretion in Prader-Willi syndrome. Acta Paediatr Scand. 1990;79(11):1059–1062. doi: 10.1111/j.1651-2227.1990.tb11383.x. [DOI] [PubMed] [Google Scholar]
- 19.Cappa M, Grossi A, Borrelli P, et al. Growth hormone (GH) response to combined pyridostigmine and GH-releasing hormone administration in patients with Prader-Labhard-Willi syndrome. Horm Res. 1993;39(1–2):51–55. doi: 10.1159/000182695. [DOI] [PubMed] [Google Scholar]
- 20.Angulo M, Castro-Magana M, Mazur B, Canas JA, Vitollo PM, Sarrantonio M. Growth hormone secretion and effects of growth hormone therapy on growth velocity and weight gain in children with Prader-Willi syndrome. J Pediatr Endocrinol Metab. 1996;9(3):393–400. doi: 10.1515/JPEM.1996.9.3.393. [DOI] [PubMed] [Google Scholar]
- 21.Grosso S, Cioni M, Buoni S, Peruzzi L, Pucci L, Berardi R. Growth hormone secretion in Prader-Willi syndrome. J Endocrinol Invest. 1998;21(7):418–422. doi: 10.1007/BF03347319. [DOI] [PubMed] [Google Scholar]
- 22.Grugni G, Guzzaloni G, Moro D, Bettio D, De Medici C, Morabito F. Reduced growth hormone (GH) responsiveness to combined GH-releasing hormone and pyridostigmine administration in the Prader-Willi syndrome. Clin Endocrinol (Oxf) 1998;48(6):769–775. doi: 10.1046/j.1365-2265.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren AC, Hagenas L, Muller J, et al. Growth hormone treatment of children with Prader-Willi syndrome affects linear growth and body composition favourably. Acta Paediatr. 1998;87(1):28–31. doi: 10.1080/08035259850157822. [DOI] [PubMed] [Google Scholar]
- 24.Thacker MJ, Hainline B, St Dennis-Feezle L, Johnson NB, Pescovitz OH. Growth failure in Prader-Willi syndrome is secondary to growth hormone deficiency. Horm Res. 1998;49(5):216–220. doi: 10.1159/000023174. [DOI] [PubMed] [Google Scholar]
- 25.Corrias A, Bellone J, Beccaria L, et al. GH/IGF-I axis in Prader-Willi syndrome: evaluation of IGF-I levels and of the somatotroph responsiveness to various provocative stimuli. Genetic Obesity Study Group of Italian Society of Pediatric Endocrinology and Diabetology. J Endocrinol Invest. 2000;23(2):84–89. doi: 10.1007/BF03343684. [DOI] [PubMed] [Google Scholar]
- 26.Eiholzer U, Stutz K, Weinmann C, Torresani T, Molinari L, Prader A. Low insulin, IGF-I and IGFBP-3 levels in children with Prader-Labhart-Willi syndrome. Eur J Pediatr. 1998;157(11):890–893. doi: 10.1007/s004310050961. [DOI] [PubMed] [Google Scholar]
- 27.Carrel AL, Myers SE, Whitman BY, Allen DB. Growth hormone improves body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome: A controlled study. J Pediatr. 1999;134(2):215–221. doi: 10.1016/s0022-3476(99)70418-x. [DOI] [PubMed] [Google Scholar]
- 28.Burman P, Ritzen EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev. 2001;22(6):787–799. doi: 10.1210/edrv.22.6.0447. [DOI] [PubMed] [Google Scholar]
- 29.Grugni G, Guzzaloni G, Morabito F. Impairment of GH responsiveness to GH-releasing hexapeptide (GHRP-6) in Prader-Willi syndrome. J Endocrinol Invest. 2001;24(5):340–348. doi: 10.1007/BF03343871. [DOI] [PubMed] [Google Scholar]
- 30.Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G. Peculiar body composition in patients with Prader-Labhart-Willi syndrome. Am J Clin Nutr. 1997;65(5):1369–1374. doi: 10.1093/ajcn/65.5.1369. [DOI] [PubMed] [Google Scholar]
- 31.Deal CL, Tony M, Höybye C, et al. Growth Hormone Research Society Workshop Summary: Consensus Guidelines for Recombinant Human Growth Hormone Therapy in Prader-Willi Syndrome. The Journal of Clinical Endocrinology & Metabolism. 2013;98(6):E1072–E1087. doi: 10.1210/jc.2012-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler MG, Lee J, Manzardo AM, et al. Growth charts for non-growth hormone treated Prader-Willi syndrome. Pediatrics. 2015;135(1):e126–135. doi: 10.1542/peds.2014-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siemensma EP, Tummers-de Lind van Wijngaarden RF, Festen DA, et al. Beneficial effects of growth hormone treatment on cognition in children with Prader-Willi syndrome: a randomized controlled trial and longitudinal study. J Clin Endocrinol Metab. 2012;97(7):2307–2314. doi: 10.1210/jc.2012-1182. [DOI] [PubMed] [Google Scholar]
