Abstract
Family history of colorectal cancer (CRC) is a known risk factor for CRC, and encompasses both genetic and shared environmental risk. We conducted a systematic review to estimate the impact of family history on the natural history of CRC and adherence to screening. We found high heterogeneity in family history definitions, the most common definition being one or more first-degree relatives. The prevalence of family history may be lower than commonly cited 10%, and confirms evidence for increasing levels of risk associated with increasing family history burden. There is evidence for higher prevalence of adenomas and of multiple adenomas in people with family history of CRC, but no evidence for differential adenoma location or adenoma progression by family history. Limited data on the natural history of CRC by family history suggests a differential age or stage at cancer diagnosis and mixed evidence on tumor location. Adherence to recommended colonoscopy screening was higher in people with family history of CRC. Stratification based on polygenic and/or multifactorial risk assessment may mature to the point of displacing family history-based approaches, but for the foreseeable future family history may remain a valuable clinical tool for identifying individuals at increased risk of CRC.
Keywords: Family history, risk stratification, colorectal cancer, systematic review
INTRODUCTION
Colorectal cancer (CRC) ranks third in cancer incidence and death in the United States. About 4% of CRCs occur in those younger than 50 years of age.1 Hereditary conditions such as familial adenomatous polyposis (FAP) and Lynch syndrome confer an extremely high lifetime risk of CRC but account for a minority of all CRCs. A much larger proportion of US adults have moderately-elevated risk of CRC due to a family history of CRC, likely due to a combination of shared polygenic and shared environmental risk.2,3,4
Early detection of CRC through screening with established modalities beginning at age 50 reduces CRC morbidity and mortality, but adherence to CRC screening remains below the CDC’s goal of 80%.5,6 Optimal screening for people with established family history of CRC is not as well defined, and screening recommendations vary and focus on earlier initiation of screening, frequency of screening, or early screening in people in racial/ethnic groups.7–12 If optimal screening strategies could be determined based on evidence-based risk, and adherence to screening could be improved, there is significant potential for further public health impact. Statistical modeling can give valuable information on how different screening practices might impact population outcomes, but population-based, high quality epidemiologic data is needed to inform such models.
We conducted a systematic review to identify evidence for the impact of family history of CRC on the risk and natural history of colorectal cancer, and on screening adherence. The review was commissioned by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group and designed to inform a decision-analytic model of optimal screening strategies conducted by the National Cancer Institute Cancer Intervention and Surveillance Modeling Network (CISNET) consortium microsimulation modeling group.13 Due to the lack of studies at the time of the review suggesting clinical utility of polygenic models of moderate-penetrance gene variants for assessing colorectal cancer risk, we focused the review on the evidence for family history.
We systematically reviewed four areas: Prevalence: What is the prevalence of a positive family history of CRC or adenoma in the population? Does prevalence vary by age, sex, and race/ethnicity of the person at risk for family history? Risk: What are the absolute and relative risks for CRC or adenoma associated with positive family history of CRC? Natural history: How does a positive family history of CRC impact the natural history of CRC and adenomas? Adherence: How does family history of CRC impact adherence to colonoscopy?
MATERIALS AND METHODS
Search strategy and study selection
Systematic literature searches were performed through February 20, 2013 in Medline, PubMed, and Centre for Reviews and Dissemination. Searches were broadly scoped, using terms for CRC, family history, natural history, and screening adherence.(supplemental material) Two investigators independently reviewed identified abstracts and articles against a priori specified inclusion criteria. Disagreements were resolved through consensus or input of a third reviewer. Detail of search strategies, study selection, analysis and results are available in the supplemental materials.
Inclusion criteria for all questions included age of the person at risk ≥18 and study reported in English language. Exclusion criteria for all questions included illness-associated CRC (e.g. Crohn disease), inherited CRC syndromes, and studies with unmeasured or poorly defined family history criteria. Only studies reporting family history in terms of both number and degree of relatedness for affected family members were included.
For risk, prevalence, and adherence, we included only population-based estimates. Case control studies were excluded to minimize bias by enrichment with people with family history of CRC. For natural history we included only studies that would allow assessment by family history of age of onset of adenoma or CRC, number of adenomas or tumors, advanced adenoma, and adenoma/tumor location. Case-only and case-control studies were permitted for this question.
We reviewed all included studies for the independence of their study populations and years of data collection. For studies from the same data source we included papers that best fit the relevance to the study questions.
Due to the large number of studies and heterogeneity of outcomes and family history definitions, we further refined our inclusion criteria using a best evidence approach, a staged method for prioritizing evidence from all potentially relevant to a set best suited to answering a question. Such approaches can be appropriate for large, heterogeneous literatures to enhance applicability of the evidence.14 For risk and prevalence we defined our best evidence set as population-based studies with a minimum sample size of 30,000, a number chosen by team consensus, and excluding screening studies as these may over-represent people with family history. For natural history we limited to studies where colonoscopy was conducted in a minimum sample size of 500 people with CRC or adenoma. For adherence, we identified an existing comprehensive review that contained data highly relevant to our study question15 and included this as a source of primary evidence,16 supplemented by subsequent US population-based studies.
Data abstraction, synthesis, and quality assessment
Histological characteristics of tumors and adenomas of interest to this review were informed by the World Health Organization histology resource17 in consultation with clinical experts (supplemental material). We abstracted estimates of prevalence or relative risk, with confidence intervals where provided. For the adherence question we limited data abstraction to adults age 50+ to reflect current screening recommendations. We did not conduct meta-analyses due the heterogeneity of outcomes measures and family history definitions. We critically appraised the best evidence set for threats to internal validity from selection; attrition, detection, and reporting bias, adapting criteria from previously published approaches.15,18
RESULTS
From 3271 abstracts, 437 articles met inclusion criteria. Of those, 224 were excluded after review of the full text for risk, prevalence, and natural history; 96 were excluded for adherence. After applying best evidence criteria our dataset included 30 unique articles: 8 addressed prevalence, 9 addressed risk, 9 addressed natural history, and 11 adherence (Figure 1).
Figure 1.
