Abstract
Musculoskeletal pain (MSP) is a common sequela of traumatic stress exposure. While biologic factors contributing to chronic MSP after motor vehicle collision (MVC) have traditionally focused on tissue injury, increasing evidence suggests that neuro/stress/immune processes mediated by stress system activation may play a more dominant role. In a previous study we found that genetic variants in the hypothalamic-pituitary-adrenal (HPA) axis-related gene FKBP5 influence vulnerability to persistent MSP 6 weeks after MVC. In the present cohort study (n = 855) we evaluated whether genetic variants in several other important HPA axis-related genes, including the glucocorticoid receptor (NR3C1), corticotropin-releasing hormone receptor R1 (CRHR1), and corticotropin-releasing hormone binding protein (CRHBP), influence risk of chronic MSP over time after MVC. Genetic polymorphism rs7718461 in the CRHBP gene showed significant association (p=0.0012) with overall pain severity during the year after MVC in regression models controlling for multiple comparisons. Two additional CRHBP alleles in high Linkage Disequilibrium with rs7718461 also showed trend-level significance. In secondary analyses, a significant interaction between this CRHBP locus (Minor Allele Frequency = 0.33) and time was observed (p = 0.015), with increasing effect observed over time following trauma. A significant CRHBP*FKBP5 interaction was also observed, with substantially increased MSP after MVC in those with a risk allele in both genes compared to either gene alone. The results of this study indicate that genetic variants in two different HPA axis genes predict chronic MSP severity following MVC, and support the hypothesis that the HPA axis is involved in chronic post-MVC MSP pathogenesis.
Keywords: Genetic variant, CRHBP, HPA axis, musculoskeletal pain, stress induced hyperalgesia, motor vehicle collision
INTRODUCTION
More than 50 million motor vehicle collisions (MVCs) occur worldwide, and more than eleven million Americans experience an MVC each year [1]. The vast majority of individuals experiencing MVC do not have identifiable acute injury, however chronic musculoskeletal pain (MSP) is commonly reported by survivors of such events[25] and results in substantial suffering and diminished health[22; 51].
The pathobiology of chronic MSP development after MVC remains poorly understood. Collision characteristics are poor predictors of MSP outcomes[24; 37]; these data and other lines of evidence indicate that tissue injury is not a dominant factor determining post-MVC MSP outcomes[35]. In contrast, increasing evidence suggests that physiologic systems which shape stress and neuro-immune responses, such as the hypothalamic-pituitary-adrenal (HPA) axis, may play an important role in influencing MSP outcomes after traumatic/stressful events such as MVC[10; 26; 34; 35; 53; 54].
If the HPA axis influences the development of chronic MSP after MVC, then genetic variants affecting key proteins modulating HPA axis function should be associated with vulnerability to chronic post-MVC MSP. In a previous study we found an association between genetic variants in the gene coding for FK506 binding protein 51 (FKBP5), an important regulator of hypothalamic-pituitary-adrenal (HPA) axis function[12], and MSP severity 6 weeks after MVC[10]. In this study we evaluated the association between post-MVC MSP and three other important HPA axis-related genes: the glucocorticoid receptor (NR3C1), corticotropin-releasing hormone (CRH) receptor 1 (CRHR1), and CRH binding protein (CRHBP). In addition, because of increasing evidence that genetic influences on pain outcomes after stress exposure are often time[15] and/or sex[38]-dependent, in secondary analyses we assessed for interactions between significant main effects and sex and time. Also, because of evidence that HPA gene effects may be interactive[16; 45; 46], we explored for interactions between main effects and the previously identified FKBP5 risk allele[10].
METHODS
Study design and population
The details of the MVC study have been reported[44]. In brief, individuals ≥ 18 and ≤ 65 years of age presenting to one of eight Emergency Departments (ED) in four no-fault insurance states within 24 hours of MVC who did not have fracture or other injury requiring hospital admission were enrolled. Patients who were not alert and oriented were excluded, as were pregnant patients, prisoners, patients unable to read and understand English, or patients taking opioids above a total daily dose of 30 mg of oral morphine or equivalent. In addition, because genetic analyses are potentially biased by population stratification, enrollment was limited to non-Hispanic whites (the most common ethnicity at study sites). Informed consent was obtained from all participants and Institutional Review Board (IRB) approval was obtained at all study sites.
DNA collection and genotyping
A blood sample (PAXgene DNA tube) was collected at the time of study enrollment. Following DNA purification (PAXgene blood DNA kit, QIAGEN), genotyping was performed using the Sequenom platform. Sixteen Single Nucleotide Polymorphisms (SNPs) were genotyped across CRHBP, CRHR1, and NR3C1 (Figure 1). These SNPs were selected because they have previously been associated with neuropsychiatric diseases or pain outcomes[23; 28; 31] or because they are tagging SNPs. Two Hapmap samples and two repeat samples were included in each genotyping batch (96 samples) to ensure genotypic accuracy and reliability.
