Abstract
Prostate epithelial cells control the potency and availability of androgen hormones in part by inactivation and elimination. UDP-glucose dehydrogenase (UGDH) catalyzes the NAD+-dependent oxidation of UDP-glucose to UDP-glucuronate, an essential precursor for androgen inactivation by the prostate glucuronidation enzymes UGT2B15 and UGT2B17. UGDH expression is androgen stimulated, which increases the production of UDP-glucuronate and fuels UGT-catalyzed glucuronidation. In this study, we compared the glucuronidation potential and its impact on androgen-mediated gene expression in an isogenic LNCaP model for androgen-dependent versus castration-resistant prostate cancer. Despite significantly lower androgen-glucuronide output, LNCaP 81 castration-resistant tumor cells expressed higher levels of UGDH, UGT2B15, and UGT2B17. However, the magnitude of androgen-activated UGDH and prostate-specific antigen (PSA) expression, as well as the androgen receptor (AR)-dependent repression of UGT2B15 and UGT2B17, was blunted several-fold in these cells. Consistent with these results, the ligand-activated binding of AR to the PSA promoter and subsequent transcriptional activation were also significantly reduced in castration-resistant cells. Analysis of the UDP-sugar pools and flux through pathways downstream of UDP-glucuronate production revealed that these glucuronidation precursor metabolites were channeled through proteoglycan and glycosaminoglycan biosynthetic pathways, leading to increased surface expression of Notch1. Knockdown of UGDH diminished Notch1 and increased glucuronide output. Overall, these results support a model in which the aberrant partitioning of UDP-glucuronate and other UDP-sugars into alternative pathways during androgen deprivation contributes to the loss of prostate tumor cell androgen sensitivity by promoting altered cell surface proteoglycan expression.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-016-0268-z) contains supplementary material, which is available to authorized users.
Keywords: Prostate cancer, Castration resistance, Dihydrotestosterone, Detoxification, LNCaP
Introduction
Prostate cancer is the most commonly diagnosed cancer in men [1]. Locally advanced cancer that is present at first diagnosis is often inoperable and treated by androgen deprivation therapy [2]. Most tumors respond initially, but recurrence is a serious clinical problem because tumor cells that resume growth despite low circulating androgen are highly aggressive [3, 4]. A major cause of mortality in prostate cancer is castration-resistant recurrence [2, 5, 6], but the underlying molecular mechanisms are not yet fully understood and are therefore an area of intense research focus. It is well accepted that tumor cells can acquire the ability to synthesize androgens locally [7] or develop aberrant androgen receptor (AR) functions through post-transcriptional and post-translational modifications that eliminate the need for exogenous androgen [8, 9]. However, less is known about the impacts of androgen elimination pathways on castration-resistant progression.
Excess androgen is normally managed by the glucuronidation pathway, in which steroids or other lipophiles are chemically inactivated and solubilized by conjugation to glucuronate moieties. UDP-glucuronosyltransferase (UGT) enzymes are expressed in hormone-sensitive target tissues and catalyze the esterification of a steroid hydroxyl to the anomeric carbon of UDP-glucuronate (UDP-glcA), yielding the androgen-glucuronide with UDP as a leaving group [10]. The net effect is to maintain levels of critical lipophilic hormones in the concentration windows at which they are most effective in engaging their receptors. Prostate epithelial cells express two UGT isoforms, UGT2B15 and UGT2B17 [11]. Cell culture experiments show that expression of these 95 % identical isoforms is androgen repressed, and their overexpression reduces the proliferation rate of cells by increasing inactivation of androgens [12–15]. Importantly, polymorphisms in both genes have been identified and correlated as genetic risk factors for prostate cancer [16, 17]. In addition, comparison of castration-resistant murine and human prostate tumor metastases to primary tumors revealed increased expression of UGT2B17 in conjunction with increases in several androgen biosynthetic enzymes [18].
UDP-glucose dehydrogenase (UGDH) is a unique, essential enzyme that catalyzes oxidation of UDP-glucose to UDP-glcA, which is the required precursor for glucuronidation. Many tissues express UGDH, but strong expression is specific to liver and prostate [19], both tissues that actively demand glucuronate conjugation to facilitate hormone excretion. We previously showed that stimulating UGDH expression and consequently increasing UDP-glcA cofactor availability can drive UGT-catalyzed glucuronidation by mass action in androgen-dependent prostate tumor cells [20]. We have also found that UGDH expression is significantly elevated in epithelial cells of cancerous prostate acini relative to those of both normal prostate or those of normal appearing acini at the tumor/normal interface in biopsied samples [21].
In the current study, our goal was to characterize the function of the glucuronidation pathway during loss of androgen dependence and determine the effects of altered androgen availability on the component enzymes and metabolite intermediates. We used an isogenic cell culture model of androgen-dependent and castration-resistant prostate cancer, LNCaP 33 and 81. LNCaP 81 was derived from LNCaP 33 by long-term passage in androgen-containing culture conditions to achieve a cell population that remained androgen responsive but was no longer androgen dependent [22]. We tested the effect of short-term and prolonged androgen deprivation on AR-dependent gene expression and activity of UGDH, UGT2B15, UGT2B17, and the UDP-sugar metabolites. We found that androgen-sensitive LNCaP 33 cells retained greater dynamic potential for AR-mediated gene expression and androgen-glucuronide secretion, while the LNCaP 81 counterparts partitioned UDP-glcA into increased synthesis of glycosaminoglycans. We confirmed these properties in 22Rv1 cells, which are also a model for castration-resistant prostate cancer. In addition, we reduced UDP-glcA by knocking down UGDH, which resulted in diminished proteoglycan production and increased androgen-glucuronide output. These results are consistent with a model in which the altered flux of UDP-sugar intermediates through multiple downstream pathways blunts the function of the glucuronidation pathway and promotes intracellular androgen accumulation to sustain cellular proliferation in the absence of exogenous steroids.
Materials and Methods
Reagents
All reagents were obtained from Fisher Scientific (Pittsburgh, PA) except where indicated. 5-α-dihydrotestosterone (DHT) and DHT-glucuronide were from Sigma (St. Louis, MO). Charcoal-stripped fetal bovine serum (FBS) was from Hyclone (Logan, UT). Antibodies were purchased and used as follows: polyclonal rabbit anti-human prostate-specific antigen (PSA; DakoCytomation, Glostrup, Denmark, 1:1500 dilution), mouse monoclonal anti-human β-tubulin (1:750,000; Sigma), IRDye 800-conjugated anti-rabbit IgG (1:5000; Rockland, Gilberstville, PA), IRDye 680-conjugated goat anti-mouse IgG (1:5000; LI-COR Biosciences, Lincoln, NE), rabbit polyclonal anti-human UGT2B17 (1:100) and rabbit polyclonal anti-human HSD17B3 (1:500; GeneTex, Irvine, CA), mouse monoclonal anti-human AR (1:500; Santa Cruz Biotechnology, Inc., Dallas, TX), rabbit monoclonal anti-human Notch1 (1:1000; Cell Signaling Technology, Danvers, MA), and mouse monoclonal anti-human HSD3B1 (1:100) and rabbit monoclonal anti-human ARv7 (1:1000; Abcam, Cambridge, MA). Rabbit polyclonal anti-human UXS1 was raised against purified recombinant human UXS1, residues 85-420 (Pocono Rabbit Farms). The UXS1 expression construct was a gift from Nicola Burgess-Brown: Addgene plasmid no. 39162.
Cell Culture and Selection of Stable Lines
LNCaP (denoted LNCaP 33 herein), 22Rv1, and PC3 human prostate adenocarcinoma cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in culture for a maximum of ten passages after thaw. LNCaP 81 androgen-independent cells were kindly provided by Dr. Ming-Fong Lin [23] (University of Nebraska Medical Center, Omaha, NE). For standard culture, PC3 was maintained in MEM supplemented with 10 % FBS, non-essential amino acids, and sodium pyruvate; LNCaP and 22Rv1 lines were maintained in RPMI-1640 supplemented with 10 % FBS, all as recommended by the vendor. To generate UGDH knockdown lines, LNCaP 33 cells were transfected with plasmids encoding short hairpin RNA (shRNA) targeted to UGDH or a scrambled non-targeting control (UGDH shRNA-pGFP-V-RS no. TG334012 and 29-mer oligo-pGFP-V-RS no. TR30008, respectively, from Origene Technologies, Rockville, MD). Stable integration of the shRNA plasmids was achieved by clonal selection in RPMI-1640 with 10 % FBS and 1 μg/ml puromycin. Once selected, clones were maintained in the same medium containing 0.5 μg/ml of puromycin. Two clones of each were chosen for full characterization. Clones designated KD1 and KD2 had >70 % reduced UGDH protein expression relative to the two control clones designated C1 and C2 with UGDH expression identical to the parental line.
