Abstract
Wnt/β-catenin signalling is a widespread cell signalling pathway with multiple roles during vertebrate development. In mouse embryonic stem (mES) cells, there is a dual role for β-catenin: it promotes differentiation when activated as part of the Wnt/β-catenin signalling pathway, and promotes stable pluripotency independently of signalling. Although mES cells resemble the preimplantation epiblast progenitors, the first requirement for Wnt/β-catenin signalling during mouse development has been reported at implantation [1,2]. The relationship between β-catenin and pluripotency and that of mES cells with epiblast progenitors suggests that β-catenin might have a functional role during preimplantation development. Here we summarize the expression and function of Wnt/β-catenin signalling elements during the early stages of mouse development and consider the reasons why the requirement in ES cells do not reflect the embryo.
Keywords: Wnt signalling, Embryogenesis, Signalling, Stem cells, Mouse
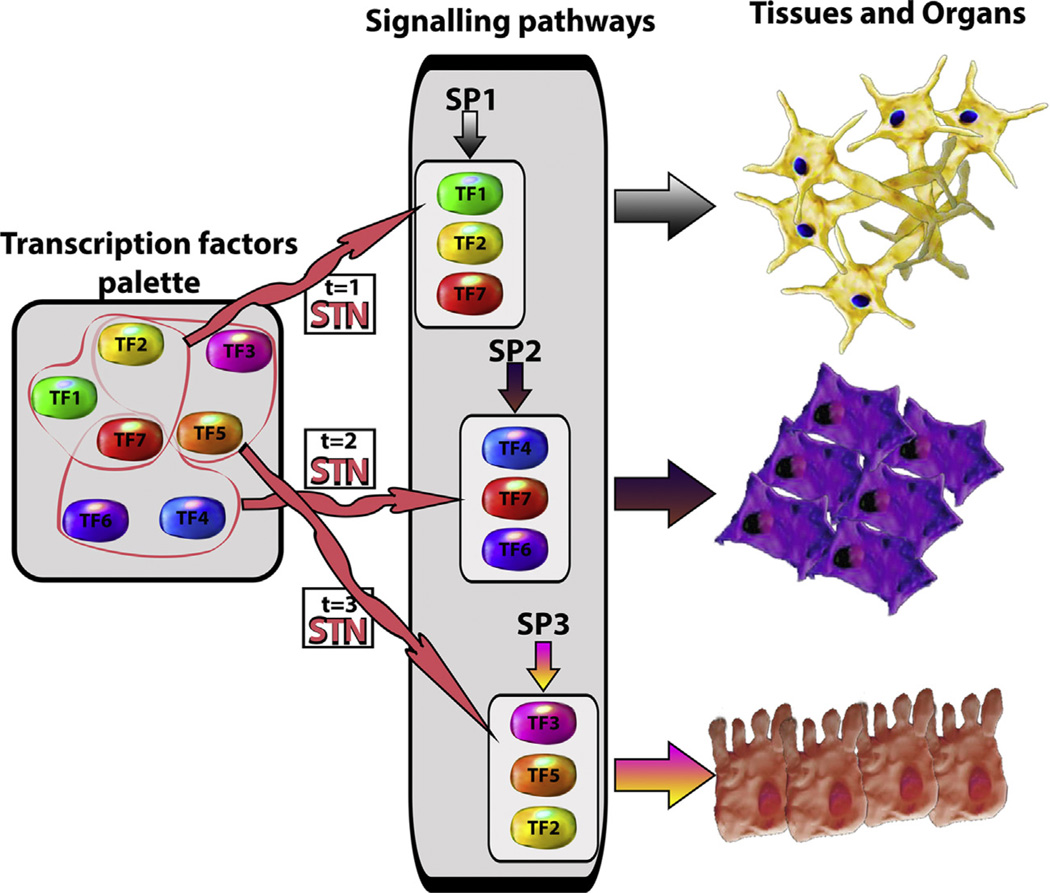
The development of an organism results from the regulated convergence of two processes: the activity of genetic programmes that fuel the generation of different kinds of cells over time, and the organization of these into three dimensional structures that are the foundation of tissues and organs. Over the last twenty years, genetic analysis has revealed that the molecular underpinning of these processes relies on two interacting functional modules: a large palette of transcription factors that are used combinatorially to generate suites of tissue and organ specific cell types, and a small set of information processing devices, signal transduction networks, that have no inherent tissue specificity and work on the transcription factor palette to modulate the combinations and their temporal dynamics (Fig. 1).
Fig. 1.
Information processing devices modulate the dynamics and combination of transcription factors to generate tissues and organs. During embryonic development, different transcription factors (TF1–TF7) are (co-)expressed to specify several fates. As development progresses, an information processing device or signal transduction network (STN in the figure) coordinates specific subsets of transcription factors. Once they are coordinated, they are regulated by signalling pathways (SP1–3 in the figure) to promote the cellular fates observed in tissues and organs.
Understanding the logic that configures interactions between the transcription factors palettes and the information processing devices during the development of an organism is an important challenge of modern biology that needs good experimental systems. While model systems like Saccharomyces cerevisiae, Caenorhabditis elegans and Drosophila have provided deep insights into the structure of biological systems and the components of both modules. The early development of mammals is emerging as one in which it is possible to study how these modules self-assemble and interact over time. Significantly, mammalian development has the added experimental value introduced by embryonic stem (ES) cells, clonal populations derived from preimplantation embryos which can be differentiated in culture under controlled conditions into all somatic and germ cells [3–5] and exhibit self-assembly properties [6–8]. These features, allow interrogation of basic processes of fate assignation in a simple system that can be related to the events taking place during embryogenesis. Hence the comparison of data obtained from embryos and ES cells can be very enlightening. Here we explore this interface by reviewing what is known about the requirements for Wnt/β-catenin signalling in embryos and ES cells and make some considerations about the relationship between both.
