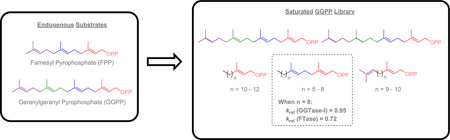
Abstract
Protein prenylation is a type of post-translational modification that aids certain proteins in localizing to the plasma member where they activate cell signaling. To better understand the isoprenoid requirements and differences of FTase and GGTase-I, a series of saturated geranylgeranyl diphosphate analogs were synthesized and screened against both mammalian FTase and GGTase-I. Of our library of compounds, several analogs proved to be substrates of GGTase-I, with 11d having a krel = 0.95 when compared to GGPP (krel = 1.0).
Keywords: Protein prenylation, Saturated GGPP analogs, Farnesylation, Geranylgeranylation, Isoprenoid Synthesis
Graphical Abstract
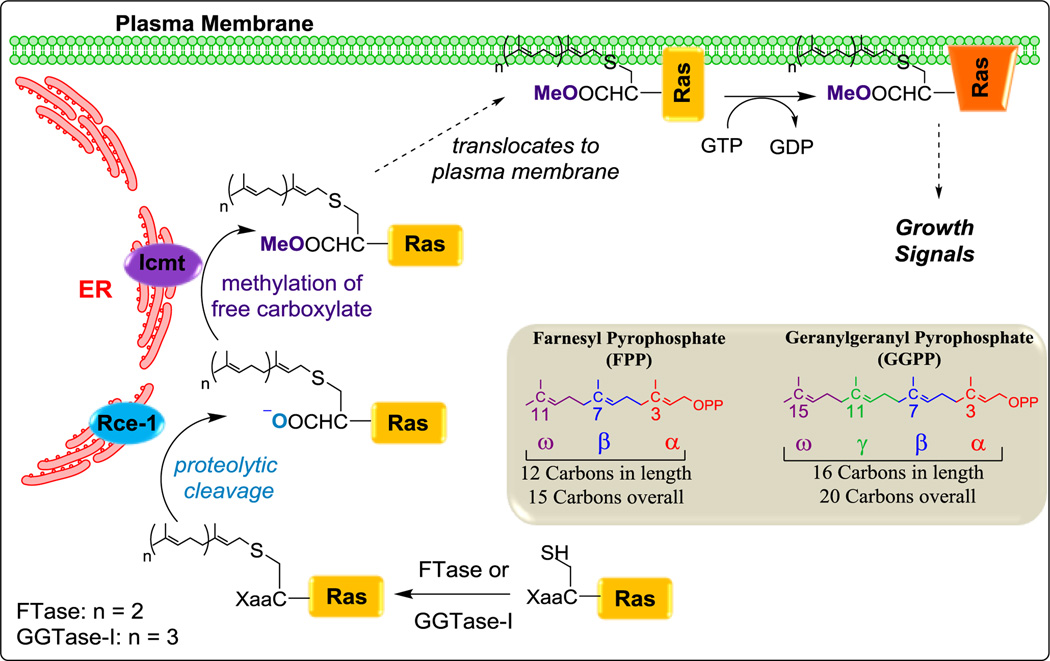
Classified as a type of lipidation, protein prenylation occurs on a cysteine residue located four residues from the C-terminus. Prenylated proteins contain a C-terminal motif known as a “CaaX box”, where ‘C’ denotes cysteine, ‘a’ is typically an aliphatic amino acid, and ‘X’ represents a small subset of amino acid residues.1 This “CaaX” motif allows proteins to be recognized by prenyl transferases located in the cytosol. The prenyl transferase enzymes (PTases) catalyze the formation of a thioether linkage between the cysteine residue of the CaaX box and isoprenyl lipids (namely farnesyl pyrophosphate, FPP; or geranylgeranyl pyrophosphate, GGPP).2 Farnesyl transferase (FTase) catalyzes the covalent attachment of a 15-carbon FPP while geranylgeranyl transferase-I (GGTase-I) catalyzes the attachment of a 20-carbon GGPP to cysteine (Figure 1).3,4 This covalent attachment then triggers the protein to relocate to the endoplasmic reticulum where it is proteolytically cleaved by Rce-1 followed by methyl esterification by Icmt.1,5,6,7 The newly modified protein is able to anchor into membranes where it regulates various important cellular functions such as cell signaling (Ras & others), cell division (CENP-E & CENP-F), and organelle structure (nuclear lamins).8
Figure 1.
Protein prenylation pathway of Ras and conventional method of isoprene unit labeling (gray box).
In the past, several other groups have investigated unnatural isoprenoid pyrophosphates as FPP mimics; however, these studies did not explore the importance of each individual isoprene unit.9–11 A previous study by our laboratory indicates that the α-isoprene is critical for substrate activity. In fact, by adding one additional methylene between the double bond of the first isoprene and the pyrophosphate of FPP turns the native substrate of FTase into an inhibitor.12 It is still unclear whether the other isoprene units are required for substrate activity.