Figure 1.
Genomic location and Linkage Disequilibrium Plots of CRHBP, CRHR1, and NR3C1 polymorphisms analyzed in this study. Color and numbers represent D’ values (dark red=high inter-SNP D’; white=low inter-SNP D’).
Assessments
Sociodemographic information was collected at the time of the ED visit using a structured interview. Overall pain intensity in the ED and average pain during the month prior to MVC were each assessed by research assistants at the time of the ED interview using a verbal 0–10 numeric rating scale (NRS). Verbal scores have advantages in acute care settings, and verbally administered NRSs have been validated as a substitute for visual analogue scales in acute pain measurement in the ED[5]. Participant follow-up evaluations were conducted six weeks, six months, and one year after MVC using a web-based questionnaire or telephone interview. Each evaluation included an assessment of overall pain intensity in the past week (0–10 NRS); this assessment was used as the primary study outcome measure.
Analyses
Linkage disequilibrium between SNPs in CRHBP, CRHR1, and NR3C1 were explored by calculating Levontin D’ and squared correlation r2 using HaploView[2]. Associations between polymorphisms in CRHBP, CRHR1 and NR3C1 and overall pain intensity in the past week, at 6 weeks, 6 months, and 1 year were assessed using a repeated measures mixed model. Based on the results of previous studies[9; 10; 30; 36], study site, sex, past pain severity, and time from trauma in weeks were included in the model as covariates. Multiple testing was controlled for using the method of principal components[39]. Haplotype frequencies were calculated for the eight possible haplotypes with CRHBP SNPs using the expectation-maximization algorithm implemented in HaploView and verified using Bayesian estimation of haplotype frequencies implemented in SAS 9.2[29]. As described below, the three SNPs genotyped from the CRHBP genomic region were in high linkage disequilibrium (D’ ≥ 0.97, Figure 1), and therefore were evaluated as a haplotype. Consistent with previous evidence[46], a dominant model of CRHBP effect provided the best fit to the data and was employed. Interactions between CRHBP haplotypes, sex, time from trauma (in weeks), and FKBP5 allele were assessed by including the corresponding product terms in the models. Based on the results of previous studies, FKBP5 tagging SNP rs3800373 was used as the FKBP5 risk allele[10; 20]. Analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Participants
Baseline characteristics of study participants are shown in Table 1. A total of 949 European Americans were enrolled in the study. Most study participants were women less than 40 years of age with some post-secondary education.
Table 1.
Baseline characteristics of study participants
| Characteristic | |
|---|---|
| Enrolled, n | 949 |
| Age, years, mean (SD) | 36(13) |
| Females, n (%) | 575(61) |
| Education, n (%) | |
| 8–11 yrs | 42(4) |
| HS | 184(19) |
| Post-HS | 57(6) |
| Some college | 311(33) |
| College | 237(25) |
| Post-college | 113(12) |
| Overall pain, 0–10 NRS, mean (SD) | |
| Past month | 0.4(0.5) |
| ED | 5.5(2.4) |
Genotyping results
All 16 selected SNPs were successfully genotyped. Genotyped SNPs were in Hardy-Weinberg equilibrium (p > 0.05) and had excellent call rates (≥99%).
Association of HPA-related SNPs with overall MSP 6 weeks, 6 months, and 1 year after MVC
Eight hundred and fifty five of 949 individuals (90%) completed pain assessments at all three timepoints following MVC and were included in the analyses. Results of analyses evaluating the association between HPA axis-related SNPs and MSP outcomes after MVC are shown in Table 2. All three CRHBP SNPs assessed (rs7718461, rs1875999, and rs1053989) were associated with MSP after MVC at the trend-level (p < .01), and one of these SNPs, CRHBP SNP rs7718461, was significantly associated with post-MVC pain burden after adjustment for multiple testing (Table 2). With each of these CRHBP SNPs, the minor allele was associated with increased chronic MSP severity. The FKBP5 tagging SNP, rs3800373, which was previously shown to be associated with MSP severity 6 weeks following MVC[10], was also significantly associated with chronic MSP severity across time in the present study, with the minor allele associated with worse MSP outcomes (Table 2).
Table 2.
