Abstract
More than 2.5 million Americans suffer from burn injuries annually, and burn management is a major public health problem. Treatments have been developed to manage wound injuries employing skin grafts, various dressings and topical and systemic agents. However, these often achieve limited degrees of success. We previously reported that targeting the interaction of thrombospondin-1 with its signaling receptor CD47 or deletion of the genes encoding either of these proteins in mice improves recovery from soft tissue ischemic injuries as well as tissue injuries caused by ionizing radiation. We now demonstrate that the absence of CD47 improves the rate of wound closure for a focal dermal second-degree thermal injury, whereas lack of thrombospondin-1 initially delays wound closure compared to healing in wild type mice. Doppler analysis of the wounded area showed increased blood flow in both CD47 and thrombospondin-1 null mice. Accelerated wound closure in the CD47 null mice was associated with increased fibrosis as demonstrated by a 4-fold increase in collagen fraction. Wound tissue of CD47 null mice showed increased thrombospondin-1 mRNA and protein expression and TGF-β1 mRNA levels. Activation of latent TGF-β1 was increased in thermally injured CD47-null tissue as assessed by phosphor-ylation of the TGF-β1 receptor-regulated transcription factors SMAD-2 and -3. Overall these results indicate that targeting CD47 may improve the speed of healing thermal injuries, but some level of CD47 expression may be required to limit the long term TGF-β1-dependent fibrosis of these wounds.
Keywords: Thrombospondin-1, CD47, TGF-β1, Wound healing
1. Introduction
Healing of cutaneous wounds requires restoration of the barrier function of skin to avoid infection. For superficial wounds, granulation tissue organizes reconstruction of the epithelial barrier and the underlying dermis and extracellular matrix (Reinke and Sorg, 2012). First degree burns undergo a similar wound repair process, but second and third degree burns present a broad range of additional challenges for restoration of function (Kagan et al., 2013; Orgill and Ogawa, 2013). Extensive hypertrophic scarring caused by inappropriate deposition of collagen matrices can impair mobility of affected joints and limbs, damage to innervation can impair sensation and cause chronic pain, and loss of underlying dermis, adipose, and muscle tissues can necessitate complex reconstructive surgery. In addition to surgical grafting of skin or engineered skin substitutes, tissue engineering research is developing approaches to employ engineered matrices to regenerate these complex tissues and use stem cells to repopulate engineered or de-cellularized natural matrices with the appropriate differentiated cell types to produce a dermis with functioning vascular beds and sensory innervation (Blais et al., 2013).
Our recent studies have identified CD47, the ubiquitous cell surface receptor for the matricellular protein thrombospondin-1 (TSP1), as a useful therapeutic target for enhancing wound repair and tissue regeneration (Soto-Pantoja et al., 2013b). Ischemic skin flaps in mice lacking either TSP1 or CD47 show enhanced survival of ischemic dorsal skin flaps (Isenberg et al., 2007a). Correspondingly, treating such skin flaps in wild type mice and miniature pigs using antibodies to TSP1 or CD47 that block their interaction or with antisense morpholinos to suppress CD47 expression enhances flap survival (Isenberg et al., 2007b, 2008d). The therapeutic benefit of blocking TSP1/CD47 signaling extends to full-thickness skin grafts (Isenberg et al., 2008c), which suggests that CD47 blockade could be applied to skin grafting in the context of surgical burn management (Orgill and Ogawa, 2013).
Mechanistically, these benefits are associated with enhanced nitric oxide/cGMP signaling, which acutely preserves blood flow in ischemic tissues and enhances vascular cell survival, motility, and proliferation to restore tissue perfusion to ischemic areas (Isenberg et al., 2006, 2007a; Soto-Pantoja et al., 2012). Further, targeting TSP1-CD47 signaling increases angiogeneic activity in wounds through NO-dependent and -independent mechanisms (Isenberg et al., 2005; Kaur et al., 2010; Roberts et al., 2012). CD47 blockade also enhances autophagy responses in cells under stress, which increases the ability of cells and tissues to survive stress caused by ionizing radiation (Soto-Pantoja et al., 2012; Soto-Pantoja et al., 2013a). At a cellular level, CD47 blockage also enhances the expression of c-Myc and the stem cell transcription factors Sox2, Oct4, and Klf4 (Kaur et al., 2013). CD47 blockade thus induces differentiated cells to reprogram into multipotent stem cells. Tissues in mice lacking CD47 exhibit increased stem cell numbers, and systemic blockade of CD47 in wild type (WT) mice increased expression of the stem cell transcription factors in vivo (Kaur et al., 2013).
These results suggest that therapeutic blockade of CD47 could improve recovery from burns in several ways including improving tissue perfusion in tissues surrounding the burn, increasing survival of cells subjected to thermal injury, and enhancing tissue regeneration through promoting stem cell numbers and function. To study the role of TSP1 and CD47 in burn injuries and explore therapeutic benefits of CD47 blockade we have established a dermal second degree thermal injury model in mice and used this model to compare healing of such injuries in WT, TSP1-null and CD47-null mice.
