Abstract
Asthma is a chronic disorder of the airways characterized by cellular infiltration, airway hyper-responsive and airway inflammation. Innate immune cells are the first line of defense against endogenous and exogenous signals in the airways and as such possess a diverse array of pattern recognition receptors. Toll-like receptors are crucial sentinels which when activated, can either promote or ameliorate the inflammatory response in predisposed individuals. The recently discovered triggering receptor expressed on myeloid cells family members are emerging mediators of inflammation. These receptors are believed to modulate inflammatory responses by collaborating with classic PRRs. Endogenous signals like HMGB-1, signaling through the receptor for advanced glycation end products, also promotes inflammation, however, its contribution to inflammation in the airways is not well known. Here, we discuss the role of each receptor in airway inflammation and highlight potential synergistic mechanisms, which contribute to disease pathogenesis in allergic asthma.
Keywords: Allergic airway inflammation, Asthma, DAP12, HMGB-1, NF-κB, RAGE, TREM, TLR
Introduction
Asthma is a chronic disorder of the conducting airways, which involves the interplay of both genetic and environmental factors, and is characterized by reversible airway obstruction, cellular infiltration, airway inflammation and airway remodeling [1, 2]. The response in asthma involves the activation of structural cells as well as cells of the innate and adaptive immune systems. Mediators released from this response result in the recruitment of inflammatory cells and causes structural changes to the airways, which ultimately result in chronic inflammation [1–3]
According to the Global Initiative for Asthma (GINA), 300 million individuals suffer from asthma with approximately 250,000 deaths annually [4]. The prevalence of allergic airway diseases in many Western countries has drastically increased over the last 30 years. This increase has been credited in part to lifestyle changes, which have resulted in reduced bacterial exposure thus promoting the Th2 phenotype associated with allergic diseases [5]. Though bacterial infections lead to a Th1 phenotype, several respiratory viral infections have been found to exacerbate the immune responses in asthma.
It is now clear that activation of the innate immune system, specifically in predisposed individuals, in response to inhaled antigens or respiratory viruses is the basic premise of disease pathogenesis in asthma [5]. The lungs are perfectly positioned to facilitate interaction with both external environment and internal blood circulation. As a result, the lung is constantly exposed to a wide array of infections agents, aeroallergens as well as host danger signals [6]. The innate immune system represents the first line of defense against pathogens entering the airways. Cells in the lung express diverse pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), which enable the recognition of exogenous pathogen associated molecular patterns (PAMPs) and endogenous danger associated molecular patterns (DAMPs) [6–8]. Upon recognition of these patterns, TLRs elicit activation of innate responses that result in the priming and engagement of the adaptive immune system, ultimately leading to elimination of pathogens and restoration of homeostasis [7, 8]. TLRs are expressed on a host of immune cells and play a role in the development and progression of allergic airway diseases. Activation of these receptors on the immune cells may promote increased airway inflammation if the endogenous regulatory mechanisms are disturbed..
Over the last decade, another family of innate receptors has been identified, triggering receptor expressed on myeloid cells (TREM). These receptors play a role in fine-tuning innate responses by amplifying or dampening signals induced by TLRs [9]. DAMPs may also activate TREM molecules such as high mobility group box (HMGB)-1 [10]. HMGB-1, a nuclear factor that enhances transcription, mediates its signaling cascade primarily through the receptor for advanced glycation end products (RAGE). It can also signal through TLRs leading to the activation of nuclear factor kappa B (NF-κB), thereby inducing cytokine production and promoting inflammation [11,12].
Clearly, there is some degree of crosstalk among TLRs, TREM molecules and RAGE. Given the role of these receptors in orchestrating and prolonging the immune response, better understanding of their interaction might prove to be pivotal in advancing our knowledge of the underlying mechanisms of airway inflammation. This review aims to highlight the role of these receptors as well as their ligands in airway diseases and to elucidate potential interactions amongst TLRs, TREM and RAGE in promoting airway inflammation associated with asthma.
Toll-like Receptors
Toll-like receptors (TLRs) are pattern recognition receptors critical for both the innate and adaptive responses to pathogens. They are composed of an amino terminal leucine rich repeat ectodomain, which is responsible for the recognition of PAMPs, as well as a transmembrane domain and a cytosolic signaling domain, called the toll-interleukin-1 receptor homology domain (TIR) [7,8]. To date, 10 human (TLR1-10) and 12 mouse (TLR1-9 and TLR11-13) toll-like receptors have been characterized. These receptors are capable of recognizing a wide range of microbial components. TLR1, TLR2, TLR6 and TLR10 reside in the plasma membrane and recognize lipoproteins and peptidoglycans. TLR4 and TLR5 also localize to the plasma membrane and engage bacterial lipopolysaccharide (LPS) and flagella, respectively. TLR3 and TLR7-9 are intracellular and can be stimulated to initiate anti-viral immunity [7,13,14]. TLR11 is expressed by bladder epithelial cells in mice and has been shown to recognize uropathogenic E. coli and a profin-like molecule from Toxoplasma [14].
Signaling through TLRs involve the myeloid differentiation response protein 88 (MyD88)-dependent or -independent pathways. When the respective ligand binds to a TLR, the MyD88 adapter protein is recruited to the TLR complex dimer. This promotes association with interleukin-1 receptor associated kinase-4 (IRAK-4) and IRAK-1. Tumor necrosis factor associated factor 6 (TRAF 6) is then recruited to IRAK-1. This causes a signal transduction cascade leading to phosphorylation of IKBα and allows NF-κB to activate the expression of pro-inflammatory genes [7,8,13] (Figure 1). All TLRs utilize the MyD88-dependent pathway, with the exception of TLR3, which mediates signaling through the TIR-domain containing adapter inducing interferon-β (TRIF). The mediators released from these responses result in neutrophil recruitment and activation of macrophages, which are involved in direct killing of pathogens, as well as the maturation of dendritic cells (DCs), which aid in the induction of the adaptive immune response [13–15].
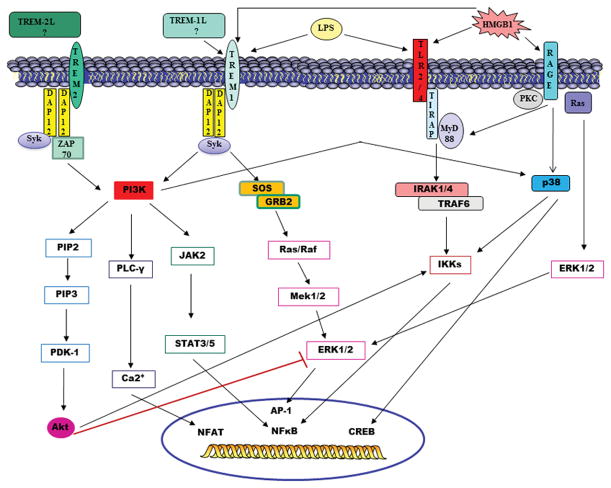
Figure 1. Signal Transduction of TLRs, TREM family members and RAGE.
Pattern recognition receptors such as TREM-1 and TLR4 can recognize both exogenous (LPS) and endogenous (HMGB-1) signals. Signaling through TLR4/2 involves activation of the MyD88 dependent pathway leading to the translocation of NFkB into the nucleus. Signaling through TREM-1 involves ligation of the DAP12 adaptor protein leading to a downstream signaling cascade, which results in the activation of pro-inflammatory transcription factors. HMGB-1 signals through its receptor RAGE, as well as TLR4 and TREM-1, which also contributes to the activation of NFkB to induce gene expression. The end result of signaling through all three pathways is the transcription of inflammatory genes, resulting in the production of mediators that contribute to increased airway inflammation. The TREM-2/DAP12 pathway may provide both inhibitory and activating signals. Phosphorylation of DAP12 leads to downstream activation of PDK-1/2, which recruits and activates Akt. Activation of PDK-1/2/Akt pathways leads to NFκB activation as well as inhibition of TLR signaling by blockade of MAPK signaling pathways.