Association of the Hypothalamic-Pituitary-Adrenal axisalleles with chronic overall pain severity
| SNP | Gene | Alleles | MAF | Overall pain | |||
|---|---|---|---|---|---|---|---|
| Homozygous Major |
Heterozygous | Homozygous Minor |
p-valuea | ||||
| rs258763 | NR3C1 | T/A | 0.43 | 3.5 (3.2, 3.8) | 3.4 (3.2, 3.7) | 3.5 (3.1, 3.8) | 0.83 |
| rs9324916 | NR3C1 | C/G | 0.23 | 3.4 (3.2, 3.6) | 3.4 (3.2, 3.7) | 4.1 (3.4, 4.7) | 0.57 |
| rs2963155 | NR3C1 | A/G | 0.21 | 3.4 (3.2, 3.6) | 3.5 (3.3, 3.8) | 3.5 (2.8, 4.2) | 0.46 |
| rs9324918 | NR3C1 | A/G | 0.14 | 3.5 (3.3, 3.6) | 3.5 (3.2, 3.9) | 3.1 (2.1, 4.0) | 0.81 |
| rs9324924 | NR3C1 | G/T | 0.33 | 3.4 (3.2, 3.6) | 3.5 (3.3, 3.8) | 3.5 (3.0, 3.9) | 0.47 |
| rs12942300 | CRHR1 | T/A | 0.15 | 3.5 (3.3, 3.7) | 3.3 (3.0, 3.6) | 3.3 (2.4, 4.2) | 0.18 |
| rs7209436 | CRHR1 | C/T | 0.43 | 3.3 (3.0, 3.6) | 3.6 (3.3, 3.8) | 3.5 (3.1, 3.8) | 0.16 |
| rs4792887 | CRHR1 | C/T | 0.09 | 3.5 (3.3, 3.7) | 3.4 (3.0, 3.8) | 3.9 (2.2, 5.6) | 0.77 |
| rs17689378 | CRHR1 | C/T | 0.23 | 3.4 (3.2, 3.6) | 3.5 (3.2, 3.8) | 3.5 (2.8, 4.1) | 0.67 |
| rs12936511 | CRHR1 | C/T | 0.03 | 3.5 (3.3, 3.6) | 3.5 (2.9, 4.2) | 3.5 (0.9, 6.2) | 0.81 |
| rs81189 | CRHR1 | G/C | 0.48 | 3.3 (3.0, 3.6) | 3.6 (3.4, 3.8) | 3.4 (3.1, 3.7) | 0.18 |
| rs16940665 | CRHR1 | T/C | 0.23 | 3.4 (3.2, 3.6) | 3.5 (3.3, 3.8) | 3.5 (2.8, 4.1) | 0.62 |
| rs16940674 | CRHR1 | C/T | 0.23 | 3.4 (3.2, 3.6) | 3.5 (3.2, 3.8) | 3.5 (2.9, 4.2) | 0.64 |
| rs7718461 | CRHBP | A/G | 0.38 | 3.1 (2.9, 3.3) | 3.5 (3.2, 3.7) | 3.5 (3.4, 3.7) | 0.0012b |
| rs1875999 | CRHBP | A/G | 0.34 | 3.2 (3.0, 3.4) | 3.5 (3.4, 3.7) | 3.4 (3.1, 3.7) | 0.0061 |
| rs1053989 | CRHBP | G/T | 0.37 | 3.1 (3.0, 3.3) | 3.5 (3.4, 3.7) | 3.4 (3.1, 3.7) | 0.0054 |
| rs3800373 | FKBP5 | T/G | 0.30 | 3.1 (2.9, 3.3) | 3.7 (3.4, 3.9) | 3.5 (2.9, 4.0) | 0.0004b |
Repeated measures regression model (6 weeks, 6 month, 1 year), dominant genetic model, adjusted for week, past pain severity, sex, study site.
p values are significant after controlling for multiple testing using the method of principal components (the number of effective tests is 12, adjusted significance threshold is 0.05/12 = 0.0042)
Secondary analyses
Evaluation for potential gene-time interactions
Because all three CRHBP SNPs were in very high Linkage Disequilibrium (LD), and because the minor allele of each SNP was associated with pain vulnerability, subsequent analyses were performed with the three CRHBP SNPs grouped into a haplotype. Of eight possible haplotypes constituted from the three SNPs, only H1, H2, and H3 had frequencies above 0.01 (Supplementary Table 1). H2 contained all three CRHBP risk alleles (G-G-T, frequency = 0.33); in a repeated measures mixed model, H2 haplotype was significantly associated with post-MVC pain severity after adjustment for assessment week and study site (F(1,900)=7.7, p=0.006). Therefore the H2 haplotype was utilized in subsequent analyses.