2. Results
2.1. Deficiency of CD47 improves wound healing after thermal injury
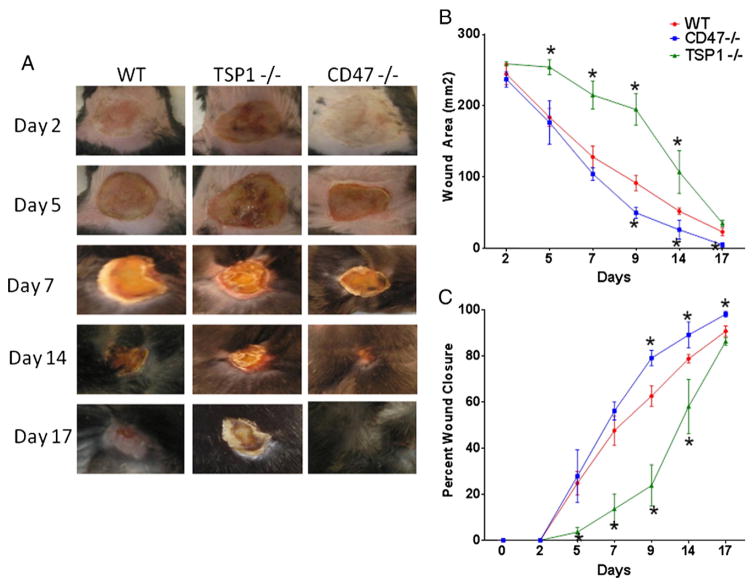
A uniform second degree thermal injury was inflicted in the dorsal dermis of 10 to 12 week old male mice of WT, CD47 null, and TSP1 null backgrounds (Fig. 1A, B, C). A delay in wound healing was observed initially in TSP1 null mice over WT and CD47 null mice. At later times after injury, CD47 null mice showed a tendency to heal faster than WT mice, with approximately 50% in wound reduction area at day 9 (80 ± 11 vs. 44 ± 2 vs. 194 ± 22 mm2 WT, CD47 null and TSP1 null respectively p < 0.05). At two weeks 40% of CD47 null mice had completely healed their wounds, with almost complete healing at day 17 (22 ± 5, 6 ± 4, 36 ± 10 mm2 WT, CD47−/− and TSP1−/− respectively). All CD47−/− animals showed complete healing by day 21 versus 20% of WT mice and none from the TSP1 null group (5 ± 3, 0 ± 0, 7 ± 3 mm2 WT, CD47−/−, TSP1−/− respective area). CD47 null animals had a higher percent rate of wound closure when compared to WT and TSP1−/−(Fig. 1C). This demonstrates that deficiency of CD47 confers an advantage in wound healing after thermal injury.
Fig. 1. Role of the TSP1/CD47 axis in thermal wound healing.
Thermal wounds were inflicted in the dorsal area of WT, CD47−/− and TSP1−/− mice, left panel (A) shows representative pictures of wound healing. The size of each wound was measured using a caliper. Area was calculated using the formula A = πa (B). Area and percent wound closure (C) were normalized from WT wounded measurements. N = 6 *p < 0.05.
2.2. CD47 deficiency increases collagen deposition to improve wound healing
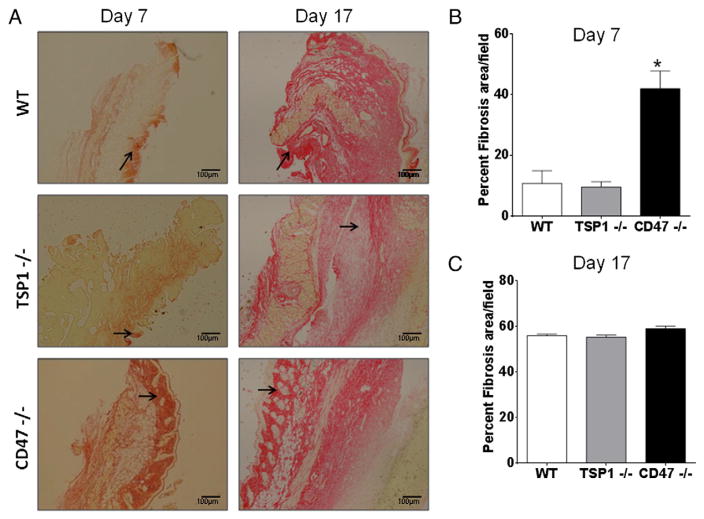
To define the mechanism that provides an advantage in wound healing in CD47-deficient mice we examined collagen deposition in skin epithelium, which is an important process for wound closure. Sections of mouse skin were stained with picrosirius red to detect collagen fibers. We quantified collagen deposition using imaging software with a pixel counter to quantify stained fibers (Cook et al., 2010). As observed in Fig. 2A, stronger deposits of collagen were observed at day 7 in wounded skin of CD47 null mice when compared to WT. Consistent with their delayed wound closure, TSP1 null mice showed the least staining of all three groups. On the other hand collagen deposition increased in WT and TSP1−/− animals by day 17. Quantification demonstrated that deficiency of CD47 increased collagen deposition by more than 30% at day 7 over that in WT skin (Fig. 2B). By the end of the study all groups had similar levels of collagen deposition (Fig. 2C). This suggests that the increased rate of wound healing in CD47−/− mice is due in part to an early deposition of collagen to improve wound closure.
Fig. 2. CD47 deficiency regulates collagen deposition to improve wound healing.
Skin sections of tissue harvested at day 7 and 17 were stained with picrosirius red to detect collagen fibers denoted by arrows (A). Collagen deposition was quantified by a computer-assisted counting technique with a pixel counter at day 7 (B) and day 17 (C) N = 3 *p < 0.05.
