Abstract
Deviance proneness models propose a multi-level interplay in which transactions among genetic, individual, and family risk factors place children at increased risk for substance use. We examined bidirectional transactions between impulsivity and family conflict from middle childhood to adolescence and their contributions to substance use in adolescence and emerging adulthood (n = 380). Moreover, we examined children’s, mothers’ and fathers’ polygenic risk scores for behavioral undercontrol, and mothers’ and fathers’ interparental conflict and substance disorder diagnoses as predictors of these transactions. Results support a developmental cascade model in which children’s polygenic risk scores predicted greater impulsivity in middle childhood. Impulsivity in middle childhood predicted greater family conflict in late childhood, which in turn predicted greater impulsivity in late adolescence. Adolescent impulsivity subsequently predicted greater substance use in emerging adulthood. Results are discussed with respect to evocative genotype-environment correlations within developmental cascades and applications to prevention efforts.
Substance use early in life significantly contributes to death and disability in adulthood, highlighting the importance of understanding the pathways within the family that contribute to substance use during adolescence and emerging adulthood (NIDA, 2014). Deviance proneness models (also referred to as “externalizing” and “disinhibition” models), propose that there is a multi-level interplay among genetic, individual, and family risk factors that places children at increased risk for substance misuse (Iacono, Malone, & McGue, 2008; King, Molina & Chassin, 2009; Krueger et al., 2002; Sher, Walitzer, Wood, & Brent, 1991; Zucker, Heitzeg, & Nigg, 2011). Two of the best known models are the deviance proneness model (Sher et al., 1991) and the disinhibition model (Iacono et al., 2008), which we collectively refer to as the deviance proneness framework. Within this framework, both models posit that children of substance-abusing parents have a genetically transmitted predisposition for behavioral undercontrol (i.e., impulsivity and sensation seeking) in childhood. The resulting undercontrolled child behavior initiates bidirectional transactions with a poor family environment, provided by substance-abusing parents. Both the poor family environment and undercontrolled behavior contribute to early problem behavior such as academic problems and deviant peer affiliation. Over time, the poor family environment and undercontrolled behavior continue to exacerbate one another, directly contributing to substance use later in life, as well as via school failure and affiliation with substance-using peers (Iacono et al., 2008; Sher et al., 1991).
Core to both these models is a genetic liability for behavioral undercontrol. Behavioral undercontrol has often been broadly conceptualized, encompassing characteristics as diverse as impulsivity, poor self-regulation, sensation seeking, and externalizing behavior. In such cases, the specific child phenotype responsible for associations with family processes and substance use can be unclear. In an effort to better understand these associations we examine impulsivity as defined by a lack of premeditation as our specific index of behavioral undercontrol (Whiteside & Lynam, 2001), which during childhood and early adolescence may be characterized by behavior such as a lack of planning and acting without forethought. Direct links between impulsivity and substance use are corroborated by findings from a meta-analysis (Charach, Yeung, Climans, & Lillie, 2008) and bidirectional transactions exist been child impulsivity and parenting (Kiff, Lengua, & Zalewski, 2011). Additionally, impulsivity is known to have a strong heritable component (Bezdjian, Baker, Tuvblad, 2011), and there is emerging evidence that genetically transmitted risk for impulsivity in childhood can evoke poor family environments (Harold et al., 2013).
This preliminary evidence fits within the deviance proneness framework and led us to examine children’s genetic disposition for behavioral undercontrol within bidirectional transactions between impulsivity and family conflict in predicting substance use in adolescence and emerging adulthood. No study has examined the longitudinal interplay between children’s impulsivity and poor family functioning in accounting for later substance use in a genetically informed design. Within the deviance proneness framework, the present study addresses this gap by investigating the bidirectional transactions between children’s impulsivity and family conflict across middle childhood and adolescence as influenced by children’s, mother’s and father’s genetic predispositions for behavioral undercontrol. We examined substance use in adolescence and emerging adulthood as outcomes of these processes, and included parents’ lifetime substance disorder diagnoses as distal predictors.
Transactions between Children’s Impulsivity and Family Processes in Predicting Substance Use
The longitudinal associations between family processes and children’s impulsivity are well studied. A review by Kiff et al. (2011) reported consistent findings that harsh, inappropriate, and controlling parenting behaviors were related to later impulsivity in early to middle childhood. Similar research by Olson and colleagues (1990, 2002) found responsive and stimulating parenting early in life to be associated with lower impulsivity at 6 years of age.
The reverse direction of effect has also been reported, that is, children’s impulsive behavior predicting parenting behavior. In middle childhood, studies have found that impulsive behavior was associated with greater levels of subsequent family conflict (see Deault, 2010 for a review). In middle to late childhood (10–16 years old), impulsivity was associated with later parenting behavior, including lower levels of constructive, positive parenting, and monitoring (Glatz, Stattin, & Kerr, 2011; Latzmann, Elkovitch, & Clark, 2009; Neumann, Barker, Koot & Maughan, 2002). These child-to-parent associations are theorized to occur because highly impulsive children may be difficult to parent, leading to negative communication patterns (Faraone & Biederman, 1998) and greater parental stress (Fischer, 1990). The resulting poor family environment can subsequently contribute to children’s impulsivity, perpetuating their impulsive behavior across time (Kiff et al., 2011). This provides evidence that bidirectional transactions may exist between impulsivity and family conflict, as hypothesized by the deviance proneness framework. Based on these findings, we examined bidirectional transactions between impulsivity and family conflict from middle childhood to adolescence.
When these negative transactions take place in childhood, they may initiate harmful developmental cascades within the family, putting the child at risk for later problem behavior and substance use (Brody & Ge, 2001; Masten & Cicchetti, 2010; Scaramella & Leve, 2004). Individually, both impulsivity and family conflict predict adolescent substance use (Baer, Garmezy, McLaughlin, Pokorny, & Wernick, 1987; Sher, Grekin, & Williams, 2005; Vakalahi, 2001; Wong, 2008; Zhou, King, & Chassin, 2006). A recent paper by Bidwell et al. (2015) examined impulsivity and sensation seeking as competing predictors of adolescent substance use. Impulsivity was the most salient predictor of substance use and also found to mediate the relation between family history of substance use and both initiation and frequency of substance use. This supports the proposition that impulsivity contributes to substance use within the deviance proneness framework through shared family origins with parental substance use.
Family conflict is also consistently associated with substance use during adolescence and adulthood. For example, greater family conflict in early adolescence predicts increases in alcohol use across adolescence (Bray, Adams, Getz, & Baer, 2001) and elevated family conflict across adolescence predicts membership in a substance using class in adulthood (Herrenkohl, Lee, Kosterman, & Hawkins, 2013). Family and interpersonal theories (e.g., family systems theory, social learning theory, emotional security theory) suggest that family conflict is both a chronic and salient stressor which contributes to poor social support and a lack of emotional security for children within the family (Cummings & Davies, 2010). This stress and resulting adolescent negative affect may increase risk for adolescent substance use. An alternative (but not necessarily mutually exclusive) explanation is that family conflict weakens the positive parent-child bond that facilitates children’s internalization of parental values, thus making them view substance use as more acceptable and making affiliation with substance-using peers more likely (Cummings & Davies, 2010). In either case, children’s perceptions of high levels of conflict in the family increase their likelihood of engaging in substance use behavior (Skeer, McCormick, Normand, Buka, & Gilman, 2009; Stone, Becker, Huber, & Catalano, 2012; Vakalahi, 2001).
Despite this evidence, relatively few studies have examined the longitudinal interplay between children’s impulsivity and negative family environments in predicting later substance use. In a cross-sectional study, retrospective reports of permissive parenting during childhood predicted lowered self-regulation in emerging adulthood, which in turn predicted poorer drinking control (Patock-Peckham, Cheong, Balhorn, & Nagoshi, 2001). In a longitudinal study, Brody and Ge (2001) examined prospective bidirectional relations between self-regulation and conflicted parenting in late childhood, and their unique influences on alcohol use in early adolescence. Self-regulation at 10-to-12 years of age predicted conflicted parenting a year later, which in turn predicted concurrent self-regulation (11-to-13 years old). Self-regulation at 11-to-13 years of age predicted alcohol use a year later in early adolescence. These findings support the deviance proneness framework in that poor self-regulation in late childhood, which is associated with impulsivity, may give rise to negative family environments, which subsequently contribute to further poor self-regulation. The resulting deficits in self-regulation in late childhood and adolescence then serve as a risk factor for later substance use. We extend previous research by examining bidirectional transactions between children’s impulsivity (as an indicator of “behavioral undercontrol”) and family conflict from middle childhood to adolescence, and their association with substance use in adolescence and emerging adulthood.
Whereas a negative family environment likely exerts a proximal influence on child behavior, children’s impulsivity may also be affected by other familial risk factors such as parental substance use (Alati et al., 2014) and interparental conflict (Heinrichs, Cronrath, Degen, & Snyder, 2010). The deviance proneness model hypothesizes that parental substance use disorder predicts children’s impulsivity both because of a genetically transmitted risk for impulsivity and through the poor parenting and poor family environment that are provided by parents with substance use disorders (Sher et al., 1991). Related research has found that parent alcoholism predicted greater initial impulsivity and less decline in impulsivity from 6 to 16 years of age (Jester et al., 2008). Similarly, Ohannessian and Hesselbrock (2009) found parent alcoholism to predict adolescent disinhibition, which subsequently predicted an earlier substance use age of onset. In the current sample there was a high prevalence of parental substance disorder, allowing us to examine mothers’ and fathers’ lifetime substance disorder diagnoses as predictors of children’s impulsivity, family conflict, and substance use in adolescence and emerging adulthood.