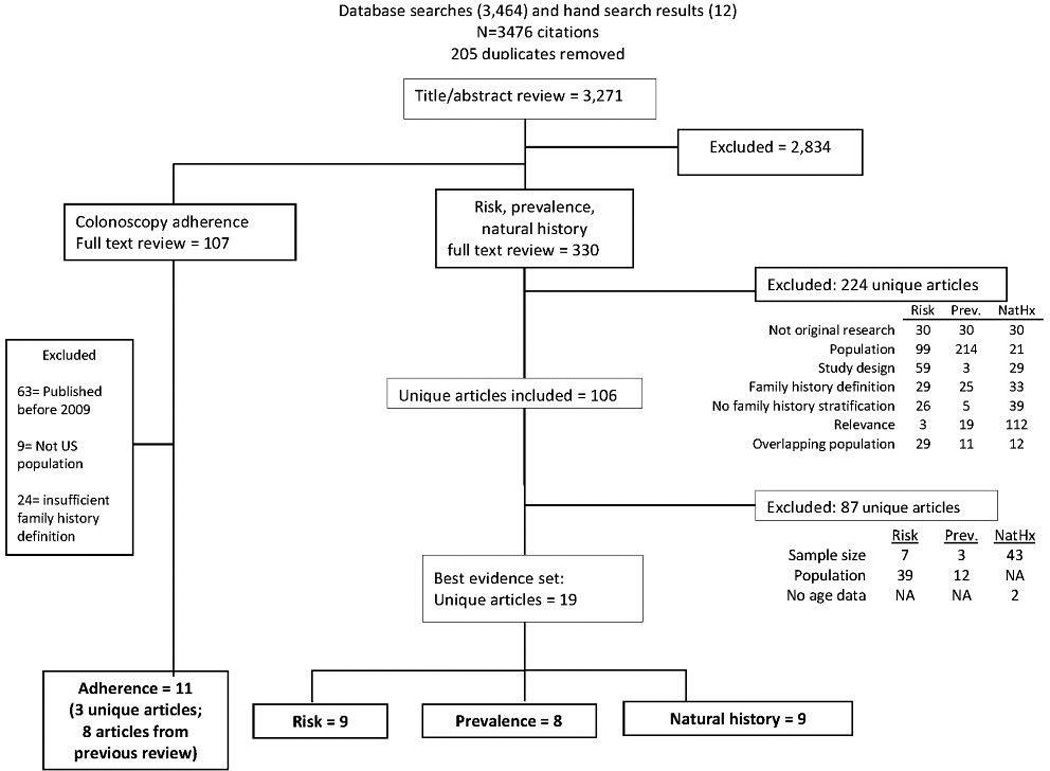
Article flow diagram
Prevalence
Summary: The prevalence of having at least one first degree relative (FDR) was estimated between 3.1% and 10%. The prevalence of having at least two FDRs was much lower (0.3% to 0.34%). Having a FDR with an early age at diagnosis (before age 45 or 50 years) was uncommon, around 0.3%, compared with having a FDR with a later diagnosis (above age 60 or 65 years), which was over 3%. Few data were available on racial and ethnic-specific prevalence of family history.
Study details
Eight studies met our best evidence criteria:2,19–25 six were from the US, one from the UK23 and one from Japan.21 All had approximately equal representation of males and females except two studies that included women only24,25 The average age in the study populations was between 50 and 60 years except one,20 which had average age of 39.3 years. All studies but one19 ascertained family history information from unverified self-report (Table 1).
Table 1.
Prevalence of family history
| Author, year | Data Source | N | Mean age (range) |
% Female | Family history categoriesf | Prevalence (%) |
|---|---|---|---|---|---|---|
| Taylor 201017 | Utah Population Database |
2 327 327 | NR (NR) | NR | 1 FDR with CRC | 3.9 |
| 1+ FDR | 4.1 | |||||
| 1+ FDR dx age: <50, <60, ≥ 60 | 0.27, 0.78, 3.38 | |||||
| 2 FDR | 0.31 | |||||
| 2+ FDR | 0.34 | |||||
| 1+ SDR dx age: <50, <60, ≥ 60 | 0.84, 2.51, 10.5 | |||||
| Scheuner 201018 | 2005 CHIS | 33 187 | 39.3 | 50% (weighted) |
EITHER no family history *or* 1 SDR dx age ≥50 with CRC or endometrial cancer *or* 1 FDR with endometrial cancer |
95.7 |
| EITHER 1 FDR *or* 2 SDRs dx age > 50 *or* 1 SDR dx age <50 with CRC and 1+ SDR dx age >50 with endometrial cancer |
4.2 | |||||
| EITHER 1+ FDR dx age <50 *or* 2+ ADR *or* hereditary syndrome |
1.1 | |||||
| Kondo 200319 | JACC | 50 864 | 57.7 | 57% | 1+ parent with CRC | 1.7 |
| Pinsky 200320 | PLCO | 149 332 | NR (55–74) | 51% | 1 FDR | 9.4b |
| 2+ FDR | 0.7 | |||||
| Sandhu 200165 | EPIC- Norfolk (UK) |
30 353c |
59.1 | 55% | 1+ FDR with CRC | 6.8d |
| 2+ FDR | 0.3d | |||||
| EITHER 2 FDR *or* 1 FDR dx age <45 | 0.6 | |||||
| 1+ FDR dx age <45, 45–64, ≥ 65 | 0.3, 2.0, 3.8d | |||||
| Poole 199922 | CPS – 1 | 429 483 | 51 | 100% | 1+ FDR with CRC | 3.7 |
| Nelson 199323 | Iowa Women's Health Study |
40 657 | NR (55–69) | 100% | 1+ mother/daughter with CC | 3.1 |
| Fuchs 19942 | NHS | 87 031 | 49.1 | 100% | 1+ FDR with CRC | 9.4e |
| Fuchs 19942 | HPFS | 32 085 | 51.5 | 0% | 1+ FDR with CRC | 10e |
Only individuals without a personal history of cancer (breast, ovarian, ednometrial, prostate, or CRC) (NOTE are weighted %s)
Reported: expected ratio by gender male 0.60 (0.57, 0.62 95% CI) female 0.76 (0.73, 0.79 95% CI).
non-cases only (n=28155)
Adjusted for sex
Values are means directly standardized according to the age distribution of the respective cohort in its entirety.
Abbreviations: FDR (first degree relative) SDR (second degree relative) ADR (any degree relative) CRC (colorectal cancer), CHIS (California Health Interview Survey), JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk), PLCO (Prostate, Lung, Colorectal, and Ovarian cancer screening trial), CPS (Cancer Prevention Study), EPIC (European Prospective Investigation into Cancer), NHS (Nurses Health Study), HPFS (Health Professional Followup Study)
The most common definition of family history was one or more first degree relatives (1+ FDR). Five studies estimated the prevalence of having 1+ FDR, with estimates ranging between 3.1% and 10%.2,19,23–25 The only study with objective measures of both family history and CRC diagnosis estimated positive family history prevalence of 4.1%.19 The lowest estimate was from a study that evaluated only mothers and daughters and likely underestimates the prevalence of a positive family history. Two studies provided estimates for the prevalence of having exactly one FDR, which were 3.9% and 9.4%.19,22 One study provided an estimate for having at least one affected parent (1.7%).21 The prevalence of having at least two affected FDRs was 0.3% to 0.34%.19,23
Scheuner et al20 estimated the prevalence of multiple family history configurations grouped together in risk groups. The “moderate risk” group, defined as either 1 FDR with late onset cancer or 2 SDRs from the same lineage with late onset cancer or one SDR with early onset cancer and other SDRs with associated cancers had a prevalence of 4.2%. Two studies considered the relative’s age at diagnosis as part of the definition of family history.19,23 As expected, having a FDR diagnosed below age 45 (prevalence 0.36%23) or 50 (prevalence 0.27%19) was much less common than having a FDR diagnosed above age 60 (3.4%19 or 65 (4.1%23).