- 34.Aycan Z, Bas VN. Prader-Willi syndrome and growth hormone deficiency. J Clin Res Pediatr Endocrinol. 2014;6(2):62–67. doi: 10.4274/jcrpe.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiholzer U, Blum WF, Molinari L. Body fat determined by skinfold measurements is elevated despite underweight in infants with Prader-Labhart-Willi syndrome. J Pediatr. 1999;134(2):222–225. doi: 10.1016/s0022-3476(99)70419-1. [DOI] [PubMed] [Google Scholar]
- 36.Bekx MT, Carrel AL, Shriver TC, Li Z, Allen DB. Decreased energy expenditure is caused by abnormal body composition in infants with Prader-Willi Syndrome. J Pediatr. 2003;143(3):372–376. doi: 10.1067/S0022-3476(03)00386-X. [DOI] [PubMed] [Google Scholar]
- 37.Festen DAM, De Lind van Wijngaarden R, Van Eekelen M, et al. Randomized controlled GH trial: effects on anthropometry, body composition and body proportions in a large group of children with Prader–Willi syndrome. Clinical Endocrinology. 2008;69(3):443–451. doi: 10.1111/j.1365-2265.2008.03228.x. [DOI] [PubMed] [Google Scholar]
- 38.Wijngaarden RFAdLv, Cianflone K, Gao Y, Leunissen RWJ, Hokken-Koelega ACS. Cardiovascular and Metabolic Risk Profile and Acylation-Stimulating Protein Levels in Children with Prader-Willi Syndrome and Effects of Growth Hormone Treatment. The Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1758–1766. doi: 10.1210/jc.2009-0656. [DOI] [PubMed] [Google Scholar]
- 39.de Lind van Wijngaarden RF, de Klerk LW, Festen DA, Duivenvoorden HJ, Otten BJ, Hokken-Koelega AC. Randomized controlled trial to investigate the effects of growth hormone treatment on scoliosis in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2009;94(4):1274–1280. doi: 10.1210/jc.2008-1844. [DOI] [PubMed] [Google Scholar]
- 40.Nagai T, Obata K, Tonoki H, et al. Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A. 2005;136(1):45–48. doi: 10.1002/ajmg.a.30777. [DOI] [PubMed] [Google Scholar]
- 41.Sacco M, Di Giorgio G. Sudden death in Prader-Willi syndrome during growth hormone therapy. Horm Res. 2005;63(1):29–32. doi: 10.1159/000082525. [DOI] [PubMed] [Google Scholar]
- 42.Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A. 2004;124A(4):333–338. doi: 10.1002/ajmg.a.20371. [DOI] [PubMed] [Google Scholar]
- 43.Vogels A, Van Den Ende J, Keymolen K, et al. Minimum prevalence, birth incidence and cause of death for Prader-Willi syndrome in Flanders. Eur J Hum Genet. 2004;12(3):238–240. doi: 10.1038/sj.ejhg.5201135. [DOI] [PubMed] [Google Scholar]
- 44.Van Vliet G, Deal CL, Crock PA, Robitaille Y, Oligny LL. Sudden death in growth hormone-treated children with Prader-Willi syndrome. J Pediatr. 2004;144(1):129–131. doi: 10.1016/j.jpeds.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 45.Oiglane E, Ounap K, Bartsch O, Rein R, Talvik T. Sudden death of a girl with Prader-Willi syndrome. Genet Couns. 2002;13(4):459–464. [PubMed] [Google Scholar]
- 46.Eiholzer U, Nordmann Y, L’Allemand D. Fatal outcome of sleep apnoea in PWS during the initial phase of growth hormone treatment. A case report. Horm Res. 2002;58(Suppl 3):24–26. doi: 10.1159/000066478. [DOI] [PubMed] [Google Scholar]
- 47.Nordmann Y, Eiholzer U, l’Allemand D, Mirjanic S, Markwalder C. Sudden death of an infant with Prader-Willi syndrome--not a unique case? Biol Neonate. 2002;82(2):139–141. doi: 10.1159/000063097. [DOI] [PubMed] [Google Scholar]
- 48.Schrander-Stumpel C, Sijstermans H, Curfs L, Fryns JP. Sudden death in children with Prader-Willy syndrome: a call for collaboration. Genet Couns. 1998;9(3):231–232. [PubMed] [Google Scholar]
- 49.Lindgren AC, Hellstrom LG, Ritzen EM, Milerad J. Growth hormone treatment increases CO(2) response, ventilation and central inspiratory drive in children with Prader-Willi syndrome. Eur J Pediatr. 1999;158(11):936–940. doi: 10.1007/s004310051246. [DOI] [PubMed] [Google Scholar]
