Abstract
Catheter ablation of atrial ablation (AF) has become an important therapy in recent years. As with all evolving techniques, unexpected complication may occur. Atrioesophageal fistula is a very rare complication of AF catheter ablation. Described for the first time in two very experienced centers in 2004, this complication is the most dreadful and lethal among all the others related to AF catheter ablation. Its clinical presentation is extremely variable. Rapid diagnosis and surgical therapy may prevent death. This review article will summarize the risk factors, diagnosis, treatment and possible preventive strategies for this condition.
Keywords: Catheter Ablation, Complications, Atrial Fibrillation
Introduction
Non-pharmacologic therapy for atrial fibrillation (AF) is increasing in popularity and can be performed with catheter based and surgical approaches. The most commonly employed strategy for ablation of AF presently involves creation of circumferential lesions around the pulmonary vein ostia or antra with or without the placement of additional ablation lesions within the left atrium (i.e. linear lesions in the left atrial roof, mitral isthmus, or ablation of sites with complex fractionated atrial electrograms).[1,2,3]
AF ablation carries a small risk of complications with the most serious being atrioesophageal fistula (AEF). Although the incidence is less than 0.1%, it is usually fatal.[4,5,6] Esophageal perforation or fistula was reported in 31 patients (0.016%) in the Global Survey of Esophageal and Gastric Injury in Atrial Fibrillation study. Symptom onset for esophageal perforation or fistula was reported on average 19.3 days after the ablation procedure but could appear as short as 6 days and as long as 59 days post ablation.[6] Esophageal injury has been observed most frequently with percutaneous radiofrequency ablation, although it has also been reported with other energy sources including cryoablation,[7] high-intensity focused ultrasound,[8] and even surgical ablation.[9]
Pathophysiology
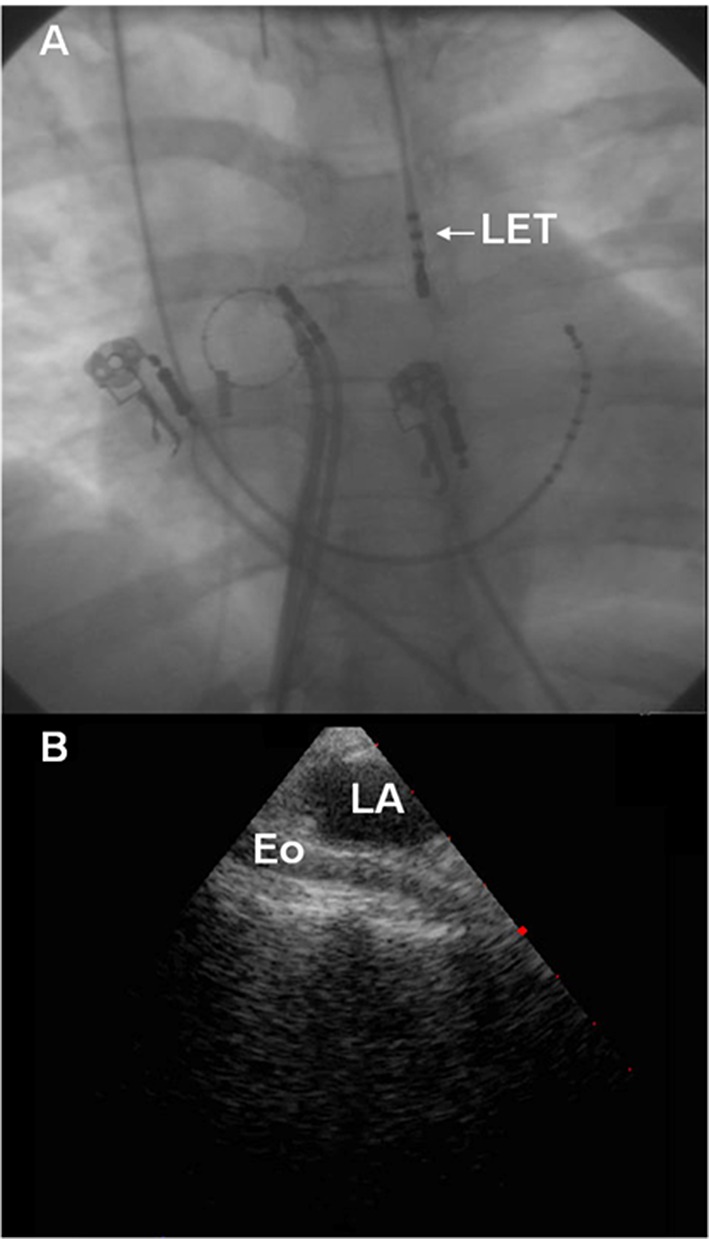
The esophagus lies in close proximity to the left atrium placing it at risk for injury during catheter ablation procedures (Figure 1). As AEF typically presents many days after ablation, direct mechanical insult during the index procedures is unlikely to be the primary culprit for fistula formation. Current theories of esophageal injury and AEF formation implicate adverse healing secondary to thermal injury to the esophagus during the index ablation procedure. The insult is believed to start at the esophageal side and extend into the mediastinum, the pericardium and then the left atrium.