Two studies considered the effect of demographic characteristics. Both found higher prevalence of family history in females compared to males (6.1% of men and 7.4% of women with at least 1 FDR23 and 4.5% of men compared to 6.2% of women in the author-defined “strong” or “moderate” risk groups.20 “Moderate” or “strong” family history was also higher among whites than other racial or ethnic groups in the same study (7.3% white, 2.5% Latino, 4.1% Asian, 6.1% AA, 4.4% other)20.
Risk
Summary: The relative risk of developing CRC varied from 0.89 (for people with no family history) to nearly a 20-fold risk (for people with likely inherited syndromes), with risk levels in between, with increasing family history burden. Risk of CRC was higher when the relative was diagnosed at an earlier age. CRC risk also depended on the age of the person at risk: people with positive family history in their 30s or 40s demonstrated a higher relative risk compared to their age-matched peers than people with the same positive family history at an older age.
Nine studies of unique populations ranging in size from 30,353 to 7 million individuals were included.2,19–21,23–27 Five studies were conducted in the US,2,19,20,24,25 four others were from Britain,23 Sweden,27 Japan,21 and China.26 Most studies considered the risk of developing colorectal cancer in those with family histories of CRC compared to those with no family history. One very large study considered standardized incidence ratios for colorectal cancer people with family history compared with the general population.19 Summarized results for risk are shown in Table 2; detailed tables are in the supplementary materials.
Table 2.
Risk of CRC associated with family history of CRC
| Author, Year | Study population | N | Age | % female | Outcome | Lowest risk reported [family history level] |
Highest risk reported) [family history level] |
|---|---|---|---|---|---|---|---|
|
Taylor 201017 |
Utah Population Database |
2 327 327 | NR | NR | Incident CRC (SMR) |
0.89 (0.87–0.91) [0 FDR with CRC] |
19.86 (7.29–43.24) [5+ FDR with CRC] |
|
Scheuner, 201018 |
California Health Interview Survey 2005 |
33 187 | mean 39.3 (18–64) |
50% (weighted) |
Incident CRC (OR)b |
2.6 (1.2–5.7) [EITHER 1 FDR *or* 2 SDRs dx age <50 *or* 1 SDR dx age <50] |
5.2 (1.7–15.8) [EITHER 1+ FDR dx age <50 *or* 2+ ADR *or* hereditary syndrome] |
|
Murphy, 200924 |
Shanghai Women’s Health Study |
73 358 | median 50 (IQR 44–60) |
100% | Incident CRC (HR)c |
0.80 (0.20–3.29) [1+ sibling with CRC] |
3.37 (1.59–7.12 [1 parent with CRC] |
| Leu 200825 | Swedish Family Cancer Database |
~7m | NR | NR | Incident CRC (RR)d |
1.81 (1.57–2.08) [1+ parent dx age ≥60] |
3.33 (2.14–4.53) [1+ parent dx age <60] |
|
Kondo 200319 |
Japan Collaborative Cohort Study (JACC) |
50 864 | mean 57.7 ±10.1 |
58.5% | Incident CRC (OR)e |
3.9 (1.7–8.4) [Second eldest] |
6.3 (3.5–11.2) [Third eldest] |
| Poole 199922 | Cancer Prevention Study (CPS)-1 |
429 483 | mean 51 | 100% | CRC Mortality (OR)f |
1.38 (0.67–2.83) [1+ FDR dx age ≥65] |
4.89 (1.07–20.24) [1+ FDR dx <45] |
| Fuchs 19942 | Nurses Health Study (NHS); Health Professionals Followup Study (HPFS) |
119 116 | Mean 49.1 (NHS); mean 51.5 (HPFS) |
100% (NHS) 0% (HPFS) |
Incident CRC (RR)g |
1.64 (1.26–2.14) [1 FDR] |
2.83 (1.33–6.02) [2+ FDR] |
|
Nelson 199323 |
Iowa Women’s Health Study |
40 657 | Range 55–69 | 100% | Incident CRC (RR)h |
- | 1.29 (0.90, 1.85) [1+ mother/daughter] |
|
Sandhu 200165 |
European Prospective Investigation into Cancer (EPIC)-Norfolk |
30 353 | Males: mean 59.4 Females: mean 58.9 |
55% | Prevalent CRC (age- adjusted) |
1.38 (0.67–2.83) [1+ FDR dx age ≥65] |
4.89 (1.07–20.24) [1+ FDR dx <45] |
Abbreviations: FDR (first degree relative) SDR (second degree relative) ADR (any degree relative) CRC (colorectal cancer)
The Utah Population Database (UPDB) is a state-wide population-based resource of genealogies of the original Utah pioneers and their modern-day descendants. This analysis used Utah Cancer Registry data (from 1952) linked to the UPDB in a subset of 2.3 million persons who were part of 3 generations of Utah genealogy data and descendants of original Utah pioneers.
Analyses from the UPDB using detailed family pedigrees found distributions of standardized incidence ratios for CRC from 0.89 (95% CI 0.87–0.91) for those with no first degree relatives with CRC to 19.86 (95% CI 9.29–43.24) for those with five or more first degree relatives, who likely have inherited syndromes.19 Significant differences in CRC incidence were present between those with confirmed negative family history (0.89), one first degree relative date of diagnosis unknown or after age 60 (1.91–1.99), one first degree relative diagnosed before age 60 (2.69), and 3 or 4 first degree relatives (4.41). The Nurses’ Health Study (NHS) found increased risk for CRC from 1+FDR to 2+ FDR.2 In the UPDB having a FDR diagnosed before age 60 years increased personal CRC risk. Other US data were consistent with an earlier diagnosis of CRC in a first degree relative conferring higher personal CRC risk, but were not consistently defined (i.e., used different ages to stratify earlier diagnosis) to contribute to precise risk estimates by age of relative’s CRC diagnosis.2,24
In the Swedish Family Cancer database, data from 7 million people representing over 2 million families was linked with the Swedish Cancer registry.27 The individuals represented offspring born after 1934, who had at least two siblings, and their parents. Having a parent with CRC was associated with a doubling of risk, and a tripling of risk if the parent was diagnosed younger than age 60.27 One Chinese study looked at risk associated with affected siblings versus parents and did not suggest greater impact of sibling CRC over parental CRC.26
Three studies provided relative risks stratified by the age of the person at risk. In the Swedish database, the presence of a parental history of CRC more than quadrupled CRC risk in adults aged 30–39 years, compared to others the same age with no family history.27 Within each age decile of the person at risk, parental cancer history was associated with increased relative risks compared to people without an affected parent of the same age. Relative risks remained elevated until at least age 70. Data from the Health Professionals Followup Study and Nurses’ Health Study populations was generally consistent.2 British data on prevalent, rather than incident cancer, suggest decreasing risk with increasing age of the person at risk but confidence intervals were wide; also this study population did not include people under 50 where the risk may be most exaggerated..23 Overall, the preponderance of data suggests that history of one or more FDR with CRC is associated with a smaller and diminishing incremental increase in relative risk in those aged 50 years and older, as the prevalence of family history in the population increases. Thus, the relative impact of family history on preventable cancers in those under ages 50–55 years will be much greater than in older adults (see supplemental tables).