1. An outline of early embryogenesis: Laying down axes and primordia
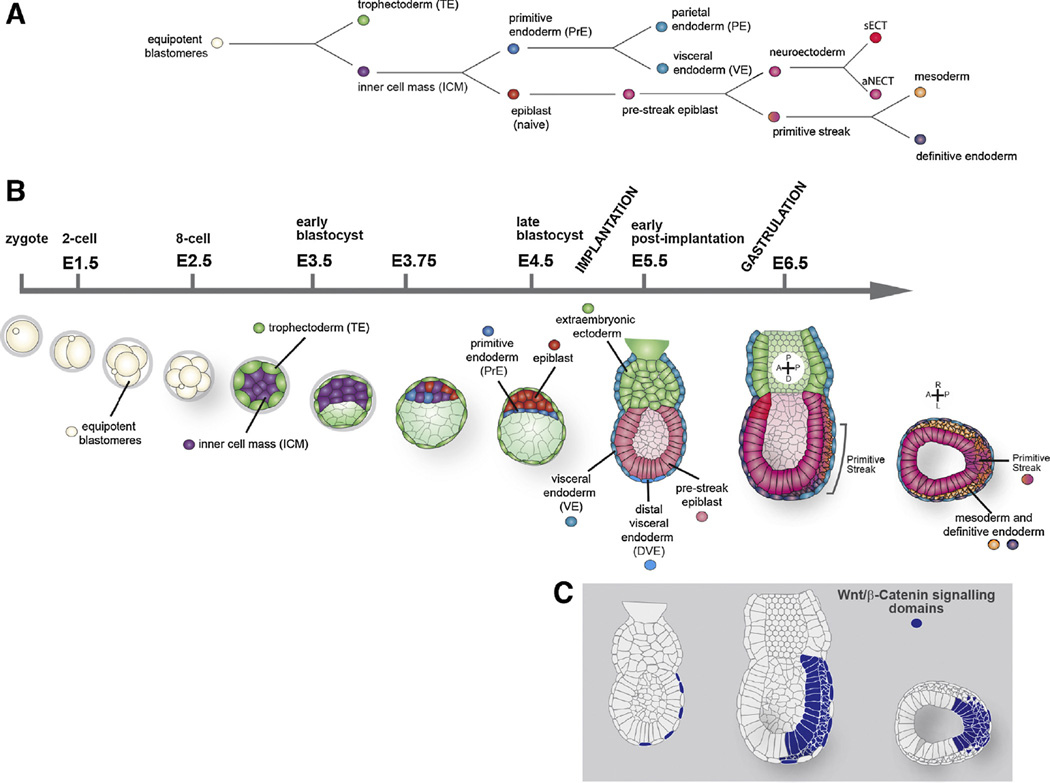
As is the case in all mammals, the early stages of the mouse embryo after fertilization are dedicated to the establishment of the extraembryonic lineages and their strategic organization [9–12]. After fertilization, the embryo undergoes 6/7 divisions over a period of 4 days during which the embryonic and extraembryonic lineages are separated from a pool of equipotent cells (Fig. 2A and B). At about day 4, as the embryo is about to implant, the precursor cells of the embryo (the epiblast, EPI) are located on one side of a cavity filled prolate spheroid bounded by the Trophectoderm (TE), which is the precursor of the foetal portion of the placenta. Between the EPI and the cavity is the primitive endoderm (PrE) which will give rise to extraembryonic membrane lineages. This cavitated preimplantation embryo is called blastocyst. After implantation, the PrE and EPI cells migrate to form a secondary cavity within the epiblast, the proamniotic cavity. At this time, the PrE will quickly differentiate two cell types: the visceral endoderm (VE), closely apposed to the embryo and together with extraembryonic mesoderm forms the visceral yolk sac, and the parietal endoderm that together with part of the TE will form the parietal yolk sac.
Fig. 2.
(A) Binary cell fate decisions made during early mouse development from the totipotent blastomeres to the extraembryonic tissues and the three germ layers at the end of gastrulation. (B) Schematic representation of the early mouse development from zygote (E0) to gastrulation (E6.5). Sagital views are shown, except the last one that shows a tranversal section across the primitive streak from the E6.5 embryo. (C) Schematic representation of Wnt/β-catenin signalling domains in E5.5 and E6.5 embryos, these include the VE, posterior epiblast, the primitive streak, mesoderm and definitive endoderm.
The mammalian embryo is patterned without maternal inputs [10,13,14] and, after the segregation of extraembryonic lineages and implantation, the remaining cells form the epiblast, a columnar epithelium of about 200 cells, will expand and become patterned into the different organs and tissues [10,15]. At about embryonic (E) day 6, the epithelium becomes subdivided into a broad anterior region and a posterior region (Fig. 2A and B). The anterior region will give rise to the anterior neuroectoderm (aNECT: the brain and parts of the head) and the surface ectoderm [16,17]. From the posterior region, the mesoderm and the endoderm (pMSEND) will emerge through the primitive streak [13,18,19]. Clonal analysis and cell transplantation experiments indicate that individual cells within the pre-streak (<E6.25) epiblast, are not committed and can give rise to any tissue of the organism [17,20–22], while cells in the early streak (~E6.5) epiblast show certain degree of commitment based on the position of the cells within the epiblast [17,20]. The regional subdivision of the epiblast depends, in part, on a symmetry breaking event that results from a sequence of inductive events that provide a proximodistal and an anteroposterior axes to the embryo. The TGF-β family member Nodal signals from the epiblast to induce the expression of Lefty1 in the distal most part of the VE, which becomes the distal visceral endoderm, DVE [23], and to recruit additional cells, which will form the anterior visceral endoderm (AVE). These cells translocate to one side of the epiblast cup and towards the proximal part of the conceptus thereby defining the anterior region of the developing embryo. This event sets up AP polarity and distinguishes the anterior region from the site of initiation of gastrulation at the posterior side. Genetic analysis suggests that the combined activities of Nodal, BMP and Wnt mediate interactions between EPI, VE and TE/extraembryonic ectoderm that establish the AP axis (Fig. 2C) [15,24–26].
There is evidence to suggest that the epiblast has a primary aNECT and that the signals from the proximal posterior impose a posterior pMSEND fate on this substrate [27,28]. Secretion of Cerberus, Lefty1 and DKK from the DVE antagonize BMP, Nodal and Wnt, respectively and protect the anterior epiblast from the posteriorizing signals [19]. Consistent with this, removal of Nodal, BMP or Wnt/β-catenin signalling in the epiblast results in a premature adoption of anterior neural fates by all cells of the epiblast [27–31] and gain of function of some of these signals e.g. Wnt/β-catenin, result in reduced aNECT development [31]. This subdivision between pMSEND and aNECT can be modelled in ES cells upon controlled culture conditions, where the role of Wnt signalling becomes explicit and paramount during differentiation [32–34].