[10] Epithelial intestinal tissue is highly susceptible to radiofrequency (RF)-induced thermal injury. Heat damage results from thermal conduction in the tissue rather than direct power application. Heat may affect esophageal endothelial cells directly, or may damage anterior esophageal arteries causing ischemia and ulceration of the mucosal layers. Morphological changes of periesophageal connective tissue and the posterior wall of the LA can be seen on endosonography after AF ablation, even in the absence of endoscopic epithelial damage.[11] The delayed appearance of AEF favors esophageal artery ischemia as the primary mechanism of injury. In addition, pre-existing esophagitis due to gastroesophageal reflux may exacerbate esophageal injury perhaps by interfering with the usual repair mechanisms after esophageal injury.[12] After esophageal tissue necrosis develops, mediastinitis and fistula formation occurs resulting in a communication between the esophageal lumen and the pericardium and subsequently with the left atrial blood pool via the oblique sinus.[10]
Figure 1. Proximity of the LA and Esophagus. A) Fluoroscopy demonstrating an esophageal temperature probe (LET). As noted, the esophagus frequently lies immediately posterior to the LA. B) Intra-cardiac Echocardiography demonstrating the relationship between the LA and esophagus.
Risk Factors For Atrioesophageal Fistula
Owing to the low incidence of AEF, esophageal endoscopic studies have been used to screen for asymptomatic epithelial injuries after ablation, with esophageal ulcerations (ESULs) serving as potential precursors of fistula formation (Figure 2). Several studies have examined predisposing risk factors for esophageal injury. Patients with persistent AF may be at higher risk due to a larger left atrial size which makes the relationship between the left atrium and esophagus more intimate. Multivariate analysis of a cohort of 260 patients undergoing AF ablation highlighted that the distance between the left atrium and esophagus was an independent predictor of ESUL.[13] Patients with persistent AF were more likely to have LA enlargement with compression of the esophagus between the left atrium and the spinal cord, potentially decreasing the distance between the left atrium and esophagus and thus the risk for esophageal injury. Interestingly, Yamasaki and colleagues[14] highlighted that, the distance between the left atrium and esophagus may also be an issue in individuals with a low body mass. Rather than direct compression of the left atrium to the esophagus seen in persistent AF patients, in this case the injury may be related to a shorter distance with less intervening tissue between the left atrium and the esophagus.[14]
Figure 2. Endoscopy demonstrating an esophageal ulcer, a possible precursor to AEF.