TREM – Triggering Receptor Expressed on Myeloid Cells, TREM-L – Putative TREM ligand, TLR – Toll-like receptor, RAGE – Receptor for Advanced Glycation End-products, HMGB – High Mobility Group Box, LPS – lipopolysaccharide, Syk - spleen tyrosine kinase, IRAK - interleukin-1 receptor associated kinase, TRAF - Tumor necrosis factor associated factor, PI3K - phosphatidylinositol 3 kinase, PKC – protein Kinase C, PIP2 - phosphatidylinositol 4,5 bisphosphate, PIP3 - phosphatidylinositol 3,4,5 trisphosphate, PDK – phosphoinositide-dependent kinase, PLC- phospholipase C, JAK – Janus Kinase, STAT – Signal Transducer and Activator of Transcription, SOS – Son of Sevenless, GRB – Growth factor Receptor Binding, IKK – I kappa B Kinase, ERK – Extracellular signal regulated kinase, AP - activation protein, NFAT – nuclear factor of activated T-cells, CREB - cAMP response element binding
TLRs in airway inflammation
The lungs are constantly being exposed to a plethora of microorganisms. TLRs are expressed by essentially all effector cells of innate immunity (Table 1) and are therefore centrally involved in orchestrating immune responses towards pathogens. Infections of the respiratory tract by microbes activate these receptors resulting in pathogen clearance. Allergic airway diseases and bacterial infections affect millions of people worldwide and continually contribute to mortality and high economic burden. Infectious diseases have a major impact on the development and severity of inflammation associated with allergic diseases. Some studies have suggested that certain types of infections may lead to predisposition for the development of asthma [3,5].
Table 1.
TLR Expression on Cells in the Airways associated with Allergic Asthma
| TLRs | Ligand | Cellular expression in the airways | Role in the airway | References |
|---|---|---|---|---|
| 1 | Triacylated lipopeptides | Monocyte B-cells DCs T-cells |
Works in conjunction with TLR2 | [8,99] |
| 2 | Viral protein envelops Lipoteichoic acid GPI anchors of protozoan Peptidoglycan |
Macrophages DCs T-cells B-cells Neutrophils |
Control of Gram positive bacterial infections | [7,8] |
| 3 | dsRNA | Innate immune cells except neutrophils and pDCs | Mediate the establishment of the anti-viral state | [8,99] |
| 4 | LPS | Neutrophils Macrophages DCs |
Control of Gram negative bacterial infection | [6,8] |
| 5 | Flagellin | Alveolar macrophages DCs Epithelial cells Eosinophils |
Recognition and control of bacterial infections (particularly Pseudomonas aeruginosa) | [6,100] |
| 6 | Diacylated lipopeptides | Monocytes/macrophages B-cells T-cells DCs |
Works in conjunction with TLR2 | [99] |
| 7 | ssRNA | B-cells pDCs |
Mediate the establishment of the anti-viral state | [6,8] |
| 8 | ssRNA | Monocyte/macrophages Myeloid DCs |
Mediate the establishment of the anti-viral state | [8] |
| 9 | CpG motifs | pDCs macrophages B-cells cDCs |
Control of infections caused by DNA viruses | [7,8] |
| 10 | Triacylated lipopeptides | B-cells pDCs |
Works in concert with TLRs 1 and 2 | [99] |
TLR-Toll like receptor; LPS-lipopolysaccharide; pDC-plasmacytoid dendritic cell; cDC-conventional dendritic cell; dsRNA-double stranded ribonucleic acid; ssRNA-single stranded ribonucleic acid; GPI-glycophosphatidylinositol
Multiple TLRs have been implicated in allergic airway inflammation (Figure 2). Exposure to bacterial lysates or administration of large doses of LPS can promote Th1 responses that reduce airway inflammation and mucus production. Conversely, low doses have been found to exacerbate the asthmatic response [6,16]. TLR2 and TLR4 are believed to be the main toll like receptors which are responsible for promoting and sustaining inflammatory responses in asthma [17]. House dust mite, a common indoor allergen, increases the development of airway hypersensitivity. Der p2, the major allergen in HDM, has been shown to share homology with myeloid differentiation 2 (MD2), the LPS binding member of the TLR4 signaling complex. Wild-type and MD2 deficient mice which were sensitized and challenged with Der p2 developed hallmark features of allergic asthma which was absent in the TLR4-deficient mice [18]. Development of AHR to HDM is dependent upon activation of TLR4/MyD88 signaling which drives Th2 polarization [19]. Mast cells, which contribute to several pathogenic features of asthma, express a number of TLRs. In a study investigating the effects of TLR ligand treatment on IgE receptor-induced mast cell activity, it was found that prolonged exposure to these ligands promoted mast cell reactivity following IgE receptor activation. Activation of TLR4 by LPS produced the most pronounced effects resulting in enhanced degranulation, secretion of leukotrienes, cytokines and chemokines [20]. These results seem to suggest that mast cells control airway inflammation and may promote exacerbations through TLR4-mediated mechanisms.
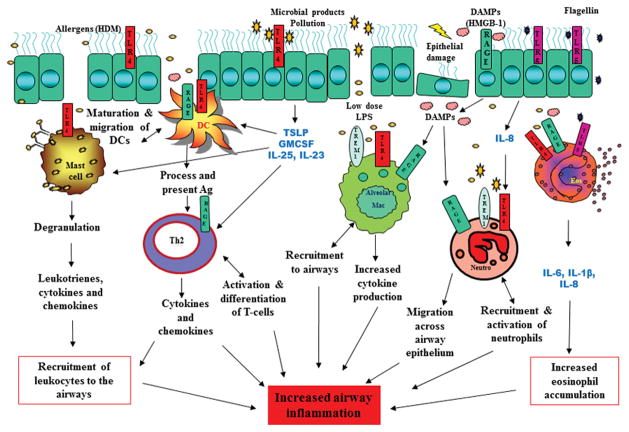
Figure 2. Interaction of TLRs, TREM-1, RAGE and HMGB-1 in airways.
The airway epithelium is exposed to a wide array of allergens and microbial products. Allergens, such as house dust mite (HDM), activate TLR4 located on airway epithelial cells. This leads to the production of TSLP, GM-CSF, IL-25 and IL-33, which activates dendritic cells and mast cells and promotes Th2 polarization. Proteases produced from allergens can also directly activate TLRs on eosinophils and neutrophils resulting in release of chemokines and cytokines leading to increased leukocyte recruitment to the airways. Allergens and microbial products can also cause epithelial damage leading to the release of DAMPs like HMGB-1. HMGB-1 binds to receptors on epithelial cells and causes further release of DAMPs, which can activate and recruit neutrophils and macrophages to the airways. The end result is increase in pro-inflammatory mediators and cellular infiltration, leading to airway inflammation and disease pathogenesis.
HDM – House dust mite, TSLP - thymic stromal lymphopoietin, GM-CSF – granulocyte monocyte colony stimulating factor, IL- interleukin, DC- dendritic cell, Th- T helper cell, DAMP - danger associated molecular patterns, Neu – neutrophil, Mac – macrophage, Eos - eosinophil
Triggering of TLR-4 expressed on structural cells in the airways have been shown to stimulate the release of thymic stromal lymphopoietin (TSLP), granulocyte-monocyte colony stimulating factor (GM-CSF), interleukin (IL)-25 and IL-33. Blockade of TLR4 on structural cells was found to abrogate HDM-driven allergic airway inflammation [21]. In fatal asthmatic patients the expression of TLR2, TLR3 and TLR4 on cells in the airways was higher than those obtained from control groups. TLR-mediated signaling was also believed to contribute to inflammation surrounding asthma deaths [22].