2.3. CD47 deficiency increases TGF-β1 expression
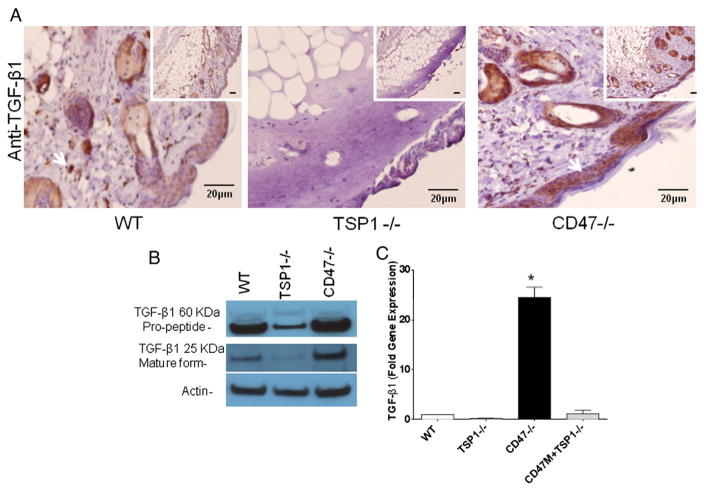
Since TGF-β1 is a strong inducer of type I collagen synthesis and activation of latent TGF-β1 can be mediated by TSP1 (Sweetwyne and Murphy-Ullrich, 2012), we assessed its expression wounded tissue. Par-affin embedded tissue sections of wounded skin were stained with an antibody to TGF-β1 to determine protein expression. As observed in Fig. 3A CD47−/− mice showed increased TGF-β1 immunoreactivity when compared to WT and TSP1−/− tissue. TSP1−/− sections had almost undetectable levels of tissue TGF-β1 expression. Because TGF-β1 is secreted from cells in a latent form, we determined whether the increased TGF-β1 mRNA and protein expression is associated with elevated levels of the cleaved mature form. As observed in Fig. 3B WT and CD47−/− mice showed similar levels of the latent propeptide form of TGF-β1, whereas TSP1−/− mice showed reduced protein levels. On the other hand wounded skin from CD47−/− mice showed increased levels of the mature form of TGF-β1 over WT and TSP−/− respectively. A 20-fold increase in TGF-β1 mRNA was observed in injured CD47 null tissue relative to WT mRNA expression in the wounds, which was consistent with the observed differences in tissue immunoreactivity. Conversely, TSP1−/− mouse tissue had a 5-fold lower TGF-β1 mRNA expression than WT (Fig. 3C). This indicates that the elevated wound closure observed in CD47 deficient mice is associated with increased TGF-β1 expression and proteolytic processing of the propeptide form of this protein.
Fig. 3. CD47 and TSP1 differentially regulate TGF-β1 expression and processing in thermal wounds.
Paraffin embedded wounded skin tissue sections were harvested at day 7 and stained using an antibody to TGF-β1 (A). Expression of the propeptide and mature form of TGF-β1 protein was measured in tissue extracts from wounded skin by western blotting (B). RNA was isolated from wounded skin from all three genotypes, TSP1−/− mice were injected with antisense morpholino to CD47 (CD47M) and TGF-β1 expression was determined by RT-PCR (C). Expression was normalized to that in injured WT tissue (mean ± SD, N = 3 *p < 0.05).
2.4. CD47 deficiency regulates SMAD signaling to improve wound healing
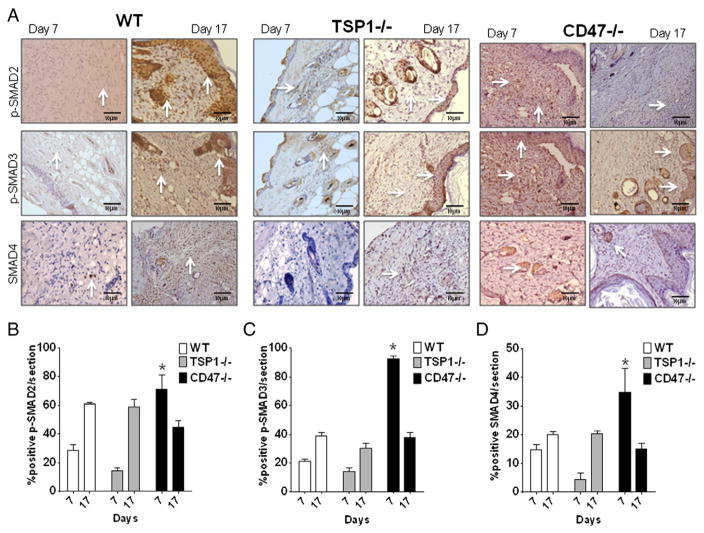
Because mature TGF-β1 remains latent while in a noncovalent complex with latency-associated peptide, activation is best assessed functionally by its signaling activity. TGF-β1 signaling was assessed by determining phosphorylation of the downstream SMAD transcription factors. Tissue sections of wounded skin at 7 days were stained with antibodies to phosphorylated SMAD2 and SMAD3. These SMADs form part of a complex that translocates to the nucleus and induces transcription of target genes including type I collagen (Penn et al., 2012). As observed in Fig. 4, p-SMAD2 and p-SMAD3 were elevated over 65% and 85%, respectively (Fig. 4A and B), in wounded CD47-deficient tissue over WT. Low immunoreactivity (10%) was observed in TSP1 null wounded skin as expected based on the role of TSP1 in latent TGF-β1 activation. Therefore, the increase in mRNA and protein expression of TGF-β1 is accompanied by increased activation of the latent complex in CD47-deficient mice, which is consistent with the increased collagen synthesis and accelerated wound healing after burn injury.