Extensive ablation on the posterior wall, such as may occur during persistent AF ablation, may predispose to esophageal heating particularly when higher powers are administered in the vicinity of the posterior wall. An increased incidence of ESUL was noted with higher power settings[15] and the use of a deflectable sheath (which may improve contact and subsequent heat transfer to the esophagus) when ablating on the posterior wall of the left atrium.[16]
General anesthesia may also increase the risk of esophageal injury.[17] The mechanism of this is unclear but may be related to decreased esophageal peristalsis and swallowing during anesthesia which might prevent physiological cooling. Additionally, it is possible that injury may be a result of the higher use of oro- or naso-gastric tubes in procedures performed under general anaesthesia. It is possible that these tubes may result in mechanical fixation of the esophagus against the LA. As is apparent, multiple factors play a role in esophageal ulcer formation. However, risk factors for the progression of an esophageal ulcer to AEF remain unknown and will be difficult to discern given the rarity of AEF.
Measures To Minimize The Risk Of Esophageal Injury
Gastric Acid Suppression
As it has been suggested that gastroesophageal reflux may play a role in aggravating the initial esophageal insult and hinder appropriate healing thereby promoting the development of AEF, prophylactic proton-pump inhibitors (PPIs) have been recommended for patients undergoing AF ablation.[12] Adequately powered clinical trials to establish the efficacy of proton pump inhibitors to reduce AEF may never be feasible given the low incidence of AEF.
Esophageal Temperature Monitoring
As it is difficult to predict the extent of heat transferred to the esophagus during catheter ablation procedures, it has been proposed that real-time luminal esophageal temperature monitoring may provide some assessment of the extent of heat transferred to the esophagus during ablation. A temperature probe placed in the esophagus at the level of the ablation catheter may allow one to detect increases in luminal esophageal temperature and may alert the operator to excessive heat transfer (Figure 1).
In a retrospective study of patients undergoing AF ablation Singh et al.[18] demonstrated that patients were less likely to experience esophageal injury after catheter ablation when using luminal esophageal temperature monitoring. Their practice was to interrupt RF applications when the luminal esophageal temperature increased to 38.5°C. In this series only 6% of patients with ablation guided by esophageal temperature monitoring developed ESUL as opposed to 36% of those without monitoring. A more recent prospective study which employed a triple-thermocouple esophageal temperature monitor and limited esophageal temperature to 40°C also hinted at a low incidence of esophageal injury with only 1.6% of 184 patients showing signs of ESUL.[19]
Leite et al[20] attempted to define an acceptable esophageal temperature rise prior to stopping RF applications.[20] In this study power on the posterior wall was limited to 25 watts and terminated when the temperature increased more than 2°C from baseline. Using this strategy, no patients demonstrated esophageal thermal injury on follow-up endoscopy. Based on the totality of this work it has been suggested that careful esophageal temperature monitoring with interrupting RF application when esophageal temperature increases may minimize the risk of esophageal injury and subsequent AEF. However, limitations inherent to luminal esophageal temperature monitoring may not always prevent esophageal injury and AEF formation.
Frequently reposition the esophageal temperature probe and mismatch of the esophageal diameter relative to that of the temperature probe may result in incorrect positioning of the esophageal temperature probe thereby limiting its ability to provide accurate local temperature readings.[21] Moreover, due to the phenomenon of thermal latency, the esophageal temperature may continue to rise even after RF is interrupted, resulting in temperature overshoot in a significant number of patients. Finally, esophageal luminal temperature may be significantly lower than esophageal mural temperature and thereby not reflect the extent of heat transfer to the outer and mid walls of the esophagus.[22] Consequently, injury may occur after ablation even when the luminal esophageal temperature is assiduously monitored. Operators must be aware of these limitations as AEF has been reported when esophageal temperature did not rise during ablation[23] clearly highlighting that esophageal temperature monitoring alone is insufficient to completely prevent esophageal thermal injuries. Other methods to actively protect the esophagus during AF ablation must be identified.