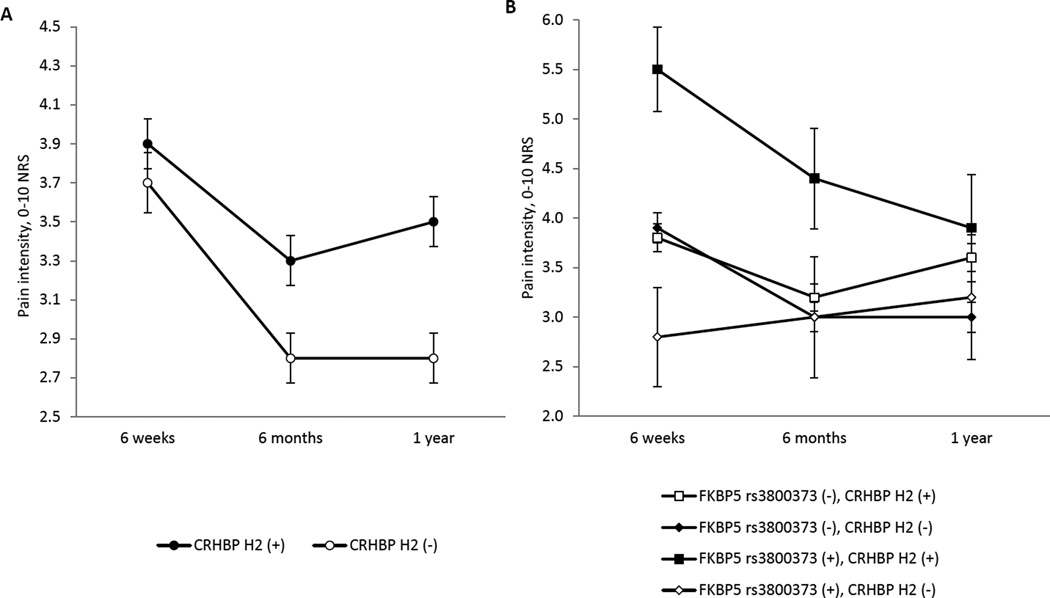
Because of evidence that genetic influences on pain outcomes after stress exposure are often time-dependent,15 we assessed for time-dependent differences in the influence of CRHBP H2 haplotype on post-MVC MSP severity, and in the influence of FKBP5 allele rs3800373 on post-MVC MSP severity. A significant time-CRHBP H2 haplotype effect was observed (F(1,897)=5.9, p=0.015, Table 3, Figure 2 Panel A), with the presence of a CRHBP H2 haplotype minor allele having an increasing effect with increasing time from trauma. No evidence for an interaction was observed between FKBP5 allele rs3800373 and time (F(1,897)=0.28, p=0.40, Table 3). These results are also presented in graphical format in Panel A of Figure 2.
Table 3.
Interaction between time after motor vehicle collision and effect of CRHBP and FKBP5 risk allele on musculoskeletal pain severity.
| Gene/SNPb | Risk allele | Pain Intensity (0–10 numeric rating scale) | |||
|---|---|---|---|---|---|
| 6 weeks | 6 months | 1 year | Interaction p-valuea |
||
| CRHBP H2 |
Present | 3.9 (3.7, 4.2) | 3.3 (3.0, 3.5) | 3.5 (3.2, 3.7) | 0.015 |
| Absent | 3.7 (3.4, 4.0) | 2.8 (2.6, 3.1) | 2.8 (2.6, 3.1) | ||
| FKBP5 rs3800373 |
Present | 4.1 (3.9, 4.4) | 3.4 (3.2, 3.7) | 3.5 (3.2, 3.8) | 0.40 |
| Absent | 3.6 (3.3, 3.9) | 2.9 (2.6, 3.1) | 3.1 (2.8, 3.4) | ||
Repeated measures regression model (6 weeks, 6 month, 1 year), dominant genetic model with a gene-week interaction term, adjusted for week, past pain severity, sex, study site
CRHBP H2 refers to haplotype 2 of the gene encoding corticotropin-releasing hormone binding protein and FKBP5 is the gene encoding FK506 binding protein 51
Figure 2.
Trajectories of pain severity by genotype. In panel A, patients are grouped by their CRHBP or FKBP5 genotype. CRHBP H2 (+) denotes the presence of at least one H2 risk haplotype; FKBP5 rs3800373 (+) denotes presence of the risk allele. In panel B, patients are grouped by the combination of their CRHBP and FKBP5 genotypes. CRHBP H2 (+) denotes the presence of at least one H2 risk haplotype; FKBP5 rs3800373 (+) denotes the presence of two risk alleles.
Evaluation for potential gene-sex interactions
As described above, because of evidence that genetic influences on pain outcomes after stress exposure are often sex-dependent14,32,33, we also evaluated for an interaction between sex and H2 haplotype and chronic post-MVC MSP severity. CRHBP analyses were stratified by timepoint because of the above evidence that CRHBP effects are time-dependent. No interaction was observed (F(1,843)=0.21, p=0.65 at 6 weeks; F(1,822)=1.73, p=0.19 at 6 months; F(1,842)=0.76, p=0.38 at 1 year). Similarly, no interaction was observed between sex and FKBP5 allele rs3800373 and pain over time after MVC (F(1,843)=0.66, p=0.42).