- 50.Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, LaFranchi SH. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88(5):2206–2212. doi: 10.1210/jc.2002-021536. [DOI] [PubMed] [Google Scholar]
- 51.Carrel AL, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab. 2002;87(4):1581–1585. doi: 10.1210/jcem.87.4.8414. [DOI] [PubMed] [Google Scholar]
- 52.Lindgren AC, Ritzen EM. Five years of growth hormone treatment in children with Prader-Willi syndrome. Swedish National Growth Hormone Advisory Group. Acta Paediatr Suppl. 1999;88(433):109–111. doi: 10.1111/j.1651-2227.1999.tb14416.x. [DOI] [PubMed] [Google Scholar]
- 53.Eiholzer U, l’Allemand D. Growth hormone normalises height, prediction of final height and hand length in children with Prader-Willi syndrome after 4 years of therapy. Horm Res. 2000;53(4):185–192. doi: 10.1159/000023565. [DOI] [PubMed] [Google Scholar]
- 54.Myers SE, Carrel AL, Whitman BY, Allen DB. Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome. J Pediatr. 2000;137(1):42–49. doi: 10.1067/mpd.2000.105369. [DOI] [PubMed] [Google Scholar]
- 55.Carrel AL, Myers SE, Whitman BY, Allen DB. Sustained benefits of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome are dose-dependent. J Pediatr Endocrinol Metab. 2001;14(8):1097–1105. doi: 10.1515/jpem-2001-0805. [DOI] [PubMed] [Google Scholar]
- 56.Lo S, Siemensma EC, Festen DM, Collin PL, Hokken-Koelega AS. Behavior in children with Prader– Willi syndrome before and during growth hormone treatment: a randomized controlled trial and 8-year longitudinal study. Eur Child Adolesc Psychiatry. 2015;24(9):1091–1101. doi: 10.1007/s00787-014-0662-4. [DOI] [PubMed] [Google Scholar]
- 57.Whitman BY, Myers S, Carrel A, Allen D. The behavioral impact of growth hormone treatment for children and adolescents with Prader-Willi syndrome: a 2-year, controlled study. Pediatrics. 2002;109(2):E35. doi: 10.1542/peds.109.2.e35. [DOI] [PubMed] [Google Scholar]
- 58.Bakker NE, Kuppens RJ, Siemensma EP, et al. Eight years of growth hormone treatment in children with Prader-Willi syndrome: maintaining the positive effects. J Clin Endocrinol Metab. 2013;98(10):4013–4022. doi: 10.1210/jc.2013-2012. [DOI] [PubMed] [Google Scholar]
- 59.Sode-Carlsen R, Farholt S, Rabben KF, et al. Growth hormone treatment for two years is safe and effective in adults with Prader-Willi syndrome. Growth Horm IGF Res. 2011;21(4):185–190. doi: 10.1016/j.ghir.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Höybye C. Growth hormone treatment of Prader–Willi syndrome has long-term, positive effects on body composition. Acta Paediatr. 2015;104(4):422–427. doi: 10.1111/apa.12898. [DOI] [PubMed] [Google Scholar]
- 61.Corrias A, Grugni G, Crino A, et al. Assessment of central adrenal insufficiency in children and adolescents with Prader-Willi syndrome. Clin Endocrinol (Oxf ) 2012;76(6):843–850. doi: 10.1111/j.1365-2265.2011.04313.x. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson DA, Anaya TM, Clayton-Smith J, et al. Unexpected death and critical illness in Prader– Willi syndrome: Report of ten individuals. American Journal of Medical Genetics Part A. 2004;124A(2):158–164. doi: 10.1002/ajmg.a.20370. [DOI] [PubMed] [Google Scholar]
- 63.Emerick J, Vogt K. Endocrine manifestations and management of Prader-Willi syndrome. International Journal of Pediatric Endocrinology. 2013;2013(1):14. doi: 10.1186/1687-9856-2013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbara DW, Hannon JD, Hartman WR. Intraoperative Adrenal Insufficiency in a Patient with Prader-Willi Syndrome. J Clin Med Res. 2012;4(5):346–348. doi: 10.4021/jocmr1039w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCandless SE. Clinical report-health supervision for children with Prader-Willi syndrome. Pediatrics. 2011;127(1):195–204. doi: 10.1542/peds.2010-2820. [DOI] [PubMed] [Google Scholar]