In the past, there have been several inhibitors of GGTase-I that contain long saturated hydrocarbon chains with some of these inhibitors displaying submicromolar IC50’s.13 Such compounds led us to question whether the α, β, γ, and ω isoprene units are essential for enzyme activity. To address the query, we synthesized a variety of diphosphate analogs in which one or more of the isoprene units were replaced with saturated hydrocarbon chains. Our goal was to select analogs that ranged in chain length between FPP (12 carbons long) and GGPP (16 carbons long).
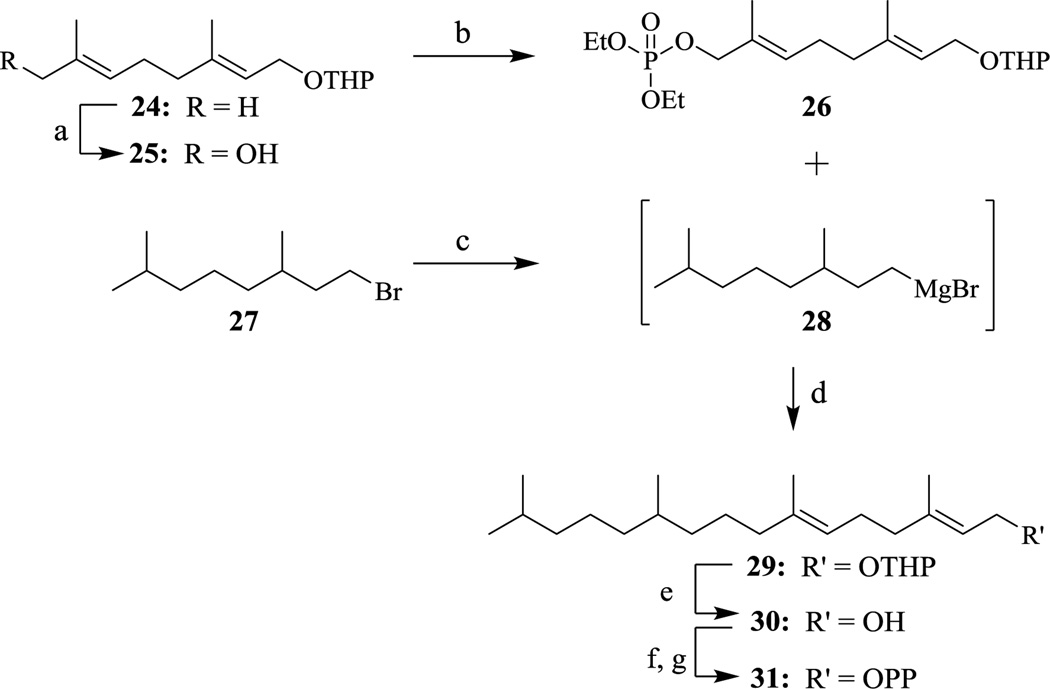
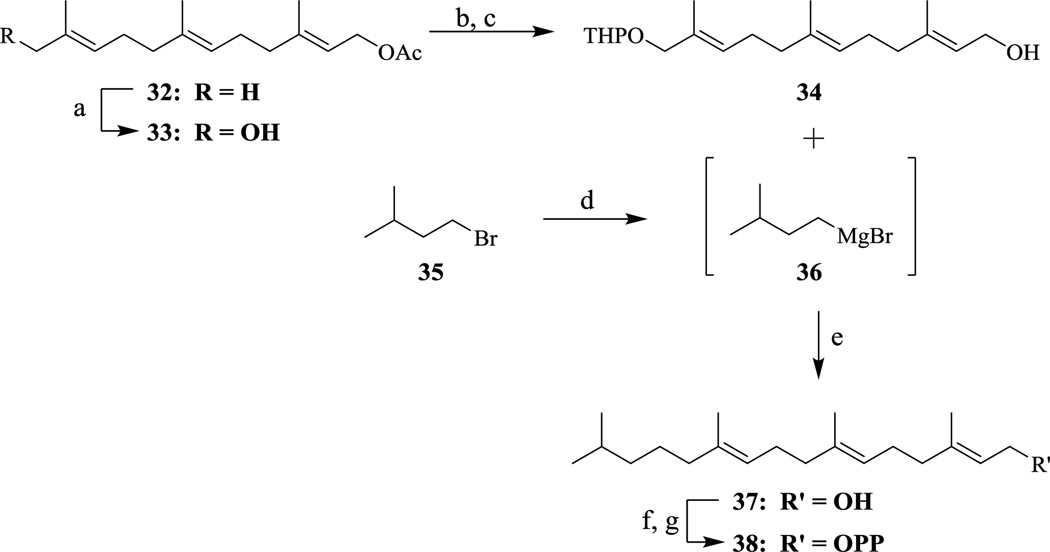
To determine if the α-isoprene is sufficient to produce substrate activity, the first set of analogs synthesized replaced the β, γ, and ω isoprene units with aliphatic chains (compounds 3a–c). Next, analogs that contain only the α and β isoprene units were synthesized (compounds 11a–d), replacing the ω isoprene of FPP and the γ and ω isoprene units of GGPP. These analogs will determine if the first two isoprene units are sufficient for substrate activity. Subsequently, the ω isoprene unit was reinstalled to obtain compounds that lack the internal β and γ isoprene units (compounds 23a–b). These compounds will examine the importance of the central isoprene units and whether they are required for catalysis. The final two compounds maintain the methylene-branching of isoprenoids but lack sp2 character to generate hydro-GGPP derivatives. The dihydro-GGPP (38) analog lacks the ω double bond but retains the methylene unit at the 15 position while the tetrahydro-GGPP (31) analog lacks both the γ and ω double bonds but retains the methylene units at the 11 and 15 positions (Schemes 4 & 5).