Studies have shown that exposure to fungal products promote airway eosinophilia, secretion of Th2 cytokines and goblet cell metaplasia. In a murine model of asthma, TLR2 was able to modulate the innate and adaptive immune responses during chronic inflammation. TLR2- deficient mice sensitized and challenged with Aspergillus fumigatus exhibited significantly lower airway hyper-responsiveness, airway inflammation and Th2 cytokine levels when compared to their wild type counterparts. Deletion of TLR2 also reduced mucus cell hyperplasia and peribronchial fibrosis up to 30 days post challenge [23]. TLR2 activation is believed to promote Th2 responses which results in a shift in the Th1/Th2 balance. This shift may be due to the production of IL-10 that inhibits Th1 cytokine production [24, 25].
Toll-like receptors also play an important role in the innate recognition of pathogens by dendritic cells (DCs). DCs are critical in determining the outcome of an allergen encounter in the lung. Inhaled antigens are constantly being sampled by these cells, which are located above and beneath the basement membrane of the respiratory epithelium [26]. Inhaled pathogens trigger TLRs on epithelial cells and macrophages leading to the production of chemokines and cytokines. TLR2 acts in concert with TLR3 to induce the synthesis of inflammatory cytokines that promote DC maturation and migration. These pathogens can also be directly processed and presented via MHC II to T-lymphocytes resulting in Th2 polarization [27].
B-cells express both a B-cell receptor (BCR) and TLRs. Engagement of both receptors can fine tune B-cell responses and link the innate and adaptive immune systems. TLR activation of B-cells has been shown to enhance proliferation, terminal differentiation to plasma cells as well as increase cytokine and immunoglobulin secretion [28,29]. Human basophils express TLR2 and TLR4 with the latter believed to be involved in the exacerbation of allergic inflammation mediated by infections [30]. Simultaneous triggering of TLR and IgE resulted in synergistic production of IL-4, IL-8, IL-13 and RANTES. Interaction mediated by IgE and TLR4 led to a pronounced Th2 response [31].
Exacerbations of asthma can be due to both aeroallergens as well as respiratory viral infections. Respiratory syncytial virus (RSV) and human rhinovirus (HRV) are two well-known examples with the former believed to play a role in pediatric asthma whereas the latter has been associated with asthma later on in life [32,33].
TLR4 is primarily responsible for recognition of infections with RSV. Studies using human airway epithelial cells showed that infection with RSV significantly increased IL-8 production from these cells. Microarray analyses suggested the involvement of a TLR4-mediated pathway that resulted in activation of transcription factor activator protein (AP)-1 [34]. Analysis of peripheral blood mononuclear cells (PBMCs) from asthmatic patients cultured with HRV, revealed lower expression of type I IFNs when compared to healthy subjects. There was also reduced expression of NF-κB family members as well as reduced responsiveness to TLR7/TLR8 activation [35]. These results suggest that antiviral signaling pathways are impaired in asthmatic patients, which may account for increased exacerbation caused by respiratory viral infections.
Exposure to CpG, the ligand for TLR9, has been found to exacerbate airway responses associated with asthma. Mice that were sensitized and challenged with ovalbumin and then exposed to CpG intranasally showed increased airway hyper-responsiveness and release of IL-5 and leukotriene B4 (LTB4) [36]. Collectively, these results highlight a crucial role of TLR-mediated signaling in promoting and exacerbating allergic airway inflammation.
TLRs are emerging innate receptors involved in the initiation and progression of airway inflammation. Though many TLRs are important in the first line host defenses against microbial products, some also play a role in exacerbating the pathogenesis of allergic airway inflammation. Understanding the complex roles of TLRs in allergic airway diseases may facilitate the development of targeted preventative and therapeutic measures for asthmatic patients.
HMGB-1 and RAGE
HMGB-1
The high mobility group box (HMGB)-1 protein is a ubiquitously expressed nuclear factor, which is an important mediator of inflammation via receptors of the innate immune system. Under homeostatic conditions, HMGB-1 enacts important features in the nucleus, which contributes to the maintenance of chromosomal architecture and regulation processes in the genome [37,38]. Nuclear HMGB-1 binds without specificity to minor grooves in the DNA helix and introduces bends. This enables interaction with various factors including p53, NF-κB and steroid hormones. HMGB-1 is also present extracellularly where it acts as a necrotic marker for immune cells as well as a signal for the production of inflammatory cytokines and chemokines through TLRs and the receptor for advanced glycation end products (RAGE) (Figure 1) [37–39].
The primary target of extracellular HMGB-1 is TLR4. Binding of ligand to the receptor leads to the translocation of NF-κB to the nucleus and activation of interferon regulatory factor 3 (IRF3) and activation protein 1 (AP-1) to produce the inflammatory cytokine repertoire [39]. Active secretion of HMGB-1 from monocytes and macrophages occurs in response to inflammatory stimuli such as LPS, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and IL-1β [38].
RAGE
The receptor for advanced glycation end products (RAGE) is a type I transmembrane protein which belong to the immunoglobulin super family. It is comprised of three immunoglobulin domains, located in the extra-cellular space, a single membrane spanning domain and a short cytoplasmic domain, which is essential for signal transduction [40]. RAGE is expressed at low basal levels in the majority of healthy tissues and is upregulated in response to a wide range of pathological events. Over the past decade, this receptor has emerged as a key pattern recognition receptor capable of binding a number of soluble and cell associated molecules involved in the host response to tissue injury, infection and inflammation [40, 41]. Ligands of RAGE include amyloid beta (Aβ), Mac-1 (which aids in adhesion of leukocytes to the endothelium), and HMGB-1. Recent studies have identified emerging ligands such as LPS, heat shock protein (HSP)-70, C3 and CpG DNA oligomers, which are well-known contributors to the onset of inflammation [42, 43]. Ligation of RAGE induces activation of multiple signaling pathways, depending on the ligand and the cellular milieu. This ultimately leads to the activation of transcription factors including AP-1, cAMP response element binding (CREB) protein, NF-κB, signal transducers and activators of transcription (STAT)-3 and early growth response (Erg)-1 [41, 42] (Figure 1).
HMGB-1 and RAGE in airway inflammation
In studies using a murine model of asthma, HMGB-1 expression in the lung tissue and bronchoalveolar lavage fluid was significantly elevated which correlated to increased airway hyper-responsiveness and eosinophilia [44]. In a chronic mouse model of allergic inflammation, eliciting both airway neutrophilia and eosinophilia, blockage of HMGB-1 was found to ameliorate these effects. It was also observed that there was a decrease in Th1 and Th2 lymphocytes and an increase in Th17 cells in the mediastinal lymph nodes and lungs [45].
Human bronchial epithelial cells (HBECs) stimulated with HMGB-1 showed increased expression and secretion of tumor necrosis factor (TNF)-α, TSLP, matrix metalloproteinases (MMP)-9 and vascular endothelial growth factor (VEGF) in a dose- and time-dependent manner. HMGB-1 also induced elevated levels of RAGE protein expression. Secretion of pro-inflammatory mediators was significantly decreased by blockade of RAGE and inhibition of p38 mitogen-activated protein kinases (MAPK) pathway. Results suggest that HMGB-1 binds to RAGE promoting activation of p38 MAPK pathways leading to increased expression of mediators that promote inflammation in asthma [46].