Another dimension of the family environment that might be influenced by parent substance use is interparental conflict (Leonard & Eiden, 2007; Whisman & Baucom, 2012). Moreover, interparental conflict, in turn, effects the quality of parenting and the level of broader family conflict (Erel & Burman, 1995), giving rise to greater levels of hostile parenting (Krishnakumar & Buehler, 2000) and parent-child conflict (Bradford, Vaughn, & Barber, 2008). Interparental conflict is proposed to affect parenting and family relationships via spillover of negative affect into the family relationship (Erel & Burman, 1995). We examined these influences by including mothers’ and fathers’ lifetime substance disorder diagnoses as predictors of interparental conflict, and interparental conflict as a predictor of children’s impulsivity and family conflict.
In summary, the deviance proneness framework predicts that a genetic predisposition for behavioral undercontrol contributes to children’s impulsive behavior. Impulsive behavior transacts with family conflict across childhood and adolescence, and escalated impulsive behavior and family conflict contribute to substance use in adolescence and emerging adulthood. Within this framework interparental conflict acts as an additional environmental risk, associated with both parental substance use and the family environment. However, whereas individual bivariate associations among children’s impulsivity, family functioning, and substance use are well researched, less is known about the developmental transactions among them. Some longitudinal data suggest that differing measures of “behavioral undercontrol” (including impulsivity and sensation seeking) in late childhood predict later negative family environments, further increasing adolescent impulsivity or sensation seeking, leading to adolescent substance use (e.g., Brody & Ge, 2001). However, the bidirectional interplay between children’s impulsivity and negative family environments in middle childhood through adolescence, and their possible contributions to adolescent and emerging adulthood substance use, has not been studied. We address this gap in the literature by examining parent’s lifetime substance disorder diagnoses and interparental conflict as predictors of the bidirectional transactions between children’s impulsivity and family conflict from middle childhood to adolescence, and their associations with substance use in adolescence and emerging adulthood. Finally, genetic influences need to be considered within these processes (King et al., 2009), which may contribute to developmental cascades of risk (e.g., Elam et al., 2014; Harold et al. 2013; Masten & Cicchetti, 2010).
Impulsivity in Genetically Informed Research
In addition to impulsivity being involved in transactions with parenting, the deviance proneness framework proposes that parents with substance use disorders transmit a genetic disposition for impulsivity to their children (Iacono et al., 2008; Sher et al., 1991). Impulsivity has a strong heritable component with evidence of genetic continuity across childhood, adolescence, and young adulthood as well as unique genetic effects at each age (Bezdjian et al., 2011; Hay, Bennet, McStephen, Rooney, & Levy, 2004; Niv, Tuvblad, Raine, & Baker, 2011). Quantitative genetic studies using twin and adoption samples have shown that impulsivity shares genetic variation with antisocial behavior and substance use disorder (Hicks, Schalet, Malone, Iacono, & McGue, 2011; Young, Stallings, Corley, Krauter, & Hewitt, 2006). A number of single nucleotide polymorphisms (SNPs) have also been associated with the overlap between behaviors such as impulsivity and substance abuse (Kreek, Nielsen, Butelman, & LaForge, 2005), and impulsivity and aggression (Pavlov, Chistiakov, & Chekhonin, 2012).
A single nucleotide polymorphism (i.e., a SNP) is a variation in a single base pair within DNA. Genetic main effects for individual SNPs exist for impulsivity and related behaviors (Hamidovic, Dlugos, Skol, Palmer, & de Wit, 2009; McGue et al., 2013; Villafuerte, Strumba, Stoltenburg, Zucker, & Burmeister, 2013; Wang, Chassin, Geiser, & Lemery-Chalfant, 2015). More recently, studies have combined multiple SNPs into indices of genetic predisposition, known as polygenic risk scores. Polygenic risk scores capture genetic variance by including multiple SNPs into a single score with each SNP proposed to exert a small, additive effect. Two studies using similar approaches have examined broad (Vrieze, McGue, Miller, Hicks, & Iacono, 2013) and developmentally specific (Salvatore et al., 2015) genetic influences related to behavioral disinhibition.
Vrieze et al. (2013) created polygenic scores for behavioral disinhibition as well as alcohol, nicotine, and drug use to examine overlap in genetic variance among these behaviors. The polygenic disinhibition score was associated with the scores for substance use, indicating shared genetic etiology underlying these behaviors. This finding supports evidence from quantitative genetic studies that disinhibited behavior may indicate genetic risk for substance use (e.g., Hicks et al., 2011).
Polygenic scores have also been used to investigate associations with specific phenotypes based on how behaviors are developmentally expressed. Salvatore et al. (2015) examined associations among a polygenic risk score for externalizing and specific developmental phenotypes including externalizing, impulsivity, conscientiousness, and sensation seeking. Broad polygenic risk for externalizing explained more variance in impulsivity in adolescence than in adulthood, and was only associated with sensation seeking in adulthood. Thus, polygenic scores can be used to examine broad genetic covariation across multiple traits, but also genetic associations that may exist with specific phenotypes based on their developmental expression.
These findings indicate that both broad and specific genetic effects exist for impulsivity during childhood and adolescence (e.g., Bezdjian et al., 2011; Niv et al., 2011; Salvatore et al., 2015). Converging evidence also indicates that impulsivity in childhood and adolescence is associated with substance use due to its effects on behavior (e.g., initiating actions without carefully considering the consequences), as well as shared family origins (Bidwell et al., 2015) and genetic covariation (Vrieze et al., 2013). We therefore examined impulsivity as a specific index of behavioral undercontrol in all longitudinal analyses. To construct our polygenic risk score we identified SNPs by conducting a review of the literature for SNPs previously found to be associated with impulsivity and related measures of behavioral undercontrol, which we then combined into a polygenic risk score for children. The same SNPs were used to create polygenic risk scores for parents to control for common genetic risk.
Given emerging evidence that genetic predispositions for impulsivity may be related to negative family environments (Harold et al., 2013), we examined evocative genotype-environment correlations (rGEs) using these polygenic risk scores relative to children’s impulsivity and family conflict. In an evocative rGE, children’s genetically influenced characteristics evoke a particular response from the environment (Plomin, DeFries, Knopik, & Neiderhiser, 2013). Previously, adoption studies have found evidence of evocative rGE in which poor behavioral motivation in toddlers evokes hostile parenting (Elam et al., 2014), and impulsive behavior in early-to-middle childhood evokes more negative and conflicted reactions from parents (Harold et al., 2013). Studies have also examined individual genetic polymorphisms as measures of genetic risk in rGE models. For example, Pener-Tessler and colleagues (2013) found that the serotonin transporter gene was related to self-control in early childhood, which evoked less positive parenting. We examined evocative rGE underlying the relation between children’s polygenic risk scores and family conflict, mediated by children’s impulsivity.
The Present Study
Deviance proneness models propose that a genetic predisposition for impulsivity is associated with substance use, and that this relation is partially mediated by negative family environments (Iacono et al., 2008; Sher et al., 1991). To date, family-based and genetic research has uncovered transactions between children’s impulsivity and the family environment. When viewed collectively, these transactions support a deviance proneness framework in which a genetic predisposition for elevated impulsivity evokes negative reactions from the family environment, which in turn contributes to disinhibited behavior, contributing to later substance use. However, the bidirectional transactions between impulsivity and family conflict across childhood and adolescence, and their respective contributions to substance use later in life, have not been tested. We extended previous research by examining the bidirectional transactions between children’s impulsivity and family conflict from middle childhood to adolescence, and their contributions to substance use in adolescence and emerging adulthood. We also extended previous research by including children’s polygenic risk scores as a predictor of these transactions and examining interparental conflict as a contributing factor (see Figure 1). We included mothers’ and fathers’ polygenic risk scores and lifetime substance disorder diagnoses as distal predictors of all primary study variables. Using this design, we were able to examine longitudinal cascades among these constructs across multiple developmental periods and their contribution to substance use outcomes in adolescence and emerging adulthood.
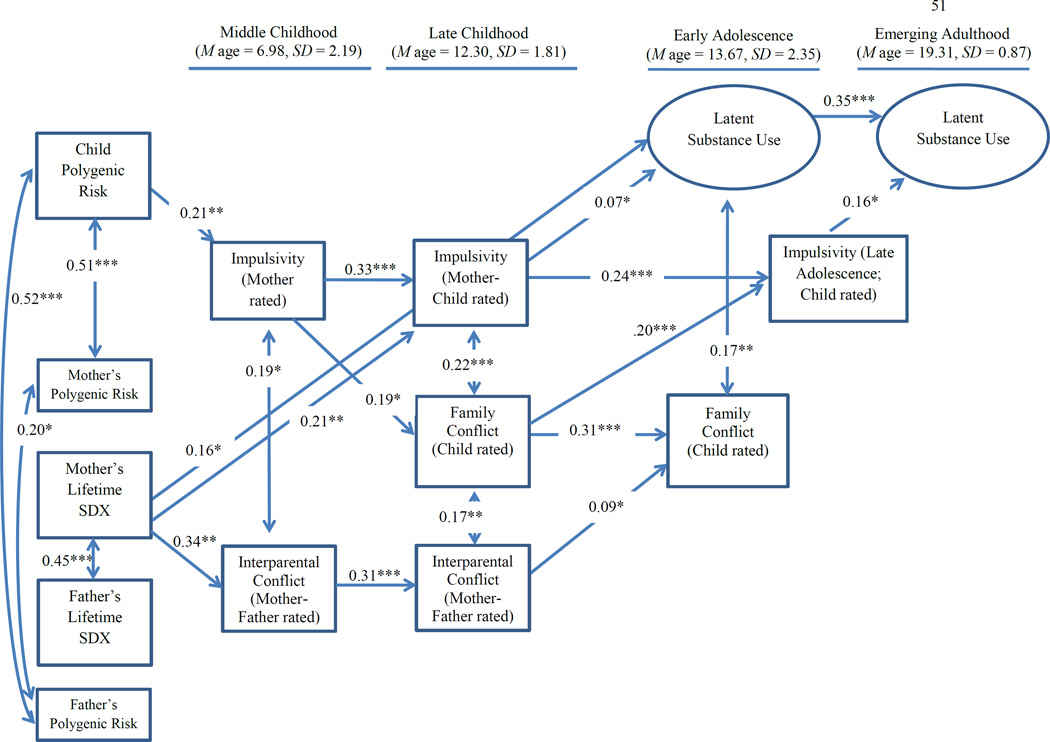
Fig. 1.