Scheme 4.
Synthesis of tetrahydro-GGPP. (a) SeO2, t-BuOOH, salicylic acid, DCM; ii. NaBH4, EtOH (27% - 2 steps); (b) DIEA, (EtO)2POCl, Et2O (76%); (c) Mg powder, Et2O; (d) 28, THF, 18 hr. (15%); (e) PPTS, EtOH, 70°C (70%); (f) NCS, DMS, DCM, 2.5 hr.; (g) (NBu4)3HP2O7, ACN, 3 hr (52% - 2 steps).
Scheme 5.
Synthesis of dihydro-GGPP. (a) SeO2, t-BuOOH, salicylic acid, DCM; ii. NaBH4, EtOH (28% - 2 steps); (b) i. DHP, PPTS, DCM; (c) saturated K2CO3/MeOH, (74% - 2 steps); (d) Mg powder, Et2O; (e) 36, Cu(I)Br, THF, −10°C, 48 hr (17%); (f) NCS, DMS, DCM, 2.5 hr; (g) (NBu4)3HP2O7, ACN, 3 hr (28%).
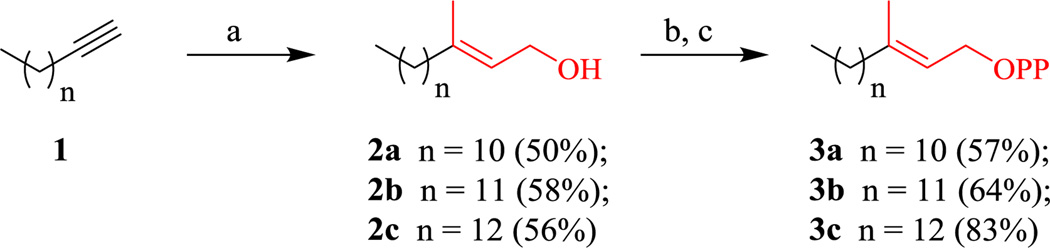
The synthesis of the saturated GGPP analogs began with the compounds containing only the α isoprene unit. The synthesis of these three analogs was simple and straight forward (Scheme 1). Briefly, commercially available alkynes 1 were subjected to Negishi’s zirconium asymmetric carbo-alumination (ZACA) reaction and quenched with paraformaldehyde to afford alcohols 2a–c in 50–58% yields.14 Next, these alcohols underwent Corey-Kim chlorination with NCS followed by pyrophosphorylation to produce the diphosphates 3a–c in moderate to good yields.15,16
Scheme 1.
Synthesis of α-containing pyrophosphates. (a) i. Me3Al, Cp2ZrCl2, DCM, 0°C, 18 hr; ii. (CH2O)n, 3 hr; (b) NCS, DMS, DCM, 0°C to rt, 2.5 hr; (c) (NBu4)3 HP2O7, ACN, 2.5 hr.
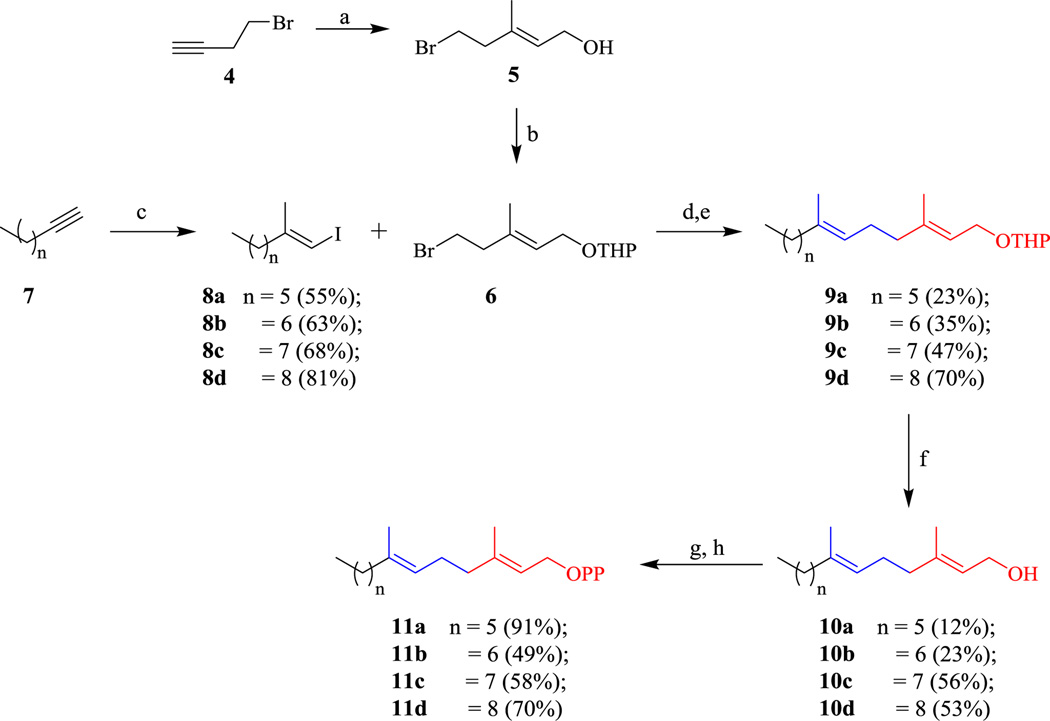
Next, we focused on the synthesis of analogs that contain only the α and β isoprene units (Scheme 2). To synthesize these compounds, commercially available alkynes underwent Negishi’s ZACA reaction followed by an iodine quench to provide vinyl iodides 8a–d. Conversion of 4-bromobut-1-yne to alcohol 5 was accomplished using the ZACA reaction.14 Next, alcohol 5 was THP-protected using standard procedures to generate compound 6 which was converted into the organoborane and Suzuki coupled to vinyl iodides 8a–d to yield compounds 9a–d.17 After deprotection, alcohols 10a–d underwent standard chlorination and pyrophosphorylation procedures to yield diphosphates 11a–d in moderate to good yields.15,16