2. Function of β-catenin in perimplantation and early patterning of the mouse embryo
An analysis of the function of Wnt/β-catenin signalling during mouse development is complicated by the number of genes encoding Wnt proteins (19) and Frizzled receptors (12) (for details on the genes and phenotypes see the Wnt page: http://www.stanford.edu/group/nusselab/cgi-bin/wnt/ and [35]) which, in addition, exhibit dynamic and overlapping patterns of expression in the preimplantation embryo [36]. Fortunately, the number of genes encoding key elements of the transduction machinery is smaller (LRP (2), Axin (2), GSK3 (2) and Dishevelled (3)) and, in the case of β-catenin, the main transcriptional effector, there is only one gene whose analysis thus provides a basal reference for the function of the Wnt/β-catenin signalling event.
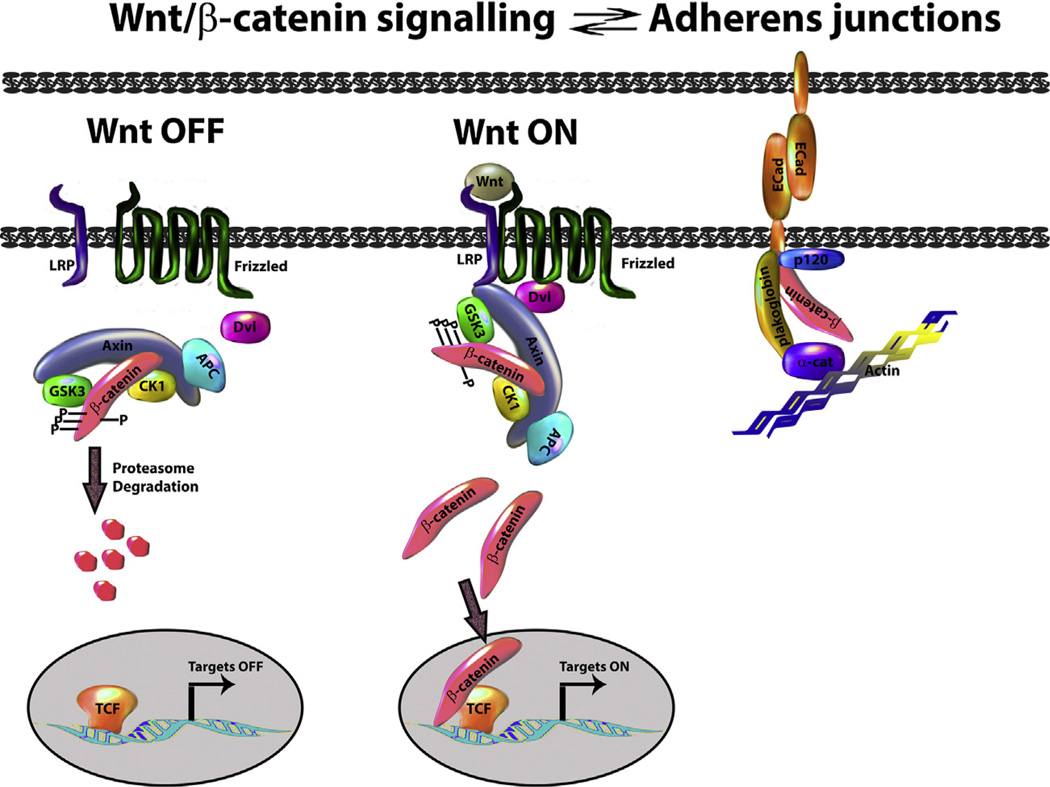
β-Catenin has a dual function as a central component of the adherens junctions and the transcriptional effector of Wnt signalling, acting through pools with different subcellular localizations (plasma membrane vs cytoplasmic/nuclear pool, respectively, Fig. 3, reviewed in [37]). In the plasma membrane, β-catenin associates with Cadherins, whose levels have been shown to affect (promoting or inhibiting depending on the cellular context) β-catenin signalling activity. These observations have led to the notion that the junctional and transcriptional functions of β-catenin are in equilibrium and therefore that the interactions between β-catenin and Cadherins should be born in mind when thinking about the consequences of gain and loss of Wnt signalling. In mouse embryos, zygotic loss of β-catenin does not affect preimplantation development nor the segregation of embryonic and extraembryonic lineages [38–40]. Although there have been reports of requirements for implantation [1], there is clear evidence that β-catenin zygotic mutant embryos can implant even if it is not efficiently. It is possible that an effect of β-catenin in the patterning of the blastocyst is obscured by the maternal contribution and the compensation effect exerted by E-Cadherin which will sequester some β-catenin thus extending its function [41]. Although this study [38] does not report any defects in the very early stages of development or blastocyst formation, it would be important to revisit this situation paying attention to early patterning effects as there is evidence that an enhancer of Nodal responds to β-catenin in the blastocyst [42], suggesting that β-catenin might have early functions.
Fig. 3.
Wnt/β-catenin signalling. In the absence of Wnt (left), β-catenin phosphorylated by casein kinase 1α (CK1) is further phosphorylated by glycogen synthase kinase 3 (GSK3) within the ‘destruction complex’ which also includes adenomatous polyposis coli (APC). The phosphorylated β-catenin is recognized by a E3 ubiquitin ligase (not shown) which targets it for proteasome degradation. In the nucleus, the target genes of the pathway are kept in an ‘OFF’ state by TCF family members. During Wnt activation (centre), the destruction complex is sequestered to the cell surface via Dishevelled (Dvl), this leads to β-catenin stabilization, which enters into the nucleus, interacts with TCF family members and promotes the transcription of target genes. Right: β-catenin can also be found as part of the adherens junctions where it interacts with E-Cadherin, this interaction can regulate the availability of β-catenin for signalling.
Zygotic β-catenin mutant embryos exhibit defects in anterior posterior (AP) patterning by stage E7.0 [38,40]. Mosaic analysis shows that these defects are due to embryonic requirements and that β-catenin is first required for the expression of Cripto and the Nodal signalling event that will define the DVE (Fig. 2C) [39]. In the absence of β-catenin, the DVE is still specified, though with some abnormalities, but it does not migrate and leads to a defective AP patterning [43,44]. The same phenotype is observed in Cripto mutants [45] suggesting that β-catenin mutant defects might be related to its requirement to activate Nodal signalling via Cripto [39]. At E7.5 it is not possible to find much embryonic tissue in β-catenin mutant embryos and it is not known whether this is due to the loss of the adhesive function or a failure to specify the different tissues, though there is evidence that plakoglobin can and does substitute for β-catenin in the adherens junctions [38,40,46]. Embryos with very reduced, but some, levels of β-catenin exhibit an excess neural tissue and no endoderm or mesoderm [29], which is consistent with the requirement for β-catenin in the specification of the primitive streak and with the observation that gain of function mutations or loss of function of Wnt antagonists result in an expansion of the posterior pMSEND fates [31]. Gain of function studies provide additional insights into the functions of β-catenin and mutations that stabilize β-catenin result in premature expression of mesoderm markers such as Snail1 and T/Brachyury and defects in DVE specification [47].