Androgen Stimulation
For short-term comparison of androgen response, LNCaP cells were subcultured to 50 % confluence in phenol red-free RPMI-1640 supplemented with 1 % charcoal-stripped FBS (CS-FBS) for 48 h (androgen-depleted conditions). DHT was serially diluted into media from concentrated stocks solubilized in ethanol as a vehicle. Androgen-depleted media were replaced with media containing the indicated concentration of DHT for 48 h and harvested for analysis. For long-term selection, cells were grown for 10 days in phenol red-free RPMI-1640 media containing 5 % CS-FBS and 0 (vehicle), 0.1, 1, or 10 nM DHT. Media were renewed every 2 days. Following selection, cells were either maintained in these conditions or subjected to 48 h in androgen-depleted media followed by 24–48 h stimulation with 10 nM DHT to quantify the magnitude of the androgen-stimulatable response.
Western Analysis
Cells were scraped into cold PBS following treatments, pelleted by centrifugation, and lysed in 1× RIPA buffer with protease inhibitor cocktail. Protein was quantified by Bradford assay, and equivalent amounts of total protein were analyzed. UGDH (rabbit polyclonal anti-human UGDH, 1:5000) [20], AR, PSA, and β-tubulin were probed simultaneously in cell lysates. UGT2B17, UXS1, Notch1, ARv7, HSD3B1, and HSD17B3 were each probed separately with β-tubulin on identical blots. Following secondary incubation, protein expression was quantified by fluorescence emission using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Signals for each wavelength were analyzed in red (700 nm, tubulin and HSD3B1) and green (800 nm, all other targets), to normalize protein expression. Statistical significance was assessed by Student’s t test. Each densitometric analysis is plotted for three technical replicates of a representative experiment that was repeated at least three times.
Detection of UDP-Glucuronosyltransferase mRNA Expression
Total RNA was isolated from LNCaP 33 and 81 cells that had been cultured in different concentrations of androgen for 3–10 days and then treated with vehicle or 10 nM DHT for another 48 h. Equivalent amounts of total RNA were reverse transcribed; the UGT2B15 and UGT2B17 coding sequences were amplified by PCR as previously published [20].
Quantification of UDP-Sugars and Androgen-Glucuronide Conjugates
Lysates from the indicated lines and treatments were extracted with butanol-saturated concentrated formic acid and analyzed for UDP-sugar content by liquid chromatography and mass spectrometry (LC-MS) according to published methods [24, 25]. Conditioned media from androgen-treated cell cultures were ethanol extracted for glucuronide analysis by LC-MS/MS, also as published [20]. Statistical significance was assessed by Student’s t test.
Chromatin Immunoprecipitation
LNCaP 33 and 81 were selected in DHT for 48 h or for 10 days. Media were removed and replaced with media-containing vehicle or 10 nM DHT for 16 h prior to chromatin immunoprecipitation (ChIP) assay. After AR immunoprecipitation, quantitative PCR (qPCR) was done using primers for the PSA enhancer element, PSA basal promoter (negative control), and actin (normalization control) [26]. AR bound to the PSA enhancer was plotted as percent input (fraction of total AR immunoprecipitated DNA that was amplified by gene-specific primers) from a single assay done in triplicate that is representative of at least three separate selection trials per condition. Amplification of DNA from the basal promoter was confirmed to be <0.02 % as expected for all conditions (not shown).
Luciferase Reporter Assay
LNCaP 33 and 81 cells were grown in RPMI-1640 media containing 5 % CS-FBS. After 10 days of selection with 0, 0.1, 1, and 10 nM of DHT, cells were transfected with pGL3-basic-Luc + pRL-SV40 Renilla plasmids or pGL3-PSA-Luc + pRL-SV40 Renilla plasmids. Twenty-four hours after transfection, cells were treated with vehicle or 10 nM DHT. After another 24 h, cells were harvested for luciferase assay using the Promega Dual Luciferase kit according to manufacturer instructions. Each experiment was done in triplicate wells and luciferase activities were normalized.
Results
Androgen-Dependent LNCaP Cells Have Lower Levels of Glucuronidation Enzymes and Greater Dynamic Range in AR-Mediated Gene Expression
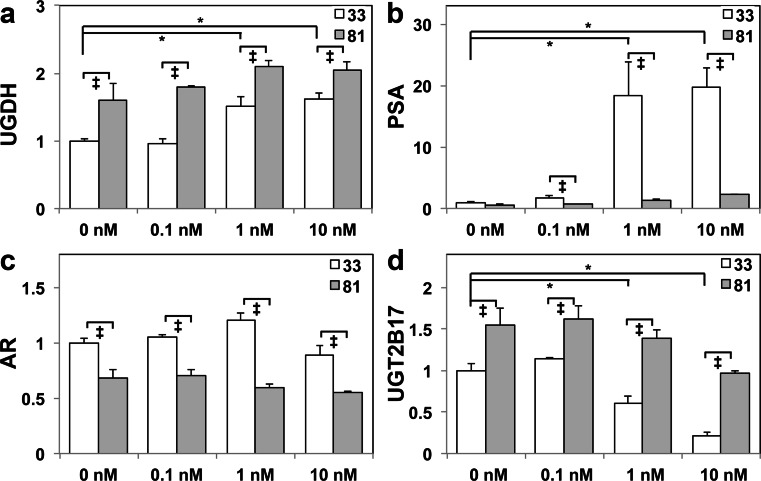
We previously showed that UGDH was an androgen-stimulated gene, and that its elevation by DHT administration produced up to 6-fold more DHT-glucuronide secretion by LNCaP 33 compared with LNCaP 81 cells [20], which model the androgen-dependent versus castration-resistant states of prostate cancer, respectively. To examine the impact of castration resistance on the glucuronidation pathway and AR-mediated gene expression, we exposed LNCaP 33 and 81 cells to 2 days of androgen-deficient media followed by an additional 2 days of vehicle (0 nM) or the indicated concentrations of DHT. As expected, UGDH expression was diminished in LNCaP 33 cells in the absence of DHT and restimulated at physiological doses (1 and 10 nM DHT, Fig 1a). UGDH levels were higher at all DHT doses in LNCaP 81 cells and were not altered by androgen withdrawal. Also as expected, PSA expression was virtually absent in both lines without DHT, and restimulated expression was >10-fold higher in androgen-sensitive LNCaP 33 cells (Fig 1b). AR expression, which is modestly suppressed by DHT, was ≈30 % lower in LNCaP 81 cells than in 33 cells (Fig 1c). UGT2B17 expression was upregulated in the absence of DHT in both LNCaP 33 and 81 cells, but while the levels were potently suppressed by DHT in a dose-dependent manner in LNCaP 33 cells, DHT treatment did not significantly reduce the elevated expression in LNCaP 81 cells (Fig 1d). Similar results were obtained using 22Rv1 cells, which express wild-type AR and are mildly androgen responsive but not androgen dependent (Fig. S1a–d). In these cells, UGDH and UGT2B17 were also elevated, while PSA and AR expression were reduced, and levels were not altered by DHT. PC3 cells, which lack AR expression, also did not have PSA, appreciable levels of UGT2B17, or DHT-sensitive UGDH upregulation (Fig. S1a–d). Collectively, these results show that the magnitude of both AR-mediated activation and repression was significantly reduced in the castration-resistant state. Moreover, despite reduced DHT-glucuronide secretion, LNCaP 81 cells had elevated expression of the glucuronidation pathway enzymes UGDH and UGT2B17.
Fig. 1.