Critical appraisal concerns: risk and prevalence studies
All but one22 study controlled for age of the person at risk, and five controlled for the sex of the person at risk.2,19,22,23,25 Only one study21 adjusted for family size, and no studies reported if or how relatedness of the study participants was addressed. Only two studies used verified family histories, both with registry-based outcomes.19,27 All the others used self-report. Studies of non-US populations may have limited generalizability to US populations.
Natural history
Adenoma summary: There are very few data with which to make a strong conclusion about adenoma and family history. Data from two studies suggest a higher prevalence of adenomas in people with a positive family history of CRC compared with people with no family history. There was a higher prevalence of two or more adenomas in people with a positive family history of CRC compared with people with no family history. No evidence suggested differential adenoma location by family history status. There is no evidence to suggest a differential prevalence of advanced adenoma in people with a family history of CRC.
Cancer summary: Very limited data were available on family history, natural history and CRC. There was no evidence for difference in age at CRC diagnosis or stage at diagnosis by family history status. There was mixed evidence on tumor location depending on family history status: Some evidence suggested that those with a positive family history are more likely to have distal tumors, whereas others reported no difference.
We identified 9 unique studies: 3 with relevant adenoma outcomes28–30, and six with relevant CRC outcomes.31–36
Adenoma study details
We identified three studies on a total of 35,590 people with family history-specific data on adenoma prevalence (two studies), advanced adenomas (three studies), multiple adenomas (three studies), or adenoma location (one study).28–30 In a study of 27,650 men enrolled in the Health Professionals Follow-up Study (HPFS) from 1986–2004,28 adenoma prevalence was increased in people with exactly one 1 FDR (15.4%) or 2+ FDRs (19.1%) compared to people with no family history (10.0%) (Table 3). Adenomas were more common in people with family history at all age groups particularly at younger ages based on slopes of weighted regression lines;, raw data not provided). A German population-based cross-sectional study of colonoscopies of 3,320 people at average risk found a similar trend, finding higher adenoma prevalence than HPFS for those with no affected FDRs (30% vs 10%) and 1+ FDR (40% vs 15%).29
Table3.
Natural history: ADENOMA
| Wark, 200926 | Hoffmeister, 201027 | ||||
|---|---|---|---|---|---|
| Population | HPFS: 1986–2004 | 33 GE clinics in Germany | |||
| % Female | 0% | 38% | |||
| Prevalence | Odds Ratios | Prevalence | Prevalence Ratios | ||
| N Screened | 0 FDR with CRC | 23 880 | 2 929 | ||
| 1 FDR | 3 445 | - | |||
| 1+ FDR | 3 770 | 391 | |||
| 2+ FDR | 325 | - | |||
| N (%) with any adenomaa |
0 FDR | 2 378 (10.0) | Refb,c | 892 (30.5) | Refb, c |
| 1 FDR | 532 (15.4) | 1.75 (1.60–1.92) | - | - | |
| 1+ FDR | 594 (15.8) | 1.75 (1.60–1.91) | 157 (40.2) | 1.17 (0.95–1.44) | |
| 2+ FDR | 62 (19.1) | 2.36 (1.84–3.04) | - | - | |
| Women | 0 FDR | - | 340 (23.1) | Refb, c | |
| 1 FDR | - | 58 (30.7) | 1.42 (1.02–1.97) | ||
| Men | 0 FDR | - | 552 (37.8) | Refb, c | |
| 1 FDR | - | 99 (49.0) | 1.42 (1.02–1.97) | ||
| N (%) with adv. adenomad |
0 FDR | 1 184 (5.0) | Refb, e | 319 (10.9) | Refb, e |
| 1 FDR | 264 (7.7) | 0.97 (0.80–1.17) | - | - | |
| 1+ FDR | 297 (7.9) | - | 63 (16.1) | 1.33 (1.00–1.76) | |
| 2+ FDR | 33 (10.2) | 1.08 (0.65–1.79) | - | - | |
| Women | 0 FDR | - | 122 (8.3) | Refb, e | |
| 1 FDR | - | 21 (11.1) | 0.96 (0.55–1.67) | ||
| Men | 0 FDR | - | 197 (13.5) | 1.46 (1.06–2.02) | |
| 1 FDR | - | 42 (20.8) | 2.22 (1.47–3.33) | ||
| N (%) with 2+ distal adenomas |
0 FDR | 470 (1.9) | Refb,f | - | |
| 1 FDR | 124 (3.4) | 1.26 (1.02–1.63) | - | ||
| 1+ FDR | 142 (3.6) | - | - | ||
| 2+ FDR | 18 (5.2) | 1.76 (0.98–3.15) | - | ||
| N (%) with 2+ proximal adenomas |
0 FDR | 363 (1.5) | - | ||
| 1 FDR | - | - | |||
| 1+ FDR | 137 (3.5) | - | |||
| 2+ FDR | - | - |
Wark: Non-advanced and advanced adenomas; Hoffmeister: Non-advanced and advanced adenomas and CRC
Wark: adjusted age, previous endoscopy, indication, aspirin, multivitamin, smoking, red meat, alcohol, folate, calcium, BMI, physical activity, total energy intake ; Hoffmeister: age at colonoscopy, gender, education, HRT use, BMI, physical activity, alcohol, red meat consumption
Wark: Odds ratio: adenoma vs. no adenoma within each family history level, Hoffmeister: Prevalence ratio between family history levels
Wark: Size ≥10mm *or* tubulo/villous structure *or* carcinoma in situ; Hoffmeister: Size ≥10mm *or* tubulo/villous structure *or* severe dysplasia *or* CRC
Wark: Odds ratio: advanced adenoma vs. non-advanced adenoma within each family history level, Hoffmeister: Prevalence ratio between family history levels
Wark: Rates of multiple vs. single adenoma
The HPFS study suggested that the odds of advanced adenoma associated with family history was similar to that of any adenoma.28 In the German study advanced adenoma was more prevalent in men than women regardless of family history (Table 3).29 The German study included CRC in an “advanced neoplasia” category (3.9% of total neoplasia, data not shown).
The HPFS analysis provided data on 2+ adenomas by family history and was limited to distal location.28 It suggested an increasing prevalence of 2+ adenomas with increasing family history. The adjusted odds of multiple adenomas remained when compared to either single adenomas or no adenomas.