3. Wnt/β-catenin signalling in early development
The Wnt/β-catenin signalling pathway is built in such a manner that the activation of the signal at the membrane via the Frizzled receptors leads to the disassembly of a ‘β-catenin destruction complex’ that shuttles between the membrane (recruited by Dishevelled, Dvl) and the cytosol. The core of this complex are the scaffolding proteins Axin and APC and the enzyme GSK3 (Fig. 3) which are encoded by multiple genes. There are 19 genes encoding Wnt proteins, many of which are expressed in preimplantation stages [36]. This situation makes it not feasible to assay the function of Wnt by mutating the ligands, however the study of the loss of Wnt chaperone Porcupine can be enlightening. Porcupine is required for secretion of all Wnt proteins and its absence is, effectively, a loss of function of all Wnt signalling. This allows to distinguish the β-catenin activities during embryogenesis that are Wnt signalling dependent, from the Wnt-independent. Embryos mutant for Porcupine exhibit a phenotype of loss of primitive streak and no defects in AP patterning [2,48,49], which is similar to that of LRP5/6 double mutants [50]. This confirms the requirement for Wnt/β-catenin signalling in the specification of the primitive streak and that the function of β-catenin in the specification of DVE is independent of Wnt [39,43,51].
Although there are multiple Wnts, genetic screens have shown that most, if not all, of the zygotic functions of Wnt/β-catenin signalling are mediated by Wnt3 as Wnt3 mutants have a phenotype similar to that of Porcupine mutant embryos [52,53]. Wnt3 is initially expressed in the posterior visceral endoderm (PVE) [54], then, as gastrulation begins, in the posterior proximal epiblast and loss of function shows that it is required for the formation of the primitive streak. However, a careful mosaic analysis of the mutant phenotype revealed that Wnt3 develops an autoregulatory loop between the PVE and the epiblast which is involved in the maintenance and not the initiation of the primitive streak. Studies of the effect of Wnt/β-catenin signalling on Brachyury expression support this possibility [55,56] and indicate that, as suggested earlier, β-catenin has more to do with the stabilization of transcriptional programmes than with their induction [57,58].
There are 10 genes encoding members of the family of the transmembrane Wnt receptors Frizzled which are expressed during early postimplantation development [59,60]. However loss-of-function studies of Fzd receptors (Fzd3, Fzd4, Fzd5, Fzd6, and Fzd9) up to now do not show gastrulation phenotypes, suggesting a functional redundancy among these receptors, which would mirror the situ-ation in Drosophila [61].
There are three dishevelled genes encoded in the mouse genome (dvl1, dvl2 and dvl3) with high degree of redundancy in their function. Although the phenotype of the single and double heterozygotes have been studied during late embryonic development, very little is known about the triple mutant or dvl2;dvl3 double mutant phenotype, only that they die before E8.5 [62] with gastrulation defects [63].
Regarding the genes encoding for elements of the destruction complex (Axin, APC and GSK3), there are two genes for each of these and one would expect that their mutations would mimic, for the most part, phenotypes of β-catenin gain of function in mouse embryonic development. This is the case for the APC mutants which show defects during DVE specification and gastrulation [64,65]. Mutations in the Axin1 gene cause axis duplication [66], the same as a mutation stabilizing Axin2 that leads to enhanced Wnt signalling in the primitive streak, while a null Axin2 mutation induces malformations in the skull [67,68]. Gsk3β is lethal at mid-gestation with no apparent axis duplication [69] and Gsk3a is viable [70]. There are no reports of the double Gsk3a; Gsk3β mutant.
4. Tcf factors
In the nucleus, β-catenin interacts with several proteins [71] but most notably with members of the Tcf family of DNA binding proteins [72,73]. There are four members of this family. Tcf4 is not expressed in the early development and genetic analysis reveals a degree of redundancy between Lef1 and Tcf1 mutants which do not show early defects but the double mutant mimics the Wnt3 mutant [74]. Tcf3 is different: a large body of evidence suggests that it cannot activate transcription and acts largely as a repressor of the signalling event. Loss-of-function results in phenotypes that resemble, but are different of, gain of function of β-catenin [75] but this phenotype might be independent of a direct interaction with β-catenin as embryos homozygous for a mutation in the β-catenin binding domain of Tcf3 undergo normal gastrulation [76].
5. Function of Wnt/β-catenin signalling in Embryonic and Epiblast stem cells
Embryonic stem (ES) cells are derivatives from early mammalian embryos that can be cultured indefinitely and differentiated into most cell types of an organism i.e. they are pluripotent [4,77]. In the case of mouse, there are two kinds of pluripotent stem cells derived from, and representing, epiblast at different embryonic stages. Naïve ES cells, which are derived from preimplantation blastocysts, and which do not exhibit lineage bias and give rise to chimeras when injected into preimplantation embryos [5], and epiblast stem cells (EpiSC) derived from the postimplantation epiblast [78,79], which exhibit lineage bias towards pMSEND and only form chimeras when injected in hosts of the same age [80,81].
The observation that GSK3β inhibition stimulates self-renewal in mouse ES cells under standard culture conditions raised the possibility that Wnt/β-catenin signalling was involved in pluripotency. In the wake of these reports, a flurry of experiments indicated that the most significant effect of the inhibition of GSK3 on pluripotency is mediated by β-catenin [82–86]. The effects of β-catenin on ES cell culture were reinforced by its central role in the activity of the 2i cocktail [87]. These experiments demonstrated a potent effect of Wnt/β-catenin signalling on the self-renewal of ES cells and on the derivation of ES cell lines from recalcitrant mouse strains [88–91] and even rats [92,93]. However, the effect of Wnt signalling is different on ES and EpiSC: whereas in ES it promotes pluripotency, in EpiSCs it promotes differentiation [94].