Castration-resistant prostate tumor cells exhibit blunted ligand-dependent AR responses, with higher levels of glucuronidation enzymes relative to androgen-sensitive controls. LNCaP 33 and LNCaP 81 cells were cultured in androgen-depleted media for 48 h, followed by 48 h of treatment with the indicated doses of DHT. Total cell lysates were analyzed in triplicate by immunoblots probed for the indicated proteins: a UGDH, b PSA, c AR, and d UGT2B17. Densitometric quantification was normalized to tubulin and plotted as mean ± SEM of fold changes relative to the LNCaP 33 0 nM treatment. *p < 0.05 relative to LNCaP 33, 0 nM DHT; ‡ p < 0.05 for LNCaP 81 relative to LNCaP 33 at the indicated DHT treatment
Prolonged Androgen Depletion Leads to Dose-Dependent Decreases in Basal and Androgen-Stimulated Gene Expression
Increased UDP-glcA production by UGDH can drive androgen inactivation and thereby modify tumor cell growth in androgen-responsive cells. If UGDH is a causative factor in these phenomena, its expression levels should be influenced by prolonged exposure to different DHT concentrations and translate to direct effects on AR-mediated gene expression. To test this, we selected LNCaP 33 and 81 cells for 10 days in androgen-free media that were supplemented with 10-fold decrements of DHT from 10 to 0 nM (vehicle). We then examined the effects of selection on gene expression and AR transcriptional activity in the unstimulated and androgen-treated states, to observe both basal impact and changes in responsiveness to hormones.
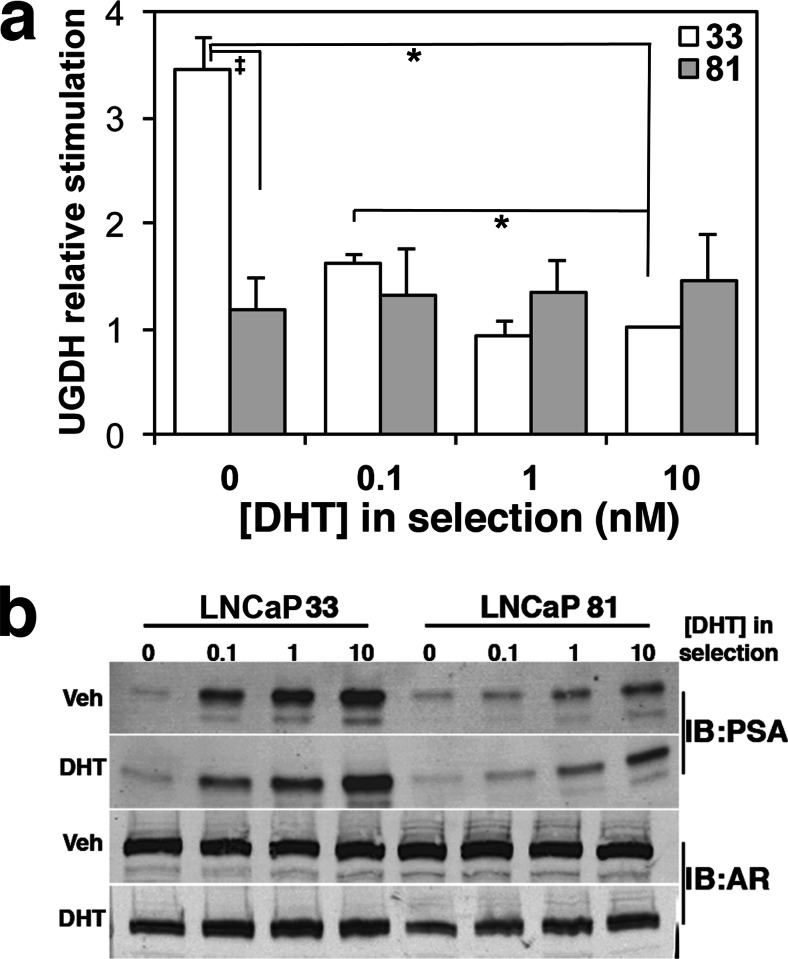
After selection for 10 days in androgen-free media, UGDH basal expression in LNCaP 33 cells was significantly reduced and could be stimulated 3.5-fold by re-introduction of DHT, while UGDH levels did not change in LNCaP 81 cells (Fig. 2a shows relative stimulation). The drop in basal UGDH expression specifically in LNCaP 33 cells suggests that the provision of androgen from extracellular sources is necessary to sustain high levels of UGDH protein in these cells. Selection in the lowest concentration of androgen (0.1 nM) preserved UGDH expression such that only ≈1.6-fold stimulation was observed upon restoration of DHT (Fig. 2a). In 1 nM or 10 nM conditions, no loss of UGDH expression was observed and no further stimulation occurred. That is, basal and stimulated UGDH expression was the same in LNCaP 33 and 81 cells that were selected in at least 1 nM DHT (Fig. 2a). Thus, 1–10 nM DHT treatment appeared to be both necessary and sufficient to maintain UGDH expression in androgen-sensitive cells. However, it is notable that this level of regulation was lost in cells that do not respond to exogenous DHT. We re-probed these western blots for PSA and for AR expression (Fig. 2b). Similar dose-dependent loss of expression was observed for PSA in both LNCaP 33 and 81 cells after selection, though as in the short-term treatments, PSA expression at all levels was significantly lower in LNCaP 81 than in LNCaP 33. Expression of AR protein was not altered by selection.
Fig. 2.
Re-expression of AR-activated genes UGDH and PSA is dose dependent following prolonged androgen depletion. LNCaP 33 and 81 cells were selected for 10 days in the indicated DHT concentrations. Prior to harvest, media were removed and replaced with media containing vehicle or 10 nM DHT for 24 h. a Equivalent amounts of protein were immunoblotted for UGDH and tubulin. UGDH expression was quantified, normalized to tubulin, and plotted as the ratio of 24 h DHT-treated versus vehicle-treated expression to illustrate stimulated expression. Mean ± SEM is plotted; *p < 0.05 relative to LNCaP 33 at 10 nM DHT; ‡ p < 0.05 for LNCaP 81 relative to LNCaP 33 at the indicated DHT treatment. b Blots in (a) were re-probed for PSA (34 kDa) and AR (102 kDa)
Extended Androgen Depletion Elevates Expression of UGT2B17 and Reduces Sensitivity of UGT2B15 and UGT2B17 to DHT Suppression
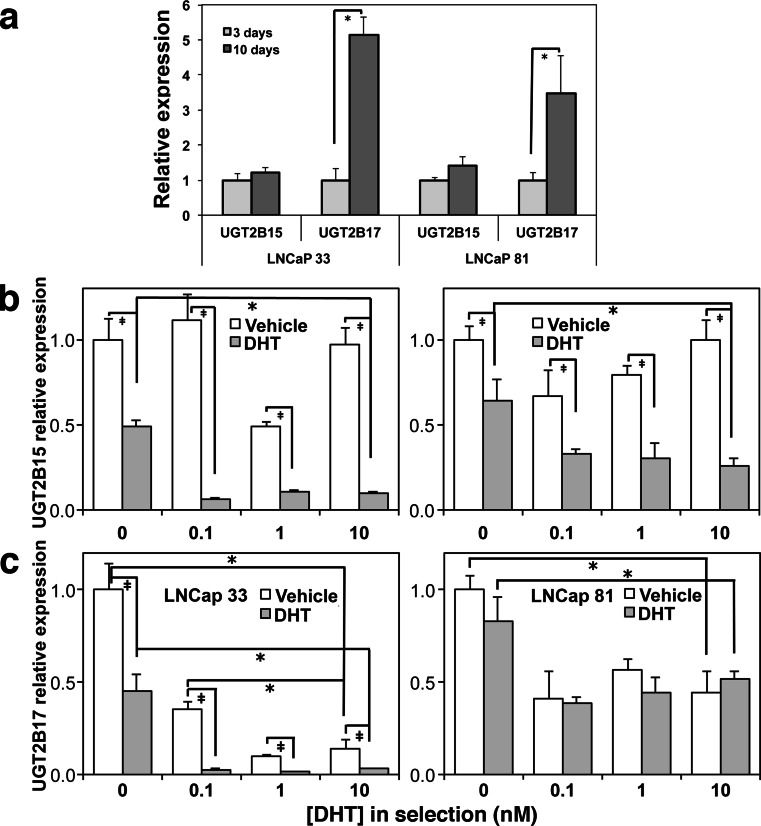
AR has been shown to mediate DHT-induced suppression of UGT isoforms UGT2B15 and UGT2B17 in dose-dependent fashion within a 24-h time period [27]. Since mass action glucuronidation driven by UGDH requires UGT activity, we next quantified effects of differential androgen selection on basal and DHT-suppressible UGT expression. LNCaP 33 and 81 cells were cultured in androgen-depleted media for 10 days, and expression was compared by qRT-PCR at an early time point (3 days) and at endpoint. Selection for 10 days modestly elevated expression of UGT2B15 in both LNCaP 33 and 81 cells relative to the 3-day time point, but UGT2B17 expression was ≈5-fold and ≈3.5-fold increased in LNCaP 33 and 81 cells, respectively (Fig. 3a). It should be noted that although levels are normalized to emphasize pairwise fold comparison in the figure, expression of both isozymes was significantly higher in LNCaP 81 cells than in LNCaP 33 cells at 3 days. This finding is consistent with the short-term results in Fig. 1 and suggests there is a loss of ligand-dependent AR activity at the UGT promoters.