- 66.Hirsch HJ, Eldar-Geva T, Bennaroch F, Pollak Y, Gross-Tsur V. Sexual dichotomy of gonadal function in Prader–Willi syndrome from early infancy through the fourth decade. Hum Reprod. 2015;30(11):2587–2596. doi: 10.1093/humrep/dev213. [DOI] [PubMed] [Google Scholar]
- 67.Siemensma EPC, Velden AAEMvA-vd, Otten BJ, Laven JSE, Hokken-Koelega ACS. Ovarian Function and Reproductive Hormone Levels in Girls with Prader-Willi Syndrome: A Longitudinal Study. The Journal of Clinical Endocrinology & Metabolism. 2012;97(9):E1766–E1773. doi: 10.1210/jc.2012-1595. [DOI] [PubMed] [Google Scholar]
- 68.Siemensma EPC, de Lind van Wijngaarden RFA, Otten BJ, de Jong FH, Hokken-Koelega ACS. Pubarche and serum dehydroepiandrosterone sulphate levels in children with Prader–Willi syndrome. Clinical Endocrinology. 2011;75(1):83–89. doi: 10.1111/j.1365-2265.2011.03989.x. [DOI] [PubMed] [Google Scholar]
- 69.Akefeldt A, Tornhage CJ, Gillberg C. A woman with Prader-Willi syndrome gives birth to a healthy baby girl. Dev Med Child Neurol. 1999;41(11):789–790. doi: 10.1017/s0012162299221573. [DOI] [PubMed] [Google Scholar]
- 70.Schulze A, Mogensen H, Hamborg-Petersen B, Graem N, Ostergaard JR, Brondum-Nielsen K. Fertility in Prader-Willi syndrome: a case report with Angelman syndrome in the offspring. Acta Paediatr. 2001;90(4):455–459. [PubMed] [Google Scholar]
- 71.Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T. Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome: a population-based study. Dev Med Child Neurol. 2002;44(4):248–255. doi: 10.1017/s001216220100202x. [DOI] [PubMed] [Google Scholar]
- 72.Diene G, Mimoun E, Feigerlova E, et al. Endocrine disorders in children with Prader-Willi syndrome--data from 142 children of the French database. Horm Res Paediatr. 2010;74(2):121–128. doi: 10.1159/000313377. [DOI] [PubMed] [Google Scholar]
- 73.Lacroix D, Moutel S, Coupaye M, et al. Metabolic and adipose tissue signatures in adults with Prader-Willi syndrome: a model of extreme adiposity. J Clin Endocrinol Metab. 2015;100(3):850–859. doi: 10.1210/jc.2014-3127. [DOI] [PubMed] [Google Scholar]
- 74.Talebizadeh Z, Butler MG. Insulin resistance and obesity-related factors in Prader-Willi syndrome: comparison with obese subjects. Clin Genet. 2005;67(3):230–239. doi: 10.1111/j.1399-0004.2004.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Purtell L, Viardot A, Sze L, et al. Postprandial metabolism in adults with Prader-Willi syndrome. Obesity (Silver Spring) 2015;23(6):1159–1165. doi: 10.1002/oby.21041. [DOI] [PubMed] [Google Scholar]
- 76.Schuster DP, Osei K, Zipf WB. Characterization of alterations in glucose and insulin metabolism in Prader-Willi subjects. Metabolism. 1996;45(12):1514–1520. doi: 10.1016/s0026-0495(96)90181-x. [DOI] [PubMed] [Google Scholar]
- 77.Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. The Prader-Willi syndrome: a study of 40 patients and a review of the literature. Medicine (Baltimore) 1983;62(2):59–80. [PubMed] [Google Scholar]
- 78.Sareen C, Ruvalcaba RH, Kelley VC. Some aspects of carbohydrate metabolism in Prader-Willi syndrome. J Ment Defic Res. 1975;19(2):113–119. doi: 10.1111/j.1365-2788.1975.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 79.Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012;59(10):889–894. doi: 10.1507/endocrj.ej12-0074. [DOI] [PubMed] [Google Scholar]
- 80.Sze L, Purtell L, Jenkins A, et al. Effects of a single dose of exenatide on appetite, gut hormones, and glucose homeostasis in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2011;96(8):E1314–1319. doi: 10.1210/jc.2011-0038. [DOI] [PubMed] [Google Scholar]
- 81.Haqq AM, Muehlbauer M, Svetkey LP, et al. Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): association with insulin sensitivity and circulating satiety peptide hormones. Clinical Endocrinology. 2007;67(6):944–951. doi: 10.1111/j.1365-2265.2007.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berthoud HR, Powley TL. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of Dil. Brain Res. 1991;553(2):336–341. doi: 10.1016/0006-8993(91)90846-n. [DOI] [PubMed] [Google Scholar]