The generally accepted linear pathway associated with Wnt/β-catenin signalling [95] led to the conclusion that the function of β-catenin in the maintenance of ES cells was mediated through its transcriptional activity [95–97]. This conclusion was supported by the effects of mutations in APC where a correlation was demonstrated between the levels of β-catenin, its transcriptional activity and the degree of pluripotency [98–100]. However, further experiments challenged the two simplest conclusions from these studies: that β-catenin is required for pluripotency and that this function acts via its transcriptional activity. β-catenin is not required for the establishment of pluripotency as ES cells can be derived from β-catenin mutant blastocysts [101] and, more significantly, β-catenin mutant cells can be maintained in culture, albeit with a certain degree of instability [29,33,85,102–104]. In addition, functional analysis of β-catenin activity in ES cells revealed that its transcriptional activity is dispensable for pluripotency [33,102,103]. This is corroborated by the observation that Tcf1, Lef1 and Tcf4 are also dispensable [87,105] and that Tcf3 represses rather than promotes pluripotency [106,107]. Despite this, although β-catenin is not required for pluripotency, it does help its maintenance as the detailed quantitative analysis of β-catenin mutant ES cells revealed that they are highly unstable and difficult to maintain in culture[29,33,108,109]. The instability of β-catenin mutant cells is associated with altered levels of Nanog and Oct4 protein [33,108]. Several studies have been conducted to investigate the mechanism by which β-catenin is involved in the maintenance of pluripotency [102,105,110,111]. These studies have led to the conclusion that the main role of β-catenin in pluripotency is to neutralize the repressive activity of Tcf3 on the pluripotency network and that it does it through a non-transcriptional activity (Fig. 4A and B′).
Fig. 4.
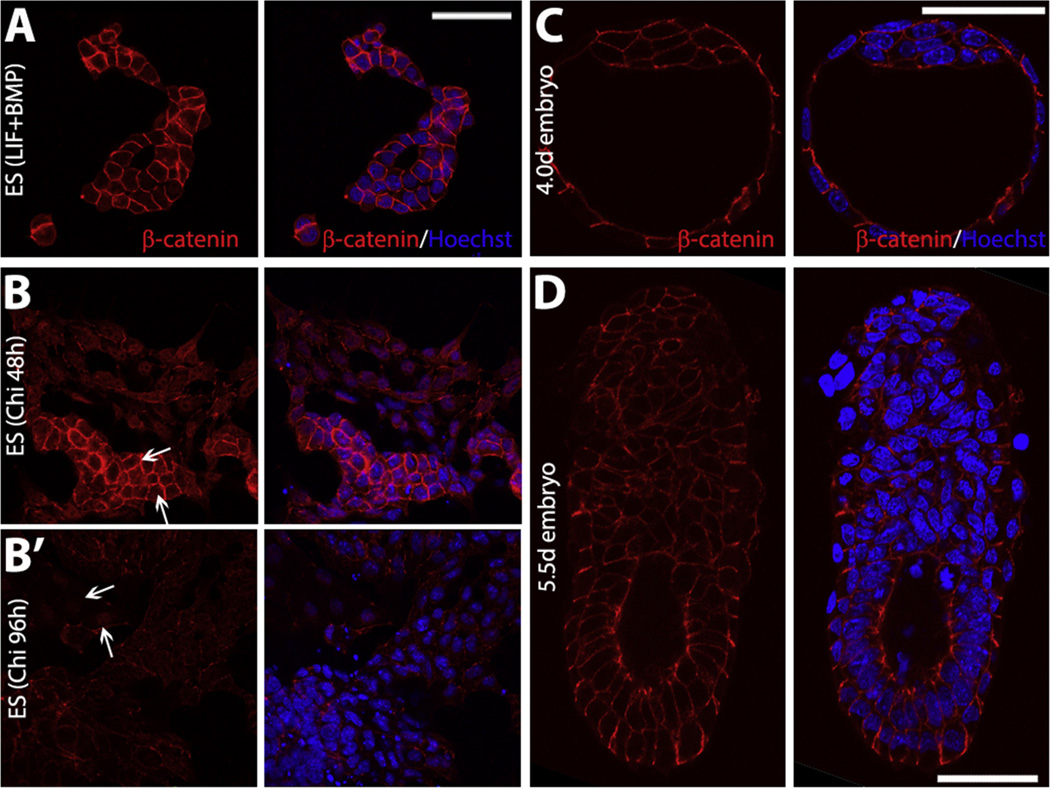
(A and B) Under self-renewing conditions β-catenin localizes at the membranes (A), and it translocates to the nucleus when cells Wnt/β-catenin is activated under differentiation culture conditions in the presence of the GSK3 inhibitor Chi for 48 h (B) or 96 h (B′). The white arrows indicate some cells with nuclear β-catenin. (C and D) β-catenin localization in E4.0d (C) and E5.5d (D) embryos. Scale bars, 50 µm.
Besides the effects of β-catenin on Tcf3, there is a clear link between β-catenin and Oct4 [33,112–114]. The situation may be intricate as defined Oct4 levels are key for the establishment of pluripotency and altered levels induce differentiation [115–117]. The effect of β-catenin on Oct4 is clear: there is evidence that β-catenin sequesters Oct4 to prevent its prodifferentiation activities [34]. Some of these interactions might be associated with the association of β-catenin with Cadherins [33].
But the controversy on the exact mechanism by which β-catenin is involved in pluripotency still remains: a new study using conditional β-catenin null cells indicates that some of the defects observed in previous studies are due to the use of long term cultured cells [118]. The short term effect of β-catenin absence is related to cell death and chromosome segregation with no effect on pluripotency markers expression.
The relationship between β-catenin and pluripotency in ES cells is mirrored in EpiSCs, where there is a low level requirement for their establishment and maintenance but where the most clear requirement is for their differentiation [94,119]. In human ES cells, which closely resemble mouse EpiSCs, similar requirements for β-catenin have been found and transcriptional activity is associated with differentiation [120,121]. These conclusions have been recently confirmed in mouse EpiSCs [94]. Further studies on reprogramming mouse EpiSCs to ES cells show that inhibition of Wnt/β-catenin signalling enhances the conversion [122].
In summary, the situation in culture is clear: β-catenin plays a central role in balancing differentiation and self-renewal. As part of a protein complex promotes self-renewal in ES cells while its transcriptional activity as part of canonical Wnt signalling promotes differentiation both in ES and EpiSCs.
6. In summary: The relationship between ES cells and embryos from the perspective of β-catenin
The first requirement for Wnt/β-catenin signalling in the mouse embryo has been reported in the differentiation of the epiblast, which develops a neural fate in the absence of β-catenin. A similar phenotype can be observed during the differentiation of ES cells and suggests that the ES cells are a good model for the events in the embryo and that Wnt/β-catenin signalling is required for the development of the mesoderm and the endoderm during differentiation.
