Abstract
The vertebrate central nervous system integrates cognition and behavior, and it also acts as both a source and target for steroid hormones like estrogens. Recent exploration of brain estrogen production in the context of learning and memory has revealed several common themes. First, across vertebrates, the enzyme that synthesizes estrogens is expressed in brain regions that are characterized by elevated neural plasticity and is also integral to the acquisition, consolidation, and retrieval of recent experiences. Second, measurement and manipulation of estrogens reveal that the period following recent sensory experience is linked to estrogenic signaling in brain circuits underlying both spatial and vocal learning. Local brain estrogen production within cognitive circuits may therefore be important for the acquisition and/or consolidation of memories, and new directions testing these ideas will be discussed.
Introduction
Historically, steroid hormones were thought to be produced exclusively in peripheral endocrine glands and to influence vertebrate behavior through long-term (hours to days) regulation of gene expression. In the case of estrogens, these ‘classical’ effects are mediated in the brain via the nuclear steroid receptors, estrogen receptor α (ERα) and ERβ. It is now clear that the brain itself is also a key site of steroid hormone synthesis and action [1]. Brain-derived steroids provide a local source of neuromodulators that can act upon neural circuits at rapid timescales akin to classical neurotransmitters (seconds to minutes) [2]. While the rapid effects of steroid hormones are often studied in the context of sexual behavior [3], the role of neurosteroids in behaviors and neural systems beyond reproduction has only recently received attention. One area in particular has been understanding how estrogen signaling may enhance or otherwise alter cognition on momentary timescales. While there are a host of hormones that modulate learning and memory [4,5], the potent endogenous estrogen 17β-estradiol (E2) has a clear influence on cognition and neural plasticity [6-8]. As such, this review will concentrate on the role of locally-synthesized brain E2 in learning and memory.
Focusing on recent findings, we evaluate three fundamental aspects of E2 and cognition: 1) the expression of estrogen synthase (aromatase) in brain regions critical for memory consolidation; 2) how measurement and manipulation of relatively rapid E2 synthesis relates to encoding recent experience; and 3) whether learning and post-learning epochs are associated with periods of E2 production and/or suppression. For the purposes of this review, we define the following terms:
Learning: active process of acquiring new information through experience.
Memory: stored information and/or consolidation of new information from a learning experience/event
Cognition: an active, sensory-dependent process that encompasses both a learning event (e.g. training) and the subsequent consolidation of the memory about that event (e.g. post-training), which can be recruited in future contexts.
Recent experience: a discrete window of time including both a potential learning event and the ~2-hour period that follows immediately after the learning event.
Encoding: the active process of memory consolidation of a recent learning event.
Does the role of E2 in brain regions associated with cognition depend on the local availability of aromatase, as well as membrane estrogen receptors, within these same regions?
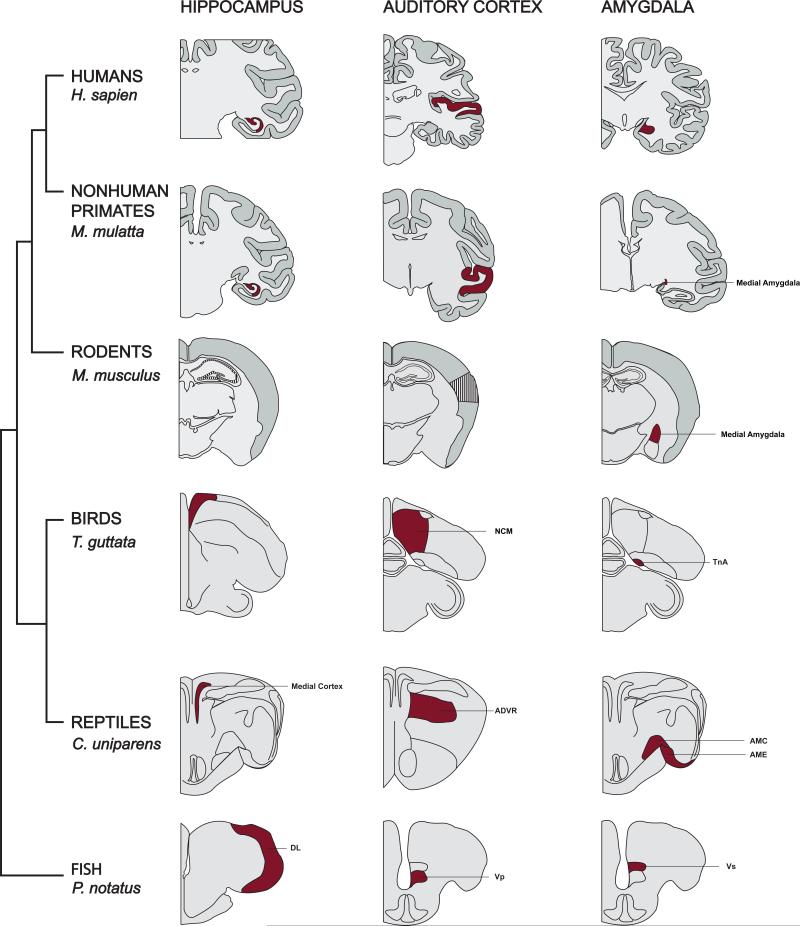