Studies involving human asthmatic subjects have shown that damage to the airway epithelium elicits chemotaxis of eosinophils and HMGB-1 was found to contribute to enhanced eosinophil survival and chemotaxis in the lungs [47]. HMGB-1 expression in sputum obtained from asthmatic patients was markedly higher when compared to normal controls. This correlated to sputum eosinophilia and expression of TNF-α, IL-5 and IL-13 [44]. Similar studies found increased levels of HMGB-1 and serum RAGE, as well as greater percentages of neutrophils in sputum from asthmatic subjects, suggesting that they might contribute to the severity of asthma pathogenesis [48, 49].
RAGE is expressed on monocytes/macrophages, B and T lymphocytes. The receptor plays an active role in the recruitment of leukocytes across the epithelium to the site of infection, a hallmark feature of inflammation. Several subsets of DCs also express RAGE, which upon receptor ligation, promotes the maturation and migration of these cells, linking the innate and adaptive immune responses [42]. In a HDM mouse model of asthma, the absence of RAGE was found to abrogate airway hyper-responsiveness, airway eosinophilia and airway remodeling [50]. Similar results were observed in mice exposed to cockroach extracts [51]. Studies using ovalbumin immunized mice showed that RAGE plays a role in the activation and differentiation of T-cells. RAGE deficient mice showed reduced cellular infiltration in bronchoalveolar lavage fluid and impaired T-cell activation in the mediastinal lymph nodes when compared to the wild type counterparts. RAGE deficient T-cells cultured in vitro demonstrated reduced production of IFN-γ but increased IL-17 production [52].
Recent genome wide association studies have identified single nucleotide polymorphisms in the human genome for RAGE ligand binding domain (G82S). This correlated with altered forced expiratory volume (FEV1), at test of lung function in asthmatics, and was associated with increase incidences of asthma [53, 54]. RAGE was found to drive allergic airway inflammation in wild type and knockout mice exposed to allergen extracts by promoting IL-33 expression and coordinating the inflammatory response downstream of IL-33. Absence of RAGE was shown to impede accumulation of type 2 innate lymphoid cells (ILCs) in the airways. Further studies from bone marrow chimeras revealed that pulmonary parenchymal RAGE has a central role in promoting allergic inflammation [55]. Studies using RAGE sufficient (RAGE+/+) and deficient (RAGE−/−) mice exposed to ovalbumin demonstrated that deficiency in RAGE resulted in reduced eosinophilic inflammation and goblet cell metaplasia as well as decreased Th2 cytokines and absolute numbers of type 2 ILCs in the lungs. Conversely, the absence of RAGE on structural cells enhanced AHR suggesting contrasting role of the receptor in airway inflammation versus airway hyper-responsiveness [56].
Apart from full length RAGE, a number of truncated forms have been identified including a soluble form known as sRAGE that acts as a decoy receptor preventing interaction of RAGE with its ligands. This is believed to afford some protection against inflammation and cell injury [57]. In a study conducted on asthmatic children, it was found that levels of sRAGE were significantly lower in asthmatic patients when compared to the healthy controls. Uncontrolled and severe asthmatic subgroups showed lower levels of sRAGE with significant negative correlation between sRAGE levels and eosinophil count as well as total IgE levels [58].
Collectively, these results suggest that HMGB-1 and its receptor RAGE could play important roles in the pathological mechanisms underlying chronic inflammation in allergic asthma. Both ligand and receptor might prove to be amenable therapeutic targets, however, further studies are needed to better understand their underlying mechanisms of action in the airways.
TREM Family Members
TREM-1 and TREM-2 are transmembrane glycoproteins of 25–30 kDa consisting of a single extracellular immunoglobulin like domain, a transmembrane region and a short cytoplasmic tail [59, 60]. The receptors are located on human chromosome 6p21 and mouse chromosome 17c3 [61]. Both TREM-1 and TREM-2 associates by a positive charge with DNAX activating protein of 12 kDa (DAP12). In TREM-1 mediated signaling, receptor ligation results in phosphorylation of tyrosine residues in DAP12/ITAM by Src kinases. This allows spleen tyrosine kinase (Syk) to bind and phosphorylate linker of activated T-cells (Lat) and non T-cell activation linker (NTAL) leading to a signal cascade which activates Akt, calcium and MAP kinases ultimately leading to cytokine rearrangement and activation of several transcription complexes [59–61]. In TREM-2 mediated signaling, phosphorylation of ITAM residues in DAP12 lead to the recruitment of Syk and ZAP70. This activates phosphatidylinositol 3 kinase (PI3K), which converts phosphatidylinositol 4,5 bisphosphate (PIP2) to phosphatidylinositol 3,4,5 trisphosphate (PIP3) leading to downstream activation of PDK-1/2, which recruits and activates Akt. Activation of PDK-1/2/Akt pathways contribute to the regulation of NF-κB and subsequent expression of inflammatory genes. The PI3K/Akt pathway can also inhibit TLR-mediated signaling by blocking the function of MAPK signaling pathways [62]. Conversely, activation of the PI3K/ERK pathways by TREM-1 signaling is pro-inflammatory and promotes survival of immune cells by inactivating pro-apoptotic family members [59] (Figure 1).
PI3K-mediated signaling pathways that promote airway inflammation are regulated by src homology 2 domain (SH2)-containing inositol phosphatase (SHIP) and SH2 containing protein tyrosine phosphatase 1/2 (SHP1/2) [63]. SHP1 has been shown to negatively modulate PI3K/Akt signaling pathways [64]. The phosphatase was found to inhibit TREM-2- and DAP12-induced signaling by binding to DAP12 and preventing PI3K from being recruited [65]. In the immune cells, downstream activation of STAT3 by PI3K is regulated by suppressor of cytokine signaling (SOCS) family members [66].
TREM-1, found highly expressed on monocyte/macrophage and neutrophils, play a role in augmenting inflammatory responses whereas TREM-2, expressed on macrophages, dendritic cells, osteoclasts and microglia is often associated with anti-inflammatory responses [10].
TREM-1
TREM-1 is expressed on both human and mouse monocyte/macrophages and polymorphic nuclear neutrophils (PMNs). Though its ligand is still unknown, several studies to ascertain its mechanism of action have relied on the use of agonistic antibodies, which induce receptor crosslinking leading to amplification of immune responses [60]. Its role as an amplifier of pro-inflammatory responses have been confirmed primarily in studies involving sepsis but it has been implicated in several other diseases including rheumatoid arthritis, cancers and inflammatory bowel syndrome [10, 67]. Though research is currently ongoing, much less is known about the role of TREM-1 in airway disease (Figure 2).
Activation of TREM-1 on neutrophils and monocytes results in the production of pro-inflammatory cytokines and chemokines, including IL-1β, IL-2, IL-6, IL-8, TNF-α, MIP-α1 and GM-CSF [9, 68]. These cells also express other pattern recognition receptors that play essential roles in innate responses to lung infection [5]. Studies using a mouse model of pneumococcal pneumonia found that infection with Streptococcus pneumonia resulted in rapid recruitment of TREM-1 positive neutrophils into the bronchoalveolar space [69]. In order to model the effects of TREM-1 blockade in humans, a TREM-1/3 deficient mouse was developed since the functional overlap of TREM-1 and TREM-3 seen in mice does not exist in humans [70, 71]. TREM-1/3 deficiency in mice led to increased mortality and a reduction in pro-inflammatory cytokines and chemokines released locally. TREM-1/3 deficient alveolar macrophages were also incapable of phagocytosing the pathogen [69]. TREM-1 expression has also been found to be strongly upregulated by LPS exposure. Studies using human neutrophils and monocytes showed that TREM-1 interacted with TLR-4 receptor complex to amplify inflammation [68]. Similar results were found using the murine macrophage cell line, RAW 264.7 [72].
In a house dust mite mouse model of asthma, injection with Aspergillus fumigatus resulted in increased TREM-1 levels in the lungs. Analysis of bronchoalveolar lavage fluid revealed significant increases in pro-inflammatory mediators. Results suggest that TREM-1 may play a role in modulating the immune response during fungal asthma [73].