The situation is different in preimplantation blastocysts, from where the ES cells are derived and are most closely associated with [123]. The requirement for β-catenin or inhibition of GSK3 in the maintenance of ES cells is underscored by the observation that GSK3 inhibitors increase the efficiency of ES cells derivation from different mouse strains and rats. However, this requirement contrasts with the lack of obvious phenotype associated with loss of β-catenin in preimplantation embryos even though β-catenin, other components of the pathway and multiple Wnt ligands are expressed in early mouse development. This is surprising and suggests two possible explanations. The first one is that a role for β-catenin in the establishment of the epiblast is obscured by its maternal component and its stability in the adherens junctions (Fig. 4C and D). This cannot be ruled out and will have to be tested with some attention to quantitative parameters as the resulting phenotype might lead to quantitative changes in the dynamics of the development of the epiblast or the balance between different cell types. For example, loss of β-catenin activity in ES cells leads to an increase in the frequency of differentiation [33,34] and an equivalent phenotype in the blastocyst might only be observed in quantitative studies as for example those performed in the role for Oct4 role in the segregation of the EPI [124]. A second possibility is that despite many suggestions and efforts, ES cells might not represent a stage in the embryo but a culture artefact and results obtained in both systems are not comparable.
A variety of studies suggest that while not all the events in embryos can be modelled in ES cells, the reverse is true i.e. what is found in ES cells usually can be observed in embryos in a very transient manner. However, there are significant differences between the control of gene expression in embryos and cells. Recent work on Nanog expression during embryogenesis has indicated that contrary to the events in ES cell culture, the levels of Nanog do not undergo large scale fluctuations in the embryo and cells will express Nanog stably on the basis of their gene expression trajectories [125]. It is possible that β-catenin plays a role in this process by altering the dynamics of the event and facilitating cell fate decisions. On this premise, there might be an early function of β-catenin and this function might be linked to the dynamics and coordination of the events, in a manner which has been suggested to be a general feature of Wnt/β-catenin signalling [57]. In this case only a quantitative analysis of the dynamics of the process will reveal its function and this will have to be analysed in the absence of both, maternal and zygotic contributions. A hint that Wnt/β-catenin might be involved in the dynamics of early events can be gauged from the phenotype of mutants in APC which result in increased Wnt/β-catenin signalling and in which fate decisions, most notably the acquisition of mesodermal and endodermal fates is anticipated [126].
Wnt/β-catenin signalling is associated with the maintenance of stem cells in many systems [127] and the role revealed in the case of ES cells might reflect the fact that epiblast cells in the blastocyst are in a very poised state to become stem cells and susceptible to be captured by β-catenin. Thus the differences between embryos and cells might be telling us something about how stem cells are set up and the role that β-catenin plays in this process.
Acknowledgments
The work of AMA is funded by an ERC Advanced investigator grant, that of AKH by the NIH and NYSTEM and that of SMD by the Royal Society and the University of Bath.
References
- 1.Xie H, Tranguch S, Jia X, Zhang H, Das SK, Dey SK, et al. Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development. 2008;135:717–727. doi: 10.1242/dev.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biechele S, Adissu HA, Cox BJ, Rossant J. Zygotic porcn paternal allele deletion in mice to model human focal dermal hypoplasia. PLoS ONE. 2013;8:e79139. doi: 10.1371/journal.pone.0079139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 4.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 5.Nichols J, Smith A. The origin and identity of embryonic stem cells. Development. 2011;138:3–8. doi: 10.1242/dev.050831. [DOI] [PubMed] [Google Scholar]
- 6.Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: self-organising stem cells. Development. 2012;139:4111–4121. doi: 10.1242/dev.079590. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 8.van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, et al. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development. 2014;141:4231–4242. doi: 10.1242/dev.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behringer RR, Eakin GS, Renfree MB. Mammalian diversity: gametes, embryos and reproduction. Reprod Fertil Dev. 2006;18:99–107. doi: 10.1071/rd05137. [DOI] [PubMed] [Google Scholar]
- 10.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 11.Martinez Arias A, Nichols J, Schroter C. A molecular basis for developmental plasticity in early mammalian embryos. Development. 2013;140:3499–3510. doi: 10.1242/dev.091959. [DOI] [PubMed] [Google Scholar]
- 12.Schrode N, Xenopoulos P, Piliszek A, Frankenberg S, Plusa B, Hadjantonakis AK. Anatomy of a blastocyst: cell behaviors driving cell fate choice and morphogenesis in the early mouse embryo. Genesis. 2013;51:219–233. doi: 10.1002/dvg.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 14.Eakin GS, Behringer RR. Diversity of germ layer and axis formation among mammals. Semin Cell Dev Biol. 2004;15:619–629. doi: 10.1016/j.semcdb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 16.Cajal M, Lawson KA, Hill B, Moreau A, Rao J, Ross A, et al. Clonal and molecular analysis of the prospective anterior neural boundary in the mouse embryo. Development. 2012;139:423–436. doi: 10.1242/dev.075499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 18.Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- 19.Arkell RM, Tam PP. Initiating head development in mouse embryos: integrating signalling and transcriptional activity. Open Biol. 2012;2:120030. doi: 10.1098/rsob.120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 21.Parameswaran M, Tam PP. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev Genet. 1995;17:16–28. doi: 10.1002/dvg.1020170104. [DOI] [PubMed] [Google Scholar]
- 22.Lawson KA, Pedersen RA. Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. Ciba Found Symp. 1992;165:3–21. doi: 10.1002/9780470514221.ch2. (Discussion-6) [DOI] [PubMed] [Google Scholar]
- 23.Takaoka K, Yamamoto M, Hamada H. Origin and role of distal visceral endoderm, a group of cells that determines anterior–posterior polarity of the mouse embryo. Nat Cell Biol. 2011;13:743–752. doi: 10.1038/ncb2251. [DOI] [PubMed] [Google Scholar]
- 24.Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- 25.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, et al. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y, et al. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- 28.Camus A, Perea-Gomez A, Moreau A, Collignon J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol. 2006;295:743–755. doi: 10.1016/j.ydbio.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Rudloff S, Kemler R. Differential requirements for beta-catenin during mouse development. Development. 2012;139:3711–3721. doi: 10.1242/dev.085597. [DOI] [PubMed] [Google Scholar]