Scheme 2.
Synthesis of α & β-containing pyrophosphates. (a) Me3Al, Cp2ZrCl2, DCM, 0°C, 18 hr then (CH2O)n, 3hr (83%); (b) PPTS, DHP, DCM (79%); (c) i. Me3Al, Cp2ZrCl2, DCM, 0°C, 18 hr, then I2, 3 hr; (d) i. t-BuLi, Et2O, −78°C, ii. β-MeO-9-BBN, THF, −78°C warming to r.t., 18 hr; (e) K3PO4, PdCl2(dppf), DMF, 85°C, 18 hr; (f) PPTS, MeOH, 60°C (Yields given for 2 steps); (g) NCS, DMS, DCM, 0°C to rt, 2.5 hr; (h) (NBu4)3HP2O7, ACN, 2.5 hr.
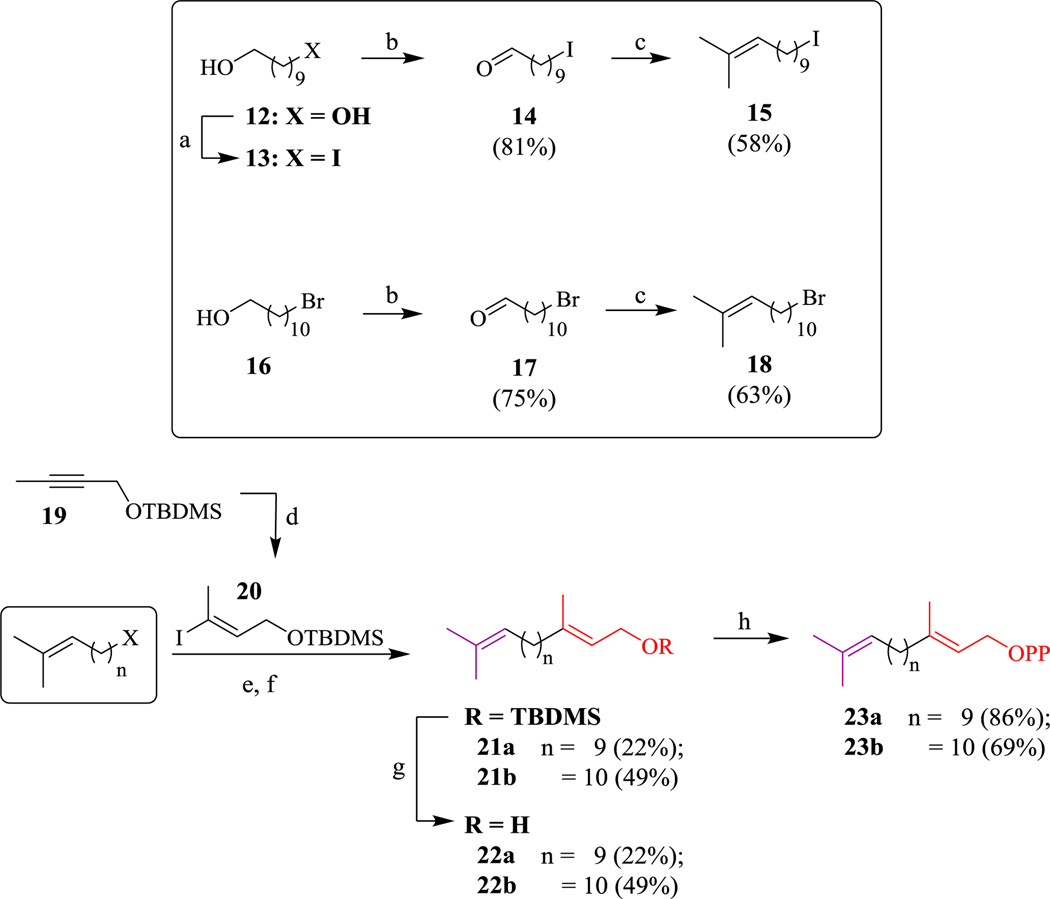
We then turned our attention to the synthesis of analogs where the ω isoprene unit was reinstalled to obtain compounds that lack the internal β and γ isoprene units (Scheme 3). In general, commercially available diol 12 was subjected to monoiodination. Intermediates 13 and 16 were subjected to Swern oxidations to afford aldehydes 14 and 17. These aldehydes then underwent Wittig reactions to install the ω isoprenes of 15 and 18 in moderate yields. Next, synthesis of vinyl-iodide 20 was accomplished by first generating the Schwartz’s reagent in situ following a method developed by Huang & Negishi.18 Following the addition of TBDMS-protected but-2-yn-1-ol (19), hydrozirconation-iodinolysis proceeds to yield vinyl iodide 20. After conversion of halides 15 and 18 into their corresponding organoboranes, Suzuki coupling to vinyl iodide 20 generated intermediates 21a–b. Following TBAF deprotection of the TBDMS group, allylic alcohols 22a–b were isolated and subjected to standard chlorination and pyrophosphorylation procedures to yield diphosphates 23a–d in moderated yields.