Fig. 3.
Strong androgen-dependent repression of UGT enzymes is maintained in androgen-sensitive LNCaP 33 cells but lost in castration-resistant LNCaP 81 cells. LNCaP 33 and 81 cells were selected for 10 days in the absence of DHT (a) or in the indicated DHT concentrations (b, c). Prior to harvest, media were removed and replaced with media containing vehicle or 10 nM DHT for 24 h. Total RNA was extracted and analyzed for UGT2B15 (b) and UGT2B17 (c) expression by qRT-PCR. Mean ± SEM of triplicate assays is plotted. In (a), expression was compared in cells cultured only in androgen-depleted media for the indicated times, without DHT stimulation; *p < 0.05 for 10 days relative to 3 days. b, c *p < 0.05, DHT-deficient selection relative to 10 nM selection; ‡ p < 0.05 for vehicle versus DHT in each selection
We next selected LNCaP 33 and 81 cells in androgen decrements for 10 days, followed by a 24-h treatment with vehicle or DHT, and evaluated UGT2B15 and UGT2B17 expression by qRT-PCR (Fig. 3b, c). Expression was normalized to its respective level at 0 nM DHT in the untreated samples. The expression of both UGT isozymes was 3–20-fold higher in LNCaP 81 than in 33 cells. In both lines, the degree to which DHT suppressed either isozyme was significantly reduced when cells were selected in the complete absence of DHT. In LNCaP 33 cells (Fig. 3b, c), both UGT2B15 and UGT2B17 were sensitive to suppression by DHT treatment in all doses. UGT2B15 basal expression was relatively stable at all selection doses and suppressed by addition of 10 nM DHT as expected (Fig. 3b, left). Inhibition of basal UGT2B17 expression in LNCaP 33 cells was ≈65 % at 0.1 nM DHT selection and further suppressed (≈90 %) at higher doses (Fig. 3c, left). Upon 24-h treatment with DHT, expression was diminished by 55 % if cells were selected in the complete absence of DHT, but up to >95 % for all selection doses. Such strong regulation was not observed for the LNCaP 81 cells, though selection in DHT reduced UGT2B17 basal and stimulated levels by ≈50 %. Thus, selection conditions profoundly impact regulation of UGT in prostate cells.
Androgen-deficient selection impacts ligand-dependent AR binding and transcriptional activation of the PSA promoter/enhancer
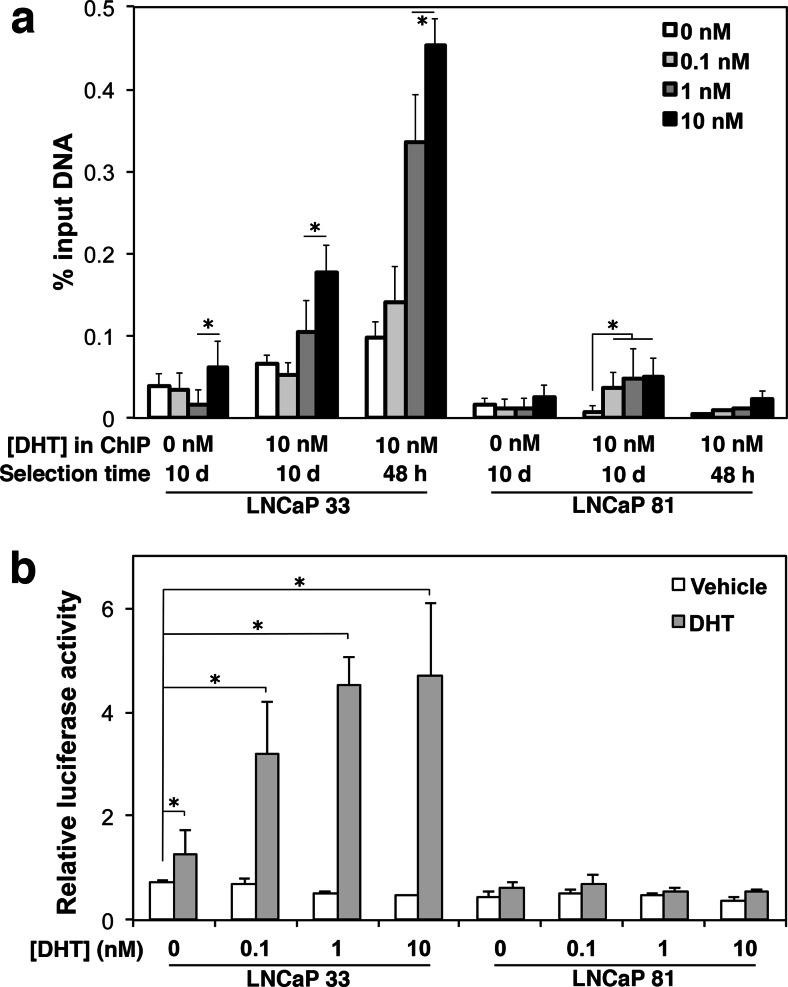
Since AR regulates PSA expression, the lack of correlation between AR protein and re-stimulation of PSA after selection suggested an impact of selection on AR activity. To examine this, we used ChIP to quantify AR bound to the PSA androgen response element as previously described [28]. Cells were selected in decreasing androgen as indicated in the legend for a 10-day period and assayed by ChIP after a 16-h treatment with or without 10 nM DHT (Fig. 4a). As a positive control, results of the 10-day selection were compared with cell cultures selected for only 48 h before final DHT treatment, which were the conditions used in prior experiments [20]. Basal AR bound to the PSA enhancer was minimal in both cell lines whether cells were selected for 48 h or 10 days unless first treated with DHT (Fig. 4a, “DHT in ChIP”: 0 nM illustrates basal AR bound, 10 nM DHT added illustrates ligand-activated AR bound, indicated on the x-axis). In LNCaP 33 cells, presence of DHT in the selection media increased AR occupancy at the PSA enhancer in a dose-dependent manner. Prolonged selection in low or no androgen strongly reduced the level of AR that could be stimulated to bind the PSA enhancer upon subsequent 10 nM DHT addition, relative to the 48 h treatment. In LNCaP 81 cells, less AR was bound to the PSA enhancer at all doses, which was only partially attributable to the reduced expression of AR in those cells, and DHT addition also did not stimulate appreciable AR binding. To examine AR transcriptional activation, we used a luciferase reporter driven by the PSA promoter. Cells selected for 10 days in the indicated DHT concentrations were transfected for 24 h with the PSA-luciferase reporter or vector control, then stimulated for 24 h with DHT before harvesting and assaying luciferase activity (Fig. 4b). As observed in the ChIP assay, selection in decreasing androgen reduced the magnitude of reporter expression upon DHT stimulation, specifically in LNCaP 33 cells. These results are consistent with dose-dependent loss of AR promoter binding and transcriptional activity after prolonged low androgen selection.
Fig. 4.