A screening study of Veterans Affairs patients (n=3121, 96.8% male; age 50–75), did not describe the family history of the entire study population but reported a higher rate of family history (1+ parent or sibling) in people with two or more adenomas (19.3%) compared to people with one adenoma (12.9%) or no polyps (12.2%) (OR 1.73, 95% CI 1.32–2.26); similarly for advanced adenoma (18.4% with advanced adenoma vs 15.8% with any adenoma (OR for advanced adenoma vs no polyp group: 1.62 (95% CI 1.16–2.26); OR for any adenoma vs no polyp group: 1.36 (1.09–1.70;).30 Only the German study reported adenoma location by family history (proximal/distal and colon/rectum) and found no significant difference in the distribution of location according to family history.28
Critical appraisal: adenoma studies
The study populations of two of the three studies were almost exclusively male, limiting their generalizability to women28,30 but otherwise included populations of screening-relevant age. Three studies assessed natural history outcomes via study colonoscopy;29,30 one used self-report but verified positive findings with medical records.28 All studies included adequate followup time to detect the findings of interest, either by cross sectional analysis of colonoscopy findings or longitudinal design. Three studies relied on self-report of family history;28,30 one with verification of self-report via medical records or death certificates.29
Cancer studies
Four studies on 4,537 people reported data on age at diagnosis by family history of CRC.31,32,34,35 A study of 3383 surgical CRC patients in Taiwan found no difference in age at detection in those with 0 or 1 FDR, but a lower age at detection for those with 2+ FDRs. However, this group included Lynch patients so may reflect differential surveillance. An analysis of 1,001 women enrolled in the Nurses’ Health Study found no difference in age at diagnosis according to family history.34
Two studies reported age of diagnosis by family history. A randomized trial for adjuvant therapy for stage III colon cancer in people within 56 days of surgery for a primary tumor did not find any baseline difference in age at trial enrollment by family history.35 A US registry-based analysis of incident cancer between 1994–1996 did not find any difference between age at diagnosis for colon cancer by family history, but younger age at diagnosis for rectal cancer in people with no family history.31
Four studies reported data by family history on location of CRC at diagnosis. Registry-based studies in Sweden and Japan suggested the majority of cancers in people with a family history were distally located.33,36 However, two other studies found a more even distribution of distally and proximally located cancers.32,34 In three studies providing data on stage at diagnosis by family history, there was no evidence of differential distributions of stage in people with a family history of CRC compared to those without.31–33
Critical appraisal concerns: cancer studies
The natural history of CRC is difficult to assess because of the known effectiveness of treatment for detected CRC. Age at diagnosis was obtained at study enrollment for two studies32,35 and from registry data for one;33,37 one study collected self-report data but verified positive results with medical records.34 Family history was assessed by interview,31,32 self-report,34,35 or from registry records.33,37 No studies reported measures of relatedness or family size.
Adherence
Summary: Individuals with a positive family history are 1.4 to 3.3 times more likely to be adherent to colorectal cancer screening recommendations than individuals with no family history. One study, which objectively measured both family history and screening behavior suggested a 7%–8% absolute increase in screening adherence in people with positive family history.
Study details
We assessed three studies published since 2009, plus 8 studies from the systematic review used as primary evidence15 for a total of 11 studies. Ten were cross-sectional studies, together representing 9 independent samples and 129,942 people age 50 years and older (Table 4). All studies but one38 used a relatively non-specific family history definition of 1+ FDR with CRC.38 Only the three newer studies included age of affected relative’s diagnosis (<age 50) as a separate analysis.38–40 Three analyzed colonoscopy adherence alone;39–41 all others considered adherence to recommended CRC screening modalities (colonoscopy, sigmoidoscopy, and/or FOBT). Eight studies published adherence rates;38–40,42–46 three published relative risks of adherence for those with family history compared with no family history.
Table 4.
Screening adherence by family history
| Author | Population, Setting | Screen-relevant sample size |
Primary outcome of interest for review |
Family history categories | Adherence % (95% CI) |
OR (95% CI) |
|---|---|---|---|---|---|---|
|
Townsend 201336 |
California Health Interview Survey 2005 |
Age 50–64 N= 10 310 (7.5% have positive family history) |
FOBT in past year, FS in past 5 years, or colonoscopy in past 10 years |
No family history of CRC Either 1 FDR or 2+ SDR Either 1 FDR or 2+ SDR dx age <50 |
44.5 (NR) (SE= 1.0) 71.5 (NR) (SE= 2.2) 65.2 (NR) (SE= 5.0) |
Ref 2.77 (2.20–3.49)a NR |
|
Zlot 201238 |
Oregon BRFSS 2008 |
Age 50+ N= 1 163 (10.2% have positive family history) |
Colonoscopy ever | No family history of CRC 1+ FDR 1+ FDR dx age <50 |
53.7 (50.3–57.1) 76.7 (67.8–83.7) 83.3 (61.4–94.0) |
refb 2.5 (1.5–4.2) 3.3 (1.0–10.7) |
| FOBT in past year *or* sigmoidoscopy in past 5 years *or* colonoscopy in past 10 years |
No family history of CRC 1+ FDR 1+ FDR dx age <50 |
63.2 (59.7–66.5) 83.3 (75.1–89.0) 87.7 (66.7–96.2) |
ref 2.2 (1.3–3.9) 2.4 (0.7–8.8) |
|||
|
Taylor 201137 |
Intermountain Healthcare/UPDB 2004–2009 |
Age 50–90 N= 71 446 (10.2% have positive family history) |
Colonoscopy in past 10 years. | No family history of CRC 1+ FDR with CRC 1+ FDR dx age <50 |
38.6 (NR) 45.6 (NR) 48.9 (NR) |
refc 1.40 (1.33–1.47) 1.53 (1.31–1.79) |
Adjusted for age, race-ethnicity, household income, health insurance, usual health-care provide, education
Adjusted for education and smoking status
Adjusted for age, sex
Adjusted for age, race, education, income, overall health score, Charlson comorbidity index score, substance abuse, psychiatric diagnosis, dual diagnosis, cognitive factors, social influence, marital status, MOS support scores, physician recommendations, access to VA services, facility complex
An analysis of the 2005 California Health Interview Survey included 10,310 adults aged 50–64, 7.5% of whom had a self-reported family history of CRC,38 those with a family history of 1+ FDR or 2+ SDRs were more than twice as likely to be adherent with CRC screening recommendations (OR 2.77, 95% CI 2.20–3.49). Absolute adherence was increased by more than 25%. In an analysis of 2008 Oregon Behavioral Risk Factor Surveillance System data, adherence was more than twice as likely in those with 1+FDR, regardless of screening criteria used, although estimates were less precise likely due to sample size.40 Absolute adherence was at least 20% greater in those with a self-reported family history, and somewhat higher when not requiring colonoscopy.
In an analysis of the Utah Population Database 2004–2009,39 cancer history was confirmed via the Utah Cancer Registry and family relationships were established using comprehensive statewide genealogy data. Adherence to colonoscopy in the previous ten years was increased in those with 1+FDR, but with somewhat attenuated relative (OR 1.40, 95% CI 1.33 to 1.47) and absolute effects (7% higher adherence) compared to the two other studies. This study is the only one to use systematic, objective approaches to assess family history and colonoscopy rather than self-reported data, which could explain the more modest effect size.
The eight older studies consistently found around a doubling of the odds of reporting compliance with recommended CRC screening, regardless of the definitions of recommended CRC screening or population, corresponding to adherence rates of 26%–50% for people with no family history compared with 44%–68% for people with 1+ FDR. Precision varied with sample size, and data to evaluate screening adherence with colonoscopy versus other modalities were limited and mixed (supplemental materials).