Full model. N = 380, Chi Square (148) = 166.19, p = 0.15, RMSEA = 0.018, CFI = 0.98, TLI = 0.96. Non-significant paths are not presented. Analyses control for age, gender, ancestry and their interactions. SDX = Substance Disorder Diagnosis.
Based on prior research, we hypothesized that children’s polygenic risk scores would be associated with their impulsivity within the longitudinal model. We also hypothesized that mothers’ and fathers’ lifetime substance disorder diagnoses would be associated with greater interparental conflict and family conflict as well as with their children’s impulsivity and substance use later in life. We also hypothesized that interparental conflict would be associated with family conflict.
Most important for our model, we hypothesized that bidirectional transactions would emerge as part of a developmental cascade between children’s impulsivity and family conflict in childhood and adolescence, which would predict substance use in adolescence and emerging adulthood. We predicted that a greater genetic predisposition for behavioral undercontrol would evoke greater family conflict in late childhood via children’s impulsivity in middle childhood (evocative rGE), and the resulting family conflict would be associated with substance use via greater children’s impulsivity in late childhood and adolescence.
Methods
Participants
Participants were part of the Adolescent/Adult Family Development Project (AFDP), a longitudinal three-generation study on familial transmission of substance use (Chassin, Barrera, Bech, & Kossak-Fuller, 1992; Chassin, Rogosch, & Barrera, 1991). Children in the current analyses were from the third generation of the study who were living with their two biological parents. Children were assessed in middle childhood (M age = 6.98, SD = 2.19), late childhood (M age = 12.30, SD = 1.81), early adolescence (M age = 13.67, SD = 2.35), late adolescence (M age = 16.80, SD = 2.48), and emerging adulthood (M age = 19.31, SD =.87). 46.7% of the children were female and 66.1% of children were non-Hispanic Caucasian. In middle childhood and late childhood children and their parents completed in-home computer-assisted interviews or telephone interviews when a family had relocated out-of-state. At the early adolescent assessment, adolescents completed measures via a web-based assessment and in late adolescent and emerging adulthood assessments were administered through phone interviews.
The current analyses (n = 380 families) only included families who had a child 5 to 10 years old at the middle childhood assessment either of non-Hispanic Caucasian or Hispanic ethnicity, who was living in a 2-parent household in which both parents were biologically related to the child. Families were only included in the current analyses if genetic data were available for the child, with missing mother (n = 14) or father (n = 53) genetic data permitted. We selected families for the current analyses in which both parents were biologically related to the child because this was necessary to account for genetic correlations among mothers’, fathers’, and children’s polygenic risk scores. We examined all study variables for selection bias in this subsample versus the larger child (third generation) sample. The only study variable significantly different in the subsample was mothers’ lifetime substance disorder diagnosis, with lower diagnosis rates present in the current subsample (only biologically related mothers and fathers) as compared to the larger sample which included non-biologically related mothers and fathers (χ2 (2) = 14.84, p = 0.001).
Biological samples (buccal and saliva) containing DNA were collected from children and parents. Genotyping was completed at the Washington University Genome Sequencing Center. The Illumina Golden Gate technology was used which is designed for genotyping 1536 SNPs with substitutions reflecting advances in the literature (Hodgkinson et al., 2008). Quality controls included cluster plots to exclude ambiguous genotype calls, examining for Mendelian inconsistencies, improper gender assignments and sample swaps, cryptic relatedness, and flagging SNPs with low call rates (p < 10−6).
Measures
Polygenic risk score
To create the literature-based polygenic risk score a systematic review of the literature was completed in Huge Navigator Database and Google Scholar to identify studies in which SNPs were significantly associated (p < 0.05) with indices of behavioral undercontrol (see Table 1 for the search terms). We matched significantly associated SNPs within the resulting studies to available SNPs within the AFDP sample or a SNP in high linkage disequilibrium at r2 >= 0.80. This resulted in 48 SNPs. Additional SNP inclusion criteria were replication in at least one other study at the gene level and partial or full sample composition of European descent. Adding these criteria resulted in 26 SNPs. SNPs were subsequently pruned for linkage disequilibrium at r2 >= 0.80 using the pruning option within PLINK, resulting in 25 SNPs. As there is much dissension in the literature regarding the identification of the risk alleles of individual SNPs (e.g., Gizer, Ficks, & Waldman, 2009), we required that a SNP’s risk allele be replicated in at least two published studies. To fulfill this requirement we completed a second, targeted literature search to investigate if the refined list of 25 SNPs were associated with broader measures of behavioral disinhibition including substance use. When contradictory risk alleles were reported across multiple studies a risk allele had to be indicated by a margin of at least 2 studies. Applying this additional criteria resulted in 6 final SNPs (see Table 1).
Table 1.
Single Nucleotide Polymorphisms Included in the Present Polygenic Risk Score
| SNP | Risk Allele |
Gene | Gene-System Function |
Phenotypes and Studies |
|---|---|---|---|---|
| rs686 | G | DRD1 | Dopamine | Cognitive Impulsivity (Oades et al., 2008); Nicotine Dependence (Huang et al., 2008); Alcohol Dependence (Batel et al., 2008); Smoking in Sx (Novak et al., 2010) |
| rs4648317 | T | DRD2 | Dopamine | Impulsivity (Hamidovic et al.,, 2009); Smoking and nicotine dependence (Laucht et al., 2008); Opiate addiction (Doehring et al., 2009) |
| rs1800497 | T | ANKK1 | Dopamine | Impulsivity (Chan et al., 2014), Reward dependence (Kazantseva, Gaysina, Malykh, & Khusnutdinova, 2011); Aggression (Zai et al., 2012); Opiate addiction (Doehring et al., 2009); Impulsivity, conduct problems (Esposito-Smythers, Spirito, Rizzo, McGeary, & Knopik, 2009); ADHD, low persistence (Nyman et al., 2007, 2012); ADHD (Gizer et al., 2009); Alcoholism (Munafo, Matheson, & Flint, 2007) |
| rs11575542 | A | DDC | Dopamine/Serotonin | Sensation seeking (Derringer et al., 2010); Binge drinking (Pan et al., 2013); Drug dependence (Hack et al., 2011) |
| rs4570625 | G | TPH2 | Serotonin | Reduced prefrontal function during inhibition task (Baehne et al., 2008); ADHD (Walitza et al., 2005); Smoking (Reuter et al., 2007) |
| rs1455858 | A | CHRM2 | Cholinergic/Muscarinic | Impulsivity and Sensation seeking (Hendershot, Bryan, Ewing, Claus, & Hutchinson, 2011); Alcohol use disorder (Dick et al., 2007); Sensation/Novelty seeking (Dick et al., 2008) |
Search terms: Impulsivity, Novelty seeking, Externalizing, Undercontrol, Delay Discounting, P300, Disinhibition, Stop Signal, Gambling Task, Sensation Seeking, Go-no-go, Risk Taking, Hyperactivity-Impulsivity, Conduct Disorder, Self-Regulation, Reward Seeking, Self-Control
Functionally, a number of the SNPs in our polygenic risk score are from the dopamine system. These SNPs may have an effect on the influx of dopamine in the midbrain and prefrontal cortex, affecting planning and inhibition and contributing to impulsive behavior (Jentsch et al., 2014; Koepp et al., 1998; Thut et al., 1997). Minor allele frequencies of all SNPs were checked in Broad Institute’s SNAP database and were below 38%. We combined these SNPs into the present literature-based polygenic risks score using the --score procedure in PLINK (Purcell et al., 2007) and created polygenic risk scores for children, and their biologically related mothers and biologically related fathers. We utilized an additive coding approach, such that for each of the 6 SNPs, participants could obtain scores between 0–2 (i.e. 0, 1, or 2 “risk” alleles; children’s scores ranged: 0.17 – 1.50; skew: 0.26; kurtosis: 0.11; mothers’ scores ranged: 0.17 – 1.33; skew: 0.33; kurtosis: −0.47; fathers’ scores ranged: 0.00 – 1.33; skew: −0.09; kurtosis: −0.26; means and standard deviations can be found in Table 3).
Table 3.
Means, Standard Deviations, and Correlations among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 0.63 (0.24) |
0.67 (0.25) |
0.61 (0.24) |
0.39 (0.67) |
0.71 (0.79) |
1.97 (0.65) |
1.85 (0.80) |
−0.04 (0.78) |
1.85 (0.72) |
3.53 (0.82) |
3.86 (0.84) |
0.00 (0.43) |
1.99 (0.50) |
0.00 (1.01) |
| 1. Child Polygenic Risk Score | — | |||||||||||||
| 2. Mother Polygenic Risk Score | 0.55*** | — | ||||||||||||
| 3. Father Polygenic Risk Score | 0.52*** | 0.21** | — | |||||||||||
| 4. Mother Substance Diagnosis | −0.09 | −0.12* | 0.004 | — | ||||||||||
| 5. Father Substance Diagnosis | 0.05 | 0.08 | 0.07 | 0.34*** | — | |||||||||
| Middle Childhood | ||||||||||||||
| 6. Impulsivity (M) | 0.20** | 0.04 | 0.09 | 0.05 | 0.16* | — | ||||||||
| 7. Interparental Conflict (MF) | 0.07 | 0.08 | 0.05 | 0.34*** | 0.25*** | 0.18** | — | |||||||
| Late Childhood | ||||||||||||||
| 8. Impulsivity (MC) | 0.07 | −0.01 | 0.01 | 0.10* | 0.07 | 0.29*** | 0.12* | — | ||||||
| 9. Family Conflict (C) | 0.05 | 0.06 | 0.02 | −0.04 | 0.11* | 0.14* | 0.10* | 0.21*** | — | |||||
| 10. Interparental Conflict (MF) | −0.07 | 0.02 | −0.02 | 0.18*** | 0.07 | 0.05 | 0.32*** | 0.08 | 0.18*** | — | ||||
| Early Adolescence | ||||||||||||||
| 11. Family Conflict (C) | −0.11 | 0.05 | 0.04 | 0.13** | 0.13** | −0.03 | 0.07 | 0.16** | 0.21*** | 0.40*** | — | |||
| 12. Child Substance Use (C) | −0.02 | −0.06 | 0.04 | 0.15** | 0.08 | −0.06 | 0.04 | 0.14** | 0.15** | 0.17*** | 0.30*** | — | ||
| Late Adolescence | ||||||||||||||
| 13. Impulsivity (C) | −0.003 | 0.05 | 0.10 | 0.11* | 0.15** | 0.14 | 0.09 | 0.24*** | 0.001 | 0.21*** | 0.14** | 0.04 | — | |
| Emerging Adulthood | ||||||||||||||
| 14. Substance Use (C) | −0.03 | −0.10 | −0.01 | 0.07 | 0.07 | −0.04 | −0.02 | 0.15** | 0.15** | 0.05 | 0.10* | 0.34*** | 0.09* | — |
Note. Parenthesis after variables refers to reporter: C = child report, M = mother report, F = father report.