In addition to the limitation of accurate esophageal monitoring, recent work has highlighted the effects of radiofrequency ablation near metallic devices. Nguyen et al.[24] highlighted esophageal temperature probes may function as “lightning rods”, attracting electrical current from the ablation catheter and potentiating heat transfer to the esophagus. Further research is needed to confirm whether esophageal temperature probes indeed exacerbate heat transfer and subsequent esophageal injury. Consistency of this finding would call into question current practices of luminal esophageal temperature monitoring during AF ablation.
Mechanical Deflection Of Esophagus
Mechanical techniques to move the esophagus away from the tip of the ablation catheter have been devised with the hope of preventing thermal injury to the esophagus. This approach is possible as the thoracic esophagus is not fixed in position by true ligaments or other significant fibrous attachments to surrounding structures. Chugh et al.[25] demonstrated the feasibility of displacing the esophagus by deflecting a transesophageal (TEE) probe placed within the esophagus during ablation. They found that it was possible to move the esophagus on average 2 cm in 10 of 12 (83%) patients. To avoid mechanical complications and shunting of RF energy towards the TEE probe, the probe was removed in all patients after the esophagus was deviated. Unfortunately the esophagus remained displaced after removal of the endoscope in only 22% of patients making this approach suboptimal.
A recent study by Koruth et al.[26] utilized an endotracheal stylet within a thoracic chest tube to deflect the esophagus away from the area of energy delivery in 20 patients undergoing AF ablation (Figure 3). Unlike Chugh’s work,[25] the stylet and chest tube remained in the esophagus to allow for sustained deviation during ablation. Leftward and rightward deflection averaged 2.8 cm each which was maintained during ablation at the posterior wall. Post-procedural endoscopy demonstrated ulceration in one patient (5%) and evidence of trauma from esophageal instrumentation without clinical consequence in 12 patients (63%). This technique did not require participation of an endoscopist during the ablation procedure as it was performed by the anaesthesiologist participating in the AF procedure. It may well be that endoluminal esophageal displacement may become an effective method of protecting the esophagus during ablation of AF.
Figure 3. Deviation of the esophagus. A) Fluoroscopy with barium contrast to highlight the baseline position of the esophagus posterior to the left atrium. Of note is the presence of a multi-electrode temperature monitor. B) Using a chest tube and stylet, the esophagus is deviated to the right and away from the left atrium and region where ablation is required. Barium highlights the course of the esophagus.

Thermal Insulation Of Esophagus
Instrumentation of the pericardial space and introduction of a balloon catheter between the LA and esophagus to move the esophagus away from the LA is an alternative approach to reduce heat transfer to the esophagus during AF ablation.[27,28] While esophageal temperature rises are limited with the introduction of a pericardial balloon, this approach does add significant complexity to an already complex AF ablation procedure.
Clinical Presentation
AEF typically occurs 1 to 4 weeks after the catheter ablation procedure, although earlier and later onsets have been reported.[29] The majority of signs and symptoms of AEF are not specific and may include fever, fatigue, malaise, chest discomfort, nausea, vomiting, dysphagia, odynophagia, hematemesis, melena and dyspnea. A high index of suspicion is recommended in patients with constitutional symptoms after AF ablation.
When entertaining the diagnosis of AEF a white blood cell count should be obtained as this is an early and sensitive laboratory marker of an AEF.[30] In addition, imaging should be performed emergently (Figure 4). Computed tomographic (CT) scan of the chest with intravenous contrast is considered by many as the test of choice as it can rapidly and safely demonstrate findings suggestive of AEF such as pneumomediastinum or pneumopericardium. This test can be considered diagnostic if intravenous contrast enters the esophagus or mediastinum from the left atrium. Transthoracic echocardiography may demonstrate air in the left heart, pericardium or the presence of a pericardial effusion. Esophageal instrumentation with endoscopy and transesophageal echocardiography are not recommended as they theoretically may worsen the situation by increasing fistula size and also increase the risk of air embolism secondary to increased esophageal pressure with instrumentation and insufflation.[31]
Figure 4. Imaging to aid with the diagnosis of AEF. A) CT chest demonstrating air in the LA, B) MRI of the brain demonstrating infarctions in multiple territories, c) Echocardiogram demonstrating air in the left heart.