Fig. 4. CD47 deficiency increases functional TGF-β activity as assessed by SMAD signaling.
Wounded tissue sections were paraffin embedded and stained using specific antibodies to phosphorylated SMAD2, phosphorylated SMAD3, and SMAD4 (A) brown stain denotes immunoreactivity (arrows). Tissue sections were scored under light microscope using cell counter mode with ImageJ software, and the percentages of cells positive for p-SMAD2 (B), p-SMAD3 (C), and SMAD4 (D) are presented as mean ± SD, N = 4.
Long term effects of TGF-β activation were determined by measuring p-SMAD2 and p-SMAD3 immunoreactivity in tissue harvested at day 17. As observed in Fig. 4A, p-SMAD2 and p-SMAD3 were attenuated in CD47 null tissue when compared to day 7. On the other hand WT and TSP1−/− tissue showed an increase in p-SMAD2 and p-SMAD3 signaling at the later time point correlated with the delayed kinetics of wound healing (Fig. 4A, B, C *p < 0.05). The TGF-β receptor-regulated SMADs act through mediator SMADs and form complexes that translocate into the nucleus to regulate gene expression. We observed over 30% increased protein expression of mediator SMAD4 in CD47 null injured skin when compared to WT and TSP1 null. The expression of SMAD4 in CD47 null wounded tissue was down to less than 20% by day 17 (Fig. 4D). On the other hand, elevated SMAD4 immunoreactivity was observed in WT tissue at day 7 over TSP1 null tissue. However, similar immunoreactivities were observed between these two groups at day 17 (Fig. 4D). This indicates that the activation of p-SMAD2/3 leads to the formation of complexes with SMAD4 to ultimately regulate gene expression. Consistent with reported defects in latent TGF-β1 activation in TSP1 null mice (Sweetwyne and Murphy-Ullrich, 2012), these mice had less collagen deposition and delayed wound closure.
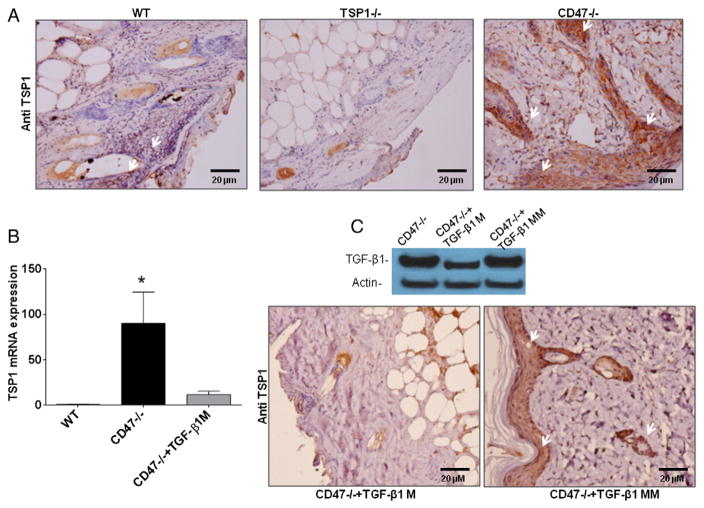
2.5. CD47 deficiency increases expression of TSP1 in wounded skin
TSP1 is a direct mediator of latent TGF-β1 activation and a target gene of TGF-β signaling (Murphy-Ullrich and Poczatek, 2000; Young and Murphy-Ullrich, 2004). Immunohistochemical analysis of wounded tissue from the CD47-deficient mice using antibody A6.1 showed stronger TSP1 inmmunoreactivity when compared to WT wounds (Fig. 5A). Further analysis indicated that TSP1 mRNA expression was up-regulated 90-fold over WT in wounded CD47 null skin tissue (Fig. 5B). This indicated that elevated levels of TSP1 could account for the increased latent TGF-β1 activation in CD47-deficient mice, conferring an advantage in the healing of thermal injury wounds. To determine whether the increased TSP1 expression in CD47−/− wound tissue is mediated by TGF-β signaling, an anti-sense morpholino to decrease TGF-β1 expression or a mismatched control morpholino was administered intradermally in CD47-null mice at day 6, and tissue was harvested at day 7. As observed in Fig. 5C, the TGF-β1 morpholino specifically decreased expression of TGF-β1 and abrogated the expression of TSP1 in injured CD47 null tissue. The mismatched control morpholino did not affect TGF-β1 protein expression, and tissue sections showed similar TGF-β1 immunoreactivity as injured CD47−/− tissue sections (Fig. 3). Therefore, the up-regulation of TSP1 in thermally injured tissue of CD47 null mice is mediated at least in part by TGF-β1 signaling.
Fig. 5. TSP1 expression is up-regulated in injured CD47 deficient skin tissue in a TGF-β1-dependent manner.
Wounded tissue sections were paraffin embedded and stained with TSP1 antibody A6.1 (A). Arrows denote positive staining in dermal epithelium. mRNA was isolated from wounded tissue extracts and subjected to quantitative RT-PCR using specific primers for TSP1. Expression is normalized to that in injured WT tissue (mean ± SD, N = 3 *p < 0.05) (B). Wounded CD47−/− mice were injected with antisense TGF-β1 morpholino (TGF-β1M) or mismatched control morpholino (TGF-β1MM), and tissue was harvested 24 h later for western analysis of TGF-β1 expression (C, upper panel) and immunohistochemical analysis of TSP1 expression (C, lower panel).
