Abstract:
Transgender individuals constitute an important focus for HIV prevention, but studies in this population present some unique methodologic and operational challenges. We consider issues related to sampling, sample size, number of sites, and trial cost. We discuss relevant design issues for evaluating interventions in both HIV-negative and HIV-infected transgender populations, as well as a method for assessing the impact of an intervention on population HIV incidence. We find that HIV-endpoint studies of transgender individuals will likely require fewer participants but more sites and have higher operational costs than HIV prevention trials in other populations. Because any intervention targeted to transgender individuals will likely include antiretroviral drugs, small scale studies looking at potential interactions between antiretroviral therapy and hormone therapy are recommended. Finally, assessing the impact of an intervention targeted to transgender individuals will require better information on the contribution of such individuals to the population HIV incidence.
Key Words: transgender, design, HIV prevention, ART
INTRODUCTION
Transgender persons constitute a hidden and often stigmatized population. Nonetheless, multiple studies have shown that transgender individuals, particularly transgender women, are at extremely high risk of HIV infection.1–4 Thus, they are a priority population for HIV prevention research. However, the design of HIV prevention studies in transgender individuals includes some unique methodologic and logistic issues. Here, we review issues related to sample selection, sample size, study design, and impact assessment for HIV prevention studies in transgender individuals.
METHODOLOGIC AND LOGISTIC ISSUES
Sampling
Because not all transgender individuals may publicly identify as such, it is impossible to develop a complete sampling frame of the entire transgender population and, therefore, traditional sampling methods would be inadequate in this context. Although studies based on convenience samples (eg, word-of-mouth or venue-based recruitment) may be internally valid (ie, provide an unbiased treatment effect in a randomized trial), such nonprobability samples often lack external validity, particularly if the goal is to estimate absolute population characteristics (eg, prevalence, incidence). One approach that has shown promise in sampling hidden populations is respondent-driven sampling (RDS).5–7 RDS relies on chain recruitment (similar to snowball sampling) but limits the number of individuals who can be recruited by each participant. In principle, RDS can provide unbiased estimates of population characteristics. However, inferences from RDS data rely on many strong assumptions raising concern about the quality of estimates derived from such samples.7,8 Development of reliable inferential methods for data arising from such samples is itself an active area of research.9
Sample Size
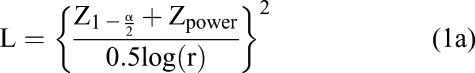
As in any HIV-endpoint trial, sample size calculations require knowledge of the incidence rate of the outcome, the expected effect size (eg, relative risk), trial duration, and expected retention rate, as well as power and type I error levels. Because background incidence rates in transgender populations are generally high, the number of individuals enrolled in HIV prevention studies of transgender individuals will likely be lower than for other populations. For an individually randomized trial, Equation 1a gives the number of HIV incident events (L) that must be observed to detect a given relative risk (r) with a given type I error rate (α) and power10; Equation 1b translates that number into the number of individuals who must be enrolled per arm (N),
 |
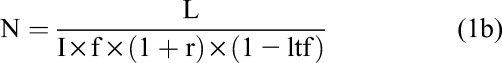 |
where Zp is the pth quantile of the standard normal distribution, I is the incidence rate in the control group, f is the duration of follow-up, and ltf is the loss to follow-up rate.
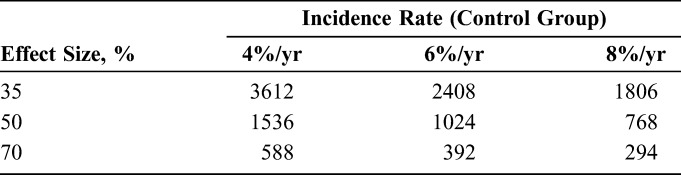
As Table 1 illustrates, an HIV-endpoint trial among transgender individuals would still be large, despite the expected high incidence rate. Furthermore, if understanding heterogeneity in the treatment effect between subgroups (ie, transgender men vs transgender women) is important, then an even larger study would be required to provide adequate power in each subgroup.
TABLE 1.