Early recognition is important, as patients often develop endocarditis with septic emboli leading to neurological manifestations such as altered mental status, seizures, and coma within hours of symptom onset.[30]
Treatment Options
Although the prevalence of AEF post-AF ablation is low, the fatality rate is high and reported between 67% and 100%.[32] The high case-fatality rate has traditionally been attributed to the lack of recognition and late presentation of this complication. The prognosis of patients who survive to obtain an accurate diagnosis and receive corrective surgical intervention is variable.
Available therapeutic options for AEF include surgical repair of the fistula (combined left atrial and esophageal repair) via thoracotomy, esophageal stenting and conservative management with aggressive chest tube drainage and treatment of sepsis. Of these three approaches, conservative treatment of esophageal fistula remains controversial, as it requires frequent radiologic assessments and is associated with very high mortality rate. Data on stenting versus surgical treatment of AEF are conflicting and at the present there is no consensus on the most effective treatment strategy for AEF. In our opinion esophageal stenting may be useful only if esophageal perforation is present without fistula formation to the left atrium.[33,34] Our preference is, if possible, early aggressive surgical repair. Our group reported on the outcomes of 29 patients undergoing AEF repair.[34] The report suggested that surgical esophageal repair with placement of tissue between the esophagus and left atrium may result in lower morbidity and mortality.[34] Attention to proper left atrial and esophageal repair is critical and may be best achieved with the use of cardiopulmonary bypass. Furthermore, surgical and medical treatment of the associated mediastinitis is important. We recommend a multidisciplinary approach to care including cardiac-thoracic surgeons, infectious disease, neurology, and critical care physicians as well as allied health care professionals such as dieticians, physiotherapists and occupational therapists.
Awareness
Due to the rarity of the complication, it is not clear if earlier detection and earlier repair will result in improved clinical outcomes; however this strategy does make sense. Given this, patient education on the signs and symptoms of AEF is of paramount importance to allow patients to present to medical attention sooner to facilitate earlier repair prior to the onset of mediastinitis or stroke. Additionally, education of primary care and emergency room physicians of this complication is also important to avoid misdiagnosis, and ensure esophageal manipulation is minimized. A recent approach by Canadian centers performing AF ablation is to provide patients who have undergone an AF ablation procedure a pocket card (Figure 5) which they present to their primary care physician with the onset of vague symptoms. The interaction when the patient receives this card educates them on the symptoms of AEF with the hope of earlier presentation. Furthermore, the instructions on the card ensure primary care physicians do not instrument the esophagus and encourages collaboration with the primary Electrophysiologist to ensure that the diagnosis is made as soon as possible. It is hoped that this strategy may minimize the morbidity and mortality associated with this complication.
Figure 5. Patient pocket card.
Conclusions
AEF is uncommon but has been reported with all approaches to AF ablation, without clearly identifiable predisposing factors. Despite our knowledge of this complication, outcomes with AEF related to AF ablation remain poor. Ongoing assessment of prevention strategies, as well as patient and physician education to recognize this complication should be encouraged as, given the growth in AF ablation procedures, AEF will continue to occur.
Disclosures
None.