In addition to amplifying inflammatory signals, TREM-1 is also involved in transepithelial migration of neutrophils in the airways. Studies using TREM-1 knockout mice demonstrated increased mortality and decreased neutrophil in the airways of these animals following Pseudomonas aeruginosa challenge. Neutrophils were found in the primary endothelium cell monolayer but failed to migrate to the airway epithelia indicating a defect in this mechanism [74].
TREM-2
The TREM-2/DAP12 pathway may provide both inhibitory and activating signals depending on the cellular and cytokine milieu. Under inflammatory conditions, TREM-2 promotes differentiation of macrophages, dendritic cells and microglial cells [62]. TREM-2 was found to promote macrophage survival and lung disease after respiratory viral infection. The mechanism believed to be driving this observation was decreased macrophage apoptosis mediated by intracellular and cell surface TREM-2 [75]. Studies using human monocyte derived DCs showed that TREM-2, in association with DAP12, promoted upregulation of CC chemokine receptor 7 (CCR7), a key mediator in homing of dendritic cells to the secondary lymphoid organs [76].
Conversely, other studies have highlighted an anti-inflammatory role of TREM-2. TREM-2 deficient bone marrow derived DCs were shown to have increased production of pro-inflammatory cytokines and type I IFNs in response to TLR ligation. These cells also showed increased TLR induced maturation and greater efficiency at inducing antigen specific T-cell responses upon CpG stimulation when compared to the wild type. The increase in IL-12 and TNF-α secretion from these cells suggests a possible Th1 polarization with TREM-2 deficiency. A potential TREM-2 ligand is expressed on the surface of BMDCs suggesting that inhibitory signals transduced by the TREM-2 receptor were due to recognition of an endogenous ligand [77]. In another study, TREM-2 expression was abrogated when macrophages were stimulated with LPS. Studies using TREM-2 deficient mice showed that TREM-2 functions to inhibit cytokine production by macrophages in response to LPS, zymosan and CpG. Results suggest that TREM-2 is expressed on newly differentiated or alternatively activated macrophages and play a role in reducing activation [78]. TREM-2 has recently been shown to play a role in attenuating inflammatory responses in alveolar macrophages. Silencing of TREM-2 on these cells enhanced the expression of TLR4, TNF-α and IL-10 following LPS stimulation [79].
sTREM-1 as a diagnostic marker
Proteolytic cleavage of membrane bound TREM-1 produces a soluble mediator, sTREM-1[10]. sTREM-1 has been detected in biological fluids of human and animals suffering from a number of infectious and inflammatory diseases such as inflammatory bowel disease (IBD), pneumonia and rheumatoid arthritis [9,10]. Most studies related to sTREM-1 have been done in relation to sepsis where it has been found to be a valuable diagnostic marker [9, 80]. Other studies have shown that there are elevated levels of sTREM-1 in bronchoalveolar lavage fluid and serum of patients with bacterial, viral and fungal infections [81–83].
Elevated levels of sTREM-1 have also been observed in patients with non-infectious inflammation such as COPD and asthma [84]. Higher levels of sTREM-1 were found in patients with exacerbated asthma and respiratory tract obstruction compared to non-exacerbated patients. Increased levels of sTREM-1 also correlated with number of neutrophils infiltrating the airways [85].
TREM family members play important roles in both the innate and adaptive immune systems. Identification of ligands for these receptors is crucial for a better understanding of the mechanisms, and thus contributing to the stimulation or amelioration of airway inflammation. The emergence of sTREM as a potential diagnostic marker may prove to be a useful tool in both infectious and non-infectious diseases.
Interactions
TLRs, HMGB-1 and RAGE interaction
There exists some degree of crosstalk among HMGB-1, RAGE and TLRs (Figure 1). RAGE was the first described receptor of HMGB-1, however TLR2 and TLR4 have also been identified as receptors. TLRs and RAGE may co-operate with each other as essential partners in strengthening the inflammatory response through the recruitment and assembly of homo and hetero-oligomers [86]. A number of TLR ligands such as CpG DNA and LPS form complexes with HMGB-1. These complexes elicit stronger inflammatory responses, compared to HMGB-1 alone, through mechanisms that seem to involve co-activation of TLR and RAGE signaling. RAGE also appears to interact with TIRAP and MyD88, both of which are intracellular adaptor proteins used by toll like receptors [86, 87].
In recent studies, HMGB-1 was found to induce neutrophil extracellular traps (NETs) formation through interaction with TLR4. TLR4 deficient mice showed diminished capacity of neutrophils to induce NET formation. Neutrophils isolated from mice exposed to LPS and HMGB-1 showed consistently greater ability to produce NETs than control groups [88]. These results provide a novel mechanism through which HMGB-1 may contribute to the severity of neutrophil inflammation associated with airway diseases like allergic asthma. Studies using recombinant human HMGB-1 (rhHMGB-1) to induce acute lung injury in rats showed that there were significant increases in IL-1β and TNF-α levels in treated animals. Cultured alveolar macrophages activated by rhHMGB-1 also showed increases in the release of these cytokines. TLR-4 expression was upregulated by rhHMGB-1 and blockade of the receptor or neutralization of HMGB-1 resulted in an attenuated inflammatory response both in vitro and in vivo. These finding suggest that HMGB-1 has the ability to activate alveolar macrophages to produce pro-inflammatory mediators through a TLR dependent manner. Alveolar macrophages have been shown to orchestrate inflammation through the release of chemokines that attract leukocytes to the airways thus promoting the development and progression of allergic airway diseases [89].
Taken together TLRs, HMGB-1 and RAGE seem to play a role in coordinating the inflammatory response as well as contributing to pathogenesis observed in acute and chronic airway inflammation (Figure 2). Our knowledge on the crosstalk between these receptors and ligand is still limited and more research is needed to ascertain the factors that enable discrimination or promote collaboration among the three. Further understanding of the molecular mechanism and pathways involved in these interactions will facilitate future studies aimed at identifying their collective roles in allergic airway inflammation.
TLRs and TREM family members
There is an increasing body of evidence to suggest that there are shared interactions between TREM family members and TLRs (Figure 1). TREM-1 has been found to be significantly upregulated by various TLR ligands, such as LPS (TLR4 ligand), lipoteichoic acid (TLR2 ligand) and polycytidylic acid (TLR3 ligand) leading to increased pro-inflammatory responses [68, 74]. Studies using bone-marrow derived macrophages from TLR2 and TLR4 knockout mice showed that these cells failed to induce expression of TREM-1 mRNA and protein in response to their respective ligands. TREM-1 expression in response to LPS was not altered in the MyD88 knockout macrophages suggesting that a downstream MyD88 independent pathway was active. Inhibition of TRIF decreased TREM-1 expression in response to LPS. Results suggest that TREM-1 expression in response to TLR ligands can occur by both MyD88 dependent and independent pathways, both of which require TLRs [90]. TLR4 and TREM-1 synergistic signaling has been shown using LPS stimulation. TREM-1 is strongly upregulated by LPS exposure which has been found to interact with the TLR4 receptor complex to amplify inflammation. Blockade of TREM-1 inhibited LPS induced TNF-α production and blocking TLR4 led to down regulation of TREM-1 crosslinking, suggesting that there may exist a TLR4/TREM-1 co-localization in human neutrophils [68]. In a model of fungal asthma, Pam3Cys (TLR2 agonist) and soluble Aspergillus antigens were shown to induce TREM-1 transcript expression in macrophages in a TLR2-dependent manner [73].