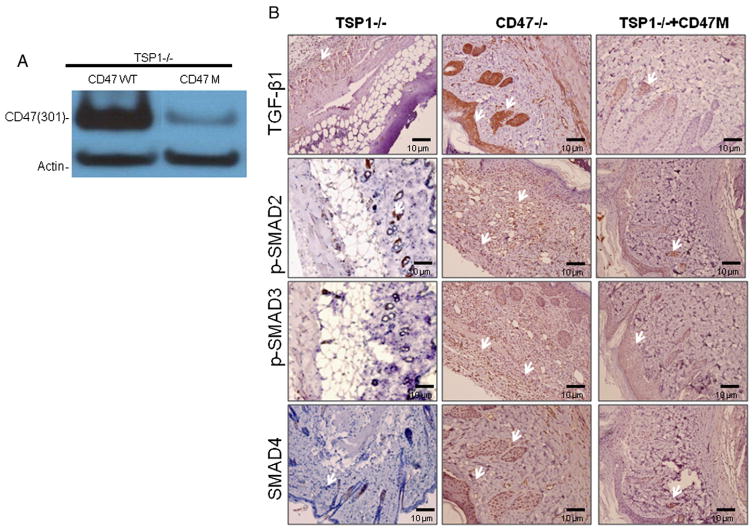
2.6. Activation of TGF-β signaling in CD47-deficient conditions is dependent on TSP1 expression
To determine whether the observed regulation of TGF-β by CD47 is dependent on TSP1 expression we injected TSP1 null mice with an anti-sense morpholino to CD47. CD47 expression knockdown was confirmed in wounded skin of the TSP1 null mice (Fig. 6A). As observed in Fig. 6B deficiency of CD47 in the absence of TSP1 did not up-regulate expression of TGF-β1 protein expression or downstream SMAD signaling in wounded skin tissue. Administration of CD47 morpholino in TSP1 null mice increased TGF-β1 mRNA levels to that of WT mice but did not reproduce the 20-fold up-regulation of TGF-β1 mRNA observed in CD47 null tissue (Fig. 3C). This shows that the up-regulation of TGF-β by decreasing CD47 expression is dependent at least in part on TSP1 expression.
Fig. 6. Activation of TGF-β1 signaling in CD47 deficient conditions is dependent on TSP1 expression.
Wounded TSP1 null mice were injected I.P. with CD47 morpholino (CD47 M) at day 5, and tissue was harvested at day 7. Efficiency of the CD47 morpholino was confirmed by western blotting using murine CD47 antibody clone map 301 (A). Tissue sections were par-affin embedded and stained with specific antibodies to TGF-β1, p-SMAD2, p-SMAD3 and SMAD4 (B). Arrows denote immunoreactivity by brown stain.
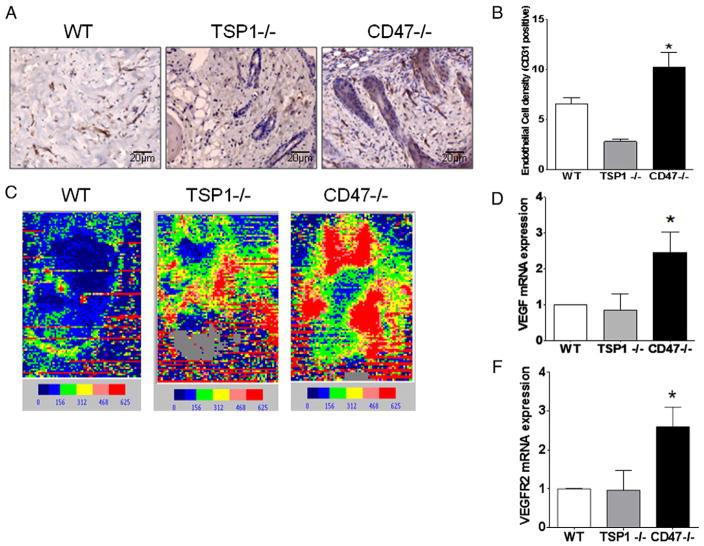
2.7. CD47 deficiency regulates blood flow to improve wound healing
Another important aspect of wound healing to restore viability of the tissue is an increase in vascularization and blood flow to the wound. As observed in Fig. 7A staining with CD31 antibody shows endo-thelial vessel formation in wounded skin. Counting vessel “hot spots” denoted by the brown stain indicated that skin from wounded CD47 deficient animals had a 47% (p < 0.05) increase in vessel density when compared to WT (Fig. 7B). Surprisingly, skin from wounded TSP1 null animals showed the lowest density among all group of animals (Fig. 7A and B). Laser Doppler analysis of tissue of thermal injury was performed under the same conditions as in our previous studies (Isenberg et al., 2007b). CD47 deficient animals demonstrated significantly more blood flow. Interestingly, increased blood flow was also observed in TSP1 null mice over WT (Fig. 7C). Analysis of the tissue indicated that deficiency of CD47 caused three-fold and two-fold increases in VEGF and VEGFR2 mRNA levels, respectively. No significant changes were observed between WT and TSP1 null tissue. Since VEGF is a strong inducer of angiogenesis and NO-dependent vasodilation, the increase in blood flow may be due in part to an increase in tissue vascularization mediated by VEGF/VEGFR2 activation (Kaur et al., 2010). In this case, TSP1 and CD47 null mice presumably have similar laser Doppler phenotypes because both null mice are known to have enhanced NO-dependent tissue perfusion under stress (Isenberg et al., 2006, 2007b). These data are consistent with our report that basal and thermally enhanced skin blood flow is limited by CD47 in young and old mice (Rogers et al., 2012).