Scales for measures follow. Polygenic Risk Scores: 0–2, greater scores indicate greater genetic risk for behavioral undercontrol. Parent Substance Diagnosis (Dx): (0) no Dx, (1) Lifetime Dx of either alcohol or drug disorder, (2) Lifetime Dx of both alcohol and drug disorder. Middle childhood impulsivity: 0–3 scale, 3 indicates high impulsivity. Late childhood impulsivity: Standardized factor scores of mother-child report of impulsivity, greater scores indicate greater impulsivity. Late adolescence impulsivity: 1–4 scale, 4 indicates high impulsivity. Middle and late childhood interparental conflict: 1–5 scale, 5 indicates high interparental conflict. Late childhood and early adolescence family conflict: 1–5 scale, 5 indicates high family conflict. Early adolescence and emerging adulthood substance use: Standardized factor scores of illicit drug use, alcohol use, and substance dependence, greater scores indicate greater substance use.
p < 0.05,
p < 0.01,
p < 0.001.
Ancestry informative markers
The larger dataset of all participants who provided genetic data included 32 ancestry marker SNPs which—in previous literature (Tian et al., 2007)—have differentiated Hispanics from non-Hispanic Caucasians. We included these 32 SNPs in a factor analysis in Mplus, using Maximum Likelihood estimation. These factor scores significantly correlated with self-reported ethnicity, both in the larger dataset (r = 0.86, p < 0.001) and in the current sample (r = 0.83, p < 0.001), suggesting that this ancestry gene score significantly differentiated between non-Hispanic Caucasians and Hispanics. Higher scores indicated lower levels of Hispanic ancestry (Caucasian M = 0.53, SD = 0.34, range: −1.00 – 1.32; skew: −0.76; kurtosis: 1.45; Hispanic M = −1.12, SD = 0.75, range: −2.87 – 0.76; skew: 0.22; kurtosis: −0.53).
Child impulsivity
Different measures and reporters of impulsivity were available in middle childhood, late childhood, and late adolescence. In middle childhood, children were too young for self-report so mother report was used. In late childhood, an average of mother and child report was used because children were old enough to give accurate ratings and to partially address method bias. In late adolescence child report was used as youth were in adolescence and accurate reporters of their own behavior (Larson & Richards, 1994).
In middle childhood, mothers rated three items (0 = never or rarely to 3 = very often) regarding levels of their child’s impulsivity using the hyperactive-impulsive subscale of the Disruptive Behavior Rating Scale (Barkley & Murphy, 1998; e.g., “Has difficulty awaiting turn”, “Blurts out answers before questions have been completed”; α = 0.64), which were averaged into a single measure of children’s impulsivity.
In late childhood, children and mothers rated six items (1 = strongly disagree to 5 = strongly agree) regarding levels of impulsivity in the child using the Junior Eysenck Impulsiveness Questionnaire (Eysenck, Easting, & Pearson, 1984; e.g., “He/She often does things on the spur of the moment”, “My child mostly speaks before thinking things out”; αs = 0.86, 0.74 for mothers and children). We examined child and mother items using confirmatory factor analysis. A one factor model had a good fit (χ2 (36) = 39.21, p = 0.33, RMSEA = 0.011, CFI = 0.99, TLI = 0.99) and was not significantly different from a model with two factors, in which mother and child items loaded onto respective latent factors (χ2 (1) = 3.71, p = 0.058). We extracted factor scores from the 1 factor model to represent combined ratings of mother-child report of impulsivity in late childhood.
In late adolescence, adolescents’ reported on six items (1 = agree strongly to 4 = disagree strongly) from the lack of premeditation subscale of the UPPS-P (Zapolski, Stairs, Settles, Combs, & Smith, 2010; e.g., “I tend to blurt out things without thinking”; α = 0.74), which were averaged into a single measure of children’s impulsivity.
Confirmatory factor analysis (CFA) was used to establish whether impulsivity items across the three assessments could be considered to assess a similar lack of premeditation (see Table 2 for impulsivity items at each assessment). To do this, we tested whether a 1-factor model of impulsivity was statistically equivalent to a 3-factor model in which each assessment represented a separate construct. The 3-factor and 1-factor models were not significantly different (χ2 (3) = 6.47, p = 0.091).
Table 2.
Item Overlap among Measures of Impulsivity
| Middle Childhood: Disruptive Behavior Rating Scale (Mother Report; Barkley & Murphy, 1998) | Late Childhood: Junior Eysenck Impulsiveness Questionnaire (Mother-Child Report; Eysenck et al., 1985) | Late Adolescence: Lack of Premeditation (Child Report; Zapolski et al., 2010) |
| Blurts outs answers before questions have been completed | My child (I) mostly speaks before thinking things out | I tend to blurt out things without thinking |
| Interrupts or intrudes on others | He/She/I generally does and says things without stopping to think | I tend to stop and think before doing things (reverse coded) |
| Has difficulty awaiting turn | My child (I) is an impulsive person; He/She usually does things on the spur of the moment | I like to stop and think about something before I do it (reverse coded) |
| He/She/I thinks that planning takes the fun out of things | I like to know what to do before I start a project (reverse coded) | |
| He/She/I usually thinks carefully before doing things (reverse coded) | I try to take a careful approach to things (reverse coded); I am very careful (reverse coded) |
Interparental conflict
In middle and late childhood, mothers and fathers reported on levels of stress and conflict within their marital/romantic relationship using three items (1 = not at all to 5 = a great deal) adapted from Todd, Chassin, Presson, and Sherman (1996; e.g., “How stressful is your relationship”, “How often do you have conflicts”; middle childhood, late childhood αs = 0.87, 0.76 and 0.70, 0.81 for mothers and fathers). Mother and father report were moderately correlated in middle childhood and late childhood, respectively (rs = 0.54, 0.52, ps < 0.001) and averaged into single measures of interparental conflict in middle and late childhood.
Family conflict
In late childhood and early adolescence, children reported on five items (1 = strongly agree to 5 = strongly disagree) regarding levels of conflict and hostility in the family environment using items from the Family Process Scale (Bloom, 1985; e.g., “We fight a lot in our family,” “Family members sometimes got so angry they threw things”; αs = 0.75, 0.81) and averaged into single measures of family conflict in late childhood and early adolescence. Child report was used because adolescent substance use should be influenced more by the child’s than the parents’ perceptions of conflict (e.g., Chassin, et al., 2005).
Adolescent/emerging adulthood substance use
We measured adolescent and emerging adult substance use using latent variables. Each latent variable had three indicators representing alcohol use, illicit drug use, and substance use-related dependence symptoms.
For alcohol use, in early adolescence and emerging adulthood, participants reported on one item assessing how often they had consumed three or more drinks at one time within the past year (1 = never to 8 = everyday). For illicit drug use, in early adolescence and emerging adulthood, adolescents reported on eight items (0 = never to 7 = every day) each assessing the frequency of a specific illicit drug used in the past year (e.g., “In the past year, how many times did you use marijuana?”). Illicit drug items were averaged into a single measure of illicit drug usage.
For substance use-related dependence, participants in early adolescence and emerging adulthood reported on 6 alcohol and 6 drug use items (1 = never to 5 = always) assessing respective dependence symptoms (e.g., “How often have you felt unable to cut down on alcohol/drugs”; αs = 0.60 – 0.74). Alcohol and drug dependence items were averaged into a single measure of substance use dependence at each time.
Parent substance disorder diagnoses
Parents’ lifetime alcohol and drug abuse and dependence diagnoses were included from the middle childhood assessment using the Substance Abuse Module of the CIDI (World Health Organization, 1990). Diagnoses for abuse and dependence were based on DSM-IV criteria. In the present study, parents were coded as having no previous lifetime diagnosis (0), having had either an alcohol or substance disorder diagnosis (1) or having had both alcohol and substance disorder diagnoses (2). This approach was used to capture any additive effects of comorbid diagnoses (e.g., McGue, Slutske, Iacono, 1999).
The larger AFDP study oversampled familial alcoholism in the initial grandparent sample producing elevated rates of parental alcohol and drug lifetime disorder diagnoses (DSM-IV). In the current sample, 29% of fathers had either an alcohol or drug lifetime disorder diagnosis, and 20% of fathers had both alcohol and drug lifetime disorder diagnoses; 18% of mothers had either an alcohol or drug lifetime disorder diagnosis, and 13% of mothers had both alcohol and drug lifetime disorder diagnoses.