- 30.Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 31.Fossat N, Jones V, Khoo PL, Bogani D, Hardy A, Steiner K, et al. Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development. 2011;138:667–676. doi: 10.1242/dev.052803. [DOI] [PubMed] [Google Scholar]
- 32.Turner DA, Hayward PC, Baillie-Johnson P, Rue P, Broome R, Faunes F, et al. Wnt/beta-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development. 2014;141:4243–4253. doi: 10.1242/dev.112979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faunes F, Hayward P, Muñoz-Descalzo S, Chatterjee SS, Balayo T, Trott J, et al. A membrane-associated β-catenin/Oct4 complex correlates with ground-state pluripotency in mouse embryonic stem cells. Development. 2013;140:1171–1183. doi: 10.1242/dev.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz Descalzo S, Rue P, Faunes F, Hayward P, Jakt LM, Balayo T, et al. A competitive protein interaction network buffers Oct4-mediated differentiation to promote pluripotency in embryonic stem cells. Mol Syst Biol. 2013;9:694. doi: 10.1038/msb.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 37.McCrea PD, Maher MT, Gottardi CJ. Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol. 2015;112:129–196. doi: 10.1016/bs.ctdb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior–posterior axis formation in mice. JCell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, et al. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- 40.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 41.De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, et al. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 42.Granier C, Gurchenkov V, Perea-Gomez A, Camus A, Ott S, Papanayotou C, et al. Nodal cis-regulatory elements reveal epiblast and primitive endoderm heterogeneity in the peri-implantation mouse embryo. Dev Biol. 2011;349:350–362. doi: 10.1016/j.ydbio.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Engert S, Burtscher I, Liao WP, Dulev S, Schotta G, Lickert H. Wnt/beta-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development. 2013;140:3128–3138. doi: 10.1242/dev.088765. [DOI] [PubMed] [Google Scholar]
- 44.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 45.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, et al. Cripto is required for correct orientation of the anterior–posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 46.Huber O, Krohn M, Kemler R. A specific domain in alpha-catenin mediates binding to beta-catenin or plakoglobin. J Cell Sci. 1997;110(Pt 15):1759–1765. doi: 10.1242/jcs.110.15.1759. [DOI] [PubMed] [Google Scholar]
- 47.Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, et al. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 48.Biechele S, Cockburn K, Lanner F, Cox BJ, Rossant J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development. 2013;140:2961–2971. doi: 10.1242/dev.094458. [DOI] [PubMed] [Google Scholar]
- 49.Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 50.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 51.Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, et al. Canonical Wnt signaling and its antagonist regulate anterior–posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 53.Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, et al. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Tortelote GG, Hernandez-Hernandez JM, Quaresma AJ, Nickerson JA, Imbalzano AN, Rivera-Perez JA. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev Biol. 2013;374:164–173. doi: 10.1016/j.ydbio.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galceran J, Hsu SC, Grosschedl R. Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression. Proc Natl Acad Sci USA. 2001;98:8668–8673. doi: 10.1073/pnas.151258098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz-Descalzo S, de Navascues J, Arias AM. Wnt–Notch signalling: an integrated mechanism regulating transitions between cell states. Bioessays. 2012;34:110–118. doi: 10.1002/bies.201100102. [DOI] [PubMed] [Google Scholar]
- 58.Martinez Arias AM, Hayward P. Filtering transcriptional noise during development: concepts and mechanisms. Nat Rev Genet. 2006;7:34–44. doi: 10.1038/nrg1750. [DOI] [PubMed] [Google Scholar]
- 59.Lu CC, Robertson EJ, Brennan J. The mouse frizzled 8 receptor is expressed in anterior organizer tissues. Gene Expr Patterns. 2004;4:569–572. doi: 10.1016/j.modgep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Kemp CR, Willems E, Wawrzak D, Hendrickx M, Agbor Agbor T, Leyns L. Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical Wnt signaling. Dev Dyn. 2007;236:2011–2019. doi: 10.1002/dvdy.21198. [DOI] [PubMed] [Google Scholar]
- 61.Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development. 1999;126:4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- 62.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wynshaw-Boris A. Dishevelled:. in vivo roles of a multifunctional gene family during development. Curr Top Dev Biol. 2012;101:213–235. doi: 10.1016/B978-0-12-394592-1.00007-7. [DOI] [PubMed] [Google Scholar]
- 64.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 65.Moser AR, Shoemaker AR, Connelly CS, Clipson L, Gould KA, Luongo C, et al. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev Dyn. 1995;203:422–433. doi: 10.1002/aja.1002030405. [DOI] [PubMed] [Google Scholar]
- 66.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 67.Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian L, Mahaffey JP, Alcorn HL, Anderson KV. Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc Natl Acad Sci USA. 2011;108:8692–8697. doi: 10.1073/pnas.1100328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 70.MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, et al. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007;6:329–337. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 71.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 72.Hoverter NP, Waterman ML. A Wnt-fall for gene regulation: repression. Sci Signal. 2008;1:e43. doi: 10.1126/scisignal.139pe43. [DOI] [PubMed] [Google Scholar]
- 73.Yi F, Merrill BJ. Stem cells and TCF proteins: a role for beta-catenin—independent functions. Stem Cell Rev. 2007;3:39–48. doi: 10.1007/s12015-007-0003-9. [DOI] [PubMed] [Google Scholar]
- 74.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, et al. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- 76.Buecker C, Srinivasan R, Wu Z, Calo E, Acampora D, Faial T, et al. Reorganization of enhancer patterns in transition from naive to primed pluripotency. Cell Stem Cell. 2014;14:838–853. doi: 10.1016/j.stem.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 78.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 79.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 80.Huang Y, Osorno R, Tsakiridis A, Wilson V. In vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2012;2:1571–1578. doi: 10.1016/j.celrep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 81.Kojima Y, Kaufman-Francis K, Studdert JB, Steiner KA, Power MD, Loebel DA, et al. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell. 2014;14:20. doi: 10.1016/j.stem.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 83.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 84.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 85.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28:1794–1804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bone HK, Damiano T, Bartlett S, Perry A, Letchford J, Ripoll YS, et al. Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides. Chem Biol. 2009;16:15–27. doi: 10.1016/j.chembiol.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiyonari H, Kaneko M, Abe S, Aizawa S. Three inhibitors of FGF receptor, ERK, and GSK3 establishes germline-competent embryonic stem cells of C57BL/6N mouse strain with high efficiency and stability. Genesis. 2010;48:317–327. doi: 10.1002/dvg.20614. [DOI] [PubMed] [Google Scholar]