Estradiol appears to influence learning and memory across a diverse group of species, including: nematodes [9], songbirds [10], rodents [6], and nonhuman [11] and human [12] primates. One interesting observation supporting the proposed role of acute neuroestrogen signaling in cognition is the presence of aromatase (estrogen synthase) in brain regions critical for memory encoding, consolidation, and recall among vertebrates. Aromatase expression is conserved across several functionally homologous neural structures in vertebrates [13]. Figure 1 presents for the first time a cross-species comparison of aromatase expression in three brain regions that facilitate distinct types of memory: 1) fear memory consolidation and social recognition (amygdala [14]); 2) spatial navigation and novel object recognition (hippocampus [8,15]); and 3) vocal communication learning, and language acquisition (auditory cortex/forebrain [8]). Neuronal aromatase is enriched in these canonical ‘memory’ regions in mammals and their functionally similar regions in nonmammalian species; we present representatives showing this in human (Homo sapiens) and nonhuman primates (Maca mulatta), rodents (Mus musculus), birds (Taeniopygia guttata), reptiles (Aspidoscelis uniparens), and fish (Porichthys notatus). While aromatase is found in the brain of amphibians [16-18], the spatial resolution and region specificity are less clear and difficult to resolve for present purposes. Of note, at present, there is a paucity of direct evidence for the presence of aromatase in mouse hippocampus [19,20], which may be explained by the promoter used to identify its presence. A recent finding in Xenopus provides intriguing evidence that there may be multiple splice variants for brain-specific aromatase [18]. Therefore, the absence of evidence for aromatase in mouse hippocampus (as well as the auditory cortex) may be due to antibody specificity. In contrast to mice, aromatase is reliably found in rat dorsal hippocampus [21].
Figure 1. Aromatase is typically expressed in brain regions crucial for cognition among vertebrates.
Aromatase expression is abundantly expressed within the hippocampus, auditory cortex/forebrain, and amygdala of several representative species across a wide range of classes. Black stripped-filled brain regions indicate no reported presence of aromatase, whereas maroon-filled brain regions indicate detectable presence of aromatase as assessed through various techniques. Briefly, 1) hippocampus - humans: [22,23]; rhesus macaques: [24]; mice: not seen in hippocampus [19,20]; but see [25]; birds: [26,27]; reptiles (medial cortex): [28,29]; fish (dorsolateral telencephalon): [30,31]; 2) auditory cortex/forebrain – humans: [32,33]; rhesus macaques: [24]; birds (caudomedial nidopallium; NCM [34]): [26,27]; reptiles (anterior dorsal ventricular ridge; ADVR [34]): [28]; fish (posterior portion of the ventral telencephalon; Vp): [30,35,36]; 3) amygdala - humans: [37]; rhesus macaques: [38]; mice: [19]; birds (nucleus taenia; TnA): [27]; reptiles: [28,29,39,40]; fish (supracommissural nucleus of the ventral telencephalon; Vs [41,42]): [30,36].
While the presence of aromatase demonstrates the capability for local E2 synthesis, acute changes in neurophysiology and behavior typically depend on membrane-bound ERs present within these same aromatase-expressing brain regions. In addition to membrane-trafficked versions of the classical nuclear ERs (ERα and ERβ), there are also several membrane-bound estrogen receptors (mERs) that rapidly modulate E2-dependent behaviors [43] and neurophysiology [44], including: mERs associated with a membrane glutamate receptor (mGluR), Gq-coupled mER (Gq-ER), GPER1 (formerly GPR30), and ER-X [as reviewed in 45]. These cognate mERs are typically co-expressed in aromatase-enriched brain regions associated with the encoding of recent experience. For example, both aromatase and GPER1 are found in the hippocampus, nucleus taeniae of the amygdala (TnA), and the caudomedial nidopallium (NCM; functionally homologous to mammalian secondary auditory cortex) of adult and developing male songbirds [46]. Regions such as NCM and hippocampus are necessary for auditory and spatial memory consolidation, respectively, across the lifespan [47-49].