Number of Individuals Who Must be Enrolled in a 2-Year Follow-up Study to Detect a 35%–70% Effect Size (Relative Risk = 0.65–0.30) if Annual HIV Incidence Is 4%–8%/yr, Assuming type I error rate = 0.05, 90% Power and Annual Loss to Follow-up of 5%
The calculations given above are for an individually randomized trial. However, some interventions are more naturally delivered at the cluster (clinic or community) level (see examples in “Prevention for Positives,” below). Cluster randomized trials are almost always larger and more complex than individually randomized trials. See Hayes and Bennett11 for sample size calculations for cluster randomized trials.
In most jurisdictions, transgender individuals form a small percentage of the population. Therefore, it is likely that an HIV-endpoint trial of transgender individuals would be conducted in multiple, perhaps many, sites. Two statistical issues are particularly relevant to such multisite trials: confounding and effect modification. In a randomized trial, it is important to randomize within site to avoid the possibility of confounding by site. In addition, depending on the intervention, the treatment effect might vary considerably across sites because of difference in sexual practices, cultural differences, or other local factors. Although it is valid to power the trial and draw inferences based on the average treatment effect, it may be difficult or impossible to understand sources of treatment effect heterogeneity because of the low power for studying statistical interactions. If assessment of regional differences in treatment efficacy is expected, this aim must be incorporated into the study design and will likely require an increased sample size.
Recruitment and Retention
Transgender persons remain a stigmatized population and may be reluctant to participate in a study. Therefore, investigators must work closely with community representatives to understand the local context and practices of transgender individuals and to develop trust between the study and the transgender population. Staff must be trained to be culturally competent (eg, www.lgbthealtheducation.org) and referral to appropriate ancillary services for study participants must be available. Innovative recruitment strategies such as RDS can potentially access individuals from segments of the transgender population that would be difficult to reach otherwise.
During recruitment, it is important to incorporate an inclusive and flexible question (or questions) to characterize the gender identity of each participant. For example, the Center for Excellence for Transgender Health12 suggests using the following 2 questions to establish gender identity: (1) What is your sex or current gender (male, female, trans male, trans female, genderqueer, additional category, declined to state); (2) What sex were you assigned at birth (male, female, declined to state).
Efforts to ensure high retention must take into consideration the life experiences of transgender individuals. Because of stigma, hostility, and discrimination, transgender people are more likely to be unemployed or underemployed; be depressed or have an anxiety disorder; or use alcohol or other substances; and thus more likely to have unstable housing.13,14 Research participants experiencing any of these issues are more difficult to retain, and effective retention may require case management or client-centered care over and above the usual study retention efforts. In addition, confidentiality may be of particular importance to transgender study participants, and discretion may be needed when contacting participants regarding follow-up.13 Rewards for retention, financial or otherwise, should be specifically tailored to this population. High retention will likely depend on the level of trust and engagement experienced by study participants, and study investigators must therefore actively cultivate a supportive and motivating relationship during the follow-up period.
Other Operational Considerations
An important operational consideration is cost—multiple sites typically lead to higher trial costs. Each site must be staffed, the staff must be trained, and equipment and supplies must be provided to each site. Costs to central resources (ie, data management, laboratory, operations, study materials translation) also increase with more sites because there are more reports to process, more site staff to communicate with and, in international trials, more languages to deal with. To the extent that participants are homeless or marginalized, more resources may be needed to ensure high study retention. All these factors lead to a higher per-participant cost and greater overall trial cost as the number of sites increases.
Prevention for Negatives
To date, there have been no HIV-endpoint trials that specifically focus on transgender individuals. The mostly likely candidate for prevention in HIV-uninfected transgender individuals is preexposure prophylaxis (PrEP). PrEP had proven effective in cisgender men15,16 and, less consistently, in cisgender women.17 However, it is unclear how these results might apply to transgender women and men, respectively. In a small subgroup of transwomen enrolled in the IPrEX study, there was no significant difference in HIV infection rates between those randomized to Truvada and those randomized to placebo.18 However, adherence among those randomized to Truvada seemed to be low.
An issue that is unique to transgender individuals is the high proportion using hormone therapy. Although there is no evidence that antiretroviral therapy (ART) is less effective in HIV-infected transgender individuals, use of female sex hormones has been implicated as a factor that increases HIV risk.19In addition, there has been little study to date of the potential interaction between use of sex hormones and antiretroviral drugs. In particular, if ART use interfered with the action of sex hormones (either in perception or reality), this could reduce uptake and adherence to PrEP among transgender individuals. This is an important area of research that could be evaluated in a small phase I pharmacokinetic study or in a substudy of a larger trial. Finally, the considerations outlined in Donnell et al20 provide a useful framework for making design decisions (ie, superiority vs noninferiority, active vs placebo-control, etc.) for an HIV-endpoint trial of PrEP in transgender individuals.