Scheme 3.
Synthesis of α & ω-containing pyrophosphates. (a) PPh3, Imidazole, I2, DCM, 0°C; (b) (COCl)2, DMSO, Et3N, DCM, −78°C; (c) i-PrPh3I, n-BuLi, THF, −78°C; (d) i. Cp2ZrCl2, DIBAL, THF, 0°C, 0.5 hr; ii. 19, warm to rt, 1.5 hr; iii. I2, THF, −78°C, 0.5 hr; (e) i. DIEA, TBDMSCl, DCM (95%); ii. DIBAL, Cp2ZrCl2, THF, 0°C (40%); (f) i. t-BuLi, Et2O, −78°C, ii. β-MeO-9-BBN, THF, −78°C warming to r.t., 18 hr; iii. K3PO4, PdCl2(dppf), DMF, 85°C, 18 hr; (g) TBAF, THF, 0°C; (h) NCS, DMS, DCM, 0°C to rt, 2.5 hr; (i) (NBu4)3 HP2O7, ACN, 2.5 hr.
To synthesize tetrahydro-GGPP we began with THP-protected geraniol (24) which underwent oxidation in the presence of SeO2 followed by a NaBH4 reduction to generate alcohol 25.19–21 Next, diethyl chlorophosphate is subjected to a displacement reaction in the presence of 25 and DIEA to generate diethyl phosphate 26. In situ formation of Grignard reagent 28 from the corresponding bromide (27) was followed by the slow addition of diethyl phosphate 26 to the reaction, which lead to a SN2 displacement of the phosphate group to afford compound 29. Following standard deprotection and pyrophosphorylation procedures, analog 31 was produced in moderate yield.
The synthesis of the final compound of this series, dihydro-GGPP (38), was first attempted in a similar manner as analog 31; however, efforts to displace the diethyl phosphate group of the corresponding farnesol derivative with Grignard reagent 36 resulted in a mixture of SN2 and SN2’ products. Thus, a new synthetic avenue was envisioned and the synthesis of analog 38 was accomplished using a Cu(I)-mediated Grignard displacement of an allylic THP-ether (Scheme 5).22,23 Briefly, acetyl-protected farnesol (32) was converted to alcohol 33 via a SeO2 oxidation followed by a NaBH4 reduction. Next, alcohol 33 was protected as the THP-ether (34) and then deacetylated using standard protocols to generate compound 34. After Grignard reagent 36 was generated from the corresponding bromide (35), it was slowly added to a cooled solution of THP-ether 34 and Cu(I)Br to yield alcohol 37. It is crucial to keep this reaction at −10°C to avoid degradation of the organocuprate intermediate. Alcohol 37 was then converted into the diphosphate following standard procedures to afford analog 38 in 28% yield.