Covariates
Covariates were child age, gender and ancestry. As described earlier, ancestry informative genetic markers were used to control for genetic variation across ethnicity. Two-way interactions among covariates were also included.
Statistical Analyses
Structural equation modeling (SEM) with full information maximum likelihood for missing data was used to conduct all primary statistical analyses using a maximum likelihood estimator. We examined all relevant statistical assumptions inherent to the application of SEM (e.g., multivariate normality) and affirmed a priori. All models were tested using Mplus 7.11 (Muthén, & Muthén, 2007).
Children’s polygenic risk scores were included as predictors of impulsivity, family conflict, interparental conflict, and substance use. Mothers’ and fathers’ polygenic risk scores were included as distal predictors of the same constructs. Mothers’ and fathers’ lifetime substance disorder diagnoses were also included as predictors of all constructs, except polygenic risk scores. The bidirectional transactions between children’s impulsivity and family conflict were included, as well as their individual effects on substance use. Interparental conflict was included as a predictor of impulsivity, family conflict, and substance use. Substance use in early adolescence was included as a predictor of late adolescent impulsivity. Correlations for distal predictors were modelled within construct, including correlations between mothers’ and fathers’ polygenic risk scores and their respective substance disorder diagnoses. All within time correlations were modelled. The three measures of substance use were conceptualized using a latent variable. Indirect effects were tested using RMediation (Tofighi & MacKinnon, 2011). Given the many parameters tested for covariates and their interactions, the significant non-hypothesized covariate effects and covariate by covariate interactions were evaluated controlling for Type 1 error using an FDR correction (Benjamini & Hochberg, 1995).
As part of the AFDP sample, multiple members were assessed from the same family at both the parent level (siblings) and at the child level (siblings, cousins). To account for this interdependence, clustering at the family level was included in Mplus, which adjusts standard errors for multilevel data.
Results
Descriptive Analyses
Correlations, means, and standard deviations are presented in Table 3. There was no evidence of multicollinearity among the independent variables. The correlations largely supported the theoretical model. Children’s polygenic risk scores were related to mothers’ ratings of the child’s impulsivity in middle childhood. Children’s polygenic risk scores were not related to family conflict at any period. Mothers’ and fathers’ substance disorder diagnoses were broadly related to children’s impulsivity, interparental conflict, family conflict, and children’s substance use (although there were exceptions). Children’s impulsivity and family conflict were related to each other within time and across time. Interparental conflict was related to family conflict within time and across time as well. Children’s impulsivity, family conflict, and interparental conflict in late childhood were related to substance use in early adolescence. Children’s substance use in early adolescence and impulsivity in late adolescence were primarily related to substance use in emerging adulthood.
Full Theoretical Model
The model was a good fit to the data, χ² (148) = 166.19, p = 0.15, RMSEA = 0.018, CFI = 0.98, TLI = 0.96. The latent measures of substance use had good loadings across the three indicators in early adolescence (alcohol use = 0.84, illicit drug use = 0.87, substance dependence symptoms = 0.74, ps < 0.001) and emerging adulthood (alcohol use = 0.85, illicit drug use = 0.96, substance dependence symptoms = 0.52, ps < 0.001).
Figure 1 presents standardized results for the model (for ease of presentation only significant paths are shown). Children’s polygenic risk scores were positively correlated with both mothers’ polygenic risk score (B = 0.027, SE B = 0.004, β = 0.51, p < 0.001) and fathers’ polygenic risk score (B = 0.026, SE B = 0.004, β = 0.52, p < 0.001). Mothers’ and fathers’ polygenic risk scores were positively correlated (B = 0.010, SE B = 0.005, β = 0.20, p = 0.043). Mothers’ and fathers’ substance disorder diagnoses were also positively correlated (B = 0.45, SE B = 0.08, β = 0.45, p < 0.001).
Polygenic risk scores and parental lifetime substance disorder diagnoses as predictors
Greater levels of children’s polygenic risk predicted greater mother-reported children’s impulsivity in middle childhood (B = 0.63, SE B = 0.20, β = 0.21, p = 0.001). Mothers’ substance disorder diagnosis predicted greater parental report of interparental conflict in middle childhood (B = 0.28, SE B = 0.10, β = 0.34, p = 0.003). Mothers’ substance disorder diagnosis also predicted greater combined mother and child report of children’s impulsivity in late childhood (B = 0.18, SE B = 0.06, β = 0.21, p = 0.006) and greater adolescent-reported substance use in early adolescence (B = 0.11, SE B = 0.04, β = 0.16, p = 0.01). Mothers’ and fathers’ polygenic risk scores did not predict any measure.
Continuity across time within measures
There was continuity across all longitudinally measured variables. There was a positive association between mothers’ report of children’s impulsivity in middle childhood and combined mother and child report of children’s impulsivity in late childhood (B = 0.40, SE B = 0.09, β = 0.33, p < 0.001), and a positive association between combined mother and child report of children’s impulsivity in late childhood and adolescent’s report of their own impulsivity in late adolescence (B = 0.15, SE B = 0.03, β = 0.24, p < 0.001). There was a positive association between children’s report of family conflict in late childhood and in early adolescence (B = 0.33, SE B = 0.06, β = 0.31, p < 0.001), as well as between parents’ report of interparental conflict in middle childhood and late childhood (B = 0.27, SE B = 0.06, β = 0.31, p < 0.001). There was a positive association between the latent measures of substance use in early adolescence and emerging adulthood, as reported by adolescents and emerging adults, respectively (B = 0.59, SE B = 0.17, β = 0.35, p < 0.001).
Cross-lagged associations
There were a number of significant cross-lagged paths. Greater mother-reported children’s impulsivity in middle childhood predicted greater child-reported family conflict in late childhood (B = 0.23, SE B = 0.09, β = 0.19, p = 0.013). Greater child-reported family conflict in late childhood subsequently predicted greater adolescent-reported impulsivity in late adolescence (B = 0.12, SE B = 0.04, β = 0.20, p < 0.001). Greater impulsivity in late childhood (combined mother and child report) and late adolescence (adolescent report) predicted greater substance use in early adolescence and emerging adult (self-report), respectively (B = 0.06, SE B = 0.03, β = 0.07, p = 0.043; B = 0.38, SE B = 0.17, β = 0.16, p = 0.027). Finally, greater parent-reported interparental conflict in late childhood predicted greater adolescent-reported family conflict in early adolescence (B = 0.11, SE B = 0.06, β = 0.09, p = 0.021).
Indirect effects
We tested patterns of indirect effects to assess possible pathways to substance use. No mediated pathways involving 5 constructs were significant so we examined pathways involving fewer constructs, and overlapping pathways leading to substance use. Greater levels of children’s polygenic risk scores predicted greater adolescent-reported substance use in early adolescence via greater parent-reported children’s impulsivity in middle childhood and combined mother and child report of impulsivity in late childhood (β = 0.015, 95% CI [0.001, 0.037], p < 0.05). Children’s greater polygenic risk scores predicted greater child-report of family conflict in late childhood via mothers’ report of children’s impulsivity in middle childhood (β = 0.146, 95% CI [0.021, 0.319], p < 0.05). Greater child-reported family conflict in late childhood predicted greater self-reported substance use in emerging adulthood via adolescent’s report of their own impulsivity in late adolescence (β = 0.046, 95% CI [0.004, 0.105], p < 0.05).
Discussion
The present study used the deviance proneness framework to investigate the longitudinal bidirectional transactions between children’s impulsivity and family conflict as predictors of substance use during adolescence and emerging adulthood. We considered children’s and parents’ polygenic risk scores for behavioral undercontrol, parents’ lifetime substance disorder diagnoses, and interparental conflict as predictors of these transactions. Few previous studies have examined longitudinal bidirectional associations between children’s impulsivity and negative family environments (see Kiff et al., 2011). Even fewer studies have investigated substance use as an outcome of their interplay (Brody & Ge 2001; Patock-Peckham et al., 2001). This study is the first, to our knowledge, to examine developmental cascades involving genetic predisposition for behavioral undercontrol, children’s impulsivity, and family conflict, in predicting substance use.
We hypothesized that our polygenic risk score would be associated with impulsivity in the longitudinal model. In support, we found a positive association between children’s polygenic risk score and their impulsivity in middle childhood, explaining 4% of the variance in impulsivity in middle childhood. Other polygenic risk scores created using this method have explained similar amounts of variance in phenotypes (Davis & Loxton, 2013; Derringer et al., 2012). This validates the polygenic risk score. Previous research has indicated that impulsivity generally declines over childhood and into early adolescence, but persists for some (Côté, Tremblay, Nagin, Zoccolillo, & Vitaro, 2002). Relatedly, genetic influences on impulsivity are strongest in childhood (Bezdjian et al., 2011) with persistent genetic effects exerting less influence over time (Bezdjian, Tuvblad, Wang, Raine, & Baker, 2014). Whereas strong genetic continuity has been found within adolescence (11 to 16 years old), these genetic effects are largely unique from genetic effects in childhood (Bezdjian et al., 2011; Niv et al., 2011). Studies have begun to discover genetic markers that have unique effects on impulsivity based upon developmental period. Salvatore et al. (2015) found their polygenic score predicted greater variance in impulsivity in adolescence vs. young adulthood, suggesting associations with unique genetic variance within age. The current finding that children’s polygenic risk score predicted impulsivity only in middle childhood may indicate that we captured genetic effects unique to impulsivity earlier in life, exclusive from any continuity in impulsivity we found as part of our longitudinal model and CFA. The small genetic effects common to multiple periods may be more difficult to identify. Finally, as genetic influences on impulsivity decrease over time environmental influences increase (Bezdjian et al., 2014). This may be indicated by our finding that family conflict, but not our polygenic risk score, was associated with impulsivity in late adolescence. However, given the dearth of literature related to SNP-level effects on impulsivity across developmental periods our polygenic risk score was not developmentally derived and these results warrant replication to more clearly understand the effects of polygenic risk on impulsivity across development.