- 89.Umehara H, Kimura T, Ohtsuka S, Nakamura T, Kitajima K, Ikawa M, et al. Efficient derivation of embryonic stem cells by inhibition of glycogen synthase kinase-3. Stem Cells. 2007;25:2705–2711. doi: 10.1634/stemcells.2007-0086. [DOI] [PubMed] [Google Scholar]
- 90.Ye S, Tan L, Yang R, Fang B, Qu S, Schulze EN, et al. Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains. PLoS ONE. 2012;7:e35892. doi: 10.1371/journal.pone.0035892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato H, Amagai K, Shimizukawa R, Tamai Y. Stable generation of serum- and feeder-free embryonic stem cell-derived mice with full germline-competency by using a GSK3 specific inhibitor. Genesis. 2009;47:414–422. doi: 10.1002/dvg.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meek S, Wei J, Sutherland L, Nilges B, Buehr M, Tomlinson SR, et al. Tuning of beta-catenin activity is required to stabilize self-renewal of rat embryonic stem cells. Stem Cells. 2013;31:2104–2115. doi: 10.1002/stem.1466. [DOI] [PubMed] [Google Scholar]
- 93.Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Kurek D, Neagu A, Tastemel M, Tuysuz N, Lehmann J, Van de Werken HJ, et al. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Rep. 2015;4:114–128. doi: 10.1016/j.stemcr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nusse R. Wnt signaling stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 96.Berge DT, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ardehali R, Ali SR, Inlay MA, Abilez OJ, Chen MQ, Blauwkamp TA, et al. Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue. Proc Natl Acad Sci USA. 2013;110:3405–3410. doi: 10.1073/pnas.1220832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 99.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 100.Atlasi Y, Noori R, Gaspar C, Franken P, Sacchetti A, Rafati H, et al. Wnt signaling regulates the lineage differentiation potential of mouse embryonic stem cells through Tcf3 down-regulation. PLoS Genet. 2013;9:e1003424. doi: 10.1371/journal.pgen.1003424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okumura N, Akutsu H, Sugawara T, Miura T, Takezawa Y, Hosoda A, et al. Beta-catenin functions pleiotropically in differentiation and tumorigenesis in mouse embryo-derived stem cells. PLoS ONE. 2013;8:e63265. doi: 10.1371/journal.pone.0063265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of beta-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anton R, Kestler HA, Kuhl M. Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007;581:5247–5254. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 105.Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, et al. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13:62–70. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Munoz Descalzo S, Rue P, Garcia-Ojalvo J, Arias AM. Correlations between the levels of oct4 and nanog as a signature for naive pluripotency in mouse embryonic stem cells. Stem Cells. 2012;30:2683–2691. doi: 10.1002/stem.1230. [DOI] [PubMed] [Google Scholar]
- 109.del Valle I, Rudloff S, Carles A, Li Y, Liszewska E, Vogt R, et al. E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development. 2013;140:1684–1692. doi: 10.1242/dev.088690. [DOI] [PubMed] [Google Scholar]
- 110.Shy BR, Wu CI, Khramtsova GF, Zhang JY, Olopade OI, Goss KH, et al. Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/beta-catenin signaling. Cell Rep. 2013;4:1–9. doi: 10.1016/j.celrep.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X, Peterson KA, Liu XS, McMahon AP, Ohba S. Gene regulatory networks mediating canonical Wnt signal directed control of pluripotency and differentiation in embryo stem cells. Stem Cells. 2013;31:2667–2679. doi: 10.1002/stem.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abu-Remaileh M, Gerson A, Farago M, Nathan G, Alkalay I, Zins Rousso S, et al. Oct-3/4 regulates stem cell identity and cell fate decisions by modulating Wnt/beta-catenin signalling. EMBO J. 2010;29:3236–3248. doi: 10.1038/emboj.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. beta-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marikawa Y, Tamashiro DA, Fujita TC, Alarcon VB. Dual roles of Oct4 in the maintenance of mouse P19 embryonal carcinoma cells: as negative regulator of Wnt/beta-catenin signaling and competence provider for Brachyury induction. Stem Cells Dev. 2011;20:621–633. doi: 10.1089/scd.2010.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Radzisheuskaya A, Le Bin Chia G, Dos Santos RL, Theunissen TW, Castro LF, Nichols J, et al. A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat Cell Biol. 2013;15:579–590. doi: 10.1038/ncb2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karwacki-Neisius V, Goke J, Osorno R, Halbritter F, Ng JH, Weisse AY, et al. Reduced Oct4 expression directs a robust pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. Cell Stem Cell. 2013;12:531–545. doi: 10.1016/j.stem.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 118.Raggioli A, Junghans D, Rudloff S, Kemler R. Beta-catenin is vital for the integrity of mouse embryonic stem cells. PLoS ONE. 2014;9:e86691. doi: 10.1371/journal.pone.0086691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sumi T, Oki S, Kitajima K, Meno C. Epiblast ground state is controlled by canonical Wnt/beta-catenin signaling in the postimplantation mouse embryo and epiblast stem cells. PLoS ONE. 2013;8:e63378. doi: 10.1371/journal.pone.0063378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, et al. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun Y, et al. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312–326. doi: 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murayama H, Masaki H, Sato H, Hayama T, Yamaguchi T, Nakauchi H. Successful reprogramming of epiblast stem cells by blocking nuclear localization of beta-catenin. Stem Cell Rep. 2015;4:103–113. doi: 10.1016/j.stemcr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boroviak T, Loos R, Bertone P, Smith A, Nichols J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat Cell Biol. 2014;16:516–528. doi: 10.1038/ncb2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le Bin GC, Munoz-Descalzo S, Kurowski A, Leitch H, Lou X, Mansfield W, et al. Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst. Development. 2014;141:1001–1010. doi: 10.1242/dev.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xenopoulos P, Kang M, Puliafito A, Di Talia S, Hadjantonakis AK. Heterogeneities in nanog expression drive stable commitment to pluripotency in the mouse blastocyst. Cell Rep. 2015:S2211–S1247. 138–132. doi: 10.1016/j.celrep.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chazaud C, Rossant J. Disruption of early proximodistal patterning and AVE formation in Apc mutants. Development. 2006;133:3379–3387. doi: 10.1242/dev.02523. [DOI] [PubMed] [Google Scholar]
- 127.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]