Fig. 7. CD47 deficiency increases blood flow and VEGF/VEGFR2 signaling to improve wound healing.
Seven days after inflicting thermal wounds, skin was excised, and paraffin embedded sections were stained using an antibody to CD31 (A). Endothelial vessel density was determined by counting vessel “hot spots” delineated by the CD31 stained cells observed at 20× magnification under light microscopy (B). One week after injury, mice were subjected to laser Doppler imaging to measure blood flow, and representative images are presented (C). mRNA was isolated from wounded tissue extracts and subjected to quantitative RT-PCR using specific primers to VEGF and VEGFR2, using mouse actin as a gene control (D and F).
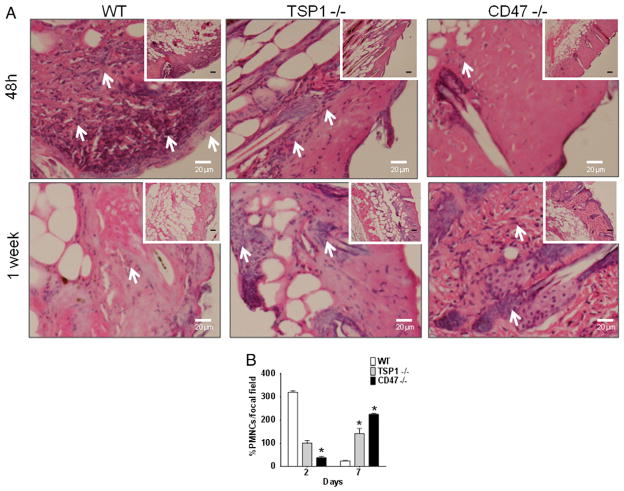
2.8. CD47 deficiency regulates wound healing inflammatory responses
The ability of injured tissue to recruit inflammatory cells during wound healing is important to remove dead cells and prevent infection. Tissue sections were paraffin embedded and stained with hematoxylin and eosin to determine tissue composition. At 48 h post-injury, WT tissue exhibited a pronounced inflammatory response characterized by the presence of polymorphonuclear cells (Fig. 8A and B). TSP1 null tissue showed an attenuated response, whereas injured CD47 null tissue showed a noticeably reduced inflammatory response at 48 h (Fig. 8A and B). On the other hand, at one week we observed a reduction in pro-inflammatory cells in WT mice and increased numbers of these cells in CD47 null tissue (Fig. 8A and B). A slight increase in proinflammatory cells was observed in the TSP1 null mice tissue at one week post-injury (Fig. 8A and B).
Fig. 8. Deficiency of CD47 regulates inflammatory response in healing wounds.
Wounded skin sections at 48 h and at one week post wound infliction were stained using hematoxylin and eosin to determine tissue structure (A). Polymorphonuclear cells present in epithelium are denoted by arrows and were quantified under light microscopy(B). N = 3 *p < 0.05.
3. Discussion
Expression of TSP1 is characteristically transiently induced during wound repair (Agah et al., 2002; Bornstein et al., 2004). Consistent with the delayed closure of dermal burn wounds that we observed in TSP1 null mice subjected to a dermal thermal injury, delayed wound closure was previously reported for TSP1 null mice and WT mice treated with antisense TSP1 oligomers in a simple excisional dermal wound model (DiPietro et al., 1996; Agah et al., 2002). In TSP1 null mice that delay was associated with decreased macrophage recruitment and levels of the chemotactic chemokine MCP1. Conversely, transgenic mice overexpressing TSP1 in skin driven by a keratin-14 promoter exhibited delayed healing of full-thickness skin wounds, which was attributed to inhibited fibroblast migration and inhibited wound angio-genesis (Streit et al., 2000). Our wound TSP1 null perfusion data is consistent with the latter study. Streit and coworkers concluded that angiogenesis was the predominant factor that governed wound closure, but despite similar enhancement of tissue perfusion in burn wounds of CD47 null and TSP1 null mice, we observed opposite effects of deleting these genes on the rate of closure of burn wounds. Therefore, factors in addition to angiogenesis must be considered to explain these divergent phenotypes.
Given the well-defined role of TSP1 in activating latent TGF-β1 (Nor et al., 2005; Sweetwyne and Murphy-Ullrich, 2012), it is not surprising that burn wounds in TSP1 null mice have decreased collagen deposition. However, the increased collagen deposition and associated TGF-β signaling in the CD47 null wounds was unexpected. Remarkably, this appears to result from an abnormal accumulation of TSP1 in the healing CD47 null wounds. Since CD47 is the necessary receptor to inhibit NO by TSP1 binding, NO signaling pathways that stimulate vascular remodeling are blind to this accumulated TSP1. Even though TSP1 can regulate vascular NO signaling through the TSP1 receptor CD36 (Isenberg et al., 2005) our previous studies established that regulation of NO signaling by engaging CD36 requires CD47 expression (Isenberg et al., 2007b). Therefore, the elevated TSP1 in the CD47 null wounds cannot limit proangiogenic NO signaling. However, the elevated TSP1 can activate latent TGF-β1 and consequently increase deposition of collagen in the healing burn wound. Increased TGF-β signaling in the CD47 null wound in turn creates positive feedback to further increase TSP1 expression.