In sum, the molecular machinery necessary to both synthesize and respond to local E2 fluctuations are found within neural structures critical for memory consolidation and encoding. It is therefore important to consider the functional significance of aromatase expression and its relationship to learning.
What is the relationship between fluctuating brain E2 levels and the acquisition vs. consolidation of recent experience?
In addition to the strong overlap of aromatase expression in functionally homologous brain regions across diverse taxa, there is ample evidence to suggest that acute neuroestrogen synthesis actively influences learning and memory. Local E2 production is implicated in learning and memory across a broad range of species, including humans, non-human primates, songbirds, rodents, and nematodes [6,8,9,45]. Research has primarily focused on hippocampal-dependent memory and E2, and mounting evidence indicates that exogenous E2 enhances hippocampal-dependent memory consolidation (which may reflect endogenous fluctuations during and after learning). For example, E2 infused into the dorsal hippocampus of adult female mice within a critical 2 hour window following a training event caused an enhancement in subsequent recognition memory performance [45]. In addition to an E2-dependent enhancement, systemic and local inhibition of aromatase activity impairs spatial and auditory memory consolidation in songbirds, as well as long-term potentiation (LTP) in rodents [10,48,50]. Therefore, exogenous manipulation of E2 availability impacts the encoding of recent experience in spatial memory tasks. However, it is less clear if pharmacologically induced changes in local E2 levels reflect physiological changes of neuroestrogen production in non-manipulated animals.
Understanding the molecular mechanisms of learning and memory has been dominated by approaches that manipulate the neurochemistry and activity of cognitive circuits. Recent approaches now allow the measurement of the on-line activity and neurochemical state of cognitive circuits. Relevant to the current topic, in vivo central E2 measurements have provided direct information about physiological changes in local steroid environments, and have been successfully adapted for songbirds [51], quail [52], rats [53], and nonhuman primates [54]. Studies using in vivo microdialysis, as well as brain content assays of macroarea homogenates, have revealed that E2 synthesis is elevated following recent learning events [51,55]. Specifically, E2 levels are elevated within 60 mins subsequent to spatial navigation and vocal communication training [51,55]. This timeframe parallels the critical window for pharmacological effects on enhancing or impairing memory consolidation by administering E2 or inhibiting aromatase, respectively [8,10,45]. One functional consequence of post-learning elevations in brain E2 may be the rapid enhancement of synaptogenesis in critical cognitive structures such the hippocampus and prefrontal cortex [56]. Thus, E2 appears to be dynamically upregulated immediately after learning events, and these increases are likely important for dendritic spine alterations and modulations of synaptic strength. In this way, modifying the strength of functional synaptic connections between neurons is a key candidate mechanism for E2 altering higher cognitive function, such as learning and memory.
A competing hypothesis – is the enhanced memory consolidation mediated by the suppression of E2 synthesis during a learning event vs. a rebound increase in E2 after training?
Work in rodents and songbirds has led to the idea that rapid post-training E2 elevations are cognitively enhancing. However, recent findings in rodents and songbirds highlight the intriguing possibility that dynamic suppression of E2 synthesis during a learning event may be a critical component of memory formation/consolidation [57]. In adult rats, systemic treatment with an aromatase inhibitor prior to and during a spatial learning task actually improves working memory in subsequent tests [58]. Furthermore, E2 levels are suppressed in the auditory forebrain of juvenile songbirds during a song learning event [51], and this suppression during tutoring is followed by a subsequent post-training elevation in E2. These findings that E2 is suppressed during a training event and subsequently elevated may explain similar observations that E2 is elevated post-training in other vertebrates [51,55]. Together, these observations lead to the hypothesis that E2 levels are “rebounding” from neuroestrogen suppression during a learning event. Therefore it is important to clarify the functional role of reduced neural E2 production in the acquisition of sensory experience, in songbirds, rats and other model systems. In particular, key future research directions include understanding the acute control mechanisms for in vivo brain aromatase activity (such as calcium-dependent phosphorylation of the enzyme [3]), as well as improving our temporal resolution for the fluctuations in neuroestradiol during and following discrete learning events.