- 83.Arens R, Gozal D, Omlin KJ, et al. Hypoxic and hypercapnic ventilatory responses in Prader-Willi syndrome. J Appl Physiol. 1994;77(5):2224–2230. doi: 10.1152/jappl.1994.77.5.2224. [DOI] [PubMed] [Google Scholar]
- 84.Cohen M, Hamilton J, Narang I. Clinically Important Age-Related Differences in Sleep Related Disordered Breathing in Infants and Children with Prader-Willi Syndrome. PLoS ONE. 2014;9(6):e101012. doi: 10.1371/journal.pone.0101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Cock VC, Diene G, Molinas C, et al. Efficacy of modafinil on excessive daytime sleepiness in Prader–Willi syndrome. American Journal of Medical Genetics Part A. 2011;155(7):1552–1557. doi: 10.1002/ajmg.a.34047. [DOI] [PubMed] [Google Scholar]
- 86.Manni R, Politini L, Nobili L, et al. Hypersomnia in the Prader Willi syndrome: clinicalelectrophysiological features and underlying factors. Clin Neurophysiol. 2001;112(5):800–805. doi: 10.1016/s1388-2457(01)00483-7. [DOI] [PubMed] [Google Scholar]
- 87.Fronczek R, Lammers GJ, Balesar R, Unmehopa UA, Swaab DF. The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader-Willi syndrome. J Clin Endocrinol Metab. 2005;90(9):5466–5470. doi: 10.1210/jc.2005-0296. [DOI] [PubMed] [Google Scholar]
- 88.Nevsimalova S, Vankova J, Stepanova I, Seemanova E, Mignot E, Nishino S. Hypocretin deficiency in Prader-Willi syndrome. Eur J Neurol. 2005;12(1):70–72. doi: 10.1111/j.1468-1331.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 89.Dykens E, Shah B. Psychiatric disorders in Prader-Willi syndrome: epidemiology and management. CNS drugs. 2003;17(3):167–178. doi: 10.2165/00023210-200317030-00003. [DOI] [PubMed] [Google Scholar]
- 90.Stein DJ, Keating J, Zar HJ, Hollander E. A survey of the phenomenology and pharmacotherapy of compulsive and impulsive-aggressive symptoms in Prader-Willi syndrome. J Neuropsychiatry Clin Neurosci. 1994;6(1):23–29. doi: 10.1176/jnp.6.1.23. [DOI] [PubMed] [Google Scholar]
- 91.Reddy LA, Pfeiffer SI. Behavioral and emotional symptoms of children and adolescents with Prader-Willi Syndrome. J Autism Dev Disord. 2007;37(5):830–839. doi: 10.1007/s10803-006-0210-2. [DOI] [PubMed] [Google Scholar]
- 92.Johnson L, Manzardo AM, Miller JL, Driscoll DJ, Butler MG. Elevated plasma oxytocin levels in children with Prader-Willi syndrome compared with healthy unrelated siblings. Am J Med Genet A. 2015 doi: 10.1002/ajmg.a.37488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nelson R, Huse D, Holman R, et al. Nutrition, Metabolism, Body composition and Response to the Ketogenic diet in Prader-Willi Syndrome. In: Holm VA, editor. Prader-Willi Syndrome. Baltimore: University Park Press; 1981. pp. 105–120. [Google Scholar]
- 94.Schoeller DA, Levitsky LL, Bandini LG, Dietz WW, Walczak A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism. 1988;37(2):115–120. doi: 10.1016/s0026-0495(98)90003-8. [DOI] [PubMed] [Google Scholar]
- 95.Butler MG. Management of obesity in Prader-Willi syndrome. Nat Clin Pract Endocrinol Metab. 2006;2(11):592–593. doi: 10.1038/ncpendmet0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abbott WG, Howard BV, Ruotolo G, Ravussin E. Energy expenditure in humans: effects of dietary fat and carbohydrate. Am J Physiol. 1990;258(2 Pt 1):E347–351. doi: 10.1152/ajpendo.1990.258.2.E347. [DOI] [PubMed] [Google Scholar]
- 97.Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2008;46(1):80–83. doi: 10.1097/01.mpg.0000304458.30294.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen KY, Sun M, Butler MG, Thompson T, Carlson MG. Development and validation of a measurement system for assessment of energy expenditure and physical activity in Prader-Willi syndrome. Obes Res. 1999;7(4):387–394. doi: 10.1002/j.1550-8528.1999.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lloret-Linares C, Faucher P, Coupaye M, et al. Comparison of body composition, basal metabolic rate and metabolic outcomes of adults with Prader Willi syndrome or lesional hypothalamic disease, with primary obesity. Int J Obes (Lond) 2013;37(9):1198–1203. doi: 10.1038/ijo.2012.228. [DOI] [PubMed] [Google Scholar]