Evaluation for potential gene-gene interactions
In addition, because of previous evidence that HPA gene effects may be interactive[16; 45; 46], we tested for an interaction between the FKBP5 genotype rs3800373 and CRHBP H2 haplotype. CRHBP analyses were stratified by timepoint because of the above evidence that CRHBP effects are time-dependent. A highly significant interaction was observed between FKBP5 and CRHBP risk allele and MSP pain outcome 6 weeks after MVC (p <0.0001, Table 4). For example, individuals with two copies of the FKBP5 risk allele and one or more copies of the CRHBP risk allele had MSP nearly double the severity of individuals without the additional CRHBP risk allele(s) (Table 4, Figure 2 Panel B). This interaction did not reach statistical significance at later timepoints, although a similar trend in effect size was observed at the 6 month timepoint.
Table 4.
CRHBP × FKBP5 interaction predicting overall pain intensity (0–10 NRS) over time following motor vehicle collision
| Time after motor vehicle collision |
FKBP5 rs3800373 genotypea |
Number of CRHBP H2 haplotypes |
Mean pain severity (95% CI) |
p-value | Gene-gene interaction p-value |
|---|---|---|---|---|---|
| Six weeks | 0 | 1 or 2 | 3.8 (3.6, 4.1) | 0.844 | <0.0001 |
| 0 | 0 | 3.9 (3.6, 4.2) | |||
| 1 | 1 or 2 | 5.5 (4.6, 6.3) | <0.0001 | ||
| 1 | 0 | 2.8 (1.8, 3.8) | |||
| Six months | 0 | 1 or 2 | 3.2 (3.0, 3.5) | 0.1514 | 0.111 |
| 0 | 0 | 3.0 (2.6, 3.2) | |||
| 1 | 1 or 2 | 4.4 (3.4, 5.4) | 0.0596 | ||
| 1 | 0 | 3.0 (1.8, 4.2) | |||
| One year | 0 | 1 or 2 | 3.6 (3.3, 3.8) | 0.0038 | 0.5774 |
| 0 | 0 | 3.0 (2.7, 3.3) | |||
| 1 | 1 or 2 | 3.9 (2.8, 4.9) | 0.3326 | ||
| 1 | 0 | 3.2 (1.9, 4.4) |
genotype is coded 0=homozygous on major allele or heterozygous, 1= homozygous on minor allele. Gene × gene × time 3 way interaction, p = 0.0031.
DISCUSSION
Currently little is known about the biological pathways involved in chronic pain development following trauma exposure or which individuals are at high risk of developing MSP following exposures such as MVC. This study is the second study to identify a genetic association between HPA axis-related genes and MSP outcomes after MVC. In our prior study using the same dataset, we reported that the same FKBP5 risk alleles predict MSP severity six weeks after both MVC and sexual assault.[10] (That sexual assault study did not include data from the later follow-up timepoints used in the present analysis.) In the present study, haplotypes within another important HPA axis gene, CRHBP, predicted MSP burden during the year after MVC, with the adverse effect of a vulnerability allele increasing over time. The effect of CRHBP also interacted with FKBP5 vulnerability alleles identified in the previous study, such that individuals with vulnerability alleles at both of these HPA axis genes had substantially greater MSP after MVC than individuals with a vulnerability allele at only one gene. This finding is consistent with the results of a previous study that found an interaction between CRHBP and FKBP5 genes and mental health outcomes following childhood trauma[46].
CRH-BP and FK506 binding protein 51 (FKBP5) are key regulatory proteins affecting HPA axis function. Neural input into the hypothalamus causes the release of Corticotropin-Releasing Hormone (CRH), which initiates activation of the HPA axis. CRH-BP has complex effects[49; 60], evidence from animal studies suggests that increased CRH-BP expression inhibits HPA axis activation[48]. HPA axis activation results in the release of cortisol which diffuses through the cell membrane of cells in the body and binds to the glucocorticoid receptor (GR)[62]. FKBP5 regulates the sensitivity of the glucocorticoid receptor (GR) to cortisol[6]. Higher expression of FKBP5 has been shown to reduce cortisol binding affinity of the GR [14] and nuclear translocation of the GR[61] (i.e., result in GR resistance to cortisol), with accompanying increased plasma cortisol levels[14; 47].
Available evidence indicates that FKBP5 variants associated with worsened chronic MSP outcomes following MVC in the present study cause GR resistance to cortisol [7; 10; 14; 47]. In contrast, the physiologic effect of CRH-BP variants associated with worsened chronic pain outcomes following MVC in the present study are not well understood. To date, no studies have identified functional effects of variants in the CRHBP locus[8]. Further studies are needed to understand the functional effects of the identified CRHBP variants.
Epistatic interactions such as the interaction observed in the present study between the FKBP5 and CRHBP risk alleles are common[42]. This interaction was observed 6 weeks after MVC but not at 6 months or 1 year. The time-dependent nature of these interactions could be due to decreased power at later timepoints, as more individuals recover and have reduced MSP scores. This hypothesis is supported by the fact that the same direction of effect was observed at the 6 month and 1 year timepoints, but the effect weaker. Another potential contributor to the different effect of these interactions over time is that stress-related biologic effects are often markedly time-dependent[15].