While suppressing E2 could facilitate learning, elevated E2 may actually interfere with the encoding of recent experience. In corvids [59] and finches [49], exogenous E2 interferes with hippocampal-dependent spatial memory, which is consistent with recent findings in the prefrontal cortex in aged nonhuman primates [11]. Thus, it may be that the plasticity-enhancing effects of E2 may be deleterious to the faithful initial encoding of a novel sensory stimulus [57]. As such, it remains important to consider the balance between potential cognitively-enhancing, as well as –impairing roles for brain-derived E2 in the encoding and consolidation of recent experience. This is especially important when considering the timing of fluctuations in local E2 levels in higher cognitive circuits.
Conclusions and future directions
Thus far, we have presented work illustrating the largely conserved expression of aromatase in brain regions associated with learning and memory, proposed functional roles for E2 synthesis within these regions as it relates to memory consolidation, and suggested an alternative possibility that local suppression of E2 may be an important modulator for experience encoding. It is clear that more work is needed to further clarify the pluripotent mechanisms by which brain E2 signaling contributes to learning and memory.
The study of estrogen signaling in learning & memory has been largely focused on spatial navigation and object recognition memory in adult animals within the hippocampus. It will be interesting and necessary to expand the study of acute E2 production in cognition to include: 1) novel memory types (e.g. sensory: auditory and olfactory [48,60]); 2) ages across the lifespan (e.g. critical periods early in development, especially in relation to sensorimotor learning); 3) aromatase-enriched regions outside of the hippocampus (e.g. medial amygdala), and 4) areas of the brain in which neurophysiological signatures of experiential learning can be readily accessed. Broadening the range of research initiatives (i.e., across neural structures, age, memory-type, and species) is now necessary to build a generalized understanding of E2's role in cognition. Moreover, there is little information about fluctuating steroid levels in oft studied brain regions involved in cognition. For example, we now have the opportunity to determine in vivo changes in central E2 levels during and following training in regions such as the hippocampus.
Other burgeoning areas of steroid-mediated learning and memory include E2's apparent effect on epigenetic alterations. Epigenetic mechanisms, namely histone acetylation and DNA methylation, appear to mediate several aspects of learning and memory, and recent evidence suggests that E2's enhancement of memory consolidation relies on local chromatin modifications [61]. While there is no direct evidence for rapid neural aromatization regulating epigenetics, future studies should begin testing the effect of aromatase inhibitors on subsequent epigenetic changes and memory retrieval.
Another exciting prospect for future work is neuroestrogens’ potential role in facilitating critical period plasticity for sensorimotor learning. HVC (proper name; functionally similar to Broca's area) is a requisite telencephalic sensorimotor nucleus for vocal learning, and integrates both auditory input and vocal output in songbirds. During development, rapid dendritic spine remodeling occurs within HVC immediately after initial tutoring experience, and the amount of spine remodeling post-tutoring is a strong predictor for vocal development and model imitation [62]. E2 is required for both the development of the sensorimotor circuit (including HVC) and for proper tutor song imitation. Therefore, acute fluctuations in brain-derived E2 may facilitate memory consolidation during development in estrogen-sensitive forebrain regions (such as NCM), which project to and modulate downstream auditory representations in HVC [63]. It is interesting to note that a role for E2 in vocal communication learning has been recently implicated in human infants, as well [64].
Research on the role of brain-derived estrogens in learning and memory has just begun. Expanding the research spotlight to include novel structures, behaviors, species, now presents an exciting jumping off point to explore the way that rapid changes in brain estrogen fluctuations regulate the encoding of recent experience.
Highlights.
We review recent literature related to rapid estrogen synthesis and cognition
Aromatase is typically found in cognitive-related brain regions across vertebrates
In vivo detection of neuroestrogens during learning provides new insights
17β-estradiol (E2) post-training can enhance memory consolidation
Central E2 suppression during training may important for encoding recent experience
Acknowledgements
Support from NSF IOS1354906 and NIH R01NS082179.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Nothing declared.