- 100.Goldstone AP, Brynes AE, Thomas EL, et al. Resting metabolic rate, plasma leptin concentrations, leptin receptor expression, and adipose tissue measured by whole-body magnetic resonance imaging in women with Prader-Willi syndrome. Am J Clin Nutr. 2002;75(3):468–475. doi: 10.1093/ajcn/75.3.468. [DOI] [PubMed] [Google Scholar]
- 101.van Mil EA, Westerterp KR, Gerver WJ, et al. Energy expenditure at rest and during sleep in children with Prader-Willi syndrome is explained by body composition. Am J Clin Nutr. 2000;71(3):752–756. doi: 10.1093/ajcn/71.3.752. [DOI] [PubMed] [Google Scholar]
- 102.Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40(3):542–552. doi: 10.1093/ajcn/40.3.542. [DOI] [PubMed] [Google Scholar]
- 103.Segal KR, Edano A, Tomas MB. Thermic effect of a meal over 3 and 6 hours in lean and obese men. Metabolism. 1990;39(9):985–992. doi: 10.1016/0026-0495(90)90312-z. [DOI] [PubMed] [Google Scholar]
- 104.Steiniger J, Karst H, Noack R, Steglich HD. Diet-induced thermogenesis in man: thermic effects of single protein and carbohydrate test meals in lean and obese subjects. Ann Nutr Metab. 1987;31(2):117–125. doi: 10.1159/000177258. [DOI] [PubMed] [Google Scholar]
- 105.Jequier E, Schutz Y. New evidence for a thermogenic defect in human obesity. Int J Obes. 1985;9(Suppl 2):1–7. [PubMed] [Google Scholar]
- 106.Maffeis C, Schutz Y, Grezzani A, Provera S, Piacentini G, Tato L. Meal-induced thermogenesis and obesity: is a fat meal a risk factor for fat gain in children? J Clin Endocrinol Metab. 2001;86(1):214–219. doi: 10.1210/jcem.86.1.7132. [DOI] [PubMed] [Google Scholar]
- 107.Tentolouris N, Alexiadou K, Kokkinos A, et al. Meal-induced thermogenesis and macronutrient oxidation in lean and obese women after consumption of carbohydrate-rich and fat-rich meals. Nutrition. 2011;27(3):310–315. doi: 10.1016/j.nut.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 108.D’Alessio DA, Kavle EC, Mozzoli MA, et al. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81(6):1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nardella MT, Sulzbacher SI, Worthington-Roberts BS. Activity levels of persons with Prader-Willi syndrome. Am J Ment Defic. 1983;87(5):498–505. [PubMed] [Google Scholar]
- 110.Davies PS, Joughin C. Using stable isotopes to assess reduced physical activity of individuals with Prader-Willi syndrome. Am J Ment Retard. 1993;98(3):349–353. [PubMed] [Google Scholar]
- 111.Rubin DA, Clark SJ, Ng J, Castner DM, Haqq AM, Judelson DA. Hormonal and metabolic responses to endurance exercise in children with Prader-Willi syndrome and non-syndromic obesity. Metabolism. 2015;64(3):391–395. doi: 10.1016/j.metabol.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 112.Castner DM, Tucker JM, Wilson KS, Rubin DA. Patterns of habitual physical activity in youth with and without Prader-Willi Syndrome. Res Dev Disabil. 2014;35(11):3081–3088. doi: 10.1016/j.ridd.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 113.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader–Willi syndrome. American Journal of Medical Genetics Part A. 2011;155(5):1040–1049. doi: 10.1002/ajmg.a.33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holm VA, Pipes PL. Food and children with Prader-Willi syndrome. Am J Dis Child. 1976;130(10):1063–1067. doi: 10.1001/archpedi.1976.02120110025003. [DOI] [PubMed] [Google Scholar]
- 115.Stadler DD. Nutritional Management. In: Greenswag LR, editor. Management of Prader-Willi Syndrome. 2. New York: Springer-Verlag; 1995. pp. 88–114. [Google Scholar]
- 116.Hoffman CJ, Aultman D, Pipes P. A nutrition survey of and recommendations for individuals with Prader-Willi syndrome who live in group homes. J Am Diet Assoc. 1992;92(7):823–830. 833. [PubMed] [Google Scholar]
- 117.Nelson R, Hayles A, Novak L, Margie J, Vernet J. Ketogenic diet and Prader-Willi syndrome. Am J Clin Nutr. 1970;23:667. [Google Scholar]