Critical appraisal concerns: adherence studies
Ten of eleven studies gathered both exposure and outcome data via self-report and only one used registries and medical records to verify family history and screening use.39 Survey-based studies found varying response rates, from quite low response rate of 29%38 to a high of 79%.44 Four studies did not report the response rate so response bias could not be assessed.40,45,47,48
DISCUSSION
We conducted a systematic review to identify the most current, highest quality evidence of the prevalence of family history of colorectal cancer in the US population, as well as its influence on CRC risk, natural history, and screening adherence. These data provide important insights to clinicians and researchers, and support further modeling of effective and efficient evidence-based screening recommendations for those at more precisely increased risk of CRC due to more completely defined family histories. Our study suggests that objective measures of prevalence of family history may be lower than commonly cited; that the increased risk associated with family history is significant and may be associated with adenoma number rather than by faster adenoma progression. People with family history were more likely to adhere to screening than those without. Taken together, these data reinforce the need for optimal screening strategies for people with family history of colorectal cancer.
Prevalence
Our findings suggest that the prevalence of family history may be lower than the commonly discussed estimate of 10%, which is likely based on self-reported data. This may reflect our emphasis on studies that focused on population samples rather than screening studies, to minimize bias due to selective volunteering of patients and families with positive family histories. Though the prevalence of second degree relatives was higher than that of first degree relatives,19 family history considering various family patterns involving second or third degree relatives was never associated with more than a doubling of CRC risk. This suggests that for clinical purposes, determining the history of cancer in all first degree relatives may be both sufficient and the most feasible. Earlier age of FDR diagnosis with CRC was relatively consistently associated with further increased risk, but current data made the degree of additional risk difficult to quantify. Only one study reported prevalence of family history by race but suggested prevalence at odds with actual increased CRC prevalence in African Americans.20 If these data are correct, family history may be underreported, less-well known, or not a primary influence in the excess CRC burden in African Americans. One study suggested higher prevalence of family history in females, suggesting gender differences in reporting consistent with other studies.18,49
Risk
Our review of population-based risk estimates suggested a range of risk from 0.89 (for individuals with no family history) to nearly a 20-fold risk for individuals with likely inherited syndromes, with risk levels in-between depending on the family history configuration. This is consistent with those in other reviews50,51 suggesting increasing risk of CRC with increasing burden of family history. Our review has the added benefit of using only population-based study populations and adds results from a high quality study using a comprehensive US-based population.19 This study used the entire population as the comparator in risk estimates, allowing subpopulation estimates that ranged from a modestly reduced risk to an exaggerated risk for the very few with five or more first degree relatives. Importantly, relative risks for CRC varied between 1.9 and 4.4 in those with at least one FDR, depending on the number of relatives and age of diagnosis. It also included relative risk estimates stratified by age of the person at risk. This study represents a population with potentially lower behavioral risk factors, such as smoking and alcohol use, than the general population. Concern is frequently raised about how genetically representative this population is, but the UPDB is genetically representative of US Caucasian and northern European populations with a low level of inbreeding.52–54 This study is of high quality and its methods should be replicated wherever possible using other data sources.
Evidence for adenoma and/or CRC risk in people with a family history of adenoma (rather than CRC) is limited; no studies of this type met our best evidence criteria. One review found only two studies, both with methodologic flaws.55 A paper published after our search ended suggests increased risk of both CRC and adenoma in people with family history of adenoma.56
Natural history
Our best evidence suggests a higher prevalence of adenomas in people with family history of CRC in men, consistent with previous work57 but does not suggest that adenoma progression (measured by advanced adenoma) is accelerated in people with family history. Small studies have suggested that adenoma growth may be accelerated in people with a family history of CRC.58,59 Future, larger studies could provide additional insight on the impact of family history on adenoma progression. Evolving temporal trends in adenoma classification, such as the sessile serrated pathway, made it challenging to pool data from multiple studies.
Two studies suggested a trend toward increased multiple adenomas in people with a family history of CRC. Our best evidence did not suggest an association between adenoma location and family history. For colorectal cancer, the data were even more limited, but suggested no difference in the age or stage at diagnosis by family history status. There was very limited, conflicting evidence on tumor location and family history. Very limited data suggest that CRC progression in people with family history of CRC is similar to that in the general population.
Adherence
Our review confirms the findings of a previous review suggesting a clear association between family history of CRC and adherence with both colonoscopy and other CRC screening modalities.15 Increased adherence likely reflects both organizational (physician recommendation, access) and patient-level factors such as risk perception, but it is worth noting that adherence remains low in all groups. Only one study provided data using objectively measured family history and screening; these data may provide the best estimates for adherence for those with a positive family history but are limited to a single geographic location with a somewhat different social environment.
Our best evidence approach allowed us to summarize an extensive, heterogeneous body of research, but may have resulted in our excluding some relevant data. To our knowledge this is the first review to limit estimates of prevalence of family history and CRC risk to population-based studies, which may be the least biased for informing modeling of population-based screening.
We were also limited by the heterogeneous nature of family history reporting in the literature. There is often analytical rationale for collapsing family history strata to increase sample size, but this makes it difficult to pool data across studies. We excluded 41 studies based on the lack of a basic definition that included number and degree of relatives affected and a further 43 for not providing data stratified by family history. In the remaining studies, more than half used our minimum family history definition of “at least one FDR.” Few studies reported age at diagnosis of the affected relative, verified the accuracy of self-reported data or used objective measures, or assessed or adjusted for family size.
We recommend that future research define family history as, at minimum, the number and degree of affected relatives, and that raw data be reported to maximize potential for aggregating study data. Family history studies should also report how history was assessed, whether and how it was verified, and family size. Measures of how often family history changes in ways that materially impact an individual’s CRC risk as they age would help reduce the uncertainty associated with family history at younger ages and the frequency with which family history for CRC should be updated. High-quality studies of family history and CRC in populations of non-European ancestry are also needed.
Family history is an imperfect and dynamic measure. As family sizes decrease over time, fewer relatives are available to define risk. Also, as endoscopic screening with curative intervention increases, family history can be hidden in families if affected persons are more likely to communicate a diagnosis of advanced cancer to their relatives than a polyp removed during endoscopy. However, family history of CRC remains a clinically meaningful way to identify individuals at increased risk of CRC, and may be the most feasible approach at present, given that multifactorial risk assessment tools are not yet validated for clinical practice60–65 and the utility of genetic risk stratification is still being investigated.66–68 In future, risk based on polygenic and/or multifactorial risk assessment may augment family history-based approaches.69 Family history collection and reporting should continue in ways that are conducive to knowledge development.