The results of this study cannot determine to what extent the HPA axis is directly involved in the pathogenesis of post-MVC MSP or is a marker for pathobiologic processes mediated by other physiologic systems. Several lines of evidence from other studies suggest however that the HPA axis may itself have a direct contributing role. First, in a well-elucidated animal model of stress-induced hyperalgesia, widespread hyperalgesia was demonstrated to be due to the direct effect of persistently elevated levels of glucocorticoids and catecholamines on primary sensory afferents[27]. In addition, glucocorticoid systems exert an important influence on immune system function, and CRHBP and FKBP5 risk haplotypes may influence post-stress outcomes in part via mechanisms which lead to the increased production of pro-inflammatory mediators (e.g. cytokines)[17; 50]. Such mediators may promote allodynia and hyperalgesia both by sensitizing peripheral and central afferents directly[18; 19; 32] and by sensitizing CNS neurons via an afferent feedback mechanism[18; 19; 32; 56–58]. CRHBP and FKBP5 risk haplotypes may also affect sensory processing after stress exposure by their effect on central nervous system glucocorticoid pathways (e.g. [52; 55]).
Findings regarding the CRHBP allele evaluated in the present study are consistent with data linking this locus with other stress-related neuropsychiatric disorders. CRHBP rs1875999, one of the three CRHBP variants comprising the CRHBP H2 haplotype in the present study and the only one of these SNPs conserved across multiple mammalian species (http://genome.ucsc.edu/ Dec. 2013 (GRCh38/hg38) Assembly), has been associated with vulnerability to attempting suicide after stress exposure[13; 46] and to stress-related drug addiction[28]. CRHBP rs1875999 has also been associated with an increased number of pain sites[23]. CRHBP rs1875999 is located in the 3’UTR of the CRHBP gene; this gene region functions as a post-transcriptional regulatory region by determining the localization, stability, and translational efficiency of a gene transcript[3]. Molecular factors currently known to influence protein translation via the 3’UTR include binding by regulatory proteins or by microRNA[4; 43]. Examination of the genomic region surrounding the two CRHBP SNPs in the 3’UTR identified in this study, do not suggest direct influence of the allele on RNA binding protein binding or microRNA binding. However, the Adenylate-uridylate (AU) content of the CRHBP 3’UTR is 66 percent (average 3’UTR AU content ~55 percent [41]) and there are a number of AU-rich elements (AREs) in the CRHBP 3’UTR[21]. Therefore one potential molecular effect mediating the association between CRHBP H2 haplotype and chronic post-MVC pain outcomes is that this polymorphism affects CRHBP protein levels via modulating regulatory protein binding to ARE regions. Indeed, one such regulatory protein, HuD (ELAVL4), has previously been shown to affect neuronal plasticity[40]. Further studies are needed to evaluate this hypothesis.
The reasons for a lack of association between NR3C1 and CRHR1 genetic variants and chronic MSP are not known. Given the relatively low MAFs for many of the NR3C1 and CRHR1 alleles evaluated in comparison to the evaluated CRHBP alleles, it is possible that our sample size was underpowered for these alleles. In addition, previous associations between NR3C1 variants and post-trauma outcomes have suggested that functional variants in this gene affect hypermethylation of the promoter region[11; 33; 59]. Because the genetic variants assessed in our study were mostly intronic and downstream of the promoter, it is possible that we did not tag alleles responsible for methylation status of NR3C1. If this is the case, then lack of adjustment for this important covariate (hypermethylation status) may have reduced our power or biased our findings towards the null.
Several limitations should be noted when interpreting the results of this study. First, to avoid confounding by population stratification our study only enrolled European Americans. The generalizability of our findings to other race/ethnic groups is unknown. Second, results regarding the association between the CRHBP allele and post-MVC MSP outcome identified in the present study have not been replicated in a second cohort. However, this is not a novel finding of a previously unstudied gene locus. Instead this is a finding of an association between a stress-related outcome (chronic post-MVC MSP) and a gene locus previously associated with other stress-related disorders, in a biologic pathway that has been implicated in the pathogenesis of chronic MSP development in both animal and human studies. Thus from a Bayesian viewpoint, we believe that the post-test probability of our allele actually contributing to risk of chronic post-MVC MSP exceeds that of a novel replicated allele, and that our finding is likely to be a true positive. Finally, another limitation is that we enrolled only about half of the potentially eligible participants. The generalizability of our results among individuals who declined enrollment is not known. These limitations are consistent with other studies enrolling subjects after an acute aftermath of trauma in an ethical manner.