References and recommended reading
- 1.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remage-Healey L. Frank Beach Award Winner: Steroids as Neuromodulators of Brain Circuits and Behavior. Hormones and Behavior. 2014 doi: 10.1016/j.yhbeh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornil CA, Seredynski AL, de Bournonville C, Dickens MJ, Charlier TD, Ball GF, Balthazart J. Rapid control of reproductive behaviour by locally synthesised oestrogens: focus on aromatase. J Neuroendocrinol. 2013;25:1070–1078. doi: 10.1111/jne.12062. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz A, Cohen SJ, Finn DA, Stackman RW., Jr. The neurosteroid allopregnanolone impairs object memory and contextual fear memory in male C57BL/6J mice. Horm Behav. 2014;66:238–246. doi: 10.1016/j.yhbeh.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. Pharmacol Biochem Behav. 2009;93:177–182. doi: 10.1016/j.pbb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013;65:1318–1350. doi: 10.1124/pr.111.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey DJ, Saldanha CJ. The importance of neural aromatization in the acquisition, recall, and integration of song and spatial memories in passerines. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. 2011;14:984–992. doi: 10.1038/nn.2854. [DOI] [PubMed] [Google Scholar]
- 10**.Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [In this study, researchers demonstrate that shunting aromatase activity in the hippocampus of adult zebra finches hinders spatial memory acquisition and subsequent retrieval using a novel food caching task. Aromatase inhibition impaired memory consolidation and retrieval to similar levels as those subjects with lesioned hippocampi.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacreuse A, Chang J, Metevier CM, LaClair M, Meyer JS, Ferris CM. Oestradiol modulation of cognition in adult female marmosets (Callithrix jacchus). J Neuroendocrinol. 2014;26:296–309. doi: 10.1111/jne.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126:123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- 14.Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. Elife. 2014;3:e02743. doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [This set of experiments provides evidence that one route of action for the E2-dependent enhancement of novel object recognition is via metabotropic glutamate receptors in the dorsal hippocampus (DH) of female mice. Additionally, their findings suggest the E2-dependent action for E2 is through membrane ER/mGluR1 coupling within the DH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coumailleau P, Kah O. Cyp19a1 (aromatase) expression in the Xenopus brain at different developmental stages. J Neuroendocrinol. 2014;26:226–236. doi: 10.1111/jne-12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwabuchi J, Koshimizu K, Nakagawa T. Expression profile of the aromatase enzyme in the Xenopus brain and localization of estradiol and estrogen receptors in each tissue. Gen Comp Endocrinol. 2013;194:286–294. doi: 10.1016/j.ygcen.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Iwabuchi J. Brain-specific promoter/exon I.f of the cyp19a1 (aromatase) gene in Xenopus laevis. J Steroid Biochem Mol Biol. 2012;132:247–255. doi: 10.1016/j.jsbmb.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda SI, Harada N, Shah NM. Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanic D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors alpha and beta, and androgen receptors. PLoS One. 2014;9:e90451. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Tabatadze N, Sato SM, Woolley CS. Quantitative Analysis of Long-Form Aromatase mRNA in the Male and Female Rat Brain. Plos One. 2014:9. doi: 10.1371/journal.pone.0100628. [This paper found pervasive expression of long-form aromatase mRNA in the brain of male and female rats, including key cognitive regions such as dorsal hippocampus and amygdala. Moreover, this group also found brain region-specific effects of sex and gonadal hormones on aromatase expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 23.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–127. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- 26.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Dias BG, Chin SG, Crews D. Steroidogenic enzyme gene expression in the brain of the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Brain Res. 2009;1253:129–138. doi: 10.1016/j.brainres.2008.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krohmer RW, Bieganski GJ, Baleckaitis DD, Harada N, Balthazart J. Distribution of aromatase immunoreactivity in the forebrain of red-sided garter snakes at the beginning of the winter dormancy. J Chem Neuroanat. 2002;23:59–71. doi: 10.1016/s0891-0618(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 30.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: Comparison with estrogen receptor alpha. J Comp Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- 32.Stoffel-Wagner B, Watzka M, Steckelbroeck S, Schwaab R, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in the human temporal lobe. Biochem Biophys Res Commun. 1998;244:768–771. doi: 10.1006/bbrc.1998.8337. [DOI] [PubMed] [Google Scholar]