Prolonged androgen depletion dampens ligand-dependent function of the AR in LNCaP 33 and 81 cells. Cells were selected in 0, 0.1, 1, or 10 nM DHT for 48 h or for 10 days. Selected cells were then treated with vehicle or 10 nM DHT prior to assay. a Genomic DNA was analyzed by anti-AR ChIP. AR occupancy of the PSA promoter was quantified by qPCR in technical duplicates and plotted as percent input (fraction of total immunoprecipitated DNA amplified by the gene-specific primers). Data shown represent mean ± SEM for one of three assays replicated with identical trends. b Following selection, cells were co-transfected with PSA-Luc and Renilla reporter constructs and subsequently treated with vehicle or 10 nM DHT for another 24 h before assaying luciferase activity. Mean ± SD of normalized activity measured in triplicate is plotted. *p < 0.05 for DHT re-stimulated relative to unstimulated at 0 nM
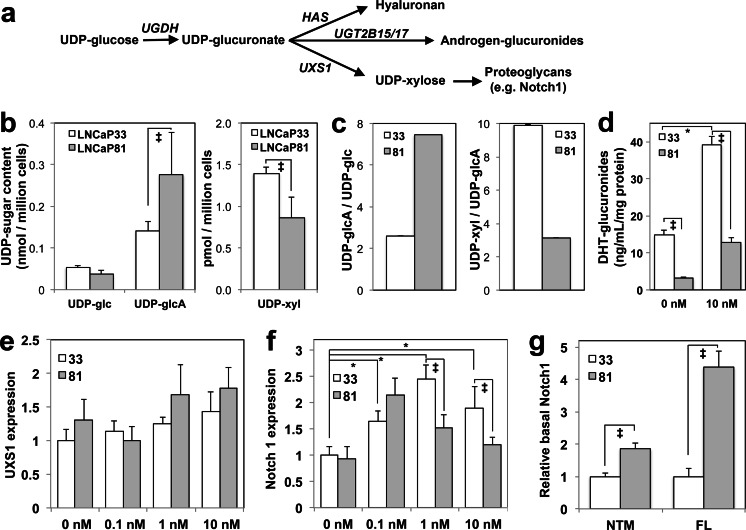
To relate loss of AR function in LNCaP 81 cells to the overall status of glucuronidation pathway function, we quantified downstream metabolites and expression levels of gene products dependent on those metabolites. Figure 5a illustrates the three direct fates of UDP-glucuronate in prostate tumor cells: (1) hyaluronan synthase (HAS) enzymes alternately polymerize glucuronate and N-acetylglucosamine using the UDP-esterified precursors; (2) UGT enzymes produce androgen-glucuronides including conjugates of testosterone, DHT, or androstanediol; and (3) UDP-xylose synthase 1 (UXS1) decarboxylates UDP-glcA to produce UDP-xylose (UDP-xyl), which is an essential initiating precursor for all chondroitin sulfate and heparan sulfate proteoglycans, as well as a precursor for specialized trisaccharides needed for surface presentation of proteoglycan receptors such as Notch1. UDP-glc and UDP-glcA are the substrate and product of UGDH, respectively. Similarly, UDP-glcA and UDP-xyl are the substrate and product of UXS1. Absolute quantification of these nucleotide sugars revealed significantly altered profiles in LNCaP 81 relative to LNCaP 33 cells (Fig. 5b). Re-plotting the ratio of UDP-glcA to UDP-glc provides a measure of the relative flux through UGDH, while the ratio of UDP-xyl to UDP-glcA indicates flux through UXS1 (Fig. 5c). From these plots, it was evident that LNCaP 81 had significantly higher levels of UDP-glcA and >2-fold higher product/substrate ratio for UGDH than LNCaP 33 cells, while the UXS1 product/substrate ratio was only ≈30 % in LNCaP 81 relative to LNCaP 33.
Fig. 5.
Metabolite analysis of glucuronidation pathway components. a Schematic of the glucuronidation pathway and its alternatives. b UDP-sugar content was quantified by LC-MS in whole lysates from LNCaP 33 and LNCaP 81 cells cultured in standard serum-containing media. Mean ± SD is plotted. c The ratios of UDP-glucuronate (UDP-glcA) to UDP-glucose (UDP-glc) and UDP-xylose (UDP-xyl) to UDP-glcA were calculated from mean values in (b) to illustrate steady-state intracellular flux through UGDH and UXS1, respectively. Note that the scale for UDP-xyl/UDP-glcA is ×10−3. d DHT-G secretion was quantified from conditioned media of each cell line and plotted as mean ± SD. e, f LNCaP 33 and LNCaP 81 cells were cultured in androgen-depleted media for 48 h, followed by 48 h of treatment with the indicated doses of DHT. Total cell lysates were analyzed in triplicate by immunoblots probed for UXS1 (e) or Notch1 (f). g Lysates from cells cultured in standard media were analyzed by immunoblot for Notch1. NTM indicates quantification of the ≈120 kDa intracellular and transmembrane domain of Notch1; FL denotes the ≈272 kDa full-length Notch1 protein. In (e)–(g), densitometric quantification was normalized to tubulin and mean ± SEM is plotted. *p < 0.05 relative to LNCaP 33, 0 nM DHT; ‡ p < 0.05 for LNCaP 81 relative to LNCaP 33 at the indicated DHT treatment
Higher UDP-glcA in LNCaP 81 cells was not secreted as DHT-glucuronide (Fig. 5d) so we measured expression of UXS1 and Notch1 to assess flux through this arm of the pathway. UXS1 levels in androgen-depleted and re-fed cells was modestly higher in LNCaP 81 cells at all DHT doses and did not exhibit significant androgen dependence (Fig. 5e). Expression of the xylose-modified mature proteoglycan Notch1 was significantly androgen stimulated at all doses in LNCaP 33 cells following a short-term androgen depletion (Fig. 5f). Importantly, overall steady-state expression of both the full-length (FL) Notch1 protein and the mature cleaved Notch1 intracellular/transmembrane domain (NTM) indicative of the correctly glycosylated state of the protein was increased ≈5- and ≈2-fold, respectively, in LNCaP 81 cells (Fig. 5g).
To confirm and extend these results, we also profiled metabolites and Notch1 expression in 22Rv1 and PC3 cells. The overall responses of 22Rv1 cells were very similar to those of LNCaP 81 cells. Specifically, steady-state UDP-sugar levels were comparable in magnitude and the ratios exhibited the same trends of higher UDP-glcA and lower UDP-xyl (Fig. S2a, b). DHT-glucuronide secretion was not detectable in either 22Rv1 or PC3 media, so we compared glucuronidation of androstanediol (A-diol), the immediate major product of DHT catabolism. A-diol-glucuronide was ≈80–100-fold lower in both lines, relative to LNCaP 33 and 81 (Fig. S2c). UXS1 expression was not different from LNCaP 33 (Fig. S2d), but Notch1 NTM and FL were also increased 2- and 6-fold, respectively, in 22Rv1 cells (Fig. S2e, f). Collectively, results of these analyses show that altered metabolite profiles indicated differences in partitioning of nucleotide sugar precursors that underlie altered glucuronidation potential of LNCaP 81 and 22Rv1 cells. Such partitioning is consistent with prioritized use of nucleotide sugars for the production of secreted proteoglycans by LNCaP 81 cells, which permits the cells to retain intracellular androgens.
Stable UGDH Knockdown Increases Basal AR-Mediated Gene Expression and DHT Glucuronidation
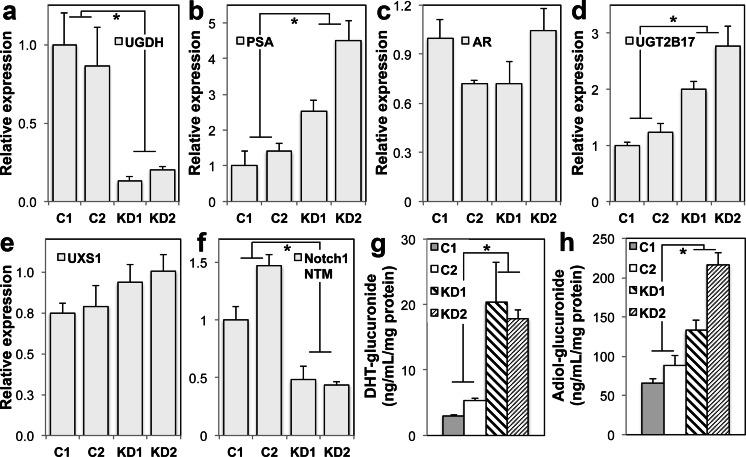
To test the role of UGDH in androgen-dependent gene expression, we compared LNCaP 33 cells clonally selected for shRNA knockdown of UGDH or expression of a non-targeting control vector. Knockdown of UGDH led to significant increases in expression of both PSA and UGT2B17, with varied impact on AR expression (Fig. 6a–d). As observed in LNCaP81 and 22Rv1 cells, UXS1 levels remained largely unchanged (Fig. 6e). However, in contrast to the profiles of these lines, the expression of cell surface Notch1 was >50 % reduced when UGDH was depleted (Fig. 6f). Furthermore, DHT-glucuronide (DHT-G) content of the cell-conditioned media from the UGDH knockdown lines was ≈4-fold higher (Fig. 6g) and A-diol-glucuronide was 1.5–2-fold higher (Fig. 6h), relative to the control lines. These results are consistent with androgen-dependent cells preferentially partitioning UDP-glcA precursors to the glucuronidation pathway in conditions during which UDP-glcA is limiting, at the expense of proteoglycan production.
Fig. 6.