CONCLUSIONS
The results of this study suggest that genetic variants in corticotropin-releasing hormone binding protein, a gene affecting glucocorticoid signal transduction, influence the severity of pain symptoms experienced one year after MVC trauma. These findings add further evidence that physiologic systems which shape stress and neuroimmune responses, such as the HPA axis, may play an important role in influencing MSP outcomes after traumatic/stressful events such as MVC. These findings also suggest that glucocorticoid pathways might be a good target of interventions aimed at improving recovery and diminishing chronic pain after trauma exposure.
Supplementary Material
Acknowledgments
We would like to thank the participants for taking part in this study.
The project described was supported by Award Number R01AR056328 from the National Institute of Arthritis and Musculoskeletal and Skin diseases.
Footnotes
Scientific Meeting Presentation: American Society for Anesthesiologists, October 2013, San Francisco, CA, Oral Presentation
DISCLOSURES
No conflicts of interest exist for any of the authors.
References
- 1.U.S. Census Bureau. Statistical Abstract of the United States: 2012. Washington, DC: 2011. [Google Scholar]
- 2.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 3.Barrett LW, Fletcher S, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cellular and molecular life sciences : CMLS. 2012;69(21):3613–3634. doi: 10.1007/s00018-012-0990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2003;10(4):390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 6.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA : the journal of the American Medical Association. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Molecular psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortsov AV, Diatchenko L, McLean SA. Complex Multilocus Effects of Catechol-O-Methyltransferase Haplotypes Predict Pain and Pain Interference 6 Weeks After Motor Vehicle Collision. Neuromolecular medicine. 2013 doi: 10.1007/s12017-013-8255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS, Holbrook D, Rathlev NK, Foley KA, Lee DC, Collette R, Domeier RM, Hendry PL, McLean SA. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013;154(8):1419–1426. doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental psychobiology. 2013;55(7):673–683. doi: 10.1002/dev.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 13.De Luca V, Tharmalingam S, Zai C, Potapova N, Strauss J, Vincent J, Kennedy JL. Association of HPA axis genes with suicidal behaviour in schizophrenia. Journal of psychopharmacology. 2010;24(5):677–682. doi: 10.1177/0269881108097817. [DOI] [PubMed] [Google Scholar]
- 14.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141(11):4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 15.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hage W, Phillips ML, Radua J, Gohier B, Zelaya FO, Collier DA, Surguladze SA. Genetic modulation of neural response during working memory in healthy individuals: interaction of glucocorticoid receptor and dopaminergic genes. Molecular psychiatry. 2013;18(2):174–182. doi: 10.1038/mp.2011.145. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 18.Ferreira SH. The role of interleukins and nitric oxide in the mediation of inflammatory pain and its control by peripheral analgesics. Drugs. 1993;46(Suppl 1):1–9. doi: 10.2165/00003495-199300461-00003. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994;657(1–2):133–140. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and anxiety. 2009;26(11):984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber AR, Fallmann J, Kratochvill F, Kovarik P, Hofacker IL. AREsite: a database for the comprehensive investigation of AU-rich elements. Nucleic acids research. 2011;39:D66–D69. doi: 10.1093/nar/gkq990. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartling L, Brison RJ, Ardern C, Pickett W. Prognostic value of the Quebec Classification of Whiplash-Associated Disorders. Spine. 2001;26(1):36–41. doi: 10.1097/00007632-200101010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Holliday KL, Nicholl BI, Macfarlane GJ, Thomson W, Davies KA, McBeth J. Genetic variation in the hypothalamic-pituitary-adrenal stress axis influences susceptibility to musculoskeletal pain: results from the EPIFUND study. Annals of the rheumatic diseases. 2010;69(3):556–560. doi: 10.1136/ard.2009.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Cote P, Guzman J, Peloso P, Nordin M, Hurwitz E, van der Velde G, Carragee E, Haldeman S. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S52–S59. doi: 10.1097/BRS.0b013e3181643ece. [DOI] [PubMed] [Google Scholar]
- 25.Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Cote P, Guzman J, Peloso P, Nordin M, Hurwitz E, van der Velde G, Carragee E, Haldeman S Bone, Joint Decade - Task Force on Neck P, Its Associated D. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S52–S59. doi: 10.1097/BRS.0b013e3181643ece. [DOI] [PubMed] [Google Scholar]
- 26.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Jeanne Kreek M. Drug Addiction and Stress-Response Genetic Variability: Association Study in African Americans. Annals of human genetics. 2014 doi: 10.1111/ahg.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin S, Cutler DJ, Zwick ME, Chakravarti A. Haplotype inference in random population samples. Am J Hum Genet. 2002;71(5):1129–1137. doi: 10.1086/344347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linnstaedt SD, Hu J, Bortsov AV, Soward AC, Swor R, Jones J, Lee D, Peak D, Domeier R, Rathlev N, Hendry P, McLean SA. mu-Opioid Receptor Gene A118 G Variants and Persistent Pain Symptoms Among Men and Women Experiencing Motor Vehicle Collision. The journal of pain : official journal of the American Pain Society. 2015 doi: 10.1016/j.jpain.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227(2):231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623(2):321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- 33.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean SA. The Potential Contribution of Stress Systems to the Transition to Chronic WAD. Spine. 2011 doi: 10.1097/BRS.0b013e3182387fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean SA. Neurobiologic mechanisms of whiplash. IASP press; 2015. [Google Scholar]