The question remains — how does the absence of CD47 lead to accumulation of TSP1 in the extracellular matrix? A previous study of TSP1 trafficking in T cells suggests one possibility (Li et al., 2006). Inhibition studies using a CD47 binding peptide derived from TSP1 suggested that CD47 cooperates with the LRP1/calreticulin pathway to clear TSP1 from the surface of activated T cells. Our data supports this model in that the absence of CD47 may similarly impair the uptake of TSP1 by cells in the healing burn wound. This could explain why TSP1 protein accumulates in the matrix of healing CD47 null burn wounds but does not directly account for the accumulation of TSP1 mRNA in these wounds. The latter presumably is secondary to the elevated TGF-β signaling, which is known to induce TSP1 mRNA levels independent of the Thbs1 promoter stabilizes the message (Okamoto et al., 2002). and by additional pathways that may be mediated by additional growth factors (Penttinen et al., 1988).
Although TSP1 is a well-known activator of latent TGF-β1, our data indicates that TSP1 and CD47 signaling can also regulate TGF-β1 mRNA and protein expression. A previous analysis in uninjured liver did not find basal differences in TGF-β1 mRNA and protein levels, although mRNA levels were moderately higher in the TSP1 null 3–6 h after partial hepatectomy (Hayashi et al., 2012). Similarly, no differences in total TGF-β1 protein levels were reported in analyses of TSP1 null bone marrow and spleen (Evrard et al., 2011). Our data indicates that CD47 signaling in the context of a burn injury negatively regulates TGF-β1 gene expression both in the absence and presence of TSP1. In addition, TSP1 appears to positively regulate TGF-β1 mRNA in this context. Further studies are needed to define a mechanism and to determine whether CD47 regulates TGF-β1 gene expression in other physiological and pathological situations.
The divergence between CD47 null and TSP1 null phenotypes in this thermal injury model differs from our previously published ischemia, ischemia-reperfusion, and radiation injury models (Isenberg et al., 2007b, 2008a, 2008b, 2008c). In dermal and hind limb fixed ischemia models and a full thickness skin grafting model TSP1 signaling through CD47 redundantly inhibits the NO-cGMP signaling cascade, which is the dominant pathway that limits restoration of tissue perfusion and ultimate survival. Therefore, lacking either the necessary receptor CD47 or its ligand TSP1 results in the same enhancement of NO-dependent tissue survival. For injuries caused by ionizing radiation, the dominant pathway is induction of autophagy, which is limited by TSP1 signaling via CD47 (Soto-Pantoja et al., 2012). Thus, CD47 and TSP1 null mice also have similar radioresistant phenotypes (Isenberg et al., 2008a). Notably, CD47 null mice display a moderately stronger radioresistant phenotype than TSP1 null mice. This could implicate roles of other TSP1 receptors that oppose the protective effects of lost CD47 signaling. Alternatively, additional indirect effects of losing CD47 such as those revealed by the present thermal injury model may play a role. Results from the thermal injury model suggest that the TGF-β pathway is regulated in unexpected ways by CD47 as well as by TSP1, and this may explain divergent responses to blocking CD47 versus TSP1 in this injury model.
4. Experimental procedures
Thrombospondin-1 null (Lawler et al., 1998) CD47 null mice (Lindberg et al., 1996) were extensively back-crossed onto a C57Bl/6J background, and WT mice C57Bl/6J were obtained from Jackson Labs. All mice were bred in the same vivarium before use to ensure consistent microbiomes. Care and handling of animals was in accordance with protocol LP-025 approved by the Animal Care and Use Committee of the National Cancer Institute.
4.1. Model of thermal injury
10 to 12 week old WT, TSP1 null and CD47 null mice in the C57Bl/6 background were subjected to general anesthesia induced with 4% isoflurane and maintained by nose cone with 2.5% isoflurane. Core temperature of the mice was maintained by using a heating pad. Animals were prepped by shaving a small area corresponding to the thermal injury location and received a full thickness (2nd degree deep dermal) contact burn to the dorsal surface causing a wound of 2.8 cm2. This corresponds to 3.5% of total body surface. Wounds were inflicted using a 1.9-cm diameter brass rod heated to 95 °C and applied to the prepped area for 10 seconds. The animals were given subcutaneous buprenorphine 2 mg/kg body weight 30 minutes prior to thermal insult and were administered lactated Ringer’s solution (0.1 ml/g body weight) immediately post burn. Burn wounds were dressed with topical triple antibiotic (bacitracin, neomycin nd polymyxin B) prilocaine, xeroform gauze and covered with adhesive bandages. Animals were allowed to recover from anesthesia in their cages with feed and water provided ad libitum. Post-burn analgesia was provided by subsequent buprenorphine injections every four hours for 24 h after wound application. A standard regime of wound care was provided, which included ongoing debridement of the burn under isoflurane and daily bacitracin application, similar to treatment human patients receive post burn injury. The diameter of each wound was measured, and area was calculated by using the formula to determine the area of an ellipse, A = πab where a is one half of the major axis and b is one half of the ellipse’s minor axis.
4.2. Morpholino knockdown of TGF-β1 and CD47
Mice were wounded and treated as specified in 4.2. For knockdown of TGF-β1, mice were injected intradermally with 10 μM TGF-β1 >antisense morpholino (AACCCTCGGCAAAGGTGGGATGC) in saline using a 27 Ga. needle. For CD47 knockdown mice were injected intraperitoneally with CD47 antisense morpholino (CGTCACAGGCAGGACCCACTGCCCA) as described previously (Soto-Pantoja et al., 2013a).