References
- 1.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 2;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 4.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2009 May 12;53 (19):1798–803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Ghia Kasturi K, Chugh Aman, Good Eric, Pelosi Frank, Jongnarangsin Krit, Bogun Frank, Morady Fred, Oral Hakan. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2009 Jan;24 (1):33–6. doi: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 6.Barbhaiya Chirag R, Kumar Saurabh, John Roy M, Tedrow Usha B, Koplan Bruce A, Epstein Laurence M, Stevenson William G, Michaud Gregory F. Global survey of esophageal and gastric injury in atrial fibrillation ablation: incidence, time to presentation, and outcomes. J. Am. Coll. Cardiol. 2015 Apr 7;65 (13):1377–8. doi: 10.1016/j.jacc.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 7.Stöckigt Florian, Schrickel Jan W, Andrié René, Lickfett Lars. Atrioesophageal fistula after cryoballoon pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2012 Nov;23 (11):1254–7. doi: 10.1111/j.1540-8167.2012.02324.x. [DOI] [PubMed] [Google Scholar]
- 8.Neven Kars, Schmidt Boris, Metzner Andreas, Otomo Kiyoshi, Nuyens Dieter, De Potter Tom, Chun K R Julian, Ouyang Feifan, Kuck Karl-Heinz. Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):260–5. doi: 10.1161/CIRCEP.109.922930. [DOI] [PubMed] [Google Scholar]
- 9.Tan Corinne, Coffey Arthur. Atrioesophageal fistula after surgical unipolar radiofrequency atrial ablation for atrial fibrillation. Ann. Thorac. Surg. 2013 Mar;95 (3):e61–2. doi: 10.1016/j.athoracsur.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Grubina Rozalina, Cha Yong-Mei, Bell Malcolm R, Sinak Lawrence J, Asirvatham Samuel J. Pneumopericardium following radiofrequency ablation for atrial fibrillation: insights into the natural history of atrial esophageal fistula formation. J. Cardiovasc. Electrophysiol. 2010 Sep;21 (9):1046–9. doi: 10.1111/j.1540-8167.2010.01740.x. [DOI] [PubMed] [Google Scholar]
- 11.Zellerhoff Stephan, Ullerich Hansjörg, Lenze Frank, Meister Tobias, Wasmer Kristina, Mönnig Gerold, Köbe Julia, Milberg Peter, Bittner Alex, Domschke Wolfram, Breithardt Günter, Eckardt Lars. Damage to the esophagus after atrial fibrillation ablation: Just the tip of the iceberg? High prevalence of mediastinal changes diagnosed by endosonography. Circ Arrhythm Electrophysiol. 2010 Apr;3 (2):155–9. doi: 10.1161/CIRCEP.109.915918. [DOI] [PubMed] [Google Scholar]
- 12.Zellerhoff Stephan, Lenze Frank, Eckardt Lars. Prophylactic proton pump inhibition after atrial fibrillation ablation: is there any evidence? Europace. 2011 Sep;13 (9):1219–21. doi: 10.1093/europace/eur139. [DOI] [PubMed] [Google Scholar]
- 13.Martinek Martin, Meyer Christian, Hassanein Said, Aichinger Josef, Bencsik Gabor, Schoefl Rainer, Boehm Gernot, Nesser Hans-Joachim, Purerfellner Helmut. Identification of a high-risk population for esophageal injury during radiofrequency catheter ablation of atrial fibrillation: procedural and anatomical considerations. Heart Rhythm. 2010 Sep;7 (9):1224–30. doi: 10.1016/j.hrthm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki Hiro, Tada Hiroshi, Sekiguchi Yukio, Igarashi Miyako, Arimoto Takanori, Machino Takeshi, Ozawa Mahito, Naruse Yoshihisa, Kuroki Kenji, Tsuneoka Hidekazu, Ito Yoko, Murakoshi Nobuyuki, Kuga Keisuke, Hyodo Ichinosuke, Aonuma Kazutaka. Prevalence and characteristics of asymptomatic excessive transmural injury after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2011 Jun;8 (6):826–32. doi: 10.1016/j.hrthm.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Martinek M, Bencsik G, Aichinger J, Hassanein S, Schoefl R, Kuchinka P, Nesser H J, Purerfellner H. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J. Cardiovasc. Electrophysiol. 2009 Jul;20 (7):726–33. doi: 10.1111/j.1540-8167.2008.01426.x. [DOI] [PubMed] [Google Scholar]