- 118.Heiman MF. The management of obesity in the post-adolescent developmentally disabled client with Prader-Willi syndrome. Adolescence. 1978;13(50):291–296. [PubMed] [Google Scholar]
- 119.Altman K, Bondy A, Hirsch G. Behavioral treatment of obesity in patients with Prader-Willi syndrome. J Behav Med. 1978;1(4):403–412. doi: 10.1007/BF00846696. [DOI] [PubMed] [Google Scholar]
- 120.Kriz JS, Cloninger BJ. Management of a patient with Prader-Willi syndrome by a dental-dietary team. Spec Care Dentist. 1981;1(4):179–182. doi: 10.1111/j.1754-4505.1981.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 121.Evans PR. Hypogenital dystrophy with diabetic tendency. Guy’s Hospital Reports. 1964;113:207–222. [PubMed] [Google Scholar]
- 122.Jancar J. Prader-Willi syndrome. (Hypotonia, obesity, hypogonadism, growth and mental retardation) J Ment Defic Res. 1971;15(1):20–29. [PubMed] [Google Scholar]
- 123.Juul J, Dupont A. Prader-Willi syndrome. J Ment Defic Res. 1967;11(1):12–22. doi: 10.1111/j.1365-2788.1967.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 124.Bistrian BR, Blackburn GL, Stanbury JB. Metabolic aspects of a protein-sparing modified fast in the dietary management of Prader-Willi obesity. N Engl J Med. 1977;296(14):774–779. doi: 10.1056/NEJM197704072961402. [DOI] [PubMed] [Google Scholar]
- 125.Collier SB, Walker WA. Parenteral protein-sparing modified fast in an obese adolescent with Prader-Willi syndrome. Nutr Rev. 1991;49(8):235–238. doi: 10.1111/j.1753-4887.1991.tb03035.x. [DOI] [PubMed] [Google Scholar]
- 126.Coplin SS, Hine J, Gormican A. Out-patient dietary management in the Prader-Willi syndrome. J Am Diet Assoc. 1976;68(4):330–334. [PubMed] [Google Scholar]
- 127.Balikcioglu PG, Balikcioglu M, Muehlbauer MJ, et al. Macronutrient Regulation of Ghrelin and Peptide YY in Pediatric Obesity and Prader-Willi Syndrome. The Journal of Clinical Endocrinology & Metabolism. 2015;100(10):3822–3831. doi: 10.1210/jc.2015-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pipes PL, Holm VA. Weight control of children with Prader-Willi syndrome. J Am Diet Assoc. 1973;62(5):520–524. [PubMed] [Google Scholar]
- 129.Caldwell ML, Taylor RL. A clinical note on food preference of individuals with Prader-Willi syndrome: the need for empirical research. J Ment Defic Res. 1983;27(Pt 1):45–49. doi: 10.1111/j.1365-2788.1983.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 130.Silverthorn KH, Hornak JE. Beneficial effects of exercise on aerobic capacity and body composition in adults with Prader-Willi syndrome. Am J Ment Retard. 1993;97(6):654–658. [PubMed] [Google Scholar]
- 131.Bakker NE, Kuppens RJ, Siemensma EP, et al. Bone mineral density in children and adolescents with Prader-Willi syndrome: a longitudinal study during puberty and 9 years of growth hormone treatment. J Clin Endocrinol Metab. 2015;100(4):1609–1618. doi: 10.1210/jc.2014-4347. [DOI] [PubMed] [Google Scholar]
- 132.Longhi S, Grugni G, Gatti D, et al. Adults with Prader–Willi Syndrome have Weaker Bones: Effect of Treatment with GH and Sex Steroids. Calcif Tissue Int. 2015;96(2):160–166. doi: 10.1007/s00223-014-9949-1. [DOI] [PubMed] [Google Scholar]
- 133.Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(11):4183–4197. doi: 10.1210/jc.2008-0649. [DOI] [PubMed] [Google Scholar]
- 134.Eldar-Geva T, Hirsch HJ, Pollak Y, Benarroch F, Gross-Tsur V. Management of hypogonadism in adolescent girls and adult women with Prader–Willi syndrome. American Journal of Medical Genetics Part A. 2013;161(12):3030–3034. doi: 10.1002/ajmg.a.36152. [DOI] [PubMed] [Google Scholar]
- 135.Einfeld SL, Smith E, McGregor IS, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. American Journal of Medical Genetics Part A. 2014;164(9):2232–2239. doi: 10.1002/ajmg.a.36653. [DOI] [PubMed] [Google Scholar]