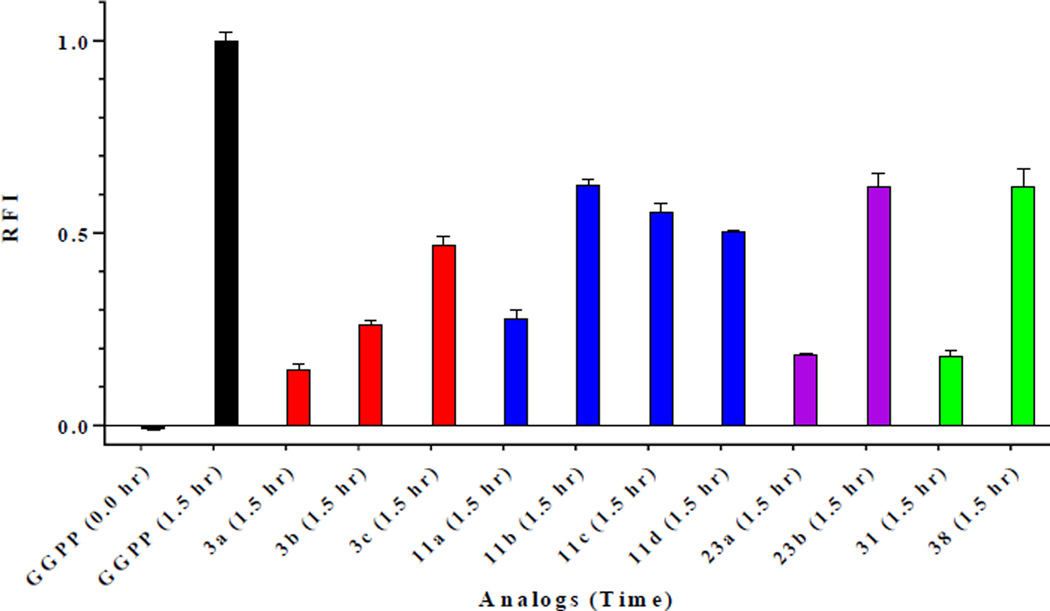
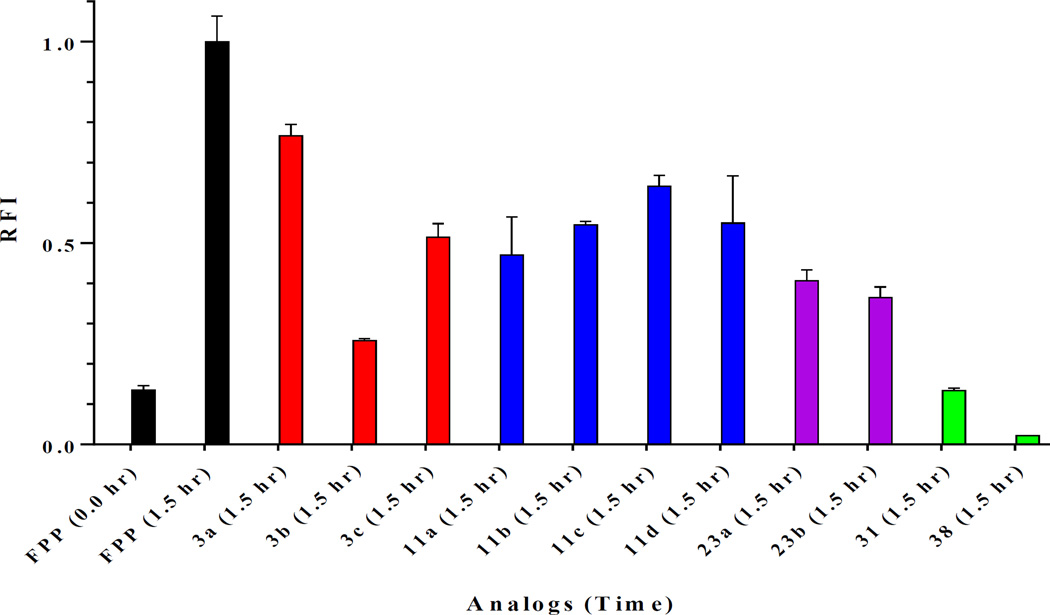
We hypothesized that the shorter chained analogs would be preferred as substrates by FTase while longer chained analogs would be preferred as substrates by GGTase-I. To test this theory, preliminary evaluation of the eleven saturated GGPP analogs was achieved utilizing an in vitro continuous spectrofluorometric assay versus GGTase with co-substrate CaaX-peptide, dansyl-GCVLL, (Figure 2) or versus FTase with co-substrate CaaX-peptide, dansyl-GCVLS (Figures 3). Analogs displaying increased fluorescence were further evaluated and their kcat/KM values were determined according to a published protocol.24 The first set of compounds containing only the α-isoprene unit (3a–c) revealed that both FTase and GGTase-I can recognize and utilize these compounds as substrates; unexpectedly, it appears the length of the carbon chain does not play as crucial a role as first anticipated. For example, one of the shortest analogs, 3a, has a krel(FTase) = 0.18 versus the krel(GGTase) = 0.08, indicating a slight preference for the enzyme whose natural substrate (FPP) is shorter. However, this trend does not appear to be true for analog 11b, which is of a similar length as 3a. In fact, 11b has a krel(FTase) = 0.15 versus the krel(GGTase) = 0.85, showing a much greater preference for GGTase-I. This is potentially due to a reduction of the amount of flexibility/rotation in the analog after the introduction of another double bond thereby decreasing its ability to find a binding mode that promotes rapid catalysis and/or product release.
Figure 2.
Preliminary screen of substrate activity represented in relative fluorescence increase (RFI) of saturated GGPP (5 µM) analogs versus GGPP (+ control) with 5 µM dansyl-GCVLL and 50 nM GGTase-I at time 1.5 hour with GGPP (1.5 hr) normalized to 1.0. Error bars represent mean ± SD (n = 3).