- 36.McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, Bortsov AV, Bair E. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain. 2013 doi: 10.1016/j.pain.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, Bortsov AV, Bair E. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain. 2014;155(2):309–321. doi: 10.1016/j.pain.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musey PI, Jr, Linnstaedt SD, Platts-Mills TF, Miner JR, Bortsov AV, Safdar B, Bijur P, Rosenau A, Tsze DS, Chang AK, Dorai S, Engel KG, Feldman JA, Fusaro AM, Lee DC, Rosenberg M, Keefe FJ, Peak DA, Nam CS, Patel RG, Fillingim RB, McLean SA. Gender differences in acute and chronic pain in the emergency department: results of the 2014 Academic Emergency Medicine consensus conference pain section. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2014;21(12):1421–1430. doi: 10.1111/acem.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. American journal of human genetics. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perrone-Bizzozero N, Bolognani F. Role of HuD and other RNA-binding proteins in neural development and plasticity. Journal of neuroscience research. 2002;68(2):121–126. doi: 10.1002/jnr.10175. [DOI] [PubMed] [Google Scholar]
- 41.Pesole G, Liuni S, Grillo G, Saccone C. Structural and compositional features of untranslated regions of eukaryotic mRNAs. Gene. 1997;205(1–2):95–102. doi: 10.1016/s0378-1119(97)00407-1. [DOI] [PubMed] [Google Scholar]
- 42.Phillips PC. Epistasis--the essential role of gene interactions in the structure and evolution of genetic systems. Nature reviews Genetics. 2008;9(11):855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichon X, Wilson LA, Stoneley M, Bastide A, King HA, Somers J, Willis AE. RNA binding protein/RNA element interactions and the control of translation. Current protein & peptide science. 2012;13(4):294–304. doi: 10.2174/138920312801619475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Platts-Mills TFBL, Bortsov AV, Soward A, Swor RA, Jones JS, Lee DC, Peak DA, Domeier RM, Rathlev NK, Hendry PL, McLean SA. Using emergency department-based inception cohorts to determine genetic characteristics associated with long term patient outcomes after motor vehicle collision: methodology of the CRASH study. BMC Emerg Med. 2011;11(14) doi: 10.1186/1471-227X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribbe K, Ackermann V, Schwitulla J, Begemann M, Papiol S, Grube S, Sperling S, Friedrichs H, Jahn O, Sillaber I, Gefeller O, Krampe H, Ehrenreich H. Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Archives of general psychiatry. 2011;68(12):1247–1256. doi: 10.1001/archgenpsychiatry.2011.100. [DOI] [PubMed] [Google Scholar]
- 46.Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. Journal of psychiatric research. 2012;46(1):72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. General and comparative endocrinology. 2001;124(2):152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 48.Seasholtz AF, Burrows HL, Karolyi IJ, Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22(5):743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 49.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature reviews Immunology. 2006;6(4):318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suissa S, Harder S, Veilleux M. The relation between initial symptoms and signs and the prognosis of whiplash. European Spine Journal. 2001;10(1):44–49. doi: 10.1007/s005860000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran L, Wiskur B, Greenwood-Van Meerveld B. The role of the anteriolateral bed nucleus of the stria terminalis in stress-induced nociception. American journal of physiology Gastrointestinal and liver physiology. 2012;302(11):G1301–G1309. doi: 10.1152/ajpgi.00501.2011. [DOI] [PubMed] [Google Scholar]
- 53.Ulirsch JC, Weaver MA, Bortsov AV, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, McLean SA. No man is an island: Living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision, and this effect is moderated by common genetic variation influencing HPA axis function. Pain. 2014 doi: 10.1016/j.pain.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walton DM, Macdermid JC, Russell E, Koren G, Van Uum S. Hair-Normalized Cortisol Waking Response as a Novel Biomarker of Hypothalamic-Pituitary-Adrenal Axis Activity following Acute Trauma: A Proof-of-Concept Study with Pilot Results. Pain research and treatment. 2013;2013:876871. doi: 10.1155/2013/876871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(39):8595–8605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watkins LR, Goehler LE, Relton J, Brewer MT, Maier SF. Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain Res. 1995;692(1–2):244–250. doi: 10.1016/0006-8993(95)00715-3. [DOI] [PubMed] [Google Scholar]
- 57.Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63(3):289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 58.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654(1):15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 59.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 60.Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Frontiers in bioscience : a journal and virtual library. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- 61.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. The Journal of biological chemistry. 2005;280(6):4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 62.Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70(5–7):407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.