- 33.Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138:389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 34.Butler AB, Reiner A, Karten HJ. Evolution of the amniote pallium and the origins of mammalian neocortex. Ann N Y Acad Sci. 2011;1225:14–27. doi: 10.1111/j.1749-6632.2011.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fergus DJ, Bass AH. Localization and divergent profiles of estrogen receptors and aromatase in the vocal and auditory networks of a fish with alternative mating tactics. J Comp Neurol. 2013;521:2850–2869. doi: 10.1002/cne.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- 37.Biegon A, Alexoff DL, Kim SW, Logan J, Pareto D, Schlyer D, Wang GJ, Fowler JS. Aromatase Imaging with [N-Methyl-11C]Vorozole PET in Healthy Men and Women. J Nucl Med. 2015;56:580–585. doi: 10.2967/jnumed.114.150383. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Hosoya T, Onoe K, Doi H, Nagata H, Hiramatsu T, Li XL, Watanabe Y, Wada Y, Takashima T, et al. 11C-cetrozole: an improved C-11C-methylated PET probe for aromatase imaging in the brain. J Nucl Med. 2014;55:852–857. doi: 10.2967/jnumed.113.131474. [DOI] [PubMed] [Google Scholar]
- 39.Cohen RE, Wade J. Aromatase mRNA in the brain of adult green anole lizards: effects of sex and season. J Neuroendocrinol. 2011;23:254–260. doi: 10.1111/j.1365-2826.2010.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen RE, Wade J. Expression of aromatase and two isozymes of 5alpha-reductase in the developing green anole forebrain. J Neuroendocrinol. 2012;24:1213–1221. doi: 10.1111/j.1365-2826.2012.02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Northcutt RG. The forebrain of gnathostomes: in search of a morphotype. Brain Behav Evol. 1995;46:275–318. doi: 10.1159/000113279. [DOI] [PubMed] [Google Scholar]
- 43.Seredynski AL, Balthazart J, Ball GF, Cornil CA. Estrogen Receptor beta Activation Rapidly Modulates Male Sexual Motivation through the Transactivation of Metabotropic Glutamate Receptor 1a. J Neurosci. 2015;35:13110–13123. doi: 10.1523/JNEUROSCI.2056-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 45.Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2011;72:1433–1446. doi: 10.1002/dneu.22004. [DOI] [PubMed] [Google Scholar]
- 47.London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;23:922–926. doi: 10.1097/WNR.0b013e3283588b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem. 2013;100:41–47. doi: 10.1016/j.nlm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–8126. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Chao A, Paon A, Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Dev Neurobiol. 2015;75:271–286. doi: 10.1002/dneu.22228. [This study revealed that E2 levels are suppressed in the auditory forebrain during tutoring for both male and female juvenile zebra finches. Post-tutoring, E2 “rebounds” and is significantly elevated, relative to baseline. This timeline is in contrast to adult zebra finches that demonstrated rapidly elevated E2 during song presentation, which return to baseline levels following cessation of the playback.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ubuka T, Haraguchi S, Tobari Y, Narihiro M, Ishikawa K, Hayashi T, Harada N, Tsutsui K. Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis. Nat Commun. 2014;5:3061. doi: 10.1038/ncomms4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato SM, Woolley CS. 44th meeting of the Society for Neuroscience. Washington D.C., USA: Nov, 2014. Abstract 639.05/OO7. [Google Scholar]
- 54.Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuscher J, Szinte J, Starrett J, Krentzel A, Fortress A, Remage-Healey L, Frick K. 43rd Annual meeting of the Society for Neuroscience. San Diego, CA, USA: Nov, 2013. 376.07/III15. [Google Scholar]
- 56.Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korol DL, Pisani SL. Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alejandre-Gomez M, Garcia-Segura LM, Gonzalez-Burgos I. Administration of an inhibitor of estrogen biosynthesis facilitates working memory acquisition in male rats. Neurosci Res. 2007;58:272–277. doi: 10.1016/j.neures.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 59*.Rensel MA, Ellis JM, Harvey B, Schlinger BA. Sex, estradiol, and spatial memory in a food-caching corvid. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.07.022. [In scrub jays, E2 implants appear to interfere with a hippocampal-dependent spatial memory tasks (food caching), consistent with the idea that in some contexts estrogens can be cognitively impairing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dillon TS, Fox LC, Han C, Linster C. 17 beta-Estradiol Enhances Memory Duration in the Main Olfactory Bulb in CD-1 Mice. Behavioral Neuroscience. 2013;127:923–931. doi: 10.1037/a0034839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts TF, Gobes SM, Murugan M, Olveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci. 2012;15:1454–1459. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaadt G, Hesse V, Friederici AD. Sex hormones in early infancy seem to predict aspects of later language development. Brain and Language. 2015;141:70–76. doi: 10.1016/j.bandl.2014.11.015. [DOI] [PubMed] [Google Scholar]