Stable UGDH knockdown increases basal AR-mediated gene expression and androgen glucuronidation, while diminishing Notch1 proteoglycan expression. Two individual clones of LNCaP 33 cells selected for stable UGDH-targeted shRNA knockdown or non-targeted shRNA controls were grown in standard steroid-replete media. Whole cell lysates were analyzed by western blot for a UGDH, b PSA, c AR, d UGT2B17, e UXS1, or f the intracellular and transmembrane domain of Notch1 (NTM). Expression was normalized to tubulin and plotted as mean ± SEM for three biological replicates of each clone. g DHT-G or h A-diol-G secretion was quantified from conditioned media of each clone and plotted as mean ± SD. In (a)–(f), *p < 0.05 relative to values for C1
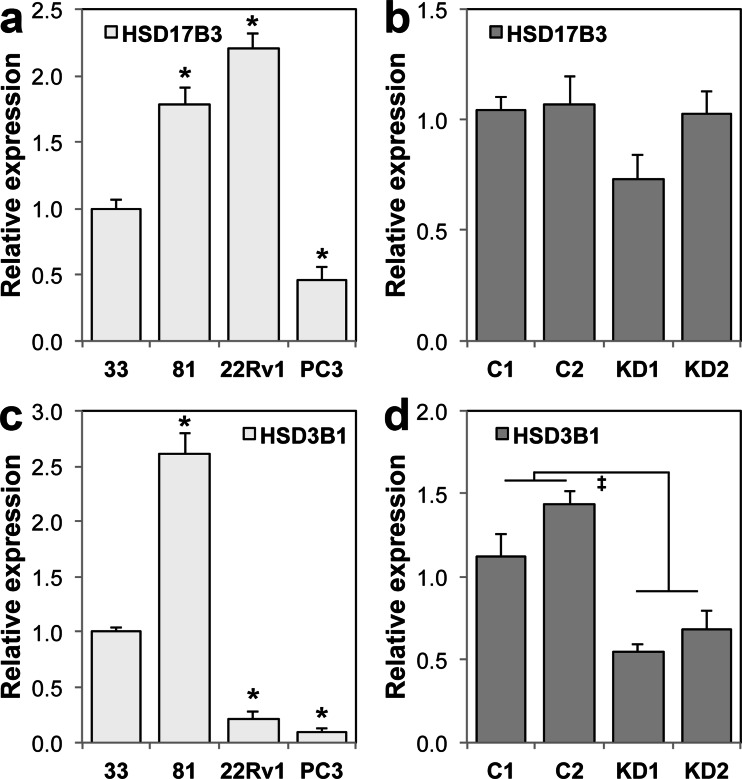
A consequence of persistent UDP-glcA deficiency could be exaggerated cellular androgen depletion, which could stimulate expression of androgen biosynthetic enzymes. Therefore, we examined expression of two representative enzymes, HSD17B3 and HSD3B1, which participate in the conversion of androgen precursors such as DHEA, estrogens, and cholesterol, into the potent AR ligand DHT. Both enzymes were expressed at detectable levels in all cell lines tested, and both were elevated in LNCaP 81 relative to LNCaP 33 (Fig. 7a, c). HSD17B3 was not affected by UGDH knockdown (Fig. 7b), but HSD3B1 was reduced ≈50 % in UGDH knockdown lines (Fig. 7d). Therefore, elevated androgen glucuronidation in the absence of UGDH, which would be expected to diminish cellular androgen levels, did not act globally to increase enzyme capacity for biosynthesis of androgens in LNCaP 33 cells. However, the increased expression of biosynthetic enzymes in LNCaP 81 cells would be predicted to increase androgens available for glucuronidation, but this was not reflected in glucuronide production (Fig. 5d). We further examined the expression of the ARv7 splice variant, which lacks the androgen binding domain and has been shown to initiate expression of a separate pool of genes from those under control of the full-length AR. Only 22Rv1 cells expressed detectable levels of ARv7 (Fig. S3a), which could contribute to the partial constitutive derepression of UGT2B17 in those cells, but does not explain the similar outcome in the LNCaP lines and UGDH knockdown sublines. Finally, we quantified basal expression of the pioneer transcription factor FoxA1, which is required for UGT2B17 expression. However, levels were unchanged in any of the lines, except PC3 (Fig. S3b). In the latter, the low levels of FoxA1 could partly account for the virtually undetectable level of UGT2B17. Collectively, the results suggest altered expression of ARv7, FoxA1, and HSD enzymes are not general underlying factors mediating metabolite partitioning, but that metabolite fate priorities are dependent on UGDH expression.
Fig. 7.
UGDH knockdown reduces HSD3B1. LNCaP 33, 81, 22Rv1, and PC3 cell lines (a, c) or LNCaP 33 control and UGDH knockdown clones (b, d) were grown in standard steroid-replete media. Total cell lysates were normalized by protein content and analyzed in triplicate by immunoblots probed for HSD17B3 (a, b) or HSD3B1 (c, d). Densitometric quantification was normalized to tubulin and plotted as mean ± SEM of fold changes relative to LNCaP 33 or C1. *p < 0.05 relative to LNCaP 33; ‡ p < 0.05 relative to C1
Discussion
Mechanisms by which prostate tumor cells lose dependence on extracellular androgens involve extensive feedback-regulated signaling and crosstalk. Maintaining narrow concentration ranges of DHT for optimal AR-mediated gene expression and function depends on a balance between exogenous hormone precursors, intracellular synthesis, and inactivation. In this study, we compared the function of the androgen-regulated inactivation pathway in an isogenic LNCaP model of androgen-dependent versus castration-resistant prostate cancer, to determine the specific role of glucuronidation precursor availability in promoting and sustaining androgen-dependent gene expression in prostate tumor cells. Despite apparent reduced glucuronide output, LNCaP 81 castration-resistant tumor cells expressed higher levels of UGDH, UGT2B15, and UGT2B17, the component enzymes of the glucuronidation pathway. However, the blunted androgen-responsive activation of UGDH and PSA, and the minimal repression of AR and UGT2B17, were consistent with the obvious loss of AR promoter binding and transcriptional activation we also measured. Analysis of the UDP-sugar pools and flux through pathways downstream of UDP-glcA production revealed that these precursors were channeled instead through proteoglycan and glycosaminoglycan biosynthetic pathways, leading to increased surface expression of Notch1. Depletion of UDP-glcA by knockdown of UGDH in androgen-dependent LNCaP cells stimulated glucuronide production and reduced Notch1. Overall, these results suggest that UDP-sugar levels may have a greater impact on androgen sensitivity than previously appreciated, by mechanisms independent of protein expression alone.
We previously found that androgen-stimulated UGDH expression increased DHT-glucuronide content of LNCaP conditioned media in androgen-dependent LNCaP 33 cells despite strong concurrent suppression of UGT2B15 and UGT2B17 [20], which demonstrated that precursor availability could drive glucuronidation with minimal glucuronosyltransferase expression. In contrast, we show here that LNCaP 81 cells have increased expression of UGDH and UGT, and elevated UDP-glcA precursors, but fail to secrete significant DHT-G. This is the more puzzling as the LNCaP 81 cells have been reported to have higher levels of intracellular androgen biosynthesis [29], which would be expected to contribute further to increased androgen-glucuronide pools. Thus, we sought to identify alternative pathways activated concurrently with the loss of androgen dependence. Gene expression profiling previously revealed alterations in ErbB2 and Cyclin H in LNCaP 81, which may promote more rapid cell proliferation independently of androgens [23]. Importantly, this report also found elevated expression of PSMA in these cells, which is a prostate-specific cell surface chondroitin sulfate proteoglycan. This is consistent with our finding that Notch1 is also upregulated and suggests a global re-patterning of UDP-glcA fates to preferentially support proteoglycan synthesis.
UDP-glcA is an immediate precursor for UDP-xyl synthesis, which is essential for all proteoglycan production. However, UGDH is strongly inhibited by UDP-xyl in vitro [30] and this is a potential endogenous regulator of UDP-glcA flux through different pathways. To investigate possible effects of UDP-xyl production on UGDH function, we quantified UDP-xyl. Levels are very low relative to the other UDP-sugars (Fig. 5b; Fig. S2). In comparing LNCaP 33 and 81 cells, UDP-xyl appears to be correlated inversely with steady-state UDP-glcA, which is consistent with the elevated UGDH and UDP-glcA in LNCaP 81 cells. Similar results were observed in 22Rv1 cells. The significantly lower UDP-xyl levels reflect rapid use of UDP-xyl in downstream proteoglycan synthesis, such that its levels do not build and inhibit UDP-glcA production, as they do in LNCaP 33. UDP-xylose is required for processing of Notch1 and presentation at the plasma membrane so fluxes in UDP-xyl levels would be predicted to affect both full-length and processed Notch1 levels [31], as we observed (Fig. 5g; Fig. S2). Since large amounts of UDP-glcA are also required for high-level proteoglycan synthesis, which occurs in the Golgi apparatus, while DHT-glucuronide formation is catalyzed in the ER, the UDP-glcA may be preferentially delivered to the Golgi, thereby restricting its availability for androgen glucuronidation in LNCaP 81 and 22Rv1 cells.