Figure 3.
Preliminary screen of substrate activity represented in relative fluorescence increase (RFI) of saturated GGPP analogs (5 µM) versus FPP (+ control) with 5 µM dansyl-GCVLS and 50 nM FTase-I at time 1.5 hours with FPP (5 µM, 1.5 hr) normalized to 1.0. Error bars represent mean ± SD (n = 3).
Perhaps the most telling evidence comes from comparing analogs of a similar length (i.e. 3c, 11d, 23b, 31, 38). These five analogs are all 16 carbons in length but differ in the number and position of double bonds present. By removing all but the α-isoprene, substrate activity is greatly diminished when compared to GGPP (krel(GGTase) = 0.16). Replacing the β-isoprene of 3c to generate 11d resulted in an increase of substrate activity with a 6-fold increase in GGTase-I reactivity (Table 1). Similarly, this modification led to an 8-fold increase in FTase reactivity. Reintroduction of the ω-isoprene of 3c to generate 23b also resulted in an increase in substrate activity of both GGTase-I and FTase; however, these increases were less substantial with a 3-fold increase in GGTase-I and a nearly 2-fold increase in FTase. While both the β- and ω-isoprene units are not required for enzyme catalysis, they are necessary for enhanced substrate activity. Based on these data, the β-isoprene unit seems to be more essential for catalysis than the ω-isoprene unit.
Table 1.
Evaluation of saturated GGPP analog substrate ability versus GGTase-I or FTase.
| Analog | GGTase-I kcat/KM (M−1s−1) |
krela (GGTase-I) |
Chain Length |
FTase kcat/KM (M−1s−1) |
krelb (FTase) |
|---|---|---|---|---|---|
| 3a | 3.20 × 103 | 0.08 | 14 | 8.30 × 103 | 0.18 |
| 3b | 8.90 × 102 | 0.02 | 15 | 1.10 × 104 | 0.24 |
| 3c | 6.20 × 103 | 0.16 | 16 | 4.10 × 103 | 0.09 |
| 11a | nd* | nd | 13 | 1.60 × 104 | 0.35 |
| 11b | 3.20 × 104 | 0.85 | 14 | 7.10 × 103 | 0.15 |
| 11c | 2.20 × 104 | 0.58 | 15 | 9.70 × 103 | 0.21 |
| 11d | 3.60 × 104 | 0.95 | 16 | 3.30 × 104 | 0.72 |
| 23a | 9.00 × 102 | 0.02 | 15 | 6.80 × 103 | 0.15 |
| 23b | 1.90 × 104 | 0.50 | 16 | 7.10 × 103 | 0.15 |
| 31 | 1.20 × 103 | 0.03 | 16 | 3.50 × 103 | 0.05 |
| 38 | 6.30 × 103 | 0.17 | 16 | nd* | nd |
| FPP | ns | ns | 12 | 4.60 × 104 | 1 |
| GGPP | 3.78 × 104 | 1.0 | 16 | - | - |
krel = relative rate in preliminary screen, with FPP and/or GGPP kcat/KM = 1.0
krel = relative rate in preliminary screen, with FPP = 1.0
Showed activity in screen but unable to attain kcat/KM value
Compounds lacking the γ and/or ω isoprene double bonds but retaining the methylene units (31 & 38) greatly decreased substrate activity with both FTase and GGTase-I (Table 1). When compared to other saturated molecules of comparable length (e.g. 11d, krel(GGTase) = 0.95; and 23b, krel(GGTase) = 0.50), analog 31 is a poorer substrate (krel(GGTase) = 0.03). Reinstalling the γ-isoprene unit to generate analog 38 results in a ~6-fold increase of substrate activity when compared to analog 31; however, analog 38 is an overall poor substrate activity with a krel(GGTase) = 0.17. Even though analog 38 contains 3 of the 4 isoprene units, its activity is comparable to analog 3a which only contains the α-isoprene. One possible explanation for the results observed for analogs 31 and 38 is molecular geometry. With the double bonds in place, the geometry of the molecule is planar; however, by removing the double bonds the molecular geometry changes from planar to tetrahedral. This change in geometry could lead to unfavorable interactions with the active site of the enzyme and ultimately reduce substrate binding.