- 16.Vijayaraman Pugazhendhi, Netrebko Pavlo, Geyfman Vitaly, Dandamudi Gopi, Casey Kevin, Ellenbogen Kenneth A. Esophageal fistula formation despite esophageal monitoring and low-power radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2009 Oct;2 (5):e31–3. doi: 10.1161/CIRCEP.109.883694. [DOI] [PubMed] [Google Scholar]
- 17.Di Biase Luigi, Saenz Luis Carlos, Burkhardt David J, Vacca Miguel, Elayi Claude S, Barrett Conor D, Horton Rodney, Bai Rong, Siu Alan, Fahmy Tamer S, Patel Dimpi, Armaganijan Luciana, Wu Chia Tung, Kai Sonne, Ching Ching Keong, Phillips Karen, Schweikert Robert A, Cummings Jennifer E, Arruda Mauricio, Saliba Walid I, Dodig Milan, Natale Andrea. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol. 2009 Apr;2 (2):108–12. doi: 10.1161/CIRCEP.108.815266. [DOI] [PubMed] [Google Scholar]
- 18.Singh Sheldon M, d'Avila Andre, Doshi Shephal K, Brugge William R, Bedford Rudolph A, Mela Theofanie, Ruskin Jeremy N, Reddy Vivek Y. Esophageal injury and temperature monitoring during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2008 Aug;1 (3):162–8. doi: 10.1161/CIRCEP.107.789552. [DOI] [PubMed] [Google Scholar]
- 19.Sause Armin, Tutdibi Osman, Pomsel Karsten, Dinh Wilfried, Füth Reiner, Lankisch Mark, Glosemeyer-Allhoff Thomas, Janssen Jan, Müller Micheal. Limiting esophageal temperature in radiofrequency ablation of left atrial tachyarrhythmias results in low incidence of thermal esophageal lesions. BMC Cardiovasc Disord. 2010;10 () doi: 10.1186/1471-2261-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leite Luiz R, Santos Simone N, Maia Henrique, Henz Benhur D, Giuseppin Fábio, Oliverira Anderson, Zanatta André R, Peres Ayrton K, Novakoski Clarissa, Barreto Jose R, Vassalo Fabrício, d'Avila Andre, Singh Sheldon M. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol. 2011 Apr;4 (2):149–56. doi: 10.1161/CIRCEP.110.960328. [DOI] [PubMed] [Google Scholar]
- 21.Perzanowski Christian, Teplitsky Liane, Hranitzky Patrick M, Bahnson Tristram D. Real-time monitoring of luminal esophageal temperature during left atrial radiofrequency catheter ablation for atrial fibrillation: observations about esophageal heating during ablation at the pulmonary vein ostia and posterior left atrium. J. Cardiovasc. Electrophysiol. 2006 Feb;17 (2):166–70. doi: 10.1111/j.1540-8167.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 22.Cummings Jennifer E, Barrett Conor D, Litwak Kenneth N, DI Biase Luigi, Chowdhury Punam, Oh Seil, Ching Chi Keong, Saliba Walid I, Schweikert Robert A, Burkhardt J David, DE Marco Shari, Armaganijan Luciana, Natale Andrea. Esophageal luminal temperature measurement underestimates esophageal tissue temperature during radiofrequency ablation within the canine left atrium: comparison between 8 mm tip and open irrigation catheters. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):641–4. doi: 10.1111/j.1540-8167.2008.01130.x. [DOI] [PubMed] [Google Scholar]
- 23.Katsiyiannis WT, Gornick CP, Alwine ZR, Gornick CC. P5-71: Esophageal-atrial fistula formation despite continuous anatomic and temperature probe monitoring during ablation for atrial fibrillation. Heart Rhythm. 2006;3:S283–S284. [Google Scholar]