Supplementary Material
Acknowledgments
This study was supported by CDC grant 5U18GD000076-02.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) [Accessed January 7, 2014]; http://seer.cancer.gov/csr/1975_2009_pops09/
- 2.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. New England Journal of Medicine. 1994 Dec 22;331(25):1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA: A Cancer Journal for Clinicians. 2001 Jan-Feb;51(1):38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Levin B, Rozen P, Young G. How should we follow up colorectal premalignant conditions? In: Rozen P, Young G, Levin B, Spann SJ, editors. Colorectal Cancer in Clinical Practice: Prevention, Early Detection, and Management. London, UK: Martin Dunitz; 2002. pp. 67–76. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use--United States, 2012. Morbidity and Mortality Weekly Report (MMWR) 2013 Nov 8;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph DA, DeGroff AS, Hayes NS, Wong FL, Plescia M. The Colorectal Cancer Control Program: partnering to increase population level screening. Gastrointestinal endoscopy. 2011 Mar;73(3):429–434. doi: 10.1016/j.gie.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Levin B, Lieberman DA, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline From the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Colorectal Cancer. [Accessed January 7, 2014];2014 http://www.cancer.org/cancer/colonandrectumcancer/index. [Google Scholar]
- 9.American Gastroenterological Association. American Gastroenterological Association Home Page. [Accessed January 7, 2014];2014 [Google Scholar]
- 10.Canadian Task Force on Preventive Health Care. Guidelines. [Accessed January 7, 2014];2014 http://canadiantaskforce.ca/guidelines/ [Google Scholar]
- 11.U.S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. [Accessed January 7, 2014];2008 AHRQ Publication 08-05124-EF-3. http://www.uspreventiveservicestaskforce.org/uspstf08/colocancer/colors.htm. [Google Scholar]
- 12.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] The American Journal of Gastroenterology. 2009 Mar;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Intervention and Surveillance Modeling Network. Colorectal Cancer Model Profiles. [Accessed January 7, 2014];2014 http://cisnet.cancer.gov/colorectal/profiles.html.
- 14.Treadwell JR, Singh S, Talati R, McPheeters ML, Reston JT. A framework for best evidence approaches can improve the transparency of systematic reviews. Journal of Clinical Epidemiology. 2012 Nov;65(11):1159–1162. doi: 10.1016/j.jclinepi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Holden DJ, Harris R, Porterfield DS, et al. Enhancing the use and quality of colorectal cancer screening. Evidence Report/Technology Assessment. 2010 Feb;2010(190):1–195. [PMC free article] [PubMed] [Google Scholar]
- 16.Whitlock EP, Lin JS, Chou R, Shekelle P, Robinson KA. Using existing systematic reviews in complex systematic reviews. Annals of Internal Medicine. 2008 May 20;148(10):776–782. doi: 10.7326/0003-4819-148-10-200805200-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000 Jun 15;88(12):2887. doi: 10.1002/1097-0142(20000615)88:12<2887::aid-cncr32>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi N, Carroll JC, Wilson B, et al. The current state of cancer family history collection tools in primary care: a systematic review. Genetics in Medicine. 2009 Jul;11(7):495–506. doi: 10.1097/GIM.0b013e3181a7e8e0. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DP, Burt RW, Williams MS, Haug PJ, Cannon-Albright LA. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010 Mar;138(3):877–885. doi: 10.1053/j.gastro.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genetics in Medicine. 2010 Nov;12(11):726–735. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Toyoshima H, Tsuzuki Y, et al. Aggregation of stomach cancer history in parents and offspring in comparison with other sites. International Journal of Epidemiology. 2003 Aug;32(4):579–583. doi: 10.1093/ije/dyg152. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky PF, Kramer BS, Reding D, Buys S, Team PP. Reported family history of cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. American Journal of Epidemiology. 2003 May 1;157(9):792–799. doi: 10.1093/aje/kwg043. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu MS, Luben R, Khaw KT. Prevalence and family history of colorectal cancer: implications for screening. Journal of Medical Screening. 2001;8(2):69–72. doi: 10.1136/jms.8.2.69. [DOI] [PubMed] [Google Scholar]
- 24.Poole CA, Byers T, Calle EE, Bondy J, Fain P, Rodriguez C. Influence of a family history of cancer within and across multiple sites on patterns of cancer mortality risk for women. American Journal of Epidemiology. 1999 Mar 1;149(5):454–462. doi: 10.1093/oxfordjournals.aje.a009833. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CL, Sellers TA, Rich SS, Potter JD, McGovern PG, Kushi LH. Familial clustering of colon, breast, uterine, and ovarian cancers as assessed by family history. Genetic Epidemiology. 1993;10(4):235–244. doi: 10.1002/gepi.1370100404. [DOI] [PubMed] [Google Scholar]
- 26.Murphy G, Shu X-O, Gao Y-T, et al. Family cancer history affecting risk of colorectal cancer in a prospective cohort of Chinese women. Cancer Causes & Control. 2009 Oct;20(8):1517–1521. doi: 10.1007/s10552-009-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leu M, Reilly M, Czene K. Evaluation of bias in familial risk estimates: a study of common cancers using Swedish population-based registers. Journal of the National Cancer Institute. 2008 Sep 17;100(18):1318–1325. doi: 10.1093/jnci/djn290. [DOI] [PubMed] [Google Scholar]
- 28.Wark PA, Wu K, van 't Veer P, Fuchs CF, Giovannucci EL. Family history of colorectal cancer: a determinant of advanced adenoma stage or adenoma multiplicity? International Journal of Cancer. 2009 Jul 15;125(2):413–420. doi: 10.1002/ijc.24288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmeister M, Schmitz S, Karmrodt E, et al. Male sex and smoking have a larger impact on the prevalence of colorectal neoplasia than family history of colorectal cancer. Clinical Gastroenteroloyg and Hepatology. 2010 Oct;8(10):870–876. doi: 10.1016/j.cgh.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Lynch KL, Ahnen DJ, Byers T, Weiss DG, Lieberman DA. First-degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clinical Gastroenterology & Hepatology. 2003 Mar;1(2):96–102. doi: 10.1053/cgh.2003.50018. [DOI] [PubMed] [Google Scholar]
- 31.Zell JA, Honda J, Ziogas A, Anton-Culver H. Survival after colorectal cancer diagnosis is associated with colorectal cancer family history. Cancer Epidemiology, Biomarkers & Prevention. 2008 Nov;17(11):3134–3140. doi: 10.1158/1055-9965.EPI-08-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao P-S, Lin J-K, Wang H-S, et al. The impact of family history on the outcome of patients with colorectal cancer in a veterans' hospital. International Journal of Colorectal Disease. 2009 Nov;24(11):1249–1254. doi: 10.1007/s00384-009-0774-3. [DOI] [PubMed] [Google Scholar]
- 33.Japanese Research Society. Clinical and pathological analyses of patients with a family history of colorectal cancer. Registry Committee, Japanese Research Society for Cancer of the Colon and Rectum. Japanese Journal of Clinical Oncology. 1993 Dec;23(6):342–349. [PubMed] [Google Scholar]
- 34.Bass AJ, Meyerhardt JA, Chan JA, Giovannucci EL, Fuchs CS. Family history and survival after colorectal cancer diagnosis. Cancer. 2008 Mar 15;112(6):1222–1229. doi: 10.1002/cncr.23294. [DOI] [PubMed] [Google Scholar]