In general, analogs with a carbon chain length of 16 (3c, 11d, 23b, & 38) are more readily turned over by GGTase-I while the shorter analog 3a (13 carbons) is more readily turned over by FTase. Analogs with intermediate carbon chain lengths (3a, 3b, 11b, 11c, 23a) display varying enzyme preferences. Comparing the FTase and GGTase-I krel values of analogs containing only the α-isoprene unit (3a & 3b) show a 2-fold and 12-fold preference for FTase, respectively. Analogs containing both α and β-isoprene unit (11b & 11c) revealed a ~6-fold and 3-fold preference for GGTase-I, respectively. The analog containing both α and ω-isoprenes with intermediate carbon chain length (23a) shows a ~8-fold preference for FTase-I. Although chain length is not the only factor driving reactivity, it is nonetheless important. Analogs with a chain length of 15-carbons (3b, 11c, and 23a) revealed 8-fold, 1.6-fold, and 25-fold decreases in GGTase-I activity, respectively, compared to GGPP. It can be speculated that shortening the length of the diphosphate chain may spatially orient the terminal methylene units of the ω-isoprene in a position where unfavorable interactions with the enzyme and/or peptide in the binding pocket could perturb catalysis or product release. It is also possible that the analog binds in a mode that reduces enzyme affinity for the peptide substrate. These results indicate that the position of the isoprene units as well as the overall length of the molecule are crucial for optimal activity. This work served as a preliminary study of the isoprene requirements and their effect on FTase and GGTase-I activity. A library of saturated GGPP compounds were synthesized and evaluated as co-substrates with dansyl-GCVLL (GGTase-I) or dansyl-GCVLS (FTase) in a fluorescence-based assay. Upon in vitro biochemical testing, it was revealed that all saturated analogs displayed some degree of substrate activity and provided us with interesting insights into FTase and GGTase-I reactivity. The saturated analogs revealed both FTase and GGTase-I can recognize and utilize compounds that contain only the α-isoprene unit; however, the other isoprene units are essential for enhanced turnover.
Supplementary Material
Acknowledgments
This work was funded by NIH Grants R01 CA78819 (R.A.G.), P30 CA21328 (Purdue University Center of Cancer Research), and GM40602 (C.A.F.). I would like to thank my post-doctoral advisor, Dr. Craig Lindsley, for his aid in publishing this article after the passing of a great scientist and even greater man, my thesis advisor, Dr. Richard A. Gibbs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Benetka W, Koranda M, Eisenhaber F. Monatsh. Chem. 2006;137:1241. [Google Scholar]
- 2.Lane KT, Beese LS. J. Lipid Res. 2006;47:681. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Long SB, Casey PJ, Beese LS. Nature. 2002;419:645. doi: 10.1038/nature00986. [DOI] [PubMed] [Google Scholar]
- 4.Taylor JS, Reid TS, Terry KL, Casey PJ, Beese LS. Embo J. 2003;22:5963. doi: 10.1093/emboj/cdg571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright LP, Philips MR. J. Lipid Res. 2006;47:883. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Baron RA, Casey PJ. BMC Bio-Chemistry. 2004;5:19. doi: 10.1186/1471-2091-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi YQ, Rando RR. J. Biol. Chem. 1992;267:9547. [PubMed] [Google Scholar]
- 8.Roskoski R. Biochem. Biophys. Res. Commun. 2003;303:1. doi: 10.1016/s0006-291x(03)00323-1. [DOI] [PubMed] [Google Scholar]
- 9.Chehade KAH, Andres DA, Spielmann HP. Biochemistry. 2000;39:79. [Google Scholar]
- 10.Kale TA, Hsieh SJ, Rose MW, Distefano MD. Curr. Top. Med. Chem. 2003;3:1043. doi: 10.2174/1568026033452087. [DOI] [PubMed] [Google Scholar]
- 11.Labadie GR, Viswanathan R, Poulter CD. J. Org. Chem. 2007;72:9291. doi: 10.1021/jo7017747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Placzek AT, Hougland JL, Gibbs RA. Org. Lett. 2012;14:4038. doi: 10.1021/ol300683r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Oualid F, Cohen LH, van der Marel GA, Overhand M. Curr. Med. Chem. 2006;13:2385. doi: 10.2174/092986706777935078. [DOI] [PubMed] [Google Scholar]
- 14.Rand CL, Vanhorn DE, Moore MW, Negishi E. J. Org. Chem. 1981;46:4093. [Google Scholar]
- 15.Davisson VJ, Woodside AB, Neal TR, Stremler KE, Muehlbacher M, Poulter CD. J. Org. Chem. 1986;51:4768. [Google Scholar]
- 16.Davisson VJ, Woodside AB, Poulter CD. Methods Enzymol. 1985;110:130. doi: 10.1016/s0076-6879(85)10068-6. [DOI] [PubMed] [Google Scholar]
- 17.Chemler SR, Trauner D, Danishefsky SJ. Angew. Chem. Int. Ed. 2001;40:4544. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Huang ZH, Negishi E. Org. Lett. 2006;8:3675. doi: 10.1021/ol061202o. [DOI] [PubMed] [Google Scholar]
- 19.Trachten.En, Nelson CH, Carver JR. J. Org. Chem. 1970;35:1653. [Google Scholar]
- 20.Krohn K, Riaz M. Tetrahedron Lett. 2004;45:293. [Google Scholar]
- 21.Guillemonat A. Ann. Chim. (France) 1939;11:143. [Google Scholar]
- 22.Mechelke MF, Wiemer DF. J. Org. Chem. 1999;64:4821. doi: 10.1021/jo990161p. [DOI] [PubMed] [Google Scholar]
- 23.Shull LW, Wiemer DF. J. Organomet. Chem. 2005;690:2521. [Google Scholar]
- 24.Hougland JL, Hicks KA, Hartman HL, Kelly RA, Watt TJ, Fierke CA. J. Mol. Biol. 2010;395:176. doi: 10.1016/j.jmb.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.