Conversely, TREM-2 interaction with TLRs plays a role in dampening immune responses. TREM-2 deficient bone-marrow dendritic cells showed increased production of inflammatory cytokines and type I IFNs in response to TLR ligation. These cells also showed increased TLR-induced maturation and were better equipped to induce antigen specific T-cell proliferation when compared to wild type cells [77]. TREM-2 has also been shown to attenuate macrophage activation by decreasing cytokine production essential to these cells [78]. Exposure to Streptococcus pneumonia following airway exposure to HDM becomes fatal due to impaired neutrophil recruitment. This is caused by desensitization of TLRs, upregulation of endogenous negative regulators of TLRs (A20, IRAK) as well as a preference of TREM-2 expression to inhibit TLR signaling [91].
Though much less data is available, other TLRs are believed to interact with TREM molecules. Flagellin, a TLR5 ligand, combined with TREM-1 engagement has led to the production of higher levels of TNF-α when compared to stimulation by flagellin alone. Stimulation with R-848, a TLR7/8 agonist, increased respiratory burst and degranulation of neutrophils by a TREM-1 and TLR mechanism [68]. CpG-ODN was found to abrogate TREM-1 LPS-induced upregulation in mouse peritoneal macrophages treated with both CpG-ODN and LPS [92]. Further research is needed to completely elucidate the interactions between TLRs and TREM molecules. The synergistic effects in either strengthening or dampening immune responses may provide new avenues for therapeutic intervention.
Expert Commentary and Five-Year View
The mechanisms of airway diseases like asthma arise from both genetic predisposition as well as exposure to environmental allergens that lead to activation of innate and adaptive immune responses. Although there have been many advances in the field over the last decade, there is still no cure for this heterogeneous disorder. Toll-like receptors have received a great deal of attention due to their possible immunomodulatory properties with regards to allergy and asthma. Several studies have been directed at shifting the Th2 response associated with the allergic phenotype towards the generation of a Th1 response by TLR association [93]. Viruses, which are common triggers of asthma, are recognized by TLR7 and TLR9. These receptors have emerged as amenable therapeutic targets. Activation of TLR7 triggers a rapid immune response which favors a subsequent Th1 phenotype. In a recent study, allergic Tlr7−/− mice infected with rhinovirus 1B (RV1B) displayed impaired IFN release, increased virus replication and airway hyper-responsiveness as well as exaggerated eosinophilic inflammation. Adoptive transfer of TLR competent pDCs was shown to block these exaggerated inflammatory responses and increase IFN-γ release [94]. Studies using synthetic TLR7 ligands (R837 and R848) have shown that activation of the receptor prevented airway hyper-responsiveness, eosinophilic infiltration and goblet cell hyperplasia in an ovalbumin model of asthma [95]. There were also decreased levels of IL-5 and LTB4 in bronchoalveolar lavage fluid, suggesting that TLR7 dampens allergic airway responses [36]. Investigators doing studies using TLR2 agonist, Pam3Cys, observed a significant reduction in eosinophil infiltration and increase production of T regulatory (Tregs) cells in the lungs of mice sensitized and challenged with ovalbumin [96].
TREM-1 plays a role in amplifying inflammatory responses and act as a direct link to the adaptive immune system. Modulation of this receptor might be crucial to dampening the inflammatory response associated with diseases like allergic asthma. Several groups are currently developing antagonists of TREM-1. A TREM-1 polypeptide, consisting of one or more sequences of the TREM-1 protein, has been shown to have anti-septic shock and anti-sepsis properties. This polypeptide was shown to down-regulate pro-inflammatory cytokines cascade associated with infection thus inhibiting hyper-responsiveness and death in animals [97]. Another group has described methods of treating inflammatory diseases by antagonizing TREM expression, signal cascade as well as DAP12 activity and/or expression [98]. This could potentially be useful in the treatment or respiratory diseases like asthma.
Although manipulation of the receptor might prove beneficial in augmenting inflammatory responses, it is important to ensure that this modulation does not alter its ability to function in bacterial clearance. Future research is definitely needed to identify the ligands for TREM family members as this will be a critical step towards gaining a better understanding of their signaling pathway and drive targeted inhibition geared at decreasing inflammation.
RAGE and HMGB-1 are emerging as contributors to the inflammatory response associated with airway diseases. Better understanding of these mechanisms is needed to clearly elucidate the role of this receptor and its ligand in asthma pathogenesis. Research to date has highlighted that there exist some degree of cross talk among these molecules. We now have the challenge of determining what factors influence collaboration of RAGE, TREM family members and TLRs and how this collaboration dictates the immune response, whether pro or anti-inflammatory.
Key Issues.
Asthma is a chronic disorder of the airways affecting approximately 300 million people globally. There is currently no cure for the disease.
PRRs on innate immune cells enable them to act as the first line defense against pathogens (viral, bacterial and fungal) that exacerbate the inflammatory response in atopic individuals.
Several TLRs have been implicated in coordinating the innate immune response and activating adaptive responses driving airway inflammation in asthma. These receptors have also been shown to be amenable targets for therapeutic intervention.
TREM-1 and −2 collaborate with TLRs to promote (the former) and dampen (the latter) inflammation.
Endogenous signals like HMGB-1 can signal through RAGE and/or TLRs. Collaborative signaling through both receptors exacerbates inflammation through MyD88-dependent pathways.
Identification of TREM ligands as well a better characterization of the mechanism driving collaboration of these PRRs is crucial for the development of targeted therapy for asthma patients.
Footnotes
Financial and competing interests disclosure
This work was supported by research grants R01 AI075315, R01 HL085680, R01 HL112597, and R01 HL120659 to DK Agrawal from the Office of the Director, National Institutes of Health, and National Heart, Lung and Blood Institute, NIH USA. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Agrawal DK, Shao Z. Pathogenesis of allergic airway inflammation. Curr Allergy Asthma Rep. 2010;10(1):39–48. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall S, Agrawal DK. Key mediators in the immunopathogenesis of allergic asthma. Int Immunopharmacol. 2014 Jun 13; doi: 10.1016/j.intimp.2014.05.034. pii: S1567–5769(14)00216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 4.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 5.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013;381(9869):861–873. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 6.Kovach MA, Standiford TJ. Toll like receptors in diseases of the lung. Int Immunopharmacol. 2011;11(10):1399–1406. doi: 10.1016/j.intimp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. Review which gives a comprehensive description of all TLRs, ligands and signaling pathways in the innate immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426(6):1246–1264. doi: 10.1016/j.jmb.2013.11.024. Article provides an in depth review of toll-like receptors, their ligands, cellular expression, signaling pathway and roles in anti-viral immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21(1):38–46. doi: 10.1016/j.coi.2009.01.009. Comprehensive analysis of TREM family members in inflammatory diseases and potential therapeutic targets for future research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelham CJ, Agrawal DK. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(2):243–256. doi: 10.1586/1744666X.2014.866519. [DOI] [PubMed] [Google Scholar]