We also found LNCaP 81 cells synthesize ≈2-fold more hyaluronan than LNCaP 33 cells [20]. This is a third alternative limiting factor for UDP-glcA availability in LNCaP 81 cells. However, we have shown that hyaluronan production suppresses cell proliferation, which is not compatible with the selected rapidly proliferating phenotype of the LNCaP 81 cells. Nonetheless, it is important to consider that prioritizing UDP-glcA to elevating proteoglycan production also requires sufficient nutrient availability to support significantly increased protein synthesis. It may be for this reason that we saw LNCaP 33 and 81 cells had similar rates of Notch1 surface expression in 1 % serum-containing media following androgen depletion (Fig. 5f) while the steady-state Notch1 levels in standard 10 % serum-containing media were higher in the LNCaP 81 cells. Overall, these results suggest the pathway favored by prostate tumor cells selected to avoid dependence on androgens is proteoglycan synthesis, because it competes with growth-slowing processes like hyaluronan production and androgen glucuronidation. This is further supported by the observation that UGDH expression is elevated in cell lines modeling CRPC, and its knockdown reprioritizes use of the limited UDP-glcA for glucuronidation, significantly diminishing proteoglycan synthesis.
While it is reasonable to propose that re-patterned metabolite use is a driver of prostate tumor cell loss of androgen dependence, other possible functional changes cannot be ruled out. Our analysis of AR expression showed no evidence of altered splice variant expression between LNCaP 33 and 81 cells (Fig. 3; Fig. S3), so we do not think ligand-independent AR function is the primary factor in the blunted responses we observed in expression of PSA and suppression of UGT (Fig. 1; Fig. S1). The AR has also been shown to undergo multiple post-translational modifications that modulate its subcellular localization and its association with transcriptional coregulation complexes [9, 32]. We did not examine or quantify these modifications so it is possible that androgen-independent gene expression changes occur by this mechanism. An additional component of androgen elimination is the organic anion transport protein family (OATP), also known as solute carrier proteins denoted SLCO, which are the transporters by which androgen conjugates leave and enter the cell. Altered expression of specific members of this transporter family has been correlated with castration-resistant and/or metastatic prostate cancer progression [33–35]. Thus, the reduced secretion of DHT-G could occur as a result of such changes, which would be expected to limit production of androgen-glucuronides and prevent use of UDP-glcA in this arm of the pathway.
Androgen glucuronidation enzymes have been implicated in prostate cancer risk, metastasis, and response to anti-androgen therapy in clinical and mechanistic studies [13, 14, 16–18, 36, 37]. Here, we found an association between dampened AR-mediated gene expression and increased glucuronidation potential as measured by UDP-glcA precursor availability and expression of enzymes in the pathway, which are hallmarks in loss of cellular androgen responsiveness. We propose a novel mechanism by which cellular metabolite flux regulates androgen dependence through partitioning of glucuronidation precursors. The knockdown of UGDH and consequent perturbation of cellular UDP-sugar metabolites yielded an overall phenotype that may be more responsive to androgen deprivation. Features of the stable UGDH knockdown included increased androgen elimination, decreased proteoglycan production, and reduced HSD3B1 expression. In prostate tumor cells, the biosynthesis of testosterone and DHT from the alternative circulating precursor DHEA-S is directly controlled by HSD3B1. Thus, the net outcome of UGDH inhibition would be to deplete all intracellular androgens while preventing the proteoglycan “escape route.” It will be interesting to determine whether loss of UGDH in castration-resistant tumor cells can reverse metabolite partitioning and restore androgen sensitivity. These insights may provide new diagnostic tools or therapeutic targets to predict castration-resistant recurrence.
Electronic Supplementary Material
Castration-resistant prostate tumor cells exhibit blunted ligand-dependent AR responses. 22Rv1 and PC3 cells were cultured in androgen-depleted media for 48 h, followed by 48 h with or without 1 nM DHT. Equivalent amounts of protein from whole cell lysates were analyzed in triplicate by immunoblots probed for the indicated proteins: a UGDH, b PSA, c AR, and d UGT2B17. Densitometric quantification was normalized to tubulin and plotted as mean ± SEM of fold changes relative to the LNCaP 33 0 nM treatment, which is also plotted for reference. *p < 0.05 relative to LNCaP 33, 0 nM DHT. (JPEG 24 kb)
Metabolite and proteoglycan partitioning analysis of 22Rv1 and PC3. a UDP-sugar content was quantified by LC-MS in whole lysates from 22Rv1 and PC3 cells cultured in standard serum-containing media. Mean ± SD is plotted. b The ratios of UDP-glcA to UDP-glc and UDP-xyl to UDP-glcA were calculated from mean values in (a) to illustrate steady-state intracellular flux through UGDH and UXS1, respectively. Note that the scale for UDP-xyl/UDP-glcA is ×10−3. c LNCaP 33, LNCaP 81, 22Rv1, and PC3 cells were cultured in androgen-depleted media for 48 h, followed by 48 h of treatment with DHT. Androstanediol (A-diol)-glucuronide secretion was quantified from conditioned media of each cell line and plotted as mean ± SD. d–f Total lysates from cells cultured in standard media were analyzed in triplicate by immunoblots probed for UXS1 (d) or Notch1 (e, f). NTM indicates quantification of the ≈120 kDa intracellular and transmembrane domain of Notch1; FL denotes the ≈272 kDa full-length Notch1 protein. In (d)–(f), densitometric quantification was normalized to tubulin and mean ± SEM is plotted. In (a–f), *p < 0.05 relative to LNCaP 33. (JPEG 52 kb)
ARv7 is only detected in 22Rv1 cells. FoxA1 expression is comparable in all AR-expressing lines and unaltered by UGDH knockdown. Total lysates from cells cultured in standard media were analyzed in triplicate by immunoblots probed for ARv7 (a) or FoxA1 (b). Densitometric quantification was normalized to tubulin and mean ± SEM is plotted. *p < 0.05 relative to LNCaP 33. (JPEG 18 kb)
Acknowledgments
This work was supported by NIH R01 CA165574 (MAS), NIH P20 GM103489 (MAS), NIH P30GM103335 (JS, JJB, MAS), and NIH R21 R21CA185993 (MAS, JJB, JEM).
Compliance with Ethical Standards
Conflict of Interest
The authors have no conflicts to declare.