- 35.Chan JA, Meyerhardt JA, Niedzwiecki D, et al. Association of family history with cancer recurrence and survival among patients with stage III colon cancer. Journal of the American Medical Association. 2008 Jun 4;299(21):2515–2523. doi: 10.1001/jama.299.21.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemminki K, Santi I, Weires M, Thomsen H, Sundquist J, Bermejo JL. Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes. BioMed Central Cancer. 2010;10:688. doi: 10.1186/1471-2407-10-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemminki K, Granstrom C, Chen B. The Swedish family-cancer database: update, application to colorectal cancer and clinical relevance. Hereditary Cancer in Clinical Practice. 2005;3(1):7–18. doi: 10.1186/1897-4287-3-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townsend JS, Steele CB, Richardson LC, Stewart SL. Health behaviors and cancer screening among Californians with a family history of cancer. Genetics in Medicine. 2013 Mar;15(3):212–221. doi: 10.1038/gim.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor DP, Cannon-Albright LA, Sweeney C, et al. Comparison of compliance for colorectal cancer screening and surveillance by colonoscopy based on risk. Genetics in Medicine. 2011 Aug;13(8):737–743. doi: 10.1097/GIM.0b013e3182180c71. [DOI] [PubMed] [Google Scholar]
- 40.Zlot AI, Silvey K, Newell N, Coates RJ, Leman R. Family history of colorectal cancer: clinicians' preventive recommendations and patient behavior. Preventing Chronic Disease. 2012;9:E21. [PMC free article] [PubMed] [Google Scholar]
- 41.Young WF, McGloin J, Zittleman L, West DR, Westfall JM. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. Journal of Rural Health. 2007;23(3):238–245. doi: 10.1111/j.1748-0361.2007.00096.x. [DOI] [PubMed] [Google Scholar]
- 42.Peterson NB, Murff HJ, Ness RM, Dittus RS. Colorectal cancer screening among men and women in the United States. Journal of women's health. 2007 Jan-Feb;16(1):57–65. doi: 10.1089/jwh.2006.0131. [DOI] [PubMed] [Google Scholar]
- 43.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004 May 15;100(10):2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008 Jul;17(7):1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher MC, Slattery ML, Lanier AP, et al. Prevalence and predictors of cancer screening among American Indian and Alaska native people: the EARTH study. Cancer causes & control : CCC. 2008 Sep;19(7):725–737. doi: 10.1007/s10552-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. American Journal of Preventive Medicine. 2002 Jul;23(1):28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 47.Wong ST, Gildengorin G, Nguyen T, Mock J. Disparities in colorectal cancer screening rates among Asian Americans and non-Latino whites. Cancer. 2005 Dec 15;104(12 Suppl):2940–2947. doi: 10.1002/cncr.21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemon S, Zapka J, Puleo E, Luckmann R, Chasan-Taber L. Colorectal cancer screening participation: comparisons with mammography and prostate-specific antigen screening. American Journal of Public Health. 2001 Aug;91(8):1264–1272. doi: 10.2105/ajph.91.8.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zallen D. To Test or Not to Test: A Guide to Genetic Screening and Risk. Rutgers University Press; 2008. [Google Scholar]
- 50.Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. European Journal of Cancer. 2006 Jan;42(2):216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. American Journal of Gastroenterology. 2001 Oct;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 52.Cannon-Albright LA, Thomas A, Goldgar DE, et al. Familiality of cancer in Utah. Cancer Research. 1994 May 1;54(9):2378–2385. [PubMed] [Google Scholar]
- 53.Jorde LB. Inbreeding in the Utah Mormons: an evaluation of estimates based on pedigrees, isonymy, and migration matrices. Annals of Human Genetics. 1989 Oct;53(Pt 4):339–355. doi: 10.1111/j.1469-1809.1989.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 54.McLellan T, Jorde LB, Skolnick MH. Genetic distances between the Utah Mormons and related populations. American Journal of Human Genetics. 1984 Jul;36(4):836–857. [PMC free article] [PubMed] [Google Scholar]
- 55.Imperiale TF, Ransohoff DF. Risk for colorectal cancer in persons with a family history of adenomatous polyps: a systematic review. Annals of Internal Medicine. 2012 May 15;156(10):703–709. doi: 10.7326/0003-4819-156-10-201205150-00006. [DOI] [PubMed] [Google Scholar]
- 56.Tuohy TM, Rowe KG, Mineau GP, Pimentel R, Burt RW, Samadder NJ. Risk of colorectal cancer and adenomas in the families of patients with adenomas: A population-based study in Utah. Cancer. 2014 Jan 1;120(1):35–42. doi: 10.1002/cncr.28227. [DOI] [PubMed] [Google Scholar]
- 57.Wilschut JA, Habbema JDF, Ramsey SD, Boer R, Looman CWN, van Ballegooijen M. Increased risk of adenomas in individuals with a family history of colorectal cancer: results of a meta-analysis. Cancer Causes & Control. 2010 Dec;21(12):2287–2293. doi: 10.1007/s10552-010-9654-y. [DOI] [PubMed] [Google Scholar]
- 58.Almendingen K, Hofstad B, Vatn MH. Does a family history of cancer increase the risk of occurrence, growth, and recurrence of colorectal adenomas? Gut. 2003 May;52(5):747–751. doi: 10.1136/gut.52.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fossi S, Bazzoli F, Ricciardiello L, et al. Incidence and recurrence rates of colorectal adenomas in first-degree asymptomatic relatives of patients with colon cancer. American Journal of Gastroenterology. 2001 May;96(5):1601–1604. doi: 10.1111/j.1572-0241.2001.03784.x. [DOI] [PubMed] [Google Scholar]
- 60.National Cancer Institute. Colorectal Cancer Risk Assessment Tool. [Accessed December 10, 2013];2010 http://www.cancer.gov/colorectalcancerrisk/
- 61.Schroy PC, 3rd, Coe AM, Mylvaganam SR, et al. The Your Disease Risk Index for colorectal cancer is an inaccurate risk stratification tool for advanced colorectal neoplasia at screening colonoscopy. Cancer prevention research (Philadelphia Pa.) 2012 Aug;5(8):1044–1052. doi: 10.1158/1940-6207.CAPR-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ClinRisk. Welcome to QCancer. [Accessed December 10, 2013];2013 http://www.qcancer.org/ [Google Scholar]
- 63.Yeoh K-G, Ho K-Y, Chiu H-M, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011 Sep;60(9):1236–1241. doi: 10.1136/gut.2010.221168. [DOI] [PubMed] [Google Scholar]
- 64.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. International Journal of Cancer. 2004 Jan 20;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei EK, Colditz GA, Giovannucci EL, Fuchs CS, Rosner BA. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses' Health Study. American Journal of Epidemiology. 2009 Oct 1;170(7):863–872. doi: 10.1093/aje/kwp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiao S, Peters U, Berndt S, et al. Estimating the Heritability of Colorectal Cancer. Human molecular genetics. 2014 Feb 21; doi: 10.1093/hmg/ddu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunlop MG, Tenesa A, Farrington SM, et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42 103 individuals. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42 103 individuals. 2012 May 22; [Google Scholar]
- 68.Khoury MJ, Janssens AC, Ransohoff DF. How can polygenic inheritance be used in population screening for common diseases? Genetics in Medicine. 2013 Feb 14; doi: 10.1038/gim.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh A, Hartge P, Kraft P, et al. Leveraging family history in population-based case-control association studies. Genetic epidemiology. 2014 Feb;38(2):114–122. doi: 10.1002/gepi.21785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