Functionally, a number of the SNPs in our polygenic risk score are from the dopamine system. These SNPs may have an effect on the influx of dopamine in the midbrain and prefrontal cortex, affecting planning and inhibition and contributing to children’s impulsive behavior (Jentsch et al., 2014; Koepp et al., 1998; Thut et al., 1997). The lack of effects of mothers’ and fathers’ polygenic risk scores on interparental or family conflict was somewhat surprising and suggests that this indicator of genetic risk for early impulsivity is not relevant to parents’ later conflict with each other or in the broader family.
We also hypothesized that mothers’ and fathers’ lifetime substance disorder diagnoses would be associated with interparental conflict, family conflict, and their children’s impulsivity and substance use. Mothers’ lifetime substance disorder diagnosis was associated with interparental conflict in middle childhood which is consistent with past research demonstrating that mothers’ substance abuse is associated with poorer marital relationships (Cranford, Floyd, Schulenberg, & Zucker, 2011). Mothers’ lifetime substance disorder diagnosis was also associated with children’s impulsivity in late childhood and with substance use in early adolescence. These effects are consistent with the deviance proneness framework and may indicate that mothers’ rather than fathers’ substance use disorder is more disruptive to family functioning because mothers traditionally serve as caregivers and thus may have more impact on family functioning. Also, unlike paternal substance disorder, maternal substance disorders can influence offspring through prenatal exposure pathways (e.g., Mick, Biederman, Faraone, Sayer, & Kleinman, 2002). Finally, families with substance-disordered mothers are also likely to be particularly high-risk because they are likely to contain two substance-disordered parents (Barnard & McKeganey, 2004). In the current data, among the families with mothers who had a lifetime substance disorder diagnosis, 71% contained two parents with a substance use disorder compared to only 29% in which only the mother had a substance use disorder. This may be partially driven by assortative mating in which substance using individuals are more accepting of such behaviors and are more likely to marry one another. Also consistent with assortative mating was the significant correlation between mothers’ and fathers’ polygenic risk scores. The possibility of mothers’ and fathers’ genetic and phenotypic assortative mating could confer elevated genetic risk to the child. This elevated genetic risk could contribute to evocative rGE, inflating estimates of evocative rGE, however the lack of evidence for evocative rGE in the current study does not support this.
In addition to mothers’ and fathers’ substance disorder diagnoses, we hypothesized that interparental conflict would be associated with family conflict. In support of this hypothesis interparental conflict in late childhood predicted family conflict in adolescence. This finding is similar to previous research finding interparental conflict to be associated with negative parenting behavior (e.g., Harold & Conger, 1997; Krishnakumar & Buehler, 2000). These findings are consistent with “spillover” effects of interparental conflict on broader family functioning and offspring outcomes in adolescence.
Finally, we hypothesized that bidirectional transactions would emerge as part of a developmental cascade between children’s impulsivity and family conflict in support of the deviance proneness framework (Iacono et al., 2008; Sher et al., 1991). Also, as hypothesized in the deviance proneness framework, we predicted that these transactions would predict later substance use. In support of this hypothesis, children’s polygenic risk scores predicted impulsivity in middle childhood, which predicted family conflict in late childhood. Family conflict in late childhood predicted impulsivity in late adolescence, which predicted substance use in emerging adulthood. This pattern of results fits within the deviance proneness framework in which early genetic predisposition for behavioral undercontrol manifests as impulsivity in middle childhood. This impulsivity evokes greater family conflict which perpetuates elevated levels of children’s impulsivity. Children’s impulsivity in adolescence subsequently contributes to substance use in emerging adulthood.
One alternative explanation for these findings may be that more impulsive children perceive more family conflict, regardless of actual levels of conflict in the family. However, we investigated this in post-hoc analyses and found that the correlation between parent and child reports of family conflict did not differ based on the level of children’s impulsivity (see Supplemental Materials). Thus, it is likely that greater impulsivity actually predicted greater family conflict, rather than reflecting children’s biased perceptions of family conflicts based on their impulsivity.
Previously, few studies have examined longitudinal transactions between impulsivity-related phenotypes and negative family environments, and their contributions to substance use (e.g., Patock-Peckham et al., 2001). The most comprehensive study by Brody and Ge (2001) found self-regulation in late childhood to predict negative parenting a year later, which in turn was correlated with poorer self-regulation. Self-regulation prospectively predicted adolescent alcohol use a year later. We broadly supported this pattern of transactions in which impulsivity, evoked poor family environments (i.e., family conflict), which contributed to continued poor impulsivity, and this impulsivity predicted later substance use over a broader scope of development. When viewed collectively, this bidirectional transaction between children’s impulsivity and family conflict constitutes a pathway to substance use in emerging adulthood consistent with the deviance proneness framework (Iacono et al., 2008; Sher et al., 1991). However this pathway was not found to be genetically mediated by our polygenic risk score.
We also considered evocative genotype-environment correlations (rGEs) in these transactions. Contrary to our hypothesis, we did not find an evocative rGE between children’s polygenic risk score and family conflict. The genetic variance captured in our polygenic risk score was not associated with family conflict, precluding firm evidence of evocative rGE. However there was an indirect effect of children’s polygenic risk score on family conflict via children’s impulsivity in middle childhood. This pattern of findings suggests that impulsivity is a genetically influenced behavior, but it is impulsive behavior and not its genetic underpinnings that evoke family conflict. The effect of impulsivity on family conflict was therefore not genetically mediated by the polygenic risk score. Few studies have investigated evocative rGE related to indices of behavioral undercontrol, but these studies have found evocative rGE for parenting behaviors (e.g., Elam et al., 2014; Harold et al., 2013). Thus, one possibility is that evocative genetic effects for child impulsivity may be more specific to parent-level behaviors rather than family-level conflict. An alternative explanation is that genetically mediated effects do exist via impulsivity on family conflict but that these effects were not captured by our polygenic risk score.
The current study had several important methodological strengths. First, we were able to examine bidirectional transactions in a developmental cascade model from middle childhood to emerging adulthood. Second, within this model, we were able to include children’s, mothers’ and fathers’ polygenic risk scores and to examine evocative rGE. Finally, we were also able to test effects of parents’ lifetime substance disorder diagnoses and incorporate multiple reporter data of study constructs.
However, the current study also had limitations that should be noted. First, the polygenic risk scores only included six SNPs, thus capturing limited genetic variance. However, this represented a conservative composite, given our requirement of replicated findings in independent published samples for inclusion in the polygenic risk score. A second limitation was that different measures and reporters of children’s impulsivity were available over the longitudinal assessments. However, our CFA showed that a one-factor model of longitudinal impulsivity did not differ from a model that considered each measure as a separate construct. Third, we lacked measurements of impulsivity and family conflict during middle to late childhood. Finally, the current analyses only included children living in two-biological parent families, which had lower rates of mothers’ substance disorder diagnosis than the full sample. These results may not reflect parent-child transactions in single parent households or alternative household structures, in which parenting may be reliant on a sole caregiver, and negative family circumstances may be more deleterious to child functioning. Additionally, results may not generalize to non-biologically related mothers. However, despite lower diagnosis rates, mothers’ substance disorder diagnosis significantly predicted impulsivity in late childhood, early adolescent substance use, and interparental conflict.
The current findings have important implications for interventions to disrupt pathways to substance use beginning in childhood. That is, it may be advantageous to target parents’ reactions to children’s impulsive behavior and to target the quality of the family environment early in life as a way of disrupting these developmental cascades and preventing later substance use (Masten & Cicchetti, 2010). Previous prevention programs have espoused the utility of focusing on parent’s reactions to difficult child behavior by providing parents training in positive and constructive responses to child behavior (e.g., Leve, Harold, Ge, Neiderhiser, & Patterson, 2010). Moreover, the association between family conflict and impulsivity in adolescence suggests that programs aimed at reducing levels of family conflict (e.g., Sanders, Kirby, Tellegen, & Day, 2014) could lead to reductions in children’s impulsivity, and subsequent substance use. Finally, given that maternal substance disorder was a distal risk factor for these cascades, these interventions are potentially useful in preventing the intergenerational transmission of substance use problems.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Alcohol Abuse and Alcoholism (AA016213, AA022097 to Laurie Chassin, AA021612 to Kaitlin Bountress, AA023128 to Frances Wang, 2P60AA011998 to the Midwest Alcohol Research Center) and the National Institute of Mental Health (T32MH018387 to Kit Elam). We thank Arpana Agrawal, Jessica Salvatore, Fazil Aliev, Danielle Dick, and Kenneth Sher for their comments on genetic analyses.
Contributor Information
Kit K. Elam, Arizona State University
Frances L. Wang, Arizona State University
Kaitlin Bountress, Medical University of South Carolina.
Laurie Chassin, Arizona State University.
Danielle Pandika, Arizona State University.
Kathryn Lemery-Chalfant, Arizona State University.