Footnotes
Brenna M. Zimmer and Michelle E. Howell contributed equally to this publication.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55(5):300–318. doi: 10.3322/canjclin.55.5.300. [DOI] [PubMed] [Google Scholar]
- 4.Katzenwadel A, Wolf P. Androgen deprivation of prostate cancer: leading to a therapeutic dead end. Cancer Lett. 2015;367(1):12–17. doi: 10.1016/j.canlet.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15(10):3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 6.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 7.Zhang A, Zhang J, Plymate S, Mostaghel EA. Classical and non-classical roles for pre-receptor control of DHT metabolism in prostate cancer progression. Horm Cancer. 2016 doi: 10.1007/s12672-016-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware KE, Garcia-Blanco MA, Armstrong AJ, Dehm SM. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr Relat Cancer. 2014;21(4):T87–T103. doi: 10.1530/ERC-13-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turgeon D, Carrier JS, Levesque E, Hum DW, Belanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142(2):778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- 11.Chouinard S, Pelletier G, Belanger A, Barbier O. Cellular specific expression of the androgen-conjugating enzymes UGT2B15 and UGT2B17 in the human prostate epithelium. Endocr Res. 2004;30(4):717–725. doi: 10.1081/ERC-200044014. [DOI] [PubMed] [Google Scholar]
- 12.Guillemette C, Hum DW, Belanger A. Regulation of steroid glucuronosyltransferase activities and transcripts by androgen in the human prostatic cancer LNCaP cell line. Endocrinology. 1996;137(7):2872–2879. doi: 10.1210/endo.137.7.8770908. [DOI] [PubMed] [Google Scholar]
- 13.Chouinard S, Barbier O, Belanger A. UDP-glucuronosyltransferase 2B15 (UGT2B15) and UGT2B17 enzymes are major determinants of the androgen response in prostate cancer LNCaP cells. J Biol Chem. 2007;282(46):33466–33474. doi: 10.1074/jbc.M703370200. [DOI] [PubMed] [Google Scholar]
- 14.Chouinard S, Yueh MF, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, Belanger A. Inactivation by UDP-glucuronosyltransferase enzymes: the end of androgen signaling. J Steroid Biochem Mol Biol. 2008;109(3–5):247–253. doi: 10.1016/j.jsbmb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Guillemette C, Levesque E, Beaulieu M, Turgeon D, Hum DW, Belanger A. Differential regulation of two uridine diphospho-glucuronosyltransferases, UGT2B15 and UGT2B17, in human prostate LNCaP cells. Endocrinology. 1997;138(7):2998–3005. doi: 10.1210/endo.138.7.5226. [DOI] [PubMed] [Google Scholar]
- 16.Park J, Chen L, Shade K, Lazarus P, Seigne J, Patterson S, Helal M, Pow-Sang J. Asp85tyr polymorphism in the udp-glucuronosyltransferase (UGT) 2B15 gene and the risk of prostate cancer. J Urol. 2004;171(6 Pt 1):2484–2488. doi: 10.1097/01.ju.0000117748.44313.43. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Chen L, Ratnashinge L, Sellers TA, Tanner JP, Lee JH, Dossett N, et al. Deletion polymorphism of UDP-glucuronosyltransferase 2B17 and risk of prostate cancer in African American and Caucasian men. Cancer Epidemiol Biomark Prev. 2006;15(8):1473–1478. doi: 10.1158/1055-9965.EPI-06-0141. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998;273(39):25117–25124. doi: 10.1074/jbc.273.39.25117. [DOI] [PubMed] [Google Scholar]
- 20.Wei Q, Galbenus R, Raza A, Cerny RL, Simpson MA. Androgen-stimulated UDP-glucose dehydrogenase expression limits prostate androgen availability without impacting hyaluronan levels. Cancer Res. 2009;69(6):2332–2339. doi: 10.1158/0008-5472.CAN-08-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang D, Casale GP, Tian J, Lele SM, Pisarev VM, Simpson MA, Hemstreet GP., 3rd Udp-glucose dehydrogenase as a novel field-specific candidate biomarker of prostate cancer. Int J Cancer. 2010;126(2):315–327. doi: 10.1002/ijc.24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50(4):222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 23.Karan D, Kelly DL, Rizzino A, Lin MF, Batra SK. Expression profile of differentially-regulated genes during progression of androgen-independent growth in human prostate cancer cells. Carcinogenesis. 2002;23(6):967–975. doi: 10.1093/carcin/23.6.967. [DOI] [PubMed] [Google Scholar]
- 24.Garcia AD, Chavez JL, Mechref Y. Sugar nucleotide quantification using multiple reaction monitoring liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2013;27(15):1794–1800. doi: 10.1002/rcm.6631. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama K, Maeda Y, Jigami Y. Interaction of GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase with GDP-mannose-4,6-dehydratase stabilizes the enzyme activity for formation of GDP-fucose from GDP-mannose. Glycobiology. 2003;13(10):673–680. doi: 10.1093/glycob/cwg099. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19(5):631–642. doi: 10.1016/j.molcel.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Bao BY, Chuang BF, Wang Q, Sartor O, Balk SP, Brown M, Kantoff PW, Lee GS. Androgen receptor mediates the expression of UDP-glucuronosyltransferase 2 B15 and B17 genes. Prostate. 2008;68(8):839–848. doi: 10.1002/pros.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi XB, Xue L, Zou JX, Gandour-Edwards R, Chen H, deVere White RW. Prolonged androgen receptor loading onto chromatin and the efficient recruitment of p160 coactivators contribute to androgen-independent growth of prostate cancer cells. Prostate. 2008;68(16):1816–1826. doi: 10.1002/pros.20849. [DOI] [PubMed] [Google Scholar]
- 29.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295(1–2):115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadirvelraj R, Sennett NC, Custer GS, Phillips RS, Wood ZA. Hysteresis and negative cooperativity in human UDP-glucose dehydrogenase. Biochemistry. 2013;52(8):1456–1465. doi: 10.1021/bi301593c. [DOI] [PubMed] [Google Scholar]
- 31.Sethi MK, Buettner FF, Ashikov A, Krylov VB, Takeuchi H, Nifantiev NE, Haltiwanger RS, Gerardy-Schahn R, Bakker H. Molecular cloning of a xylosyltransferase that transfers the second xylose to O-glucosylated epidermal growth factor repeats of notch. J Biol Chem. 2012;287(4):2739–2748. doi: 10.1074/jbc.M111.302406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprenger CC, Plymate SR. The link between androgen receptor splice variants and castration-resistant prostate cancer. Horm Cancer. 2014;5(4):207–217. doi: 10.1007/s12672-014-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A, Namiki M, Kawai K, Tamai I. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem Pharmacol. 2012;84(8):1070–1077. doi: 10.1016/j.bcp.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto N, Kubo T, Inatomi H, Bui HT, Shiota M, Sho T, Matsumoto T. Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 2013;16(4):336–340. doi: 10.1038/pcan.2013.23. [DOI] [PubMed] [Google Scholar]
- 35.Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, Stanford JL, Mostaghel EA. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomark Prev. 2011;20(4):619–627. doi: 10.1158/1055-9965.EPI-10-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai L, Huang W, Chou KC. Prostate cancer with variants in CYP17 and UGT2B17 genes: a meta-analysis. Protein Pept Lett. 2012;19(1):62–69. doi: 10.2174/092986612798472848. [DOI] [PubMed] [Google Scholar]
- 37.Levesque E, Beaulieu M, Green MD, Tephly TR, Belanger A, Hum DW. Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics. 1997;7(4):317–325. doi: 10.1097/00008571-199708000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Castration-resistant prostate tumor cells exhibit blunted ligand-dependent AR responses. 22Rv1 and PC3 cells were cultured in androgen-depleted media for 48 h, followed by 48 h with or without 1 nM DHT. Equivalent amounts of protein from whole cell lysates were analyzed in triplicate by immunoblots probed for the indicated proteins: a UGDH, b PSA, c AR, and d UGT2B17. Densitometric quantification was normalized to tubulin and plotted as mean ± SEM of fold changes relative to the LNCaP 33 0 nM treatment, which is also plotted for reference. *p < 0.05 relative to LNCaP 33, 0 nM DHT. (JPEG 24 kb)
Metabolite and proteoglycan partitioning analysis of 22Rv1 and PC3. a UDP-sugar content was quantified by LC-MS in whole lysates from 22Rv1 and PC3 cells cultured in standard serum-containing media. Mean ± SD is plotted. b The ratios of UDP-glcA to UDP-glc and UDP-xyl to UDP-glcA were calculated from mean values in (a) to illustrate steady-state intracellular flux through UGDH and UXS1, respectively. Note that the scale for UDP-xyl/UDP-glcA is ×10−3. c LNCaP 33, LNCaP 81, 22Rv1, and PC3 cells were cultured in androgen-depleted media for 48 h, followed by 48 h of treatment with DHT. Androstanediol (A-diol)-glucuronide secretion was quantified from conditioned media of each cell line and plotted as mean ± SD. d–f Total lysates from cells cultured in standard media were analyzed in triplicate by immunoblots probed for UXS1 (d) or Notch1 (e, f). NTM indicates quantification of the ≈120 kDa intracellular and transmembrane domain of Notch1; FL denotes the ≈272 kDa full-length Notch1 protein. In (d)–(f), densitometric quantification was normalized to tubulin and mean ± SEM is plotted. In (a–f), *p < 0.05 relative to LNCaP 33. (JPEG 52 kb)
ARv7 is only detected in 22Rv1 cells. FoxA1 expression is comparable in all AR-expressing lines and unaltered by UGDH knockdown. Total lysates from cells cultured in standard media were analyzed in triplicate by immunoblots probed for ARv7 (a) or FoxA1 (b). Densitometric quantification was normalized to tubulin and mean ± SEM is plotted. *p < 0.05 relative to LNCaP 33. (JPEG 18 kb)