- 24.Nguyen Duy T, Barham Waseem, Zheng Lijun, Dinegar Sarah, Tzou Wendy S, Sauer William H. Effect of radiofrequency energy delivery in proximity to metallic medical device components. Heart Rhythm. 2015 Oct;12 (10):2162–9. doi: 10.1016/j.hrthm.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Chugh Aman, Rubenstein Joel, Good Eric, Ebinger Matthew, Jongnarangsin Krit, Fortino Jackie, Bogun Frank, Pelosi Frank, Oral Hakan, Nostrant Timothy, Morady Fred. Mechanical displacement of the esophagus in patients undergoing left atrial ablation of atrial fibrillation. Heart Rhythm. 2009 Mar;6 (3):319–22. doi: 10.1016/j.hrthm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Koruth Jacob S, Reddy Vivek Y, Miller Marc A, Patel Kalpesh K, Coffey James O, Fischer Avi, Gomes J Anthony, Dukkipati Srinivas, D'Avila Andre, Mittnacht Alexander. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012 Feb;23 (2):147–54. doi: 10.1111/j.1540-8167.2011.02162.x. [DOI] [PubMed] [Google Scholar]
- 27.Buch Eric, Nakahara Shiro, Shivkumar Kalyanam. Intra-pericardial balloon retraction of the left atrium: a novel method to prevent esophageal injury during catheter ablation. Heart Rhythm. 2008 Oct;5 (10):1473–5. doi: 10.1016/j.hrthm.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahara Shiro, Ramirez Rafael J, Buch Eric, Michowitz Yoav, Vaseghi Marmar, de Diego Carlos, Boyle Noel G, Mahajan Aman, Shivkumar Kalyanam. Intrapericardial balloon placement for prevention of collateral injury during catheter ablation of the left atrium in a porcine model. Heart Rhythm. 2010 Jan;7 (1):81–7. doi: 10.1016/j.hrthm.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scanavacca Mauricio, Hachul Denise, Sosa Eduardo. Atrioesophageal fistula--a dangerous complication of catheter ablation for atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007 Nov;4 (11):578–9. doi: 10.1038/ncpcardio1010. [DOI] [PubMed] [Google Scholar]
- 30.Dagres Nikolaos, Kottkamp Hans, Piorkowski Christopher, Doll Nicolas, Mohr Friedrich, Horlitz Marc, Kremastinos Dimitrios Th, Hindricks Gerhard. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J. Cardiovasc. Electrophysiol. 2006 Nov;17 (11):1213–5. doi: 10.1111/j.1540-8167.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 31.Sonmez Bingur, Demirsoy Ergun, Yagan Naci, Unal Mehmet, Arbatli Harun, Sener Deniz, Baran Turker, Ilkova Feryal. A fatal complication due to radiofrequency ablation for atrial fibrillation: atrio-esophageal fistula. Ann. Thorac. Surg. 2003 Jul;76 (1):281–3. doi: 10.1016/s0003-4975(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 32.Cummings Jennifer E, Schweikert Robert A, Saliba Walid I, Burkhardt J David, Kilikaslan Fethi, Saad Eduardo, Natale Andrea. Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann. Intern. Med. 2006 Apr 18;144 (8):572–4. doi: 10.7326/0003-4819-144-8-200604180-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bunch T Jared, Nelson Jennifer, Foley Tom, Allison Scott, Crandall Brian G, Osborn Jeffrey S, Weiss J Peter, Anderson Jeffrey L, Nielsen Peter, Anderson Lars, Lappe Donald L, Day John D. Temporary esophageal stenting allows healing of esophageal perforations following atrial fibrillation ablation procedures. J. Cardiovasc. Electrophysiol. 2006 Apr;17 (4):435–9. doi: 10.1111/j.1540-8167.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- 34.Singh Sheldon M, d'Avila Andre, Singh Steve K, Stelzer Paul, Saad Eduardo B, Skanes Allan, Aryana Arash, Chinitz Jason S, Kulina Robert, Miller Marc A, Reddy Vivek Y. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm. 2013 Nov;10 (11):1591–7. doi: 10.1016/j.hrthm.2013.08.012. [DOI] [PubMed] [Google Scholar]