- 136.Tauber M, Mantoulan C, Copet P, et al. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: a randomised placebo-controlled trial in 24 patients. Orphanet J Rare Dis. 2011;6:47. doi: 10.1186/1750-1172-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Artuch R, Salviati L, Jackson S, Hirano M, Navas P. Coenzyme Q(10) Deficiencies in Neuromuscular Diseases. Adv Exp Med Biol. 2009;652:117–128. doi: 10.1007/978-90-481-2813-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Butler MG, Dasouki M, Bittel D, Hunter S, Naini A, DiMauro S. Coenzyme Q10 levels in Prader-Willi syndrome: Comparison with obese and non-obese subjects. American Journal of Medical Genetics Part A. 2003;119A(2):168–171. doi: 10.1002/ajmg.a.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eiholzer U, Meinhardt U, Rousson V, Petrovic N, Schlumpf M, l’Allemand D. Developmental profiles in young children with Prader-Labhart-Willi syndrome: effects of weight and therapy with growth hormone or coenzyme Q10. Am J Med Genet A. 2008;146a(7):873–880. doi: 10.1002/ajmg.a.32137. [DOI] [PubMed] [Google Scholar]
- 140.Miller JL, Lynn CH, Shuster J, Driscoll DJ. Carnitine and coenzyme Q10 levels in individuals with Prader-Willi syndrome. Am J Med Genet A. 2011;155a(3):569–573. doi: 10.1002/ajmg.a.33887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoffman KL. Animal models of obsessive compulsive disorder: recent findings and future directions. Expert opinion on drug discovery. 2011;6(7):725–737. doi: 10.1517/17460441.2011.577772. [DOI] [PubMed] [Google Scholar]
- 142.Ramirez-Nino AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine selfadministration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl ) 2013;225(2):473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miller JL, Angulo M. An open-label pilot study of N-acetylcysteine for skin-picking in Prader-Willi syndrome. Am J Med Genet A. 2014;164a(2):421–424. doi: 10.1002/ajmg.a.36306. [DOI] [PubMed] [Google Scholar]
- 144.Joharapurkar AA, Dhanesha NA, Jain MR. Inhibition of the methionine aminopeptidase 2 enzyme for the treatment of obesity. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2014;7:73–84. doi: 10.2147/DMSO.S56924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miller J, Drisoll D, Chen A, Hughes T, Kim DD. The Obesity Society. Boston, MA, USA: 2014. Randomized, Double-blind, Placebo Controlled 4 week proof of concept trial of Beloranib, A novel treatment for Prader-Willi Syndrome. [Google Scholar]
- 146.Shoemaker APJ, Abuzzahab J, Markovic T, Malloy J, Kim DD. Endocrine Society. San Diego, CA, USA: 2015. Randomized, double-bline, placebo-controlled 4 week proof of concept trial of beloranib resulted in rapid and significant weight loss in patients with hypothalamic injury associated obesity. [Google Scholar]
- 147.Alqahtani AR, Elahmedi MO, Al Qahtani AR, Lee J, Butler MG. Laparoscopic sleeve gastrectomy in children and adolescents with Prader-Willi syndrome: a matched-control study. Surg Obes Relat Dis. doi: 10.1016/j.soard.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fong AW, Wong SH, Lam CH, Ng EW. Ghrelin Level and Weight Loss After Laparoscopic Sleeve Gastrectomy and Gastric Mini-Bypass for Prader–Willi Syndrome in Chinese. Obes Surg. 2012;22(11):1742–1745. doi: 10.1007/s11695-012-0725-x. [DOI] [PubMed] [Google Scholar]
- 149.Whittington JE, Holland AJ, Webb T. Ageing in people with Prader-Willi syndrome: mortality in the UK population cohort and morbidity in an older sample of adults. Psychol Med. 2015;45(3):615–621. doi: 10.1017/S0033291714001755. [DOI] [PubMed] [Google Scholar]
- 150.Sinnema M, Schrander-Stumpel CT, Maaskant MA, Boer H, Curfs LM. Aging in Prader-Willi syndrome: twelve persons over the age of 50 years. Am J Med Genet A. 2012;158a(6):1326–1336. doi: 10.1002/ajmg.a.35333. [DOI] [PubMed] [Google Scholar]
- 151.Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T. Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome: a population-based study. Dev Med Child Neurol. 2002;44(4):248–255. doi: 10.1017/s001216220100202x. [DOI] [PubMed] [Google Scholar]