4.3. Doppler analysis
Mice with inflicted wounds were subjected to Doppler imaging a week after injury to measuring blood flow in the wound. Core temperature was monitored via rectal probe and maintained at 37 °C by a heated stage. Anesthesia was obtained with 1.5% isoflurane. A MoorLD1–2λ scanner (Moor Instruments) was used with the following parameters: scan area, 1.6 × 2.5 cm; scan speed, 4 ms per pixel; scan time, 1 minute 54 seconds; override distance, 25 cm.
4.4. Histochemistry and immunohistochemistry
Tissue was harvested, paraffin embedded, and stained using antibodies specific for TGF-β, p-SMAD2 (s465/467), p-SMAD3 (s423/425), SMAD4 (Cell Signaling), CD31 (Abcam) or TSP1 (A6.1 Neomarkers) and was processed as reported previously (Soto-Pantoja et al., 2013a). To determine endothelial vessel density only cells delineated by immu-nostaining were counted in areas of high density or “hot spots” as shown previously (Soto-Pantoja et al., 2009) To determine collagen deposition, tissue sections were stained with picrosirius red for one h. Sections were visualized under light microscopy and quantified with computer assisted software as demonstrated previously (Cook et al., 2010). To quantify immunoreactivity to p-SMAD2, p-SMAD3 and SMAD4 sections were visualized under light microscopy, pictures were taken at 20× magnification, and positive nuclei were counted using the ImageJ software counter tool.
4.5. Quantitative RT-PCR
Wounded skin of mice was excised, and total RNA was isolated using the TRIzol following the manufacturer’s instructions. For each analysis, 5 μg of RNA was reverse-transcribed using the SuperScript III first strand synthesis kit using specific primers to mouse TGF-β, TSP1, VEGF and VEGFR2, and normalized against mouse actin. Quantitative real-time PCR was performed using SYBR Green on an MJ Research Opticon I instrument as shown previously (Soto-Pantoja et al., 2012).
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (D.D.R.), the Howard Hughes Medical Institute–NIH Research Scholars Program (H.B.S.), and by a DOD Breast Cancer Research Program Postdoctoral Fellowship BC112023 (K.L.C.).
Abbreviations
- SMAD
mothers against decapentaplegic homolog
- TGF-β1
transforming growth factor β1
- TSP1
thrombospondin-1
- WT
wild type
References
- Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais M, Parenteau-Bareil R, Cadau S, Berthod F. Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med. 2013;2:545–551. doi: 10.5966/sctm.2012-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Cook KL, Metheny-Barlow LJ, Tallant EA, Gallagher PE. Angiotensin-(1-7) reduces fibrosis in orthotopic breast tumors. Cancer Res. 2010;70:8319–8328. doi: 10.1158/0008-5472.CAN-10-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- Evrard S, Bluteau O, Tulliez M, Rameau P, Gonin P, Zetterberg E, Palmblad J, Bonnefoy A, Villeval JL, Vainchenker W, Giraudier S, Wagner-Ballon O. Thrombospondin-1 is not the major activator of TGF-beta1 in thrombopoietin-induced myelofibrosis. Blood. 2011;117:246–249. doi: 10.1182/blood-2010-07-294447. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sakai K, Baba H, Sakai T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology. 2012;55:1562–1573. doi: 10.1002/hep.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007a;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007b;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, Tsokos M, Wink DA, Roberts DD. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008a;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008b;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg. 2008c;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg. 2008d;247:860–868. doi: 10.1097/SLA.0b013e31816c4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan RJ, Peck MD, Ahrenholz DH, Hickerson WL, Holmes Jt, Korentager R, Kraatz J, Pollock K, Kotoski G. Surgical management of the burn wound and use of skin substitutes: an expert panel white paper. J Burn Care Res. 2013;34:e60–e79. doi: 10.1097/BCR.0b013e31827039a6. [DOI] [PubMed] [Google Scholar]
- Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006;108:3112–3120. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Nor JE, Dipietro L, Murphy-Ullrich JE, Hynes RO, Lawler J, Polverini PJ. Activation of latent TGF-beta1 by thrombospondin-1 is a major component of wound repair. Oral Biosci Med. 2005;2:153–161. [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Ono M, Uchiumi T, Ueno H, Kohno K, Sugimachi K, Kuwano M. Up-regulation of thrombospondin-1 gene by epidermal growth factor and transforming growth factor beta in human cancer cells — transcriptional activation and messenger RNA stabilization. Biochim Biophys Acta. 2002;1574:24–34. doi: 10.1016/s0167-4781(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Orgill DP, Ogawa R. Current methods of burn reconstruction. Plast Reconstr Surg. 2013;131:827e–836e. doi: 10.1097/PRS.0b013e31828e2138. [DOI] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- Penttinen RP, Kobayashi S, Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988;85:1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NM, Thomson AW, Isenberg JS. Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:1538–1550. doi: 10.1681/ASN.2012020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Menon J, Gallagher PE, Tallant EA. Angiotensin-(1-7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular en-dothelial growth factor. Mol Cancer Ther. 2009;8:1676–1683. doi: 10.1158/1535-7163.MCT-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Miller TW, Pendrak ML, Degraff WG, Sullivan C, Ridnour LA, Abu-Asab M, Wink DA, Tsokos M, Roberts DD. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–1642. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep. 2013a;3:1038. doi: 10.1038/srep01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Stein EV, Rogers NM, Sharifi-Sanjani M, Isenberg JS, Roberts DD. Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther Targets. 2013b;17:89–103. doi: 10.1517/14728222.2013.733699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GD, Murphy-Ullrich JE. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004;279:47633–47642. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]