References
- Alati R, Baker P, Betts KS, Connor JP, Little K, Sanson A, Olsson CA. The role of parental alcohol use, parental discipline and antisocial behaviour on adolescent drinking trajectories. Drug and Alcohol Dependence. 2014;134:178–184. doi: 10.1016/j.drugalcdep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Baehne CG, Ehlis AC, Plichta MM, Conzelmann A, Pauli P, Jacob C, Fallgatter AJ. Tph2 gene variants modulate response control processes in adult ADHD patients and healthy individuals. Molecular Psychiatry. 2009;14:1032–1039. doi: 10.1038/mp.2008.39. [DOI] [PubMed] [Google Scholar]
- Baer PE, Garmezy LB, McLaughlin RJ, Pokorny AD, Wernick MJ. Stress, coping, family conflict, and adolescent alcohol use. Journal of Behavioral Medicine. 1987;10:449–466. doi: 10.1007/BF00846144. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Attention-deficit hyperactivity disorder: A clinical workbook. Guilford Press; 1998. [Google Scholar]
- Barnard M, McKeganey N. The impact of parental problem drug use on children: what is the problem and what can be done to help? Addiction. 2004;99:552–559. doi: 10.1111/j.1360-0443.2003.00664.x. [DOI] [PubMed] [Google Scholar]
- Batel P, Houchi H, Daoust M, Ramoz N, Naassila M, Gorwood P. A haplotype of the DRD1 gene is associated with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2008;32:567–572. doi: 10.1111/j.1530-0277.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B Methodological. 1995:289–300. [Google Scholar]
- Bezdjian S, Baker LA, Tuvblad C. Genetic and environmental influences on impulsivity: a meta-analysis of twin, family and adoption studies. Clinical Psychology Review. 2011;31:1209–1223. doi: 10.1016/j.cpr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S, Tuvblad C, Wang P, Raine A, Baker LA. Motor impulsivity during childhood and adolescence: A longitudinal biometric analysis of the go/no-go task in 9-to 18-year-old twins. Developmental Psychology. 2014;50:2549–2557. doi: 10.1037/a0038037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Knopik VS, Audrain-McGovern J, Glynn TR, Spillane NS, Ray LA, Leventhal AM. Novelty seeking as a phenotypic marker of adolescent substance use. Substance Abuse: Research and Treatment. 2015;9:1–10. doi: 10.4137/SART.S22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom BL. A factor analysis of self-report measures of family functioning. Family Process. 1985;24:225–239. doi: 10.1111/j.1545-5300.1985.00225.x. [DOI] [PubMed] [Google Scholar]
- Bradford K, Vaughn LB, Barber BK. When there is conflict interparental conflict, parent-child conflict, and youth problem behaviors. Journal of Family Issues. 2008;29:780–805. [Google Scholar]
- Bray JH, Adams GJ, Getz JG, Baer PE. Developmental, family, and ethnic in influences on adolescent alcohol usage: A growth curve approach. Journal of Family Psychology. 2001;15:301–314. doi: 10.1037//0893-3200.15.2.301. [DOI] [PubMed] [Google Scholar]
- Brody GH, Ge X. Linking parenting processes and self-regulation to psychological functioning and alcohol use during early adolescence. Journal of Family Psychology. 2001;15:82–94. doi: 10.1037//0893-3200.15.1.82. [DOI] [PubMed] [Google Scholar]
- Chan TWS, Bates JE, Lansford JE, Dodge KA, Pettit GS, Dick DM, Latendresse SJ. Impulsivity and genetic variants in DRD2 and ANKK1 moderate longitudinal associations between sleep problems and overweight from ages 5 to 11. International Journal of Obesity. 2014;38:404–410. doi: 10.1038/ijo.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chassin L, Barrera M, Jr, Bech K, Kossak-Fuller J. Recruiting a community sample of adolescent children of alcoholics: a comparison of three subject sources. Journal of Studies on Alcohol. 1992;53:316–319. doi: 10.15288/jsa.1992.53.316. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose J, Sherman SJ, Davis MJ, Gonzalez JL. Parenting style and smoking-specific parenting practices as predictors of adolescent smoking onset. Journal of Pediatric Psychology. 2005;30:333–344. doi: 10.1093/jpepsy/jsi028. [DOI] [PubMed] [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. Journal of Abnormal Psychology. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Côté S, Tremblay RE, Nagin D, Zoccolillo M, Vitaro F. The development of impulsivity, fearfulness, and helpfulness during childhood: Patterns of consistency and change in the trajectories of boys and girls. Journal of Child Psychology and Psychiatry. 2002;43:609–618. doi: 10.1111/1469-7610.00050. [DOI] [PubMed] [Google Scholar]
- Cranford JA, Floyd FJ, Schulenberg JE, Zucker RA. Husbands’ and wives’ alcohol use disorders and marital interactions as longitudinal predictors of marital adjustment. Journal of Abnormal Psychology. 2011;120:210–222. doi: 10.1037/a0021349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Marital conflict and children: An emotional security perspective. New York: Guilford; 2010. [Google Scholar]
- Davis C, Loxton NJ. Addictive behaviors and addiction-prone personality traits: Associations with a dopamine multilocus genetic profile. Addictive Behaviors. 2013;38:2306–2312. doi: 10.1016/j.addbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD) Child Psychiatry & Human Development. 2010;41:168–192. doi: 10.1007/s10578-009-0159-4. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Dick DM, Aliev F, Grucza RA, Saccone S, et al. The aggregate effect of dopamine genes on dependence symptoms among cocaine users: Cross-validation of a candidate system scoring approach. Behavior Genetics. 2012;42:626–635. doi: 10.1007/s10519-012-9531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Dick DM, Saccone S, Grucza RA, Agrawal A Gene Environment Association Studies (GENEVA) Consortium. Predicting sensation seeking from dopamine genes a candidate-system approach. Psychological Science. 2010;21:1282–1290. doi: 10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Wang JC, Hinrichs A, Bertelsen S, Bucholz KK, Bierut LJ. Alcohol dependence with comorbid drug dependence: genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contribution to risk. Addiction. 2007;102:1131–1139. doi: 10.1111/j.1360-0443.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Goate A. Using dimensional models of externalizing psychopathology to aid in gene identification. Archives of General Psychiatry. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Doehring A, von Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G, Lötsch J. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenetics and Genomics. 2009;19:407–414. doi: 10.1097/FPC.0b013e328320a3fd. [DOI] [PubMed] [Google Scholar]
- Elam KK, Harold GT, Neiderhiser JM, Reiss D, Shaw DS, Natsuaki MN, Leve LD. Adoptive parent hostility and children’s peer behavior problems: Examining the role of genetically informed child attributes on adoptive parent behavior. Developmental Psychology. 2014;50:1543–1552. doi: 10.1037/a0035470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O, Burman B. Interrelatedness of marital relations and parent-child relations: a meta-analytic review. Psychological Bulletin. 1995;118:108–132. doi: 10.1037/0033-2909.118.1.108. [DOI] [PubMed] [Google Scholar]
- Esposito-Smythers C, Spirito A, Rizzo C, McGeary JE, Knopik VS. Associations of the DRD2 TaqIA polymorphism with impulsivity and substance use: preliminary results from a clinical sample of adolescents. Pharmacology Biochemistry and Behavior. 2009;93:306–312. doi: 10.1016/j.pbb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SB, Easting G, Pearson PR. Age norms for impulsiveness, venturesomeness and empathy in children. Personality and Individual Differences. 1984;5:315–321. [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biological Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Fischer M. Parenting stress and the child with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology. 1990;19:337–346. [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Glatz T, Stattin H, Kerr M. Parents’ reactions to youths’ hyperactivity, impulsivity, and attention problems. Journal of Abnormal Child Psychology. 2011;39:1125–1135. doi: 10.1007/s10802-011-9541-3. [DOI] [PubMed] [Google Scholar]
- Hack LM, Kalsi G, Aliev F, Kuo PH, Prescott CA, Patterson DG, Kendler KS. Limited associations of dopamine system genes with alcohol dependence and related traits in the Irish Affected Sib Pair Study of Alcohol Dependence (IASPSAD) Alcoholism: Clinical and Experimental Research. 2011;35:376–385. doi: 10.1111/j.1530-0277.2010.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Experimental and Clinical Psychopharmacology. 2009;17:374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Conger RD. Marital conflict and adolescent distress: The role of adolescent awareness. Child Development. 1997;68:333–350. doi: 10.1111/j.1467-8624.1997.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, Thapar A. Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. Journal of Child Psychology and Psychiatry. 2013;54:1038–1046. doi: 10.1111/jcpp.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DA, Bennett KS, McStephen M, Rooney R, Levy F. Attention deficit-hyperactivity disorder in twins: A developmental genetic analysis. Australian Journal of Psychology. 2004;56:99–107. [Google Scholar]
- Heinrichs N, Cronrath AL, Degen M, Snyder DK. The link between child emotional and behavioral problems and couple functioning. Family Science. 2010;1:152–172. [Google Scholar]
- Hendershot CS, Bryan AD, Ewing SWF, Claus ED, Hutchison KE. Preliminary evidence for associations of CHRM2 with substance use and disinhibition in adolescence. Journal of Abnormal Child Psychology. 2011;39:671–681. doi: 10.1007/s10802-011-9511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrenkohl TI, Lee JO, Kosterman R, Hawkins JD. Family influences related to adult substance use and mental health problems: A developmental analysis of child and adolescent predictors. Journal of Adolescent Health. 2013;51:129–135. doi: 10.1016/j.jadohealth.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior Genetics. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol and Alcoholism. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD. Significant association of DRD1 with nicotine dependence. Human Genetics. 2008;123:133–140. doi: 10.1007/s00439-007-0453-9. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Annals of the New York Academy of Sciences. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JM, Nigg JT, Buu A, Puttler LI, Glass JM, Heitzeg MM, Zucker RA. Trajectories of childhood aggression and inattention/hyperactivity: Differential effects on substance abuse in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1158–1165. doi: 10.1097/CHI.0b013e3181825a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantseva A, Gaysina D, Malykh S, Khusnutdinova E. The role of dopamine transporter (SLC6A3) and dopamine D2 receptor/ankyrin repeat and kinase domain containing 1 (DRD2/ANKK1) gene polymorphisms in personality traits. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1033–1040. doi: 10.1016/j.pnpbp.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, Zalewski M. Nature and nurturing: Parenting in the context of child temperament. Clinical Child and Family Psychology Review. 2011;14:251–301. doi: 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Molina BS, Chassin L. Prospective relations between growth in drinking and familial stressors across adolescence. Journal of Abnormal Psychology. 2009;118:610–622. doi: 10.1037/a0016315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krishnakumar A, Buehler C. Interparental conflict and parenting behaviors: A meta-analytic review. Family Relations. 2000;49:25–44. [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Larson R, Richards MH. Divergent realities: The emotional lives of mothers, fathers, and adolescents. New York, NY: Basic Books; 1994. [Google Scholar]
- Latzman RD, Elkovitch N, Clark LA. Predicting parenting practices from maternal and adolescent sons’ personality. Journal of Research in Personality. 2009;43:847–855. [Google Scholar]
- Laucht M, Becker K, Frank J, Schmidt MH, Esser G, Treutlein J, Schumann G. Genetic variation in dopamine pathways differentially associated with smoking progression in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:673–681. doi: 10.1097/CHI.0b013e31816bff77. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Eiden RD. Marital and family processes in the context of alcohol use and alcohol disorders. Annual Review of Clinical Psychology. 2007;3:285–310. doi: 10.1146/annurev.clinpsy.3.022806.091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Harold GT, Ge X, Neiderhiser JM, Patterson G. Refining intervention targets in family-based research lessons from quantitative behavioral genetics. Perspectives on Psychological Science. 2010;5:516–526. doi: 10.1177/1745691610383506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Iacono WG. Personality and substance use disorders: II. Alcoholism versus drug use disorders. Journal of Consulting and Clinical Psychology. 1999;67:394–404. doi: 10.1037//0022-006x.67.3.394. [DOI] [PubMed] [Google Scholar]
- McGue M, Zhang Y, Miller MB, Basu S, Vrieze S, Hicks B, Iacono WG. A genome-wide association study of behavioral disinhibition. Behavior Genetics. 2013;43:363–373. doi: 10.1007/s10519-013-9606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Molecular psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén B. Mplus 5. Los Angeles: Muthén & Muthén; 2007. [Google Scholar]
- National Institute of Drug Abuse. Principles of adolescent substance use disorder treatment: A research-based guide (NIH Publication No. 14-7953) 2014 Retrieved July 22, from https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/podata_1_17_14.pdf.