Prevention for Positives
HIV transmission risk is a complex function of behavioral and biological factors. Prevention in positive interventions may seek to reduce HIV transmission risk behaviors or reduce infectivity by reducing viral load in the positive partner. The ideal endpoint for such an intervention is the number of HIV transmissions. For example, HPTN 052 enrolled over 1700 HIV-discordant heterosexual couples in stable partnerships and showed that the use of antiretroviral drugs by the HIV-infected individual could prevent transmission to the uninfected partner.21 However, that study design would be difficult to replicate in a transgender population. Thus, a positive prevention intervention for HIV-infected transgender individuals will likely rely on surrogate outcomes. The availability of a valid surrogate for HIV transmission will depend on the intervention.
Interventions that target sexual risk behavior to reduce transmission are particularly challenging to evaluate. Self-reported outcomes (eg, numbers of unprotected acts) may be unreliable and subject to social desirability bias. Surrogate biological measures, such as incident sexually transmitted infection, are also imperfect because their validity as a surrogate for HIV transmission depends on the prevalence of sexually transmitted infection, behaviors such as serosorting, and other factors.
Interventions that seek to reduce viral load are easier to evaluate. It is generally believed that the biological results of HPTN 052 are applicable to anal and vaginal sex22 (although this may not apply to those who have had sex reassignment surgery). Thus, interventions that can lower viral load in HIV-infected individuals through increased testing, linkage to care and adherence to daily ART are likely to reduce HIV transmission in transgender men and women. Approaches to increase testing and linkage rates may be evaluated at the individual or community level, depending on the intervention. For example, the iKnow project23 randomized men who have sex with men (MSM) to the use of home HIV testing kits vs clinic-based testing to evaluate the effect of such kits on HIV testing frequency. The Linkages Study24 randomized HIV-infected individuals to one of 3 linkage strategies to improve the percent of individuals who were linked to care in a timely manner. HPTN 06525 evaluated the use of financial incentives to increase rates of testing and linkage to care using a clinic randomized design. All these trials used a standard parallel trial design. However, a stepped wedge design could be used to evaluate a program to increase testing, linkage, and/or suppression during rollout based on immediately available surrogate outcomes, such as number tested, number linked to care, or number virally suppressed.26
Assessing Impact
Although not a necessary component of trial design, assessing the potential impact of a proposed intervention on population-level HIV incidence is an important consideration in setting research priorities given limited resources. Assessing potential impact requires not only knowledge of the efficacy of the intervention but also information on the percent of the (HIV incidence) population that would be targeted by the intervention and the potential coverage of the intervention. Figure 1 illustrates the calculation for PrEP in US men who have sex with men. A similar exercise could be conducted for an intervention targeted to the transgender population although data for such an exercise (particularly on the overall contribution of the transgender population to HIV incidence in the United States) are lacking. Clearly, however, low coverage, low efficacy, or a small target subgroup all reduce potential impact of a proposed intervention on population-level HIV incidence.
FIGURE 1.
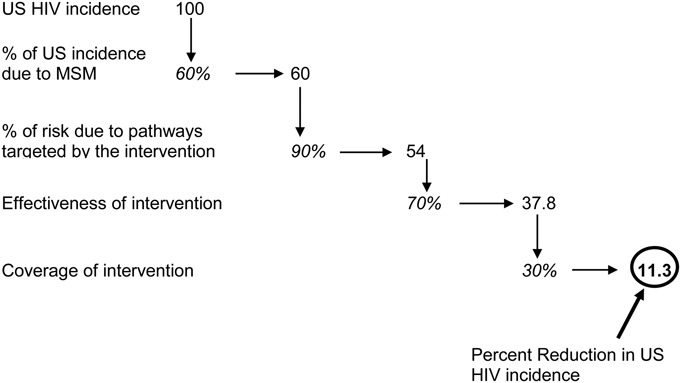
Calculation of potential impact of PrEP for men who have sex with men (MSM) on US HIV epidemic. The figure starts with 100 incident HIV cases and computes the number of cases that would be prevented if PrEP is rolled out to MSM. Assumptions: 60% of incident HIV cases in the United States are in MSM; 90% of those cases are sexually transmitted; PrEP is 70% effective against sexual transmission of HIV; and 30% of MSM adopt use of PrEP.
CONCLUSIONS
Although the nature of the intervention and the scientific questions under study will ultimately determine overall trial design, we find that HIV-endpoint studies of transgender individuals will likely require fewer participants but more sites and have higher operational costs than HIV prevention trials in other populations. Trials for prevention in negatives will likely focus on PrEP, but preliminary pharmacokinetic studies are needed to evaluate interactions (if any) between hormone use and ART. Prevention for positive trials should focus on increasing testing, linkage to care, and adherence to ART. Viral suppression can serve as a valid surrogate for HIV transmission in these trials. Finally, the impact of any intervention on the HIV epidemic depends not only on the efficacy of the intervention but also on the size of the target population and the uptake/coverage of the intervention.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Baral SD, Poteat T, Stromdahl S, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:214–222. [DOI] [PubMed] [Google Scholar]
- 2.Clements-Nolle K, Marx R, Guzman R, et al. HIV prevalence, risk behaviors, health care use, and mental health status of transgender persons: implications for public health intervention. Am J Public Health. 2001;91:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guadamuz TE, Wimonsate W, Varangrat A, et al. HIV prevalence, risk behavior, hormone use and surgical history among transgender persons in Thailand. AIDS Behav. 2011;15:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1–17. [DOI] [PubMed] [Google Scholar]
- 5.Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Social Probl. 1997;44:174–199. [Google Scholar]
- 6.Heckathorn D. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Social Probl. 2002;49:11–34. [Google Scholar]
- 7.Magnani R, Sabin K, Saidel T, et al. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(suppl 2):S67–S72. [DOI] [PubMed] [Google Scholar]
- 8.Gile KJ, Johnston LG, Salganik MJ. Diagnostics for respondent-driven sampling. J R Stat Soc Ser A. 2015;178:241–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volz EH, Heckathorn DD. Probability based estimation theory for respondent driven sampling. J Offical Stat. 2008;24:79–97. [Google Scholar]
- 10.Fleming TR, Harrington DP. Counting Processes in and Survival Analysis. New York, NY: John Wiley and Sons; 1991. [Google Scholar]
- 11.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. [DOI] [PubMed] [Google Scholar]
- 12.Center for Excellance for Transgender HIV Prevention. Recommendations for Inclusive Data Collection of Trans People in HIV Prevention, Care and Services. San Francisco, CA: University of California, San Francisco; 2009. [Google Scholar]
- 13.Institute of Medicine. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 14.Xavier J, Honnold J, Bradford J. The health, health-related needs, and lifecourse experiences of transgender Virginians. Available at: https://www.vdh.virginia.gov/epidemiology/DiseasePrevention/documents/pdf/THISFINALREPORTVol1.pdf. Accessed June 1, 2016. [Google Scholar]
- 15.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2:e512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnell D, Hughes JP, Wang L, et al. Study design considerations for evaluating efficacy of systemic preexposure prophylaxis interventions. J Acquir Immune Defic Syndr. 2013;63(suppl 2):S130–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization WHO and U.S. NIH Working Group Meeting on Treatment for HIV Prevention Among MSM: What Additional Evidence Is Required? Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 23.Katz DA, Swanson F, Stekler JD. Why do men who have sex with men test for HIV infection? Results from a community-based testing program in Seattle. Sex Transm Dis. 2013;40:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnabus R, van Rooyen H, Tumweigye E, Brantley J, et al. Uptake of antiretroviral therapy and male circumcision following community-based HIV testing linkage strategies versus referral: a randomized, multisite, open-label, individual trial in South Africa and Uganda. The Lancet HIV. 2016;3:e212–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnell DJ, Hall HI, Gamble T, et al. Use of HIV case surveillance system to design and evaluate site-randomized interventions in an HIV prevention study: HPTN 065. Open AIDS J. 2012;6:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes JP, Granston TS, Heagerty PJ. Current issues in the design and analysis of stepped wedge trials. Contemp Clin Trials. 2015;45:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