- 11.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira-Machado JA, de Oliveira Volpe CM. HMGB-1 as a target for inflammation controlling. Recent Pat Endocr Metab Immune Drug Discov. 2012;6(3):201–209. doi: 10.2174/187221412802481784. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Xiang Y, Yao X, et al. The active contribution of Toll-like receptors to allergic airway inflammation. Int Immunopharmacol. 2011;11(10):1391–1398. doi: 10.1016/j.intimp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42(4):506–518. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Jacquet A. Innate immune responses in house dust mite allergy. ISRN Allergy. 2013;2013:735031. doi: 10.1155/2013/735031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo L, Lucas K, Fortuna CA, Chuang CC, Best TM. Molecular Regulation of Toll-like Receptors in Asthma and COPD. Front Physiol. 2015;6:312. doi: 10.3389/fphys.2015.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trompette A, Divanovic S, Visintin A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phipps S, Lam CE, Kaiko GE, et al. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am J Respir Crit Care Med. 2009;179(10):883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 20.Saluja R, Delin I, Nilsson GP, Adner M. FcepsilonR1-mediated mast cell reactivity is amplified through prolonged Toll-like receptor-ligand treatment. PLoS One. 2012;7(8):e43547. doi: 10.1371/journal.pone.0043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira DS, Annoni R, Silva LF, et al. Toll-like receptors 2, 3 and 4 and thymic stromal lymphopoietin expression in fatal asthma. Clin Exp Allergy. 2012;42(10):1459–1471. doi: 10.1111/j.1365-2222.2012.04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckland KF, O’Connor E, Murray LA, Hogaboam CM. Toll like receptor-2 modulates both innate and adaptive immune responses during chronic fungal asthma in mice. Inflamm Res. 2008;57(8):379–387. doi: 10.1007/s00011-008-8004-y. [DOI] [PubMed] [Google Scholar]
- 24.Re F, Strominger JL. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol. 2004;173(12):7548–7555. doi: 10.4049/jimmunol.173.12.7548. [DOI] [PubMed] [Google Scholar]
- 25.Phipps S, Lam CE, Foster PS, Matthaei KI. The contribution of toll-like receptors to the pathogenesis of asthma. Immunol Cell Biol. 2007;85(6):463–470. doi: 10.1038/sj.icb.7100104. [DOI] [PubMed] [Google Scholar]
- 26.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18(5):684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 27.Vanhoutte F, Paget C, Breuilh L, et al. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol Lett. 2008;116(1):86–94. doi: 10.1016/j.imlet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Buchta CM, Bishop GA. Toll-like receptors and B cells: functions and mechanisms. Immunol Res. 2014;59(1–3):12–22. doi: 10.1007/s12026-014-8523-2. [DOI] [PubMed] [Google Scholar]
- 29.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12(4):282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komiya A, Nagase H, Okugawa S, et al. Expression and function of toll-like receptors in human basophils. Int Arch Allergy Immunol. 2006;140(Suppl 1):23–27. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 31.Suurmond J, Stoop JN, Rivellese F, Bakker AM, Huizinga TW, Toes RE. Activation of human basophils by combined toll-like receptor- and FcepsilonRI-triggering can promote Th2 skewing of naive T helper cells. Eur J Immunol. 2014;44(2):386–396. doi: 10.1002/eji.201343617. [DOI] [PubMed] [Google Scholar]
- 32.Brar T, Nagaraj S, Mohapatra S. Microbes and asthma: the missing cellular and molecular links. Curr Opin Pulm Med. 2012;18(1):14–22. doi: 10.1097/MCP.0b013e32834dccc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto K, Inoue H. Viral infections in asthma and COPD. Respir Investig. 2014;52(2):92–100. doi: 10.1016/j.resinv.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Yang J, Deng H, et al. Respiratory syncytial virus infection modulates interleukin8 production in respiratory epithelial cells through a transcription factoractivator protein1 signaling pathway. Mol Med Rep. 2014;10(3):1443–1447. doi: 10.3892/mmr.2014.2357. [DOI] [PubMed] [Google Scholar]
- 35.Pritchard AL, White OJ, Burel JG, Carroll ML, Phipps S, Upham JW. Asthma is associated with multiple alterations in anti-viral innate signalling pathways. PLoS One. 2014;9(9):e106501. doi: 10.1371/journal.pone.0106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adner M, Starkhammar M, Georen SK, Dahlen SE, Cardell LO. Toll-like receptor (TLR) 7 decreases and TLR9 increases the airway responses in mice with established allergic inflammation. Eur J Pharmacol. 2013;718(1–3):544–551. doi: 10.1016/j.ejphar.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 38.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26(7):381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141(3):347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez I, Romero J, Rodriguez BL, Perez-Castro R, Rojas A. The immunobiology of the receptor of advanced glycation end-products: trends and challenges. Immunobiology. 2013;218(5):790–797. doi: 10.1016/j.imbio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Sukkar MB, Ullah MA, Gan WJ, et al. RAGE: a new frontier in chronic airways disease. Br J Pharmacol. 2012;167(6):1161–1176. doi: 10.1111/j.1476-5381.2012.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kierdorf K, Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94(1):55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 43.Rouhiainen A, Kuja-Panula J, Tumova S, Rauvala H. RAGE-mediated cell signaling. Methods Mol Biol. 2013;963:239–263. doi: 10.1007/978-1-62703-230-8_15. [DOI] [PubMed] [Google Scholar]
- 44.Shim EJ, Chun E, Lee HS, et al. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012;42(6):958–965. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee CC, Lai YT, Chang HT, et al. Inhibition of high-mobility group box 1 in lung reduced airway inflammation and remodeling in a mouse model of chronic asthma. Biochem Pharmacol. 2013;86(7):940–949. doi: 10.1016/j.bcp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Liang Y, Hou C, Kong J, et al. HMGB1 binding to receptor for advanced glycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol Cell Biochem. 2015;405(1–2):63–71. doi: 10.1007/s11010-015-2396-0. [DOI] [PubMed] [Google Scholar]
- 47.Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112(4):605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105(4):519–525. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y, Jiang YQ, Wang WX, et al. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum Immunol. 2012;73(11):1171–1174. doi: 10.1016/j.humimm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Milutinovic PS, Alcorn JF, Englert JM, Crum LT, Oury TD. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. Am J Pathol. 2012;181(4):1215–1225. doi: 10.1016/j.ajpath.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Ullah MA, Loh Z, Gan WJ, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014 Aug;134(2):440–50. doi: 10.1016/j.jaci.2013.12.1035. Most recent study which highlights the role of HMGB-1 and RAGE in airway inflammation. [DOI] [PubMed] [Google Scholar]
- 52.Akirav EM, Henegariu O, Preston-Hurlburt P, Schmidt AM, Clynes R, Herold KC. The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma. PLoS One. 2014;9(4):e95678. doi: 10.1371/journal.pone.0095678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hancock DB, Eijgelsheim M, Wilk JB, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42(1):45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42(1):36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oczypok EA, Milutinovic PS, Alcorn JF, et al. Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells. J Allergy Clin Immunol. 2015 Sep;136(3):747–756.e4. doi: 10.1016/j.jaci.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taniguchi A, Miyahara N, Waseda K, et al. Contrasting roles for the receptor for advanced glycation end-products on structural cells in allergic airway inflammation vs. airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L789–800. doi: 10.1152/ajplung.00087.2015. [DOI] [PubMed] [Google Scholar]
- 57.Mahajan N, Dhawan V. Receptor for advanced glycation end products (RAGE) in vascular and inflammatory diseases. Int J Cardiol. 2013;168(3):1788–1794. doi: 10.1016/j.ijcard.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 58.El-Seify MY, Fouda EM, Nabih ES. Serum level of soluble receptor for advanced glycation end products in asthmatic children and its correlation to severity and pulmonary functions. Clin Lab. 2014;60(6):957–962. doi: 10.7754/clin.lab.2013.130418. [DOI] [PubMed] [Google Scholar]
- 59.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3(6):445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 60.Molloy EJ. Triggering Receptor Expressed on Myeloid Cells (TREM) family and the application of its antagonists. Recent Pat Antiinfect Drug Discov. 2009;4(1):51–56. doi: 10.2174/157489109787236292. [DOI] [PubMed] [Google Scholar]