- Neumann A, Barker ED, Koot HM, Maughan B. The role of contextual risk, impulsivity, and parental knowledge in the development of adolescent antisocial behavior. Journal of Abnormal Psychology. 2010;119:534–545. doi: 10.1037/a0019860. [DOI] [PubMed] [Google Scholar]
- Niv S, Tuvblad C, Raine A, Baker LA. Aggression and rule-breaking: Heritability and stability of antisocial behavior problems in childhood and adolescence. Journal of Criminal Justice. 2013;41:285–291. doi: 10.1016/j.jcrimjus.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, LeBlanc M, Zai C, Shaikh S, Renou J, DeLuca V, Le Foll B. Association of polymorphisms in the BDNF, DRD1 and DRD3 genes with tobacco smoking in schizophrenia. Annals of Human Genetics. 2010;74:291–298. doi: 10.1111/j.1469-1809.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- Nyman ES, Loukola A, Varilo T, Taanila A, Hurtig T, Moilanen I, Peltonen L. Sex-specific influence of DRD2 on ADHD-type temperament in a large population-based birth cohort. Psychiatric Genetics. 2012;22:197–201. doi: 10.1097/YPG.0b013e32834c0cc8. [DOI] [PubMed] [Google Scholar]
- Nyman ES, Ogdie MN, Loukola A, Varilo T, Taanila A, Hurtig T, Peltonen L. ADHD candidate gene study in a population-based birth cohort: association with DBH and DRD2. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1614–1621. doi: 10.1097/chi.0b013e3181579682. [DOI] [PubMed] [Google Scholar]
- Oades RD, Lasky-Su J, Christiansen H, Faraone SV, Sonuga-Barke EJ, Banaschewski T, Asherson P. The influence of serotonin-and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behavioral and Brain Functions. 2008;4:48–62. doi: 10.1186/1744-9081-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM. A finer examination of the role that negative affect plays in the relationship between paternal alcoholism and the onset of alcohol and marijuana use. Journal of Studies on Alcohol and Drugs. 2009;70:400–408. doi: 10.15288/jsad.2009.70.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SL, Bates JE, Bayles K. Early antecedents of childhood impulsivity: The role of parent-child interaction, cognitive competence, and temperament. Journal of Abnormal Child Psychology. 1990;18:317–334. doi: 10.1007/BF00916568. [DOI] [PubMed] [Google Scholar]
- Olson SL, Bates JE, Sandy JM, Schilling EM. Early developmental precursors of impulsive and inattentive behavior: From infancy to middle childhood. Journal of Child Psychology and Psychiatry. 2002;43:435–447. doi: 10.1111/1469-7610.00035. [DOI] [PubMed] [Google Scholar]
- Pan Y, Luo X, Liu X, Wu LY, Zhang Q, Wang L, Wang KS. Genome-wide association studies of maximum number of drinks. Journal of Psychiatric Research. 2013;47:1717–1724. doi: 10.1016/j.jpsychires.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patock-Peckham JA, Cheong J, Balhorn ME, Nagoshi CT. A social learning perspective: a model of parenting styles, self-regulation, perceived drinking control, and alcohol use and problems. Alcoholism: Clinical and Experimental Research. 2001;25:1284–1292. [PubMed] [Google Scholar]
- Pavlov KA, Chistiakov DA, Chekhonin VP. Genetic determinants of aggression and impulsivity in humans. Journal of Applied Genetics. 2012;53:61–82. doi: 10.1007/s13353-011-0069-6. [DOI] [PubMed] [Google Scholar]
- Pener-Tessler R, Avinun R, Uzefovsky F, Edelman S, Ebstein RP, Knafo A. Boys’ serotonin transporter genotype affects maternal behavior through self-control: A case of evocative gene-environment correlation. Development and Psychopathology. 2013;25:151–162. doi: 10.1017/S095457941200096X. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser J. Behavioral Genetics. London, UK: Palgrave Macmillan; 2013. [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Hennig J, Amelang M, Montag C, Korkut T, Hueweler A, Stürmer T. The role of the TPH1 and TPH2 genes for nicotine dependence: a genetic association study in two different age cohorts. Neuropsychobiology. 2007;56:47–54. doi: 10.1159/000110728. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Bucholz K, Agrawal A, Hesselbrock V, Hesselbrock M, Dick DM. Polygenic risk for externalizing disorders gene-by-development and gene-by-environment effects in adolescents and young adults. Clinical Psychological Science. 2015;15:189–201. doi: 10.1177/2167702614534211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MR, Kirby JN, Tellegen CL, Day JJ. The Triple P-Positive Parenting Program: A systematic review and meta-analysis of a multi-level system of parenting support. Clinical Psychology Review. 2014;34:337–357. doi: 10.1016/j.cpr.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Scaramella LV, Leve LD. Clarifying parent-child reciprocities during early childhood: The early childhood coercion model. Clinical Child and Family Psychology Review. 2004;7:89–107. doi: 10.1023/b:ccfp.0000030287.13160.a3. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Skeer M, McCormick MC, Normand SLT, Buka SL, Gilman SE. A prospective study of familial conflict, psychological stress, and the development of substance use disorders in adolescence. Drug and Alcohol Dependence. 2009;104:65–72. doi: 10.1016/j.drugalcdep.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AL, Becker LG, Huber AM, Catalano RF. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addictive Behaviors. 2012;37:747–775. doi: 10.1016/j.addbeh.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tian C, Hinds DA, Shigeta R, Adler SG, Lee A, Pahl MV, Seldin MF. A genomewide single-nucleotide-polymorphism panel for Mexican American admixture mapping. The American Journal of Human Genetics. 2007;80:1014–1023. doi: 10.1086/513522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M, Chassin L, Presson CC, Sherman SJ. Role stress, role socialization, and cigarette smoking: Examining multiple roles and moderating variables. Psychology of Addictive Behaviors. 1996;10:211–221. [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakalahi HF. Adolescent substance use and family-based risk and protective factors: A literature review. Journal of Drug Education. 2001;31:29–46. doi: 10.2190/QP75-P9AR-NUVJ-FJCB. [DOI] [PubMed] [Google Scholar]
- Villafuerte S, Strumba V, Stoltenberg SF, Zucker RA, Burmeister M. Impulsiveness mediates the association between GABRA2 SNPs and lifetime alcohol problems. Genes, Brain and Behavior. 2013;12:525–531. doi: 10.1111/gbb.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: twin biometry, GCTA, and genome-wide scoring. Behavior Genetics. 2013;43:97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitza S, Renner TJ, Dempfle A, Konrad K, Wewetzer C, Halbach A, Lesch KP. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in attention-deficit/hyperactivity disorder. Molecular Psychiatry. 2005;10:1126–1132. doi: 10.1038/sj.mp.4001734. [DOI] [PubMed] [Google Scholar]
- Wang FL, Chassin L, Geiser C, Lemery-Chalfant K. Mechanisms in the relation between GABRA2 and adolescent externalizing problems. European Child & Adolescent Psychiatry. 2015:1–14. doi: 10.1007/s00787-015-0703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Baucom DH. Intimate relationships and psychopathology. Clinical Child and Family Psychology Review. 2012;15:4–13. doi: 10.1007/s10567-011-0107-2. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Wong MM. Perceptions of parental involvement and autonomy support: Their relations with self-regulation, academic performance, substance use and resilience among adolescents. North American Journal of Psychology. 2008;10:497–518. [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI) (Version 1.0) Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]
- Zai CC, Muir KE, Nowrouzi B, Shaikh SA, Choi E, Berall L, Kennedy JL. Possible genetic association between vasopressin receptor 1B and child aggression. Psychiatry Research. 2012;200:784–788. doi: 10.1016/j.psychres.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Zapolski TC, Stairs AM, Settles RF, Combs JL, Smith GT. The measurement of dispositions to rash action in children. Assessment. 2010;17:116–125. doi: 10.1177/1073191109351372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, King KM, Chassin L. The roles of familial alcoholism and adolescent family harmony in young adults’ substance dependence disorders: Mediated and moderated relations. Journal of Abnormal Psychology. 2006;115:320–331. doi: 10.1037/0021-843X.115.2.320. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol-disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Development Perspectives. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.