- 61.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213(9–10):701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Paradowska-Gorycka A, Jurkowska M. Structure, expression pattern and biological activity of molecular complex TREM-2/DAP12. Hum Immunol. 2013;74(6):730–737. doi: 10.1016/j.humimm.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Z, Oh SY, Cho YS, Zhang L, Kim YK, Zheng T. Tyrosine phosphatase SHP-1 in allergic and anaphylactic inflammation. Immunol Res. 2010;47(1–3):3–13. doi: 10.1007/s12026-009-8134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mittal Y, Pavlova Y, Garcia-Marcos M, Ghosh P. Src homology domain 2-containing protein-tyrosine phosphatase-1 (SHP-1) binds and dephosphorylates G(alpha)-interacting, vesicle-associated protein (GIV)/Girdin and attenuates the GIV-phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway. J Biol Chem. 2011;286(37):32404–32415. doi: 10.1074/jbc.M111.275685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3(122):ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan Z, Syed MA, Panchal D, et al. Triggering receptor expressed on myeloid cells 1 (TREM-1)-mediated Bcl-2 induction prolongs macrophage survival. J Biol Chem. 2014;289(21):15118–15129. doi: 10.1074/jbc.M113.536490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Arts RJ, Joosten LA, van der Meer JW, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93(2):209–215. doi: 10.1189/jlb.0312145. Article highlights shared interactions and signaling mechanisms of TLRs and TREM family members. [DOI] [PubMed] [Google Scholar]
- 69.Hommes TJ, Hoogendijk AJ, Dessing MC, et al. Triggering receptor expressed on myeloid cells-1 (TREM-1) improves host defence in pneumococcal pneumonia. J Pathol. 2014;233(4):357–367. doi: 10.1002/path.4361. [DOI] [PubMed] [Google Scholar]
- 70.Chung DH, Seaman WE, Daws MR. Characterization of TREM-3, an activating receptor on mouse macrophages: definition of a family of single Ig domain receptors on mouse chromosome 17. Eur J Immunol. 2002;32(1):59–66. doi: 10.1002/1521-4141(200201)32:1<59::AID-IMMU59>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 71.Klesney-Tait J, Colonna M. Uncovering the TREM-1-TLR connection. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1374–6. doi: 10.1152/ajplung.00415.2007. [DOI] [PubMed] [Google Scholar]
- 72.Syed MA, Joo M, Abbas Z, et al. Expression of TREM-1 is inhibited by PGD2 and PGJ2 in macrophages. Exp Cell Res. 2010;316(19):3140–3149. doi: 10.1016/j.yexcr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Buckland KF, Ramaprakash H, Murray LA, et al. Triggering receptor expressed on myeloid cells-1 (TREM-1) modulates immune responses to Aspergillus fumigatus during fungal asthma in mice. Immunol Invest. 2011;40(7–8):692–722. doi: 10.3109/08820139.2011.578270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klesney-Tait J, Keck K, Li X, et al. Transepithelial migration of neutrophils into the lung requires TREM-1. J Clin Invest. 2013;123(1):138–149. doi: 10.1172/JCI64181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu K, Byers DE, Jin X, et al. TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J Exp Med. 2015;212(5):681–697. doi: 10.1084/jem.20141732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194(8):1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ito H, Hamerman JA. TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur J Immunol. 2012;42(1):176–185. doi: 10.1002/eji.201141679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turnbull IR, Gilfillan S, Cella M, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177(6):3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 79.Gao X, Dong Y, Liu Z, Niu B. Silencing of triggering receptor expressed on myeloid cells-2 enhances the inflammatory responses of alveolar macrophages to lipopolysaccharide. Mol Med Rep. 2013;7(3):921–926. doi: 10.3892/mmr.2013.1268. [DOI] [PubMed] [Google Scholar]
- 80.Sandquist M, Wong HR. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev Clin Immunol. 2014;10(10):1349–1356. doi: 10.1586/1744666X.2014.949675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ten Oever J, Tromp M, Bleeker-Rovers CP, et al. Combination of biomarkers for the discrimination between bacterial and viral lower respiratory tract infections. J Infect. 2012;65(6):490–495. doi: 10.1016/j.jinf.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Shi JX, Li JS, Hu R, et al. Diagnostic value of sTREM-1 in bronchoalveolar lavage fluid in ICU patients with bacterial lung infections: a bivariate meta-analysis. PLoS One. 2013;8(5):e65436. doi: 10.1371/journal.pone.0065436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye W, Hu Y, Zhang R, Ying K. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in lower respiratory tract infections: a meta-analysis. Respirology. 2014;19(4):501–507. doi: 10.1111/resp.12270. [DOI] [PubMed] [Google Scholar]
- 84.Phua J, Koay ES, Zhang D, et al. Soluble triggering receptor expressed on myeloid cells-1 in acute respiratory infections. Eur Respir J. 2006;28(4):695–702. doi: 10.1183/09031936.06.00005606. [DOI] [PubMed] [Google Scholar]
- 85.Bucova M, Suchankova M, Dzurilla M, et al. Inflammatory marker sTREM-1 reflects the clinical stage and respiratory tract obstruction in allergic asthma bronchiale patients and correlates with number of neutrophils. Mediators Inflamm. 2012;2012:628754. doi: 10.1155/2012/628754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol. 2013;56(4):739–744. doi: 10.1016/j.molimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 87•.Sakaguchi M, Murata H, Yamamoto K, et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6(8):e23132. doi: 10.1371/journal.pone.0023132. Study indicating a functional interaction between RAGE and TLRs in inflammation and the immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tadie JM, Bae HB, Jiang S, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. 2013;304(5):L342–9. doi: 10.1152/ajplung.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y, Yang Z, Gao Y, et al. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One. 2013;8(5):e64375. doi: 10.1371/journal.pone.0064375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng H, Heiderscheidt CA, Joo M, et al. MYD88-dependent and -independent activation of TREM-1 via specific TLR ligands. Eur J Immunol. 2010;40(1):162–171. doi: 10.1002/eji.200839156. [DOI] [PubMed] [Google Scholar]
- 91.Habibzay M, Saldana JI, Goulding J, Lloyd CM, Hussell T. Altered regulation of Toll-like receptor responses impairs antibacterial immunity in the allergic lung. Mucosal Immunol. 2012;5(5):524–534. doi: 10.1038/mi.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Molad Y, Pokroy-Shapira E, Carmon V. CpG-oligodeoxynucleotide-induced TLR9 activation regulates macrophage TREM-1 expression and shedding. Innate Immun. 2013;19(6):623–630. doi: 10.1177/1753425913476970. [DOI] [PubMed] [Google Scholar]
- 93.Bezemer GF, Sagar S, van Bergenhenegouwen J, et al. Dual role of Toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2012;64(2):337–358. doi: 10.1124/pr.111.004622. [DOI] [PubMed] [Google Scholar]
- 94.Hatchwell L, Collison A, Girkin J, et al. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax. 2015;70(9):854–61. doi: 10.1136/thoraxjnl-2014-205465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drake MG, Kaufman EH, Fryer AD, Jacoby DB. The therapeutic potential of Toll-like receptor 7 stimulation in asthma. Inflamm Allergy Drug Targets. 2012;11(6):484–491. doi: 10.2174/187152812803589967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nawijn MC, Motta AC, Gras R, Shirinbak S, Maazi H, van Oosterhout AJ. TLR-2 activation induces regulatory T cells and long-term suppression of asthma manifestations in mice. PLoS One. 2013;8(2):e55307. doi: 10.1371/journal.pone.0055307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gibot S, Alauzet C, Massin F, et al. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. 2006;194(7):975–983. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- 98.Derive M, Massin F, Gibot S. Triggering receptor expressed on myeloid cells-1 as a new therapeutic target during inflammatory diseases. Self Nonself. 2010;1(3):225–230. doi: 10.4161/self.1.3.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ospelt C, Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol. 2010;42(4):495–505. doi: 10.1016/j.biocel.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011;3(6):920–940. doi: 10.3390/v3060920. [DOI] [PMC free article] [PubMed